Abstract
Case summary
This work describes the diagnosis and successful treatment of a 2-year-old domestic cat infected with Leishmania species and presenting fever, and ulcerative and nodular skin lesions after being treated for pyodermatitis for 1 year without clinical improvement. After anamnesis the cat was submitted to a complete clinical examination. Blood was collected for determination of haematological and biochemical parameters, detection of feline leukaemia virus (FeLV), feline immunodeficiency virus (FIV), feline coronavirus (FCoV) and Leishmania amastigotes. Fine-needle aspiration puncture from the skin nodules was also performed. After definitive diagnosis the animal was treated and followed up over a 2 year period. The animal tested negative for FIV-specific antibodies, FeLV antigen and feline coronavirus RNA. Leishmania amastigotes in the skin nodules were confirmed by cytology and molecular diagnosis. Treatment was initiated with allopurinol, resulting in a slight clinical improvement. Thus, N-methyl-glucamine antimoniate was added and administered for 30 days, with complete closure of the ulcerative lesions in the hindlimbs requiring a surgical approach. Close monitoring of the patient in the following 24 months indicated that combined therapy was safe and clinical cure was achieved without further relapses or side effects.
Relevance and novel information
Considering the increasing number of feline leishmaniosis cases and the inconsistent results of most therapeutic protocols described in the literature, the use of new approaches, especially in refractory cases, is essential. Although the use of allopurinol and N-methyl-glucamine antimoniate is off-label in cats, in this case the combination treatment was followed by an extensive analytical monitoring, supporting their safety and effectiveness.
Introduction
The occurrence of feline leishmaniosis (FeL) has increased worldwide, revealing a possible role of cats in leishmaniosis epidemiology. Better accuracy of laboratory techniques has improved the diagnosis of this disease, and changes in ecosystems over the last few years associated with the use of repellents on dogs might play a role in sandflies’ altered feeding patterns in urban areas, leading to infections in other mammalian hosts.1–3
Worldwide epidemiological surveys of Leishmania species causing feline asymptomatic infection and FeL have been reported.4,5 In Europe, Spain had the first described FeL case in 1932.6 Some authors claim that low prevalence of FeL in endemic areas may be related to a natural resistance to the parasite in cats.7 Moreover, the number of clinical cases may be underestimated, leading to few reports and lack of information by clinicians and owners. Although Portugal is an endemic country for canine leishmaniosis caused by Leishmania infantum, there are few published clinical reports of FeL, with the first description of it in 1994 in Sesimbra, followed by others in Porto and Lisbon.8–10
Despite occasional reports of ocular and visceral disease, dermatological, mucocutaneous/mucosal presentations and lymph node enlargement are the most frequent clinical features.11–14 Clinical suspicion is based on history, clinical signs and clinicopathological abnormalities. Diagnosis is confirmed with parasitological and serological methods.15
A long-term administration of allopurinol (10–20 mg/kg q12h or q24h) is the most recommended treatment protocol for FeL,10,11,15 however, similarly to dogs, recurrence can occur if the infection is not cleared. Some authors have reported a good clinical response using N-methyl-glucamine antimoniate monotherapy (5–100 mg/kg or 375 mg/cat q24h SC or IM under different protocols).8,15
This study aims to describe the treatment of a recurring clinical case of FeL in a cat receiving combined therapy (allopurinol and N-methyl-glucamine antimoniate).
Case description
A 2-year-old domestic European neutered male cat was referred to the Veterinary Teaching Hospital of the Faculty of Veterinary Medicine, University of Lisbon, with multiple nodular skin lesions (Figure 1), ulcerative dermatitis involving the four limbs (Figure 2) and fever. The cat was referred 1 year after the onset of skin lesions and was previously treated for a presumptive pyodermatitis with prednisolone (1 mg/kg PO q12h for 12 days) and amoxicillin/clavulanic acid (20 mg/kg PO q12h for 6 days), followed by doxycycline (5 mg/kg PO q12h for 8 days), and showed no clinical improvement.
Figure 1.
