Abstract
Purpose
Neoadjuvant chemotherapy has been shown to improve survival in locally advanced gastric cancer, but it is associated with significant toxicity. Sarcopenia and sarcopenic obesity have been studied in several types of cancers and have been reported to be associated with higher chemotherapy toxicity and morbi-mortality. The aim of this study was to assess the prevalence of sarcopenia/sarcopenic obesity in patients with gastric cancer, as well as its association with chemotherapy toxicity and long-term outcomes.
Materials and Methods
A retrospective analysis was performed using an academic cancer center patient cohort diagnosed with locally advanced gastric cancer between January 2012 and December 2014 and treated with neoadjuvant chemotherapy. We analyzed body composition (skeletal muscle and visceral fat index) in axial computed tomography images.
Results
A total of 48 patients met the inclusion criteria. The mean age was 68±10 years, and 33 patients (69%) were men. Dose-limiting toxicity was observed in 22 patients (46%), and treatment was terminated early owing to toxicity in 17 patients (35%). Median follow-up was 17 months. Sarcopenia and sarcopenic obesity were found at diagnosis in 23% and 10% of patients, respectively. We observed an association between termination of chemotherapy and both sarcopenia (P=0.069) and sarcopenic obesity (P=0.004). On multivariate analysis, the odds of treatment termination were higher in patients with sarcopenia (odds ratio=4.23; P=0.050). Patients with sarcopenic obesity showed lower overall survival (median survival of 6 months [95% confidence interval {CI}=3.9–8.5] vs. 25 months [95% CI=20.2–38.2]; log-rank test P=0.000).
Conclusions
Sarcopenia and sarcopenic obesity were associated with early termination of neoadjuvant chemotherapy in patients with gastric cancer; additionally, sarcopenic obesity was associated with poor survival.
Keywords: Stomach neoplasms, Body composition, Sarcopenia, Neoadjuvant therapy, Prognosis
INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer worldwide and the third leading cause of cancer-related death [1]. It is often diagnosed at an advanced stage and has a low 5-year survival rate [2]. Neoadjuvant chemotherapy (ChT) improves survival in locally advanced GC [3]. In 2006, the MRC Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial showed that in patients with operable esophagogastric adenocarcinomas, a perioperative regimen of epirubicin, cisplatin, and infused 5-fluorouracil (ECF) resulted in downstaging of the disease and significantly improved both disease-free and overall survival when compared with surgery alone [3]. However, in the MAGIC trial, only 41.6% of the patients assigned to perioperative ChT completed all 6 cycles of ChT, with some discontinuation owing to toxic effects [4]. Therefore, there is a great need to identify host or tumor factors that might explain individual variation in therapeutic efficacy and toxicity.
Body composition (i.e., the proportions of skeletal muscle and fat) has been studied in several types of tumors in the context of various anti-cancer treatments. The evaluation of skeletal muscle and fat using cross-sectional computed tomography (CT) imaging is gaining popularity due to its wide availability, high precision, and low incremental costs [5]. Sarcopenia, which is the depletion of skeletal muscle, is associated with higher ChT toxicity and higher morbi-mortality in cancer patients, with an overall worse prognosis [6,7,8]. Recent reports in patients with GC demonstrate that sarcopenia is a significant predictor of ChT toxicity [4], worse postoperative outcomes [9,10,11,12,13], and reduced overall survival [11,14,15]. One of the reasons for the variable ChT toxicity among individuals may be different body composition, which is not currently taken into account when prescribing ChT.
Not only skeletal muscle mass depletion but also the distribution of adipose tissue might influence survival [16]. The presence of both sarcopenia and obesity has been associated with worse prognosis in a series of reports [5,16,17]. In the specific setting of GC, sarcopenic obesity was shown to be an independent predictive factor of postoperative complications in patients undergoing radical gastrectomy [18,19].
The aim of this study was to assess the prevalence of sarcopenia and sarcopenic obesity in a population of patients with GC, as well as its association with ChT toxicity, response, and long-term outcomes.
MATERIALS AND METHODS
We conducted a single-center retrospective study in a secondary care hospital — Hospital Beatriz Ângelo (HBA). The study protocol was approved by the Scientific and Ethics Committee of HBA. The requirement for informed consent from patients was waived because of the retrospective design of the study.
Patients
We selected all patients diagnosed between January 2012 and December 2014 with locally advanced adenocarcinoma from the stomach or gastroesophageal junction (GEJ, Siewert type III only) who received neoadjuvant ChT in our institution. Locally advanced gastric/GEJ cancer was defined as tumor stage greater than cT2 or positive locoregional lymph nodes (cN+), according to the tumor, node, and metastasis (TNM) staging classification (American Joint Committee on Cancer). Included patients had histologically confirmed adenocarcinoma with no evidence of distant metastasis on preoperative staging. CT was performed at diagnosis in all cases. Endoscopic ultrasound was used to confirm T stage in the absence of suspicious lymph nodes. Staging laparoscopy was not uniformly used in this cohort of patients because it was not yet systematically included in the staging protocol of our unit at that time.
