Abstract
Case summary
A 7-year-old spayed domestic longhair cat from Perth, Western Australia, presented with left-sided head tilt, dysphonia, head shaking, inappetence and weight loss. A polypoid lesion had previously been removed from the external ear canal. Otitis media with extension into the external ear canal was suspected and investigated using video-otoscopy and computed tomography examination. Invasive disease with extension from the middle ear to the base of the skull, and intracranial extension into the caudal fossa and cranial cervical vertebral canal was detected. Cytology of external ear canal exudate showed capsulated budding yeasts and Cryptococcus gattii VGII was cultured. Treatment with amphotericin B infusions and oral fluconazole was prescribed, with nutritional support via oesophagostomy tube. The cat clinically recovered 12 months after treatment commenced.
Relevance and novel information
This case report describes the successful medical treatment of otogenic meningoencephalomyelitis due to C gattii (VGII) infection in a cat.
Introduction
Cryptococcosis is a common systemic mycosis affecting cats worldwide and can affect cats of any age and sex.1 The two most common species causing disease in animals are Cryptococcus neoformans and Cryptococcus gattii. Historically, Cryptococcus was classified into five serotypes (A, B, C, D and AD) on the basis of antigenic differences in capsular polysaccharides.2 Using a modern molecular typing scheme, cryptococcal strains are now divided into eight multilocus sequence types (VNI, VNII, VNIII, VNIV, VGI, VGII, VGIII, VGIV), which are likely to be cryptic species.3
C gattii has been associated with tropical and subtropical climates, with an outbreak of disease affecting both humans and animals in the Pacific Northwest of the USA, emanating from an initial focus of infection on Vancouver Island, British Columbia, Canada.4,5 C gattii has unique features, which distinguish it from C neoformans.4–6 C gattii primarily infects immunocompetent hosts and the organism can cause disease in unusual host species (eg, horses, ferrets, goats, dolphins).7,8 VGII has a high environmental presence in Perth, Western Australia, and areas of the Northern Territory, in contrast to eastern Australia where VGI isolates predominate. The genetic diversity of VGII environmental and clinical isolates obtained from the Perth environs suggests there is a sexually recombining population structure in this geographical niche.9,10
While C gattii VGI is strongly associated with detritus in mature eucalyptus tree hollows, the ecological niche of VGII is largely unknown.11 In Vancouver Island, where an outbreak of VGIIa was diagnosed in humans and animals, C gattii VGII colonises coniferous, evergreen and deciduous trees in the Douglas fir tree bioclimatic zone, with transient isolation from soil, saltwater, freshwater and air.12
Infection most likely occurs subsequent to inhalation of airborne infectious propagules such as basidiospores or desiccated yeast cells with clinical signs of sneezing, stertor, nasal deformity and discharge reflecting nasal cavity infection.11 It is common for the infection to spread, both locally to contiguous adjacent structures and haematogenously to other sites.
We present an unusual case of otogenic infection with intracranial extension attributable to C gattii VGIIb in a cat for which signs resolved with aggressive medical therapy.
Case description
A 7-year-old spayed domestic longhair cat was presented to Murdoch University Veterinary Hospital for evaluation of left head tilt, dysphonia, head shaking, inappetance and weight loss of 2 weeks’ duration.
Initial investigations by the referring veterinarian identified an aural mass in the left horizontal ear canal, which had been removed. Sabouraud’s dextrose agar culture of an ear swab had resulted in heavy growth of C gattii. Serum latex cryptococcal antigen agglutination test (LCAT) was positive, with a titre of 1:256. ELISA testing for feline immunodeficiency virus antibodies and feline leukaemia virus antigen were negative.
The cat was treated with enrofloxacin (5 mg/kg PO q24h [Baytril; Bayer Animal Health]), itraconazole (10 mg/kg PO q24h [Sporanox; Janssen Pharmaceutica]), meloxicam (0.05 mg/kg PO q24h [Meloxicam; Troy Laboratories Australia]) and a topical compounded enrofloxacin/dexamethasone otic preparation. No improvement in the clinical signs was seen.