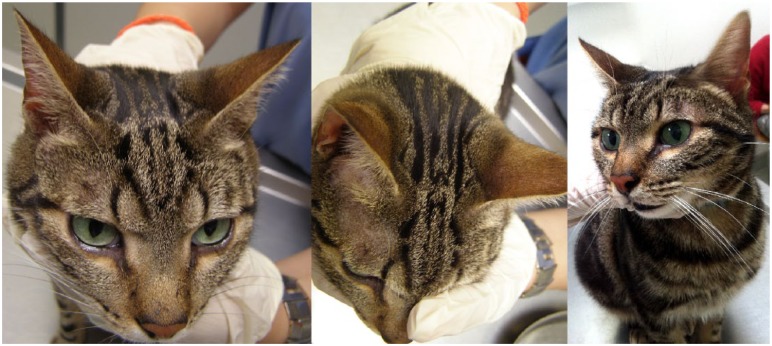
Skin nodules measuring 2–10 mm in diameter were distributed on the (a) outer pinnal surfaces and (b) orbital regions. Most of them were observed on the right pinna, where they gathered with coalescing patterns
Figure 2.
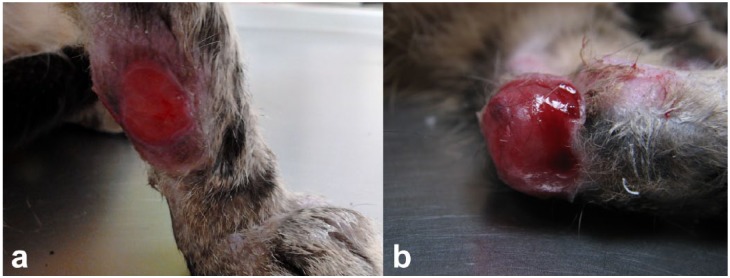
Single spots (between 20 and 50 mm in diameter) of ulcerative dermatitis that had raised margins affecting the (a) antebrachial and (b) hock regions after disinfection of lesions
The cat was not vaccinated, but had been regularly protected against ectoparasites with fipronil and imidacloprid. At first admission, mild fever (39.5ºC) and dermatological lesions on the head and limbs were the only abnormalities on physical examination. About 20–25 skin nodules measuring 2–10 mm in diameter were distributed on the outer pinnal surfaces (Figure 1a) and orbital regions (Figure 1b). Most of them were observed on the pinnae, where they gathered with coalescing patterns. Differential diagnosis was pyodermatitis, allergic reaction, autoimmune disease, neoplastic disease or leishmaniosis. Single spots (20–50 mm in diameter) of ulcerative dermatitis, which had raised margins, affected the antebrachial (Figure 2a) and hock (Figure 2b) regions.
A complete blood count and biochemical profile were used to evaluate the clinical status of the cat before treatment, after treatment and at all follow-up examinations. Urinalysis and urinary protein: creatinine ratio were evaluated 6 months after treatment. Haematological and biochemical values were within the reference intervals. Serum protein electrophoresis showed an albumin: globulin ratio, but a slight hypergammaglobulinaemia was evident (Table 1).
Table 1.
Haematological, biochemical and serological results obtained before, during and after treatment
| Day 0 | Day 45 | Month 6 | Month 12 | Month 18 | Month 24 | Reference intervals | |
|---|---|---|---|---|---|---|---|
| Haemogram | |||||||
| Leukocytes (×10³/µl) | 13.3 | 15.0 | 10.0 | NA | NA | 15.6 | 5.5–19.5 |
| Erythrocytes (×106/µl) | 6.6 | 9.2 | 10.2 | NA | NA | 9.5 | 5.0–10.0 |
| Platelets (×10³/µl) | 185 | 327 | 129 | NA | NA | 223 | 300–700 |
| Haemoglobin (g/dl) | 9 | 12 | 14 | NA | NA | 13 | 8–15 |
| PCV (%) | 29 | 40 | 40 | NA | NA | 43 | 24–45 |
| Non-segmented neutrophils (/µl) | 0 | 0 | 0 | NA | NA | 0 | 0–300 |
| Segmented neutrophils (/µl) | 9.520 | 11,000 | 6.468 | NA | NA | 7.000 | 2.500–12.500 |
| Lymphocytes (/µl) | 2.856 | 3000 | 2.980 | NA | NA | 2.800 | 1.500–7.000 |
| Monocytes (/µl) | 0.5 | 0.3 | 0.1 | NA | NA | 4.7 | 0–850.0 |
| Eosinophils (/µl) | 0.680 | 0.600 | 0.390 | NA | NA | 0 | 0–1.500 |
| Basophils (/µl) | 0 | 0 | 0 | NA | NA | 0 | Rare |
| Biochemical parameters | |||||||
| Urea (mg/dl) | 48.7 | 52.9 | 50.0 | NA | 52.3 | 59.9 | 0–82.0 |