Patient data were obtained from the electronic records at HBA. Demographic and clinical data, such as age, sex, tumor site and histological type, ChT regimens used, and ChT response and toxicity, were retrieved. ChT toxicity was graded according to National Cancer Institute Common Toxicity Criteria. Dose-limiting toxicity (DLT) was defined as any grade 3/4 toxicity associated with physician-ordered dose reduction or termination of therapy. Response to ChT was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria [20] and confirmed on surgical specimens (when available). Date of the last follow-up and date and cause of death were collected. Overall survival was measured from the date of histologic diagnosis until the date of death from any cause.
Body composition evaluation
Weight and height at diagnosis were recorded by hospital staff. Body mass index (BMI) was calculated as weight (kg)/height (m2).
CT scans were obtained and evaluated for body composition data by one investigator (SV) who was blinded to clinical and endoscopic data to ensure objective interpretation of image findings. Skeletal muscle and fat tissue cross-sectional areas were measured on axial CT images, at the level of the third lumbar vertebra (L3) with the patient lying supine (Fig. 1). Skeletal muscle area (SMA), visceral fat area (VFA), and subcutaneous fat area (SFA) were measured in square centimeters on the basis of the pixel count using appropriate software [21]. Mean muscle radiation attenuation (MA) was calculated for muscle area and was also recorded. Skeletal muscle and visceral fat were normalized for height to obtain the skeletal muscle index (SMI) and visceral fat mass index (VFI) — cm2/m2. Sarcopenia was defined as SMI lower than 41 cm2/m2 in women or lower than 43 cm2/m2 in men with BMI <25 kg/m2 and <53 cm2/m2 in men with BMI ≥25 kg/m2, as described by Martin et al. [21]. Sarcopenic obesity was defined as sarcopenia in patients with BMI ≥25 kg/m2.
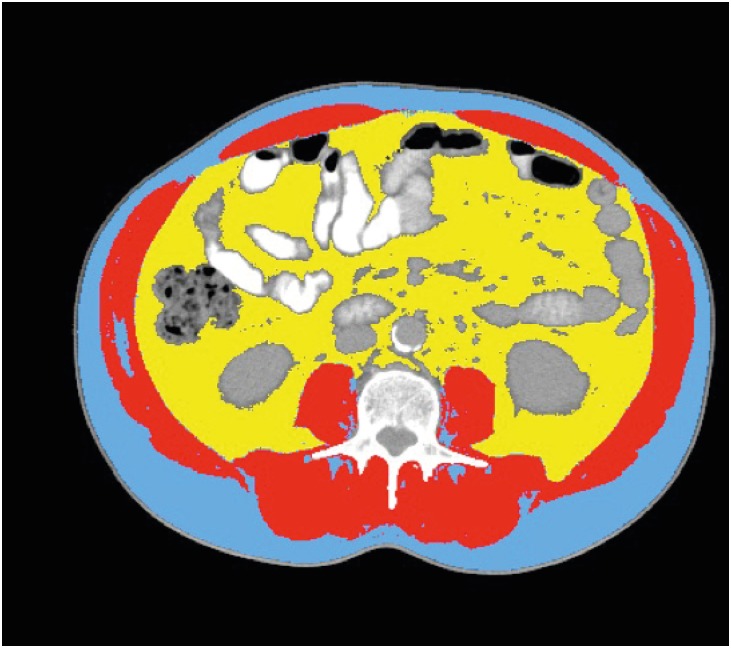
Fig. 1.
Axial CT images of the third lumbar vertebra region. CT was analyzed for muscle and fat tissue cross sectional areas and analyzed using appropriate software. SMA (shown in red) was quantified within a HU range of −29 to 150, VFA (shown in yellow) ranged from −150 to −50 HU, and SFA (shown in blue) ranged from −190 to −30 HU.
CT = computed tomography; SMA = skeletal muscle area; HU = Hounsfield unit; VFA = visceral fat area; SFA = subcutaneous fat area.
Body composition data were evaluated at the time of cancer diagnosis using the CT scan performed for staging. We were also able to access a follow-up CT scan after completion of neoadjuvant ChT in a subset of 43 patients. These scans were used to perform a longitudinal analysis of body composition over time. The mean interval (± standard deviation [SD]) between CT scans was 86.4±29.0 days.
Statistical analysis
All continuous variables were described as median and range, while categorical variables were expressed as frequency and percentage. Differences in mean continuous variables with a normal distribution were analyzed using an independent Student's t-test. The other continuous variables were compared using the Mann-Whitney U test. To explore univariate associations in the distribution of categorical data, the χ2 test or Fisher's exact test was used as appropriate. On multivariate analysis, treatment termination was used as the dependent variable, since we considered this variable the most clinically relevant. Any variable with a P-value <0.250 on univariate analysis or that was considered clinically relevant was included, and variable selection was performed with a stepwise analysis. Logistic regression was used for dichotomous outcomes, in order to determine the effect estimates, which are presented as odds ratio (OR) and 95% confidence intervals (CI). It is noteworthy that all patients with sarcopenic obesity experienced early treatment termination, so a high OR was obtained, and it was not possible to calculate the upper 95% CI limit. For this reason, this variable was not included in the final model to avoid error in parameter estimation. For continuous variables in the model, cubic spline graphs and the Wald test of linearity were used to test linearity in the logit model. For the Wald test of linearity, age and MA did not demonstrate statistically significant P-values (P=0.430 and P=0.270, respectively). However, linearity was not clear for both MA and age on cubic spline graphs, so these variables were categorized. Age was categorized using 65 years as a cut-off. MA was categorized with a cut-off (35 Hounsfield units [HUs]) provided by cubic spline graph analysis. Survival curves were estimated using the Kaplan-Meier method and compared using a log-rank test. A P-value <0.05 was considered statistically significant. Statistical analysis was performed using Statistical Package for Social Sciences version 22 (SPSS Inc., Chicago, IL, USA) and R software (R Foundation, Vienna, Austria).
