Abstract
Case summary
A 6-month-old female domestic shorthair cat was presented with acute onset non-ambulatory right hemiparesis and horizontal nystagmus following an injection attempt in the neck, during which the cat did not cooperate. Magnetic resonance imaging (MRI) revealed a well-defined intra-axial lesion on the right side of the myelencephalon. The lesion was T2-weighted hypointense and T1-weighted hypointense to isointense to grey matter, non-contrast enhancing, with perilesional oedema and signal void on T2*-weighted images. A linear hyperintense lesion in the muscles of the right dorsolateral aspect of the neck on short tau inversion recovery images was also observed. These MRI findings were consistent with iatrogenic brainstem haemorrhage and a muscle needle tract. The cat made a good recovery with just mild residual neurological deficits 6 weeks after the injury.
Relevance and novel information
To our knowledge, this is the first report of an iatrogenic brainstem needle injury in a cat and the first report of a central nervous system iatrogenic trauma after a parenteral injection. Care should be taken with neck parenteral injections, especially in the cranial cervical area.
Introduction
Subcutaneous and intramuscular (IM) injections are common routes of drug administration and the quadriceps, lumbodorsal, trapezius and triceps muscles are recommended IM injection sites.1 Incorrect administration can result in complications such as damage to the sciatic nerve, which has been reported after an IM injection in the hamstring and gluteal muscles.2,3 This paper describes an unusual and severe complication in a cat following a parenteral injection in the cranial cervical area, and it illustrates why it is important to be careful even when performing minimally invasive procedures.
Case description
A 6-month-old female entire domestic shorthair cat was presented to the referring veterinary surgery for neutering. Following an attempt to give parenteral premedication with medetomidine and butorphanol in the neck region of a non-compliant cat using a syringe with a 23 G × 16 mm needle, the cat collapsed, became instantly non-ambulatory and was referred. It was reported that none of the premedication was injected, as the same volume remained in the syringe after the injection attempt. The cat was reported to be previously healthy. Vaccination and worming status were current, and the cat was housed strictly indoors.
On referral, physical examination did not reveal any significant findings other than a small erythematous skin lesion at the injection site in the right cranial cervical area. Neurological examination revealed mild obtundation with intermittent horizontal nystagmus, decreased bilateral nasal stimulation response and ptosis of the right upper eyelid. The cat displayed non-ambulatory right-sided hemiparesis. Paw replacement, hopping, tactile and visual placing on the right thoracic and pelvic limbs were absent. There was increased muscle tone on the left thoracic and pelvic limbs, and a decreased withdrawal reflex on the right thoracic limb. The cutaneous trunci muscle reflex (CTR) was absent. There was no evidence of pain on palpation of the vertebral column or on passive flexion of the head and neck.
Based on the neurological findings, a multi-focal neuroanatomical localisation affecting the brainstem (the vestibular nuclei, the vestibulospinal tract, the sympathetic innervation to the eye, the ascending reticular activating system and the proprioceptive tracts) and C6–T2 spinal cord segments or a single brainstem lesion with spinal shock was suspected. The CTR can be absent in neurologically normal cats.4 The response to stimulation of the nasal mucosa is a cortically mediated withdrawal of the head and was thought to be absent as a result of impairment of the ascending reticular activating system. Main differential diagnoses included a traumatic iatrogenic lesion, vascular accident or inflammatory disease.
Complete blood count and serum biochemistry were unremarkable. Prothrombin and thromboplastin times were normal. Serial blood pressure measurements were within the normal interval. Dynamic radiographic study (flexed and extended views) of the cervical vertebral column obtained under general anaesthesia did not show any abnormalities.
Magnetic resonance imaging (MRI) of the cervical vertebral column and the brain was performed using a 1.5 Tesla unit (Siemens Magnetom Essenza; Siemens). A well-defined, 2.5 mm in diameter, rounded intra-axial lesion was identified at the right ventro-lateral aspect of the myelencephalon. The lesion was hypointense on T2-weighted (T2W) and iso- to hypo-intense on T1-weighted (T1W) images when compared with normal grey matter. A rounded signal void corresponding to the lesion described above was identified on T2*-weighted images. A perilesional rim hyperintensity on T2W and fluid-attenuated inversion recovery images was also observed (Figures 1 and 2a). There was no contrast enhancement after intravenous injection of gadolinium (0.1 mmol/kg, Gadovist; Bayer). A caudodorsal to cranioventral linear hyperintense lesion was observed in the muscles of the right dorsolateral aspect of the neck at the level of C1–C2 on parasagittal short tau inversion recovery images in the direction of the cisterna cerebellomedullaris (CMC) (Figure 2b). The above description was consistent with a haemorrhagic brainstem lesion associated with perilesional oedema. Changes observed in the musculature of the neck were compatible with a needle track. Cerebrospinal fluid (CSF) collection was not attempted