| Creatinine (mg/dl) | 0.73 | 0.97 | 1.07 | NA | 1.23 | 1.25 | 0.70– 2.20 |
| ALT (U/l) | 37 | 37 | 35 | NA | 45 | 37 | 10–130 |
| AST (U/l) | NA | NA | NA | NA | 28 | 28 | 8–35 |
| Alkaline phosphatase (U/l) | NA | NA | 43 | NA | 21 | 32 | 20–220 |
| Calcium (mmol/l) | NA | NA | NA | NA | 9.52 | 8.92 | 9.40–11.20 |
| Total bilirubin (mg/dl) | NA | NA | NA | NA | 0.06 | 0.05 | 0.08–0.30 |
| Potassium (mmol/l) | NA | NA | NA | NA | 4.4 | 4.8 | 3.5–5.2 |
| Chloride (mmol/l) | NA | NA | NA | NA | 123 | 137 | 108–128 |
| Sodium (mmol/l) | NA | NA | NA | NA | 156 | 156 | 143–153 |
| Biliary acids (µmol/l) | NA | NA | NA | NA | 3.68 | 3.53 | 1–20.00 |
| Inorganic phosphorus (mmol/l) | NA | NA | NA | NA | 4.4 | 4.8 | 2.4–6.0 |
| Urinary protein: creatinine ratio (%) | NA | NA | 0.04 | NA | NA | NA | <0.50 |
| Serum protein electrophoresis | |||||||
| Total protein (g/dl) | 6.9 | 7.1 | 6.8 | NA | 7.2 | NA | 5.7–7.9 |
| Albumin (g/dl) | 2.6 | 2.7 | 3.1 | NA | 3.2 | NA | 2.1–4.0 |
| Alpha 1 (g/dl) | 0.4 | 0.1 | 0.1 | NA | 0.1 | NA | 0.2–1.1 |
| Alpha 2 (g/dl) | 1.1 | 1.4 | 1.6 | NA | 1.7 | NA | 0.4–0.9 |
| Beta (g/dl) | 0.4 | 1.1 | 0.9 | NA | 1.1 | NA | 0.9–1.9 |
| Gama (g/dl) | 2.4 | 1.9 | 1.1 | NA | 1.1 | NA | 1.3–2.2 |
| Albumin: globulin ratio (%) | 0.60 | 0.60 | 0.90 | NA | 0.80 | NA | 0.45–1.30 |
| Antibody titre (IFAT) | |||||||
| Anti-Leishmania antibodies | ⩾1:1280 | 1:320 | 1:80 | 1:80 | 1:40 | 1:40 | |
PCV = packed cell volume; ALT = alanine aminotransferase; AST = aspartate aminotransferase; NA = not available
The patient was tested for FIV-specific anti-bodies, FeLV antigens by ELISA (ViraCHEK/FIV and ViraCHEK/FeLV, respectively; Synbiotics) and immunoflourescence antibody test (IFAT) using an adaptation of Leishmania Spot IFI kit (bioMérieux) with a fluorescently labelled anticat IgG conjugate (anticat IgG MegaCor; MegaCor Diagnostik), which was used to quantify anti-Leishmania antibodies (cut-off 1:40). Previous studies, including more than 100 cats, validated this latter test in inter-laboratory assays using known positive and negative sera, with results as expected. Repeatability and reproducibility were also assessed (unpublished results). The cat tested negatively for FIV antibodies and FeLV antigens, but was positive for anti-Leishmania antibodies, with a titre ⩾1:1280 (Table 1).
Fine-needle aspiration puncture from the ear skin nodules was performed. Smears were stained with Giemsa (Merck) and examined under an optical microscope (Olympus BX51). The aspirate was also used for further molecular and parasitological analysis. The smears revealed Leishmania amastigotes (Figure 3), and absence of bacteria/neutrophilic infiltration or characteristic cells of autoimmune diseases.
Figure 3.