RESULTS
A total of 160 cases of gastric/GEJ (Siewert type III) cancers were diagnosed in our institution during the study period, of which 48 were locally advanced cancers treated with neoadjuvant ChT. Survival was 93.8% at 3 months, 62.5% at 1 year, and 41.7% at 2 years. Response to neoadjuvant ChT was observed in 30 patients (63%), with 3 cases of complete pathological response (6%). DLT was observed in 22 patients (46%), among whom 17 patients (35%) terminated ChT early (i.e., before completion of 3 cycles of neoadjuvant ChT).
Mean BMI at diagnosis was 23.8±3.5 kg/m2, with 42% of patients categorized as overweight (BMI 25–29 kg/m2) or obese (BMI ≥30 kg/m2). Body composition data at diagnosis are shown in Table 1. Sarcopenia was present in 23% and sarcopenic obesity in 10% of patients at diagnosis. We did not find a significantly higher proportion of sarcopenia in older patients, but patients older than 65 years had a lower value of MA (30.0±6.0 vs. 39.8±8.1 HU; P=0.001). We also assessed patient demographics and body composition characteristics according to the presence/absence of sarcopenia (Table 2). Sarcopenic patients were more frequently female (64% vs. 22%; P=0.023), but were otherwise similar with respect to age, tumor site, histology and stage of disease, BMI, VFI, and MA. We found no significant association between ChT response and the presence of sarcopenia or sarcopenic obesity.
Table 1. Body composition data at diagnosis.
| Variables | Values (n=48) | |
|---|---|---|
| BMI (kg/m2) | 23.8±3.5 | |
| Underweight (BMI <20) | 5 (10) | |
| Normal (BMI 20–24) | 23 (48) | |
| Overweight (BMI 25–29) | 18 (38) | |
| Obese (BMI ≥30) | 2 (4) | |
| SMI (cm2/m2) | 48.7±9.7 | |
| Men | 52±9 | |
| Women | 41±7 | |
| FMI (cm2/m2) | 50.4±36.3 | |
| Men | 59±39 | |
| Women | 33±23 | |
| MA (HU) | 34.2±7.9 | |
| Sarcopenia | 11/47 (23) | |
| Sarcopenic obesity | 5/47 (10) | |
Values are presented as number of patients (%) or mean±SD.
BMI = body mass index; SMI = skeletal muscle index; FMI = fat mass index; MA = muscle radiation attenuation; HU = Hounsfield unit; SD = standard deviation.
Table 2. Patient demographics according to the presence/absence of sarcopenia.
| Variables | Sarcopenia (n=11) | Non-sarcopenia (n=36) | P-value | |
|---|---|---|---|---|
| Age (yr)* | 69.3±9.1 | 67.1±10.4 | 0.534 | |
| Sex | 0.023 | |||
| Male | 4 | 28 | ||
| Female | 7 | 8 | ||
| Tumor site | 0.924 | |||
| Body | 5 | 18 | ||
| Antrum | 5 | 14 | ||
| Esophagogastric junction (Siewert III) | 1 | 4 | ||
| Histology | 0.111 | |||
| Intestinal | 6 | 27 | ||
| Diffuse | 5 | 6 | ||
| Mixed | 0 | 3 | ||
| Clinical TNM stage | 0.322 | |||
| II | 0 | 5 | ||
| III | 11 | 31 | ||
| Type of ChT used | 0.388 | |||
| ECF/EOF/EOX/ECX | 2/0/6/0 | 9/2/17/1 | ||
| XELOX/FOLFOX/Xeloda/DCF | 1/0/1/1 | 6/1/0/0 | ||
| ChT response | 1.000 | |||
| Yes | 7 | 22 | ||
| No | 4 | 14 | ||
| ChT toxicity | ||||
| Grade 2/3/4 | 3/6/0 | 11/14/0 | 0.455 | |
| Type: GI/hematological/other | 5/2/3 | 12/12/4 | 0.135 | |
| DLT | 7 | 14 | 0.181 | |
| Early termination of ChT due to toxicity | 7 | 10 | 0.069 | |
| BMI(kg/m2) | 22.7±3.6 | 24.0±3.3 | 0.278 | |
| FMI(cm2/m2) | 39.1±34.0 | 53.9±36.7 | 0.241 | |
| MA (HU) | 31.2±7.4 | 35.1±8.0 | 0.151 | |
| Follow-up (mo)† | 10 [6–36] | 20 [9.25–33.75] | 0.551 | |
TNM = tumor, node, and metastasis; ChT = chemotherapy; ECF = epirubicin, cisplatin, and 5-fluorouracil; EOF = epirubicin, oxaliplatin, and 5-fluorouracil; EOX = epirubicin, oxaliplatin, and capecitabine; ECX = epirubicin, cisplatin, and capecitabine; XELOX = capecitabine plus oxaliplatin; FOLFOX = folinic acid plus 5-fluorouracil plus oxaliplatin; Xeloda = capecitabine; DCF = docetaxel, cisplatin, and 5-fluorouracil; GI = gastrointestinal; DLT = dose-limiting toxicity; BMI = body mass index; FMI = fat mass index; MA = muscle radiation attenuation; HU = Hounsfield unit; SD = standard deviation; IQR = interquartile range.