The cat was thin (body condition score [BCS] 3/9; weight 4.9 kg) and had a left-sided head tilt. The remainder of the clinical examination, including full ophthalmic and neurological examinations, was unremarkable. The presence of a head tilt without ipsilateral postural reaction deficits was consistent with left peripheral vestibular disease. Hand-held otoscopic examination demonstrated a mass occluding the lumen of the left horizontal ear canal, and ceruminous discharge.
Preanaesthetic blood tests were clinically insignificant. The cat was premedicated with buprenorphine (0.018 mg/kg IM [Temgesic; Symbion Pharmacy Services]) and acepromazine (0.04 mg/kg IM [ACP; Westralian Holdings]), anaesthetised with propofol and maintained with isoflurane in 100% oxygen. A CT (Siemens Emotion Duo 2 slice CT scanner; GE Healthcare Australia) examination of the head was performed using soft tissue and bone spatial reconstruction algorithm and 1 mm slice thickness pre- and postintravenous contrast (iohexol; 10 ml [300 mmol/l8] IV) (Figure 1), followed by video-otoscopic examination.
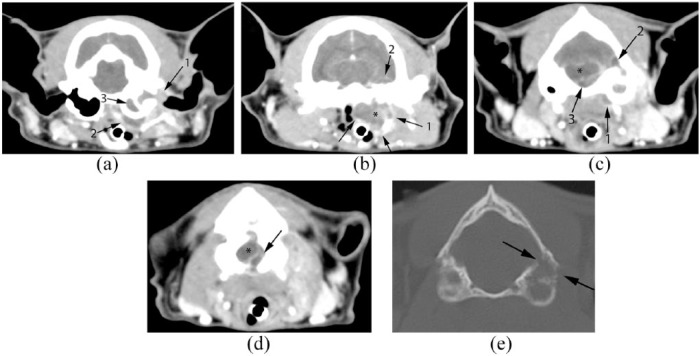
Figure 1.
(a) The medial portion of the left external ear canal is filled with strongly and homogeneously contrast-enhancing soft tissue attenuating material (1). The ear canal lining is moderately contrast enhancing (compared with the right). The left tympanic bulla is filled with mildly and homogeneously contrast-enhancing material (3). Similar moderately enhancing material is seen extending ventromedially from the left bulla tympanica. A rim of strong contrast enhancement outlines this region (2). (b) The material seen ventromedial to the tympanic bulla continues caudally and forms a 1.6 cm (width) × 1.3 cm (height) × 2.5 cm (length) soft tissue structure, poorly enhancing centrally but strongly enhancing peripherally (1). This mass (*) distorts local tissue architecture such that the nasopharyngeal lumen is narrowed >50%, and the hyoid bones are displaced laterally. Additionally, at this level, there is a rim of contrast enhancement seen in the ventral aspect of the left temporal lobe of the cerebrum (2). (c) At the level of the caudal aspect of the left tympanic bulla, the soft tissue lesion can still be seen ventromedial to the bulla (1). Additionally, there is a rim of contrast enhancement outlining a hypoattenuating area in the left lateral cerebellum (2). There is poorly contrast-enhancing soft tissue attenuating material in the caudal fossa that is displacing the brainstem dorsally and to the right (3). Additionally, there is strong meningeal contrast enhancement in this region. (d) The poorly contrast-enhancing material in the cranial cavity can be followed further caudally, to the level of the atlas. This image, at the level of the foramen magnum, demonstrates abnormal tissue (arrow) displacing and compressing the cervical spinal cord (*) dorsally and to the right. (e) Bone window computed tomography image at the most caudal aspect of the bulla. Note the discontinuity of the temporal bone (black arrows), indicative of bony lysis
CT showed moderate contrast enhancement of the left ear canal lining. The medial portion of the left external ear canal and left tympanic bulla were filled with homogenous, contrast-enhancing soft tissue attenuating material (Figure 1a). Ventromedial and rostral to the tympanic bulla, a region (1.6 cm width × 1.3 cm height × 2.5 cm length) of poorly contrast-enhancing tissue, outlined by a rim of strong contrast enhancement (Figure 1b) causing a mass effect, was seen, which compressed the nasopharyngeal lumen by >50% and displaced the hyoid bones laterally (Figure 1b). Ventromedial and caudal to the bulla, a similarly enhancing region was seen (0.6 cm height × 0.3 cm width × 0.6 cm length). In the caudal left bulla, there was bony lysis of the temporal bone (Figure 1e). Lateral to the oval foramen and dorsal to the larger parapharyngeal lesion, an intracranial rim of contrast enhancement was seen in the ventral aspect of the left temporal lobe. Adjacent to the lytic region of the temporal bone, contrast enhancement outlining a hypoattenuating area with broad dural base was seen (Figure 1c). Poorly enhancing material extended caudally along the left ventral portion of the brainstem to mid first cervical vertebra, displacing and compressing the brainstem and cranial spinal cord, accompanied by strong meningeal contrast enhancement (Figure 1d).