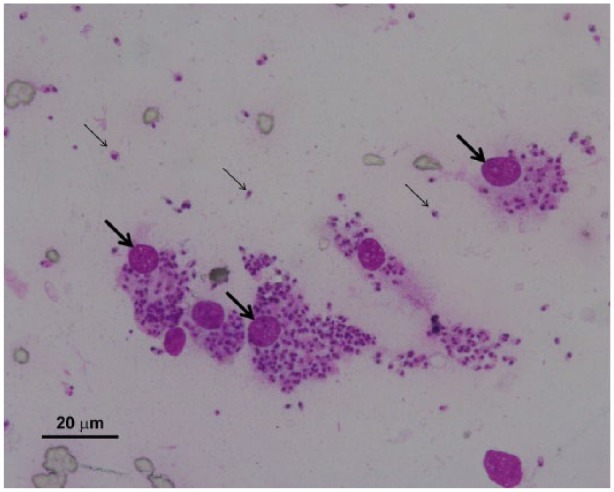
Fine-needle aspiration smear of a skin nodule showing many extracellular (small arrows) and intracellular Leishmania amastigotes in the cytoplasm of macrophages (large arrows). Giemsa stain, scale bar = 20 µm
DNA extraction from EDTA blood samples, skin nodules and lymph node aspirates was carried out with a DNeasy Blood & Tissue kit (Qiagen). Detection of feline coronavirus from plasma was performed by reverse transcription quantitative PCR (RT-qPCR),16 targeting the 3′ end of the virus genome. Additionally, qPCR using L infantum-specific Taqman probes targeting the kinetoplast minicircle DNA (kDNA) was also performed to determine the initial and follow-up parasitic load.17
RT-qPCR was negative for coronavirus, while qPCR of skin nodules and blood samples were positive for L infantum kDNA, showing a parasitic load of 1700 and 0.1 parasites per 50 ng DNA, respectively (Table 2).
Table 2.
Leishmania infantum parasitic load detected by quantitative PCR before, during and after treatment
| Parasitic load (number of parasites/50 ng total DNA) | ||||
|---|---|---|---|---|
| Day 0 | Day 30 | Day 45 | Month 18 | |
| Skin nodules | 1700 | 10 | NA | NA |
| Blood | 0.10 | 0.05 | 0.05 | 0 |
| Lymph node | NA | 100.0 | 0.1 | 0 |
NA = not available
Nodular and lymph node aspirates were inoculated in Schneider’s Insect Medium (Sigma-Aldrich) supplemented with 0.6% (w/v) of CaCL2, 5 mM Hepes, 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin.18 Cultures were maintained at 24ºC in a refrigerated incubator (Lovibond) and observed by optical microscopy after 8 days of incubation. Viable promastigotes were observed.
The cat was treated orally with 10 mg/kg allopurinol q12h and a three-layered bandage was applied to the four limbs. Medical follow-up was performed every 2 days during the first fortnight. After 15 days of treatment with allopurinol only a slight clinical improvement was noted. Fever persisted and skin ulcers seemed equally profound. Therefore, a combined therapeutic protocol not yet approved for cats was suggested to the owner. N-methyl-glucamine antimoniate administration is off-label in cats, with only a few published cases of FeL treated with this drug. After owner consent was obtained, treatment was reinforced with 50 mg/kg N-methyl-glucamine antimoniate (Glucantime; Merial) q24h SC for 30 consecutive days. This study was conducted in the framework of FCT Project PTDC/CVT/118566/2010, with the agreement of the Ethics Committee and Animal Welfare of the Faculty of Veterinary Medicine, University of Lisbon.
During and after treatment there were no abnormalities reported by the owner. The cat maintained a normal physical condition19 and examination apart from the skin lesions.