Values are presented differently follow as *mean±SD; †median [IQR].
Although not statistically significant, we found a trend toward a higher percentage of DLT in patients with sarcopenia (64% vs. 39%; P=0.181) and sarcopenic obesity (80% vs. 42%; P=0.165). We found an association between early termination of ChT and the presence of sarcopenia (64% vs. 28%; P=0.069) and sarcopenic obesity (100% vs. 28%; P=0.004). Seven of 11 patients with sarcopenia (64%) and all patients with sarcopenic obesity (n=5) required early ChT termination.
Univariate and multivariate analysis were performed to assess factors that could contribute to termination of treatment (Table 3). On univariate analysis, both sarcopenia and MA were associated with treatment termination. Additionally, a strong effect was found for sarcopenic obesity, since treatment termination was observed in all patients with sarcopenic obesity. There was no significant association between disease stage and termination of treatment.
Table 3. Univariate and multivariate analysis assessing the OR of treatment termination associated with clinical variables and body composition markers in patients with GC.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||
| Age (yr) | |||||||
| <65 | 1.00 | 0.920 | 1.00 | 0.308 | |||
| ≥65 | 1.06 | 0.30–3.81 | 0.44 | 0.08–2.08 | |||
| Sex | Excluded | ||||||
| Male | 1.00 | 0.700 | |||||
| Female | 1.27 | 0.34–4.50 | |||||
| Tumor site | |||||||
| Body | 1.00 | 0.420 | |||||
| Antrum/GEJ | 1.63 | 0.49–5.61 | |||||
| Stage | Excluded | ||||||
| II | 1.00 | 0.260 | |||||
| III | 0.33 | 0.04–2.23 | |||||
| Histology type | Excluded | ||||||
| Intestinal | 1.00 | 0.640 | |||||
| Diffuse/mixed | 1.36 | 0.34–5.24 | |||||
| SMA | 0.98 | 0.96–1.00 | 0.250 | Excluded | |||
| VFA | 1.00 | 0.99–1.01 | 0.120 | Excluded | |||
| SFA | 1.00 | 0.99–1.01 | 0.280 | Excluded | |||
| MA (HU) | |||||||
| <35 | 1.00 | 0.040 | 1.00 | 0.040 | |||
| ≥35 | 0.27 | 0.06–0.95 | 0.20 | 0.33–0.95 | |||
| Sarcopenia | |||||||
| No | 1.00 | 0.030 | 1.00 | 0.050 | |||
| Yes | 4.55 | 1.13–20.70 | 4.23 | 0.98–20.80 | |||
| Sarcopenic obesity | Not included* | ||||||
| No | 1.00 | ||||||
| Yes | 106,362,030.9 | 2.81×10−72-NA | <0.001 | ||||
OR = odds ratio; GC = gastric cancer; CI = confidence interval; GEJ = gastroesophageal junction; SMA = skeletal muscle area; VFA = visceral fat area; SFA = subcutaneous fat area; MA = muscle radiation attenuation; HU = Hounsfield unit.
*All patients with sarcopenic obesity experienced treatment termination, and for that reason a high OR was obtained, and the upper 95% CI limit was not possible to calculate. This variable was not included in the final model to avoid error in parameter estimation.
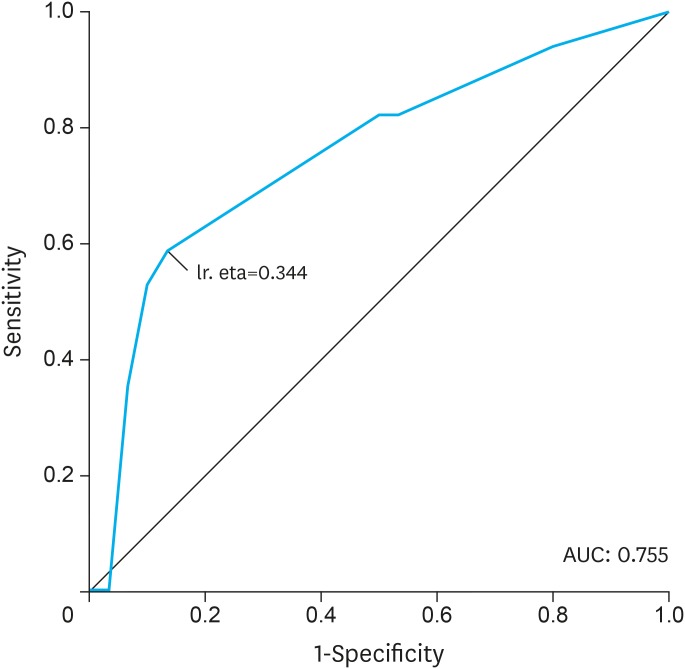
On multivariate analysis, the odds of treatment termination were reduced in patients with higher MA, as compared to patients with lower MA (OR=0.20; P=0.040). In addition, the odds of treatment termination were higher in patients with sarcopenia as compared to patients without sarcopenia (OR=4.23; P=0.050). The receiver operating characteristic (ROC) curve showed an acceptable power of discrimination of treatment termination using a model with age, sarcopenia, and MA as independent variables (area under the curve [AUC] of 0.755) (Fig. 2).