Video otoscopy (MedRx Video Otoscope) identified a large pink fleshy mass obscuring the lumen of the horizontal ear canal. The mass traversed the tympanic membrane, and was contiguous the tympanic bulla (Figure 2), in agreement with CT findings. Pinch biopsies and material near the mass was collected for fungal culture. The mass was debulked using curettes and biopsy forceps passed through the video otoscope. An oesophageal feeding tube was placed for nutritional support.
Figure 2.
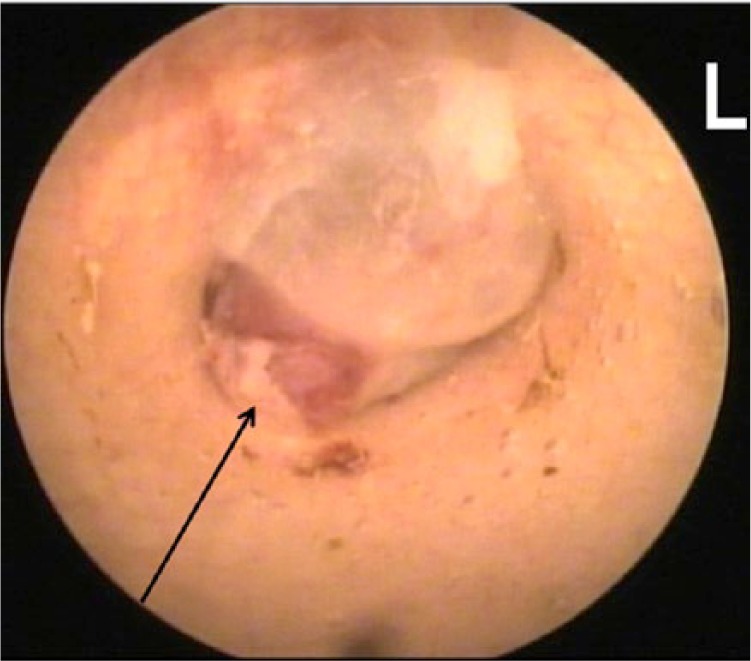
Video-otoscopic photograph of the left tympanic membrane, which appears to have been breached by abnormal inflammatory tissue (arrows), which extends into the tympanic bulla
Impression smears of the mass revealed large numbers of round yeast, surrounded by a prominent clear halo and narrow-necked budding consistent with cryptococcal infection. A heavy pure growth of a Cryptococcus species resulted, which was identified as C gattii by conventional phenotypic mycology testing. Further analysis by sequencing of the ribosomal internal transcribed spacer region identified C gattii VGII by comparing the sequence with published signature sequences;13 however, the isolate was not available for further molecular typing. The isolate was susceptible to fluconazole, itraconazole, posaconazole, flucytosine and amphotericin B, but resistant to caspofungin. The minimum inhibitory concentration (MIC) for fluconazole was 8 mg/l (Table 1).
Table 1.
Antifungal susceptibility data for the Cryptococcus gattii VGII isolate cultured from the cat
| Antifungal agent | MIC (mg/l) | Interpretation |
|---|---|---|
| Amphotericin B | 0.25 | S |
| Fluconazole | 8.00 | S |
| Itraconazole | 0.06 | S |
| Ketoconazole | 0.03 | S |
| Posaconazole | 0.12 | S |
| 5-Flucytosine | 0.50 | S |
| Voriconazole | 0.06 | S |
| Caspofungin | 16.00 | R |
S = susceptible; R = resistant; MIC = minimum inhibitory concentration
Antifungal therapy was commenced with fluconazole (10 mg/kg PO q12h [Symbion Pharmacy Services]) and amphotericin deoxycholate (0.5 mg/kg; subcutaneous infusion in 350 ml 0.45% NaCl and 2.5% dextrose three times weekly).14 Monitoring for potential nephrotoxicity included weekly serum urea and creatinine determinations and urinalyses.