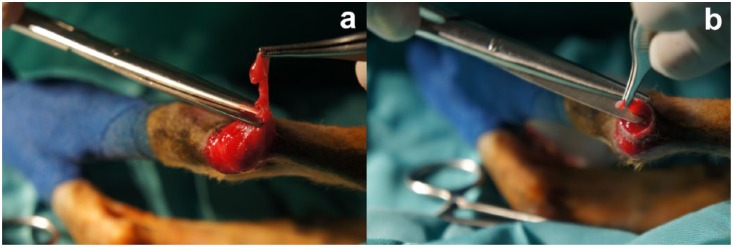
Total remission of skin nodules on the head, as well as the foreleg ulcers, was achieved (Figure 4), with body temperature being normalised on day 45. Complete closure of the hindlimb ulcerative lesions did not occur owing to an extreme tension in those regions, which required surgery (Figure 5). One hour prior to surgery, the cat received a therapeutic protocol of Ringer’s lactate fluid therapy, amoxicillin with clavulanic acid (8.75 mg/kg IM), buprenorphine (0.02 mg/kg IM) and methylprednisolone succinate (1 mg/kg IM). Propofol (4 mg/kg IV) was used for the induction of anaesthesia followed by isoflurane for its maintenance. Debridement and excision of the wound edges were performed and a horizontal mattress suture was used for closure. The excised edges of the defect were immersed in a 10% formalin solution to allow for histopathological study.
Figure 4.
Cat 18 months after treatment with allopurinol and N-methyl-glucamine antimoniate (Glucantime; Merial), illustrating the absence of skin nodules on the ears and head
Figure 5.
Intraoperative images showing the excision of the defect edges in the (a) right and (b) left hindlimb
The cat was medicated with antibiotic (amoxicillin/clavulanic acid, 12.5 mg/kg PO q12h for 10 days) and a non-steroidal anti-inflammatory (tolfenamic acid 4 mg/kg PO q 24h for 4 days), and was assessed 48 h after surgery in order to evaluate the regions operated on; stitches were removed on days 6 and 12. As an integral part of the healing process, the cat was maintained for 2 weeks with Robert-Jones bandages. The complete healing of hindlimb lesions was achieved 1 month after surgery (Figure 6).
Figure 6.
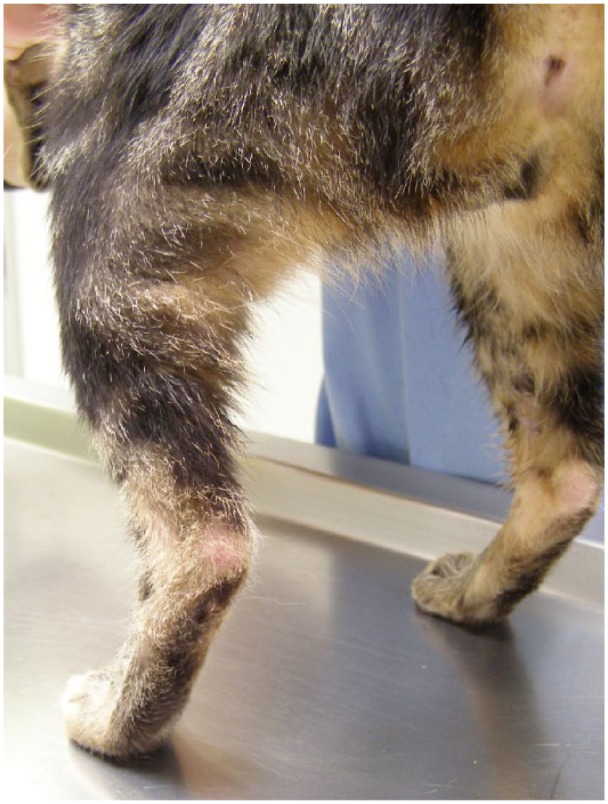
Hindlimbs 4 weeks after surgery
Histological examination of the removed tissues showed a granulomatous-like inflammatory response with some foci of lymphocytes, activated plasmacytes and occasional intracellular amastigote forms of Leishmania species in macrophages.
The slight hypergammaglobulinaemia observed at admission returned to normal values. No further clinical or laboratory changes were detected 6, 18 and 24 months after the end of treatment (Table 1). Allopurinol was stopped when antibody titres decreased to 1:40 (18 months). At month 24, the antibody level remained unchanged.
Parasitic load decreased in both blood and skin nodules after 15 days of combined treatment. The lymph node parasitic load showed a pronounced decrease after day 30 (Table 2).