Fig. 2.
ROC curve using treatment termination as dependent variable and age, sarcopenia, and MA as independent variables. Sensitivity: 58.8%; Specificity: 86.7%; Positive predictive value: 21.2%; Negative predictive value: 28.6%.
ROC = receiver operating characteristic; MA = muscle radiation attenuation; AUC = area under the curve.
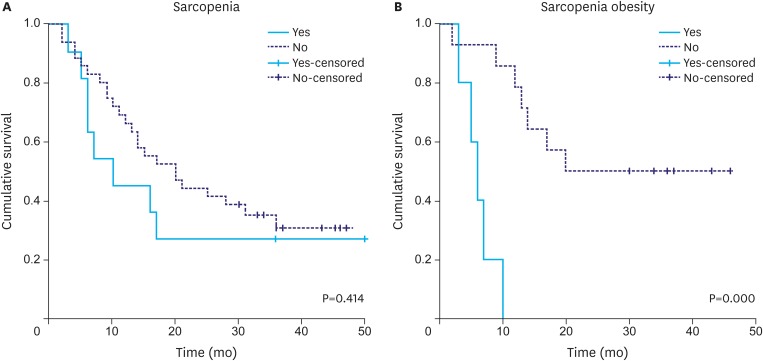
There was no significant difference in overall survival between patients with and without sarcopenia (Fig. 3A). However, patients with sarcopenic obesity showed reduced survival (median survival 6 months [95% CI=3.9–8.5] vs. 25 months for patients who were obese and did not have sarcopenia [95% CI=20.2–38.2]; log-rank test P=0.000) (Fig. 3B).
Fig. 3.
Kaplan-Meier survival curves of patients with and without sarcopenia (A) and of obese patients with and without sarcopenia (B) (log-rank P-value).
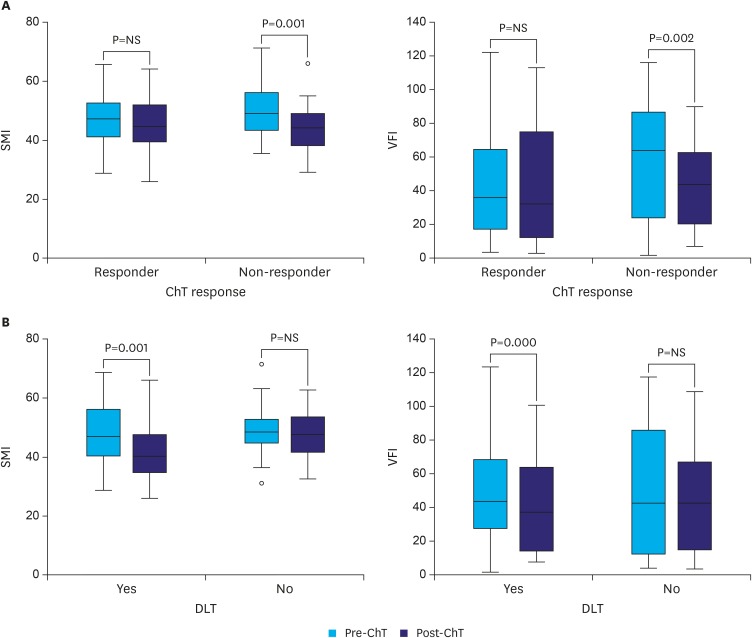
A second CT scan after completion of neoadjuvant ChT was available in a subset of 43 patients. No second CT scan was available for 5 patients, either owing to obvious clinical disease progression or because either magnetic resonance imaging or ultrasonography was used. The mean interval (± SD) between CT scans was 86.4±29.0 days. The mean loss of SMA during follow-up (mean 86 days) was 15.4±2.8 cm2. In the second CT scan, sarcopenia and sarcopenic obesity were found in 38% and 17% of patients, respectively, after neoadjuvant ChT. Over time, there was a significant loss of skeletal muscle and adipose tissue (Table 4). Stratification of patients according to ChT response demonstrated that only those patients who did not respond to ChT experienced a significant reduction in SMI (49.9±10.1 to 44.6±9.5 cm2/m2; P=0.001) and VFI (57.5±33.6 to 42.1±26.3 cm2/m2; P=0.002) (Fig. 4A). Similarly, only those patients who experienced DLT had a significant reduction in SMI (47.0±10.2 to 43.0±10.8 cm2/m2; P=0.001) and VFI (52.7±31.2 to 39.2±28.5 cm2/m2; P=0.000) (Fig. 4B).