Four weeks after treatment commenced, the cat was brighter, with complete resolution of the head tilt, although inappetence persisted. Combination therapy continued. At 8 weeks, the cat had received a cumulative amphotericin B dose of 12.5 mg/kg, and was starting to eat and gain weight. Owing to finances, the frequency of amphotericin B infusion was reduced to twice weekly (dose 0.7 mg/kg/infusion). Fluconazole was continued (50 mg PO q12h). No azotaemia developed during therapy.
Three months after diagnosis, the cat was appetant and BCS was 5/9. The frequency of amphotericin B infusion was reduced to once weekly (0.7 mg/kg/infusion). Four months after diagnosis, LCAT was 1:256 and the plasma concentration of fluconazole was 45 mg/l. Seven months after diagnosis, the LCAT was 1:64 and was 1:32 a further 3 months later, with a corresponding plasma concentration of fluconazole of 47 mg/l. After 12 months, the weekly amphotericin B infusions (0.7 mg/kg) were discontinued and the cat continued to be clinically well, receiving only fluconazole (50 mg q12h). At the time of writing, 21 months after starting therapy, the LCAT was 1:8 and the cat continued to be clinically well.
Discussion
Peripheral vestibular disease associated with cryptococcosis has been previously reported in three cats.15 One cat had a nasopharyngeal cryptococcal granuloma caused by C gattii VGI. Treatment involved removal of the granuloma followed by oral itraconazole. In another cat, otitis media due to C neoformans var grubii infection was managed by a total ear canal ablation and lateral bulla osteotomy, followed by oral itraconazole. In the third cat ventral bulla osteotomy and twice weekly amphotericin B subcutaneous infusions, with oral flucytosine and fluconazole, were used. In these latter two cats, diagnosis was made on cytological and histological evaluation of material removed via bulla osteotomy, culture and serum antigen testing.
The cat presented initially with unilateral peripheral vestibular disease. As a mass in the left external ear canal was detected during preliminary examination, an aural polyp arising from the middle ear was initially suspected. Presence of additional signs, including dysphonia, inappetence and weight loss, may have suggested a more sinister underlying aetiology. Cytology from the external ear canal resulted in a prompt diagnosis of cryptococcosis; however, failure to improve clinically to azole monotherapy prompted referral. Video otoscopy and CT of the head demonstrated extensive and invasive disease of the ear, skull, cervical spinal cord and ipsilateral intracranial dural space.
The primary site of infection and the chronology of disease progression are speculative. Inhalation of air-borne basidiospores or desiccated yeast cells into the nasal cavity is considered the primary route of infection in cats.14 Colonisation of the caudal nasal cavity, with subsequent epithelial invasion, possibly in the vicinity of the nasopharynx, may have been the primary route of infection, with extension via the auditory tube to the middle ear. Possible pathways from the inner ear into the brainstem include erosion through the medial aspect of the petrous temporal bone (although this was not apparent in the CT scans), along the nerves and vessels of the internal acoustic meatus, or via haematogenous spread.16,17 Inoculation of cryptococcal organisms directly into the external ear canal, with subsequent spread to the middle ear by penetration of the tympanic membrane is considered unlikely and usually results in only superficial lesions.18 A grass seed heavily contaminated with cryptococcal cells may provide an explanation for how this might have occurred, although these are less commonly found in the ear canal of cats than dogs. This case is quite distinct from a recent feline case of bilateral cryptococcal otitis interna, which was thought to have arisen haematogenously as there was no evidence of rhinosinusitis disease or otitis media, and a small cryptococcal lesion was also present in the thalamus; this patient was infected with C neoformans var grubii.19
The pathogenesis of cryptococcus in this cat is unusual and supports the contention by Sykes and Malik that VGII and VGIII infections are more invasive than C gattii VGI and C neoformans var grubii infections.14
All clinical signs resolved after aggressive medical therapy and initial de-bulking within the ear canal. While recent papers concerning C gattii VGI infections of the nasal cavity and contiguous tissues in koalas have emphasised the need for surgical intervention and/or intralesional therapy because antifungal agents may not penetrate well into poorly perfused tissues, the requirement for this probably should be made on a case-by-case basis.20,21 Surgical excision is not always possible, as in this cat.