Discussion
FeL is rarely reported in various parts of the world.4,20,21 In Europe, a total of 46 clinical cases have been published between 1989 and 2014, namely in Italy, France, Spain and Switzerland, with regards to cats imported from Spain.4,20,22–24 In Portugal, although the first clinical case was reported in the 1990s,8 only in recent years have epidemiological studies been conducted to better understand the role of cats in the biological cycle of Leishmania species. In the metropolitan region of Lisbon, although the detection of anti-Leishmania antibody titres points to a low prevalence or, at least, low cat–parasite contact (1.3%), the detection of Leishmania DNA indicates high levels of infection in stray (30.4%) and domestic cats (20.3%). Even so, most of the animals are asymptomatic.3
This disease is seldom considered during the differential diagnosis in cats, as in the present clinical case, where the animal had been previously treated for other diseases for 1 year.
Dermatological findings in FeL usually include erythema, alopecia, papules, nodules, ulcers or crusts, with the head being the most affected region, but other body parts may also be affected.17,25,26 In the present clinical case, as the ulcers in the hindlimbs were severely contaminated at first examination, it was not possible to determine whether the fever was a systemic sign of FeL or a consequence of secondary bacterial infection. Concerning the available therapeutics for FeL, this cat was first treated with allopurinol, which was insufficient despite its use being common in the treatment of canine leishmaniosis. Even though the use of allopurinol is off-label in both dogs and cats, there is a more extensive clinical experience of efficacy and safety in dogs. Under the scope of ‘cascade’ a combined therapeutic not yet approved for cats was suggested to the owner.
Initially, this cat showed very high antibody titres by IFAT as seen in other reported cases of FeL.12,13,23 After 2 weeks of the combined protocol the decrease in antibody titre and parasitic load was evident. Similarly to canine leishmaniosis, there was a gradual decline in antibody titre and it took around 18 months to attain the lowest titre. Clinical remission was accomplished together with surgical recovery in less than 2 months. Furthermore, this therapeutic protocol seemed to be safe for the cat as no analytical change or side effect was observed.
By 24 months of follow-up, no relapses had occurred. Regarding the albumin levels, although within the reference interval, the increase noted, associated with the decrease in gamma(γ)-globulin values, kept the total protein concentration within the reference interval, which is in agreement with the remission of clinical signs.
Cats may live for years with a slowly progressing chronic disease, even without a specific anti-Leishmania treatment.4,5,15 However, in this case, the severity of cutaneous lesions was the clinician’s main concern leading her to search for another therapeutic protocol when monotherapy with allopurinol proved to be insufficient.
Although full clinical recovery was achieved, it is possible that a much reduced number of viable parasites may have persisted (mainly in the lymph nodes) after the end of the treatment. However, as it is not possible to guarantee total elimination or its dispersion to the skin, parasite transmission should be kept in mind. These facts highlight the need for new topical insecticides aiming to prevent sandfly bites in cats, as all insecticide products currently registered against sandflies contain permethrin, which is extremely toxic to cats.
Conclusions
The present work describes a successful case of FeL treatment in a young cat, negative for FIV antibodies and FeLV antigenaemia, after a 15 day refractory period using allopurinol monotherapy. To the best of our knowledge, this is the first report of a combined therapy, using allopurinol and N-methyl-glucamine antimoniate with no relapses or adverse effects, resulting in a possible new treatment for use in FeL refractory cases.
Acknowledgments
We thank Merial (Portugal) for supplying the Glucantime used during the cat’s treatment.
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Funding for this work was provided by the Portuguese Foundation for Science and Technology (FCT) with co-participation of European Union Fund (FEDER) through the research project PTDC/CVT/118566/2010 and UID/CVT/00276/2013, and grants SFRH/BD/77886/2011 and SFRH/BD/101467/2014.