Table 4. Change in body composition over time in patients with GC (n=43).
| Variables | First CT scan | Second CT scan | P-value |
|---|---|---|---|
| SMA (cm2) | 132.1±30.9 | 123.9±29.6 | 0.001 |
| MA (HU) | 34.0±7.2 | 31.7±6.7 | 0.006 |
| VFA (cm2) | 137.8±96.2 | 113.5±86.4 | 0.001 |
| SFA (cm2) | 136.2±76.3 | 119.6±78.7 | 0.002 |
| SMI (cm2/m2) | 48.2±9.6 | 45.3±9.5 | 0.001 |
| VFI (cm2/m2) | 49.8±34.0 | 41.0±30.1 | 0.001 |
Mean interval (± SD) between CT scans: 86.4±29.0 days.
GC = gastric cancer; CT = computed tomography; SMA = skeletal muscle area; MA = muscle radiation attenuation; HU = Hounsfield unit; VFA = visceral fat area; SFA = subcutaneous fat area; SMI = skeletal muscle index; VFI = visceral fat mass index; SD = standard deviation.
Fig. 4.
Body composition changes (namely SMI and VFI in cm2/m2) before and after neoadjuvant ChT, according to ChT response (A) and DLT (B).
SMI = skeletal muscle index; VFI = visceral fat mass index; ChT = chemotherapy; DLT = dose-limiting toxicity; NS = non-significant.
DISCUSSION
In this observational study in patients with locally advanced GC who underwent neoadjuvant ChT, we observed that sarcopenic obesity was associated with poorer outcome, with a high likelihood of early termination of treatment and lower overall survival. Additionally, we confirmed that neoadjuvant ChT was associated with important catabolic losses of muscle and fat over time, although these occurred only in patients who did not respond to ChT or in those who developed toxicity.
Several recent studies have reported a high prevalence of sarcopenia at diagnosis in patients with gastrointestinal cancers (Table 5) [22,23,24,25,26,27,28]. Comparison of sarcopenia prevalence among studies is rather difficult because of the use of different methodologies: axial CT cross-sectional imaging of SMAs, muscle mass using dual-energy X-ray absorptiometry, or a combination of anthropometric and physical performance measurements [29,30,31,32]. Furthermore, even when the same methodology is employed, different cut-offs for sarcopenia are often used (Table 5).
Table 5. Sarcopenia prevalence at diagnosis of gastrointestinal tumors.
| Study reference | Country | Type of tumor | No. of patients | Sarcopenia (%) | Sarcopenia definition |
|---|---|---|---|---|---|
| Yip et al. [28] | United Kingdom | Esophageal cancer | 35 | 26.0 | 1 |
| Reisinger et al. [27] | The Netherlands | Esophageal cancer | 108 | 56.0 | 1 |
| Awad et al. [6] | United Kingdom | Esophagogastric cancer | 47 | 57.0 | 1 |
| Tan et al. [4] | United Kingdom | Esophagogastric cancer | 89 | 49.4 | 1 |
| Tegels et al. [36] | The Netherlands | GC | 152 | 57.7 | 2 |
| Huang et al. [9] | China | GC | 470 | 16.8 | 3 |
| Chen et al. [10] | China | GC | 158 | 24.7 | 3 |
| Zhuang et al. [11] | China | GC | 937 | 41.5 | 4 |
| Wang et al. [12] | China | GC | 255 | 12.5 | 5 |
| Hayashi et al. [14] | Japan | GC | 53 | 69.8 | 2 |
| Huang et al. [15] | China | GC | 173 | 30.1 | 3 |
| Fukuda et al. [13] | Japan | GC | 99 | 21.2 | 6 |
| Nishigori et al. [19] | Japan | GC | 157 | 57.0 | 1 |
| Lieffers et al. [22] | Canada | Colorectal cancer | 234 | 39.0 | 1 |
| Reisinger et al. [24] | The Netherlands | Colorectal cancer | 310 | 47.7 | 1 |
| Huang et al. [30] | China | Colorectal cancer | 142 | 12.0 | 5 |
| Tan et al. [23] | Canada | Pancreatic cancer | 111 | 56.0 | 1 |
| Peng et al. [8] | USA | Pancreatic cancer | 557 | 25.0 | 7 |
| Joglekar et al. [25] | USA | Pancreatic cancer | 118 | 26.3 | 8 |
| Harimoto et al. [26] | Japan | Hepatocellular carcinoma | 186 | 40.3 | 9 |
Sarcopenia definition:
SMI ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men
SMI <41 cm2/m2 for women and <43 cm2/m2 in men with BMI <25 kg/m2 and <53 cm2/m2 in men with BMI ≥25 kg/m2
SMI <34.9 cm2/m2 for women and ≤40.8 cm2/m2 for men plus handgrip strength <18 kg for women and <26 kg for men and/or 6-m usual gait speed <0.8 m/s
SMI <34.9 cm2/m2 for women and ≤40.8 cm2/m2 for men
SMI <29 cm2/m2 for women and ≤36 cm2/m2 for men plus handgrip strength <18 kg for women and <26 kg for men and/or 6-m usual gait speed <0.8 m/s
SMI <6.42 kg/m2 for women and ≤8.87 kg/m2 for men plus handgrip strength <20 kg for women and <30 kg for men and/or 6-m usual gait speed <0.8 m/s
Total psoas muscle index <362 mm2/m2 for women and <492 mm2/m2 for men
Total psoas muscle index <4.0 cm2/m2 for women and <5.2 mm2/m2 for men
SMI ≤41.1 cm2/m2 for women and ≤43.75 cm2/m2 for men
GC = gastric cancer; SMI = skeletal muscle index; BMI = body mass index.