C gattii VGII can be divided into VGIIa, VGIIb and VGIIc strains, with VGIIa being the major genotype in the Vancouver Island outbreak. Determining the specific biotype is critical because C gattii VGII and VGIII isolates tend to demonstrate heteroresistance to fluconazole.22–25 The epidemiological cut-off values for fluconazole in VGII and VGIII infections are therefore higher than for VGI and C neoformans var grubii isolates.22–25 In this instance the causal VGII isolate was susceptible to fluconazole in vitro, with a MIC of 8 mg/l. Given the pharmacokinetics of fluconazole in the cat, a dose of 10 mg/kg PO q12h should produce peak blood concentrations of fluconazole at steady state in the order of 20–80 mg/l.20
In eastern Australia, about 20% of cryptococcosis in cats and dogs is caused by C gattii (predominantly VGI).1 In Western Australia, VGIIb (mating type α) is the predominant species of C gattii, although VGI and VGII (mating type a) also occur. Studies on the C gattii VGIIa isolates from the Vancouver Island outbreak and VGIIb isolates from Australia have demonstrated that such strains are highly virulent in experimental infections of mice and rats.26,27
Although central nervous system (CNS) involvement is an important negative prognostic indicator in feline cryptococcosis, this cat improved markedly with combination therapy using amphotericin B and fluconazole.28,29 Cross-sectional imaging was not often undertaken when cases of cryptococcosis were first recorded in the veterinary literature. In this case, clinical findings and plain radiographs would not have provided indication of the extensiveness of the disease, and the success of medical therapy in this cat testifies that good outcomes may occur despite extensive invasive disease.
Treatment for CNS cryptococcosis is prolonged and should be continued until the LCAT titre reaches zero, which may take 1–2 years.14 In recovered cats, the titre can be monitored every 3–6 months to allow early detection of recurrences, which is more common than re-infection with a new strain or antifungal resistance.29,30
Conclusions
This case report describes the successful medical treatment of otogenic meningoencephalomyelitis due to C gattii (VGII) infection in a cat.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
Conflict of interest: The authors do not have any potential conflicts of interest to declare.
References
- 1. O’Brien CR, Krockenberger MB, Wigney DI, et al. Retrospective study of feline and canine cryptocococcus in Australia from 1981 to 2001: 195 cases. Med Mycol 2004; 42: 449–460. [DOI] [PubMed] [Google Scholar]
- 2. Kwon-Chung KJ, Polacheck I, Bennett JE. Improved diagnostic medium for separation of Cryptococcus neoformans var neoformans (serotypes A and D) and Cryptococcus neoformans var gattii (Serotypes B and C). J Clin Microbiol 1982; 15: 535–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ngamskulrungroj P, Gilgado F, Faganello J, et al. Genetic diversity of the Cryptococcus species complex suggests that Cryptococcus gattii deserves to have varieties. PLoS One 2009; 4: e5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byrnes EJ, Li W, Lewit Y, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 2010; 6: e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartlett KH, Cheng PY, Duncan C, et al. A decade of experience: Cryptococcus gattii in British Columbia. Mycopathologia 2012; 173: 311–319. [DOI] [PubMed] [Google Scholar]
- 6. Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev 2014; 27: 980–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell DH, Sorrell TC, Allworth AM, et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis 1995; 20: 611–616. [DOI] [PubMed] [Google Scholar]
- 8. Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis 1995; 21: 28–34. [DOI] [PubMed] [Google Scholar]
- 9. Carriconde F, Gilgado F, Arthur I, et al. Clonality and alpha-a recombination in the Australian Cryptococcus gattii VGII population an emerging outbreak in Australia. PLoS One 2011; 6: e16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGill S, Malik R, Saul N, et al. Cryptococcosis in domestic animals in Western Australia: a retrospective study from 1995–2006. Med Mycol 2009; 47: 625–639. [DOI] [PubMed] [Google Scholar]