References
- 1. WHO. Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. WHO Technical Report Series, 2010, p 186. [Google Scholar]
- 2. Campino L, Maia C. Epidemiologia das leishmanioses em Portugal. Acta Med Port 2010, 23: 859–864. [PubMed] [Google Scholar]
- 3. Maia C, Gomes J, Cristóvão J, et al. Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet Parasitol 2010; 174: 336–340. [DOI] [PubMed] [Google Scholar]
- 4. Pennisi MG, Cardoso L, Baneth G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors 2015; 8: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soares CSA, Duarte SC, Sousa SR. What do we know about feline leishmaniosis? J Feline Med Surg. Epub ahead of print 26 June 2015. DOI: 1098612X15589358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayllon T, Tesouro MA, Amusategui I, et al. Serologic and molecular evaluation of Leishmania infantum in cats from Central Spain. Ann N Y Acad Sci 2008; 1149: 361–364. [DOI] [PubMed] [Google Scholar]
- 7. Solano-Gallego L, Rodríguez-Cortés A, Iniesta L, et al. Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the northwestern Mediterranean. Am J Trop Med Hyg 2007; 76: 676–680. [PubMed] [Google Scholar]
- 8. Costa-Durão J, Rebelo E, Peleteiro M, et al. First case of leishmaniosis in domestic cat (Felis catus domesticus) detected in Portugal (Sesimbra). Rev Port Cienc Vet 1994; 511: 140–144. [Google Scholar]
- 9. Maia C, Sousa C, Ramos C, et al. First case of leishmaniosis caused by Leishmania infantum genotype E in a cat with a concurrent nasal squamous cell carcinoma. J Feline Med Surg Open Rep 2015; 1 (2). DOI: 10.1177/20551169155939690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcos R, Santos M, Malhão F, et al. Pancytopenia in a cat with visceral leishmaniasis. Vet Clin Path 2009; 38: 201–205. [DOI] [PubMed] [Google Scholar]
- 11. Leiva M, Lloret A, Peña T, et al. Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol 2005; 8: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verneuil M. Ocular leishmaniasis in a cat: case report. J Fr Ophtalmol 2013; 36: e67–e72. [DOI] [PubMed] [Google Scholar]
- 13. Pennisi M, Venza M, Reale S, et al. Case report of leishmaniasis in four cats. Vet Res Commun 2004; 28: 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simões-Mattos L, Bevilaqua C, Mattos M, et al. Feline leishmaniasis: uncommon or unknown? Rev Port Cienc Vet 2004; 9: 79–97. [Google Scholar]
- 15. Pennisi MG, Hartmann K, Lloret A, et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gil S, Leal RO, Duarte A, et al. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Res Vet Sci 2013; 94: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helhazar M, Leitão J, Duarte A, et al. Natural infection of synathropic rodent species Mus musculus and Ratus norvegicus by Leishmania infantum in Sesimbra and Sintra – Portugal. Parasit Vectors 2013; 6: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hockmeyer WT, Kager PA, Rees PH, et al. The culture of Leishmania donovani in Schneider’s insect medium: its value in the diagnosis and management of patients with visceral leishmaniasis. Trans R Soc Trop Med Hyg 1981; 75: 861–863. [DOI] [PubMed] [Google Scholar]
- 19. Bjornvad CR, Laflamme DP. Cat body condition score. http://www.wsava.org/sites/default/files/Body%20condition%20score%20chart%20cats.pdf (2013, accessed August 2, 2015).
- 20. Ozon C, Marty P, Pratlong F, et al. Disseminated feline leishmaniosis due to Leishmania infantum in Southern France. Vet Parasitol 1998; 75: 273–277. [DOI] [PubMed] [Google Scholar]
- 21. Rougeron V, Catzeflis F, Hide M, et al. First clinical case of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis in a domestic cat from French Guiana. Vet Parasitol 2011; 181: 325–328. [DOI] [PubMed] [Google Scholar]
- 22. Poli A, Abramo F, Barsotti P, et al. Feline leishmaniosis due to Leishmania infantum in Italy. Vet Parasitol 2002; 106: 181–191. [DOI] [PubMed] [Google Scholar]
- 23. Rüfenacht S, Sager H, Müller N, et al. Two cases of feline leishmaniosis in Switzerland. Vet Rec 2005; 156: 542–545. [DOI] [PubMed] [Google Scholar]
- 24. Pennisi M. Feline leishmaniosis from A to Z [in Italian]. In: Veterinarie E. (ed). Leishmaniosi canina: recenti acquisizioni su pidemiologia, implicazioni cliniche, diagnosi, terapia e prevenzione. Cremona: Edizioni Veterinarie, 2010, pp 59–64. [Google Scholar]
- 25. Hervás J, Chacón-Manrique de, Lara F, Sanchez-Isarria M, et al. Two cases of feline visceral and cutaneous leishmaniosis in Spain. J Feline Med Surg 1999; 1: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pennisi MG, Lupo T, Malara D, et al. Serological and molecular prevalence of Leishmania infantum infection in cats from southern Italy. J Feline Med Surg 2012; 14: 656–657. [Google Scholar]