In the specific setting of patients with GC, the reported prevalence in the literature of sarcopenia ranges from 12.5% to 69.8% at diagnosis (Table 5). In our population, a quarter of the patients had sarcopenia at diagnosis. Additionally, the prevalence of sarcopenia increased following neoadjuvant ChT. Similarly, Awad et al. [6] reported an increase in sarcopenia prevalence from 57% pre-ChT to 79% in patients with esophagogastric cancer.
Several recent studies show a relationship between skeletal muscle mass depletion and treatment toxicity. In a recent systematic review by Kazemi-Bajestani et al. [33], there were 14 published articles relating CT-based body composition to the prevalence of ChT-induced toxicity. These were mainly single-center investigations with small samples, [33] and only one addressed patients with GC [4]. In a prospective study in patients with colon cancer by Prado et al. [7], the authors found an increase in DLT in patients with lower muscle mass treated with 5-fluorouracil (5-FU). Likewise, in a prospective randomized trial in patients with colon cancer by Ali et al. [34], low lean body mass was an independent predictor of DLT and neuropathy in patients administered folinic acid plus 5-fluorouracil plus oxaliplatin (FOLFOX)-based regimens. More recently, data analysis from a randomized controlled trial in advanced non-small cell lung cancer also showed an association between low muscle mass and ChT-induced hematological toxicity [35].
However, studies addressing the impact of sarcopenia in ChT toxicity in patients with GC are scarce. Recently, Tan et al. [4] demonstrated that sarcopenia at the time of diagnosis in patients with esophagogastric cancer was a significant predictor of DLT. In the present study, we found that the odds of treatment termination were higher in patients with sarcopenia. The mechanism that links sarcopenia with increased ChT toxicity is currently unknown. Some authors speculate that different proportions of lean and adipose tissue compartments may be associated with alterations in the distribution, metabolism, and clearance of ChT agents [7].
Although the impact of sarcopenia in DLT in patients with GC needs further prospective characterization, there are several recent reports demonstrating the important predictive role of low muscle mass in short- and long-term outcomes in patients with GC. In patients who undergo radical gastrectomy for GC, there is a significant relationship between sarcopenia and postoperative complications [10,11,12,13,30]. In a recent prospective study by Huang et al. [15] including 173 elderly patients undergoing curative gastrectomy for GC, sarcopenia was predictive of higher 1-year mortality (hazard ratio [HR]=3.615; 95% CI=1.459–8.957). Similarly, a large retrospective study of 937 patients with GC showed that sarcopenia was an independent predictor of low overall survival after gastrectomy [11]. Nonetheless, it is important to highlight that the impact of sarcopenia in postoperative and long-term outcomes has not been universally reported [36]. Further prospective studies using a consensual definition for sarcopenia are needed.
Although cachexia is a frequent feature of patients with advanced cancer, a substantial increase has been observed in the past decades in the proportion of cancer patients with a BMI in the overweight range [37]. In the present study, 42% of patients were overweight or obese at the time of diagnosis of GC. Abdominal adipose fat distribution might have an influence on tumor growth and therefore on cancer outcome [16]. The negative impact of visceral obesity has been previously reported in patients with colon, pancreatic, and renal cancer [38,39,40]; however, few studies in GC have addressed this issue.
The simultaneous presence of sarcopenia and obesity (especially visceral obesity) is a worst-case scenario associated with poorer prognosis [5]. The inflammatory cytokines produced by adipose cells are thought to play an important role in insulin resistance, resulting in an increase in muscle protein loss [41]. In addition, in patients with sarcopenic obesity, the increased body mass inflates the overall administered ChT dose, which is then distributed within a reduced lean tissue compartment, thus resulting in a disproportionately small volume of drug distribution and hence higher toxicity [5]. A population-based study by Prado et al. [5] that included 250 obese patients with solid tumors of the respiratory and gastrointestinal tracts showed that patients with sarcopenic obesity had poorer functional status and lower survival. In a recent prospective study of 206 overweight or obese patients with GC after radical gastrectomy, sarcopenic obesity was an independent predictor of postoperative complications [18]. Likewise, Nishigori et al. [19] retrospectively reported a prevalence of 24% of sarcopenic obesity (45 of 157 patients with GC) and found an association between sarcopenic obesity and surgical site infection.
Limitations of our study include its retrospective design, single-center recruitment, and small sample size. One important limitation was the lack of staging laparoscopy that is now routinely used in our unit. Some of the patients included in the study might have already had peritoneal disease, and this may explain the notable percentage of patients with disease progression during neoadjuvant ChT. Another important limitation, associated with the retrospective nature of the study, was the use of different regimens of ChT drugs according to patient characteristics. Nonetheless, 77% of our cohort received ECF or a similar regimen, with no difference between patients with and without sarcopenia. The relationship between ChT toxicity and body composition has been observed in patients receiving a variety of different ChT regimens [35,42]. One possible explanation is that the measurements of body composition reveal reduced fitness and low ability to tolerate cancer therapy independently of the type of ChT.