- 11. Ellis DH, Pfeiffer TJ. Natural habitat of Cryptococcus neoformans var. gattii. J Clin Microbiol 1990; 28: 1642–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidd SE, Chow Y, Mak S, et al. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl Environ Microbiol 2007; 73: 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katsu M, Kidd S, Ando A, et al. The internal transcribed spacers and 5.8S rRNA gene show extensive diversity among isolates of the species complex. FEMS Yeast Research 2004; 4: 377–388. [DOI] [PubMed] [Google Scholar]
- 14. Sykes JE, Malik R. Cryptococcosis. In: Greene CE. (ed). Infectious diseases of the dog and cat. St Louis, MO: Elsevier, 2012, pp 621–634. [Google Scholar]
- 15. Beatty JA, Barrs VR, Swinney GR, et al. Peripheral vestibular disease associated with cryptococcosis in three cats. J Feline Med Surg 2000; 2: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sturges BK, Dickinson PJ, Kortz GD, et al. Clinical signs, magnetic resonance imaging features, and outcome after surgical and medical treatment of otogenic intracranial infection in 11 cats and 4 dogs. J Vet Intern Med 2006; 20: 648–656. [DOI] [PubMed] [Google Scholar]
- 17. Spangler EA, Dewey CW. Meningoencephalitis secondary to bacterial otitis media/interna in a dog. J Am Anim Hosp Assoc 2000; 36: 239–243. [DOI] [PubMed] [Google Scholar]
- 18. Martin CL, Stiles J, Willis M. Ocular adnexal cryptococcosis in a cat. Vet Comp Opthalmol 1996; 6: 225–229. [Google Scholar]
- 19. Paulin J, Morshed M, Armien AG. Otitis interna induced by Cryptococcus neoformans var grubii in a cat. Vet Pathol 2013; 50: 260–263. [DOI] [PubMed] [Google Scholar]
- 20. Malik R, Wigney DI, Muir DB, et al. Cryptococcus in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol 1993; 30: 133–144. [DOI] [PubMed] [Google Scholar]
- 21. Byrnes EJ, 3rd, Bildfell RJ, Frank SA, et al. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis 2009; 199: 1081– 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brandt ME, Pfaller MA, Hajjeh RA, et al. Molecular subtypes and antifungal susceptibilities of serial Cryptococcus neoformans isolates in human immunodeficiency virus-associated cryptococcosis. J Infect Dis 1996; 174: 812–820. [DOI] [PubMed] [Google Scholar]
- 23. Chong HS, Dagg R, Malik R, et al. In vitro susceptibility of the yeast pathogen cryptococcus to fluconazole and other azoles varies with molecular genotype. J Clin Microbiol 2010; 48: 4115–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chowdhary A, Randhawa HS, Sundar G, et al. In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii serotype B from north-western India. J Med Microbiol 2011; 60: 961–967. [DOI] [PubMed] [Google Scholar]
- 25. Espinel-Ingroff A, Chowdhary A, Cuenca-Estrella M, et al. Cryptococcus neoformans–Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for amphotericin B and flucytosine. Antimicrob Agents Chemother 2012; 56: 3107–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fraser JA, Giles SS, Wenink EC, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 2005; 437: 1360–1364. [DOI] [PubMed] [Google Scholar]
- 27. Ngamskulrungroj P, Serena C, Gilgado F, et al. Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver Island outbreak genotype. Clin Microbiol Infect 2011; 17: 251–258. [DOI] [PubMed] [Google Scholar]
- 28. Duncan C, Stephen C, Campbell J. Clinical characteristics and predictors of mortality for Cryptococcus gattii infection in dogs and cats of southwestern British Columbia. Can Vet J 2006; 47: 993–998. [PMC free article] [PubMed] [Google Scholar]
- 29. O’Brien CR, Krockenberger MB, Martin P, et al. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Aust Vet J 2006; 84: 384–392. [DOI] [PubMed] [Google Scholar]
- 30. Kluger EK, Karaoglu K, Krockenberger MB, et al. Recrudescent cryptococcosis caused by Cryptococcus gattii (molecular type VGII), over a 13-year period in a Birman Cat. Med Mycol 2006; 44: 561–566. [DOI] [PubMed] [Google Scholar]