CONCLUSIONS
Sarcopenic obesity is a strong risk factor for ChT toxicity, premature termination of ChT, and reduced overall survival. Future studies are required to define ChT dosages that are within the limits of tolerability for this unique and vulnerable subgroup.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Tan BH, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41:333–338. doi: 10.1016/j.ejso.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 6.Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–3268. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 8.Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang DD, Zhou CJ, Wang SL, et al. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery. 2017;161:680–693. doi: 10.1016/j.surg.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Chen FF, Zhang FY, Zhou XY, Shen X, Yu Z, Zhuang CL. Role of frailty and nutritional status in predicting complications following total gastrectomy with D2 lymphadenectomy in patients with gastric cancer: a prospective study. Langenbecks Arch Surg. 2016;401:813–822. doi: 10.1007/s00423-016-1490-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore) 2016;95:e3164. doi: 10.1097/MD.0000000000003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SL, Zhuang CL, Huang DD, Pang WY, Lou N, Chen FF, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol. 2016;23:556–564. doi: 10.1245/s10434-015-4887-3. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016;19:986–993. doi: 10.1007/s10120-015-0546-4. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi N, Ando Y, Gyawali B, Shimokata T, Maeda O, Fukaya M, et al. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol Rep. 2016;35:1727–1731. doi: 10.3892/or.2015.4475. [DOI] [PubMed] [Google Scholar]
- 15.Huang DD, Chen XX, Chen XY, Wang SL, Shen X, Chen XL, et al. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: a prospective study. J Cancer Res Clin Oncol. 2016;142:2347–2356. doi: 10.1007/s00432-016-2230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XT, Tang L, Chen Y, Li YL, Zhang XP, Sun YS. Visceral and subcutaneous fat as new independent predictive factors of survival in locally advanced gastric carcinoma patients treated with neo-adjuvant chemotherapy. J Cancer Res Clin Oncol. 2015;141:1237–1247. doi: 10.1007/s00432-014-1893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103:572–580. doi: 10.1002/bjs.10075. [DOI] [PubMed] [Google Scholar]
- 18.Lou N, Chi CH, Chen XD, et al. Sarcopenia in overweight and obese patients is a predictive factor for postoperative complication in gastric cancer: a prospective study. Eur J Surg Oncol. 2017;43:188–195. doi: 10.1016/j.ejso.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Nishigori T, Tsunoda S, Okabe H, Tanaka E, Hisamori S, Hosogi H, et al. Impact of sarcopenic obesity on surgical site infection after laparoscopic total gastrectomy. Ann Surg Oncol. 2016;23:524–531. doi: 10.1245/s10434-016-5385-y. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 22.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–6979. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 24.Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 25.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 27.Reisinger KW, Bosmans JW, Uittenbogaart M, Alsoumali A, Poeze M, Sosef MN, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol. 2015;22:4445–4452. doi: 10.1245/s10434-015-4558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 29.Wagner D, DeMarco MM, Amini N, Buttner S, Segev D, Gani F, et al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg. 2016;8:27–40. doi: 10.4240/wjgs.v8.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17:O256–O264. doi: 10.1111/codi.13067. [DOI] [PubMed] [Google Scholar]
- 31.Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, et al. Impact total psoas volume on short- and long-term outcomes in patients undergoing curative resection for pancreatic adenocarcinoma: a new tool to assess sarcopenia. J Gastrointest Surg. 2015;19:1593–1602. doi: 10.1007/s11605-015-2835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen RD, Williamson DA, Finnegan TP, Lloyd BD, Grady JN, Diamond TH, et al. Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr. 2007;86:952–958. doi: 10.1093/ajcn/86.4.952. [DOI] [PubMed] [Google Scholar]
- 33.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016;54:2–10. doi: 10.1016/j.semcdb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5:607–616. doi: 10.1002/cam4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjøblom B, Grønberg BH, Benth JS, Baracos VE, Fløtten Ø, Hjermstad MJ, et al. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer. 2015;90:85–91. doi: 10.1016/j.lungcan.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Tegels JJ, van Vugt JL, Reisinger KW, Hulsewé KW, Hoofwijk AG, Derikx JP, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112:403–407. doi: 10.1002/jso.24015. [DOI] [PubMed] [Google Scholar]
- 37.Irigaray P, Newby JA, Lacomme S, Belpomme D. Overweight/obesity and cancer genesis: more than a biological link. Biomed Pharmacother. 2007;61:665–678. doi: 10.1016/j.biopha.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 39.Gaujoux S, Torres J, Olson S, Winston C, Gonen M, Brennan MF, et al. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2908–2916. doi: 10.1245/s10434-012-2301-y. [DOI] [PubMed] [Google Scholar]
- 40.Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist. 2011;16:71–81. doi: 10.1634/theoncologist.2010-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 42.Sjøblom B, Benth JS, Grønberg BH, Baracos VE, Sawyer MB, Fløtten Ø, et al. Drug dose per kilogram lean body mass predicts hematologic toxicity from carboplatin-doublet chemotherapy in advanced non-small-cell lung cancer. Clin Lung Cancer. 2016 doi: 10.1016/j.cllc.2016.09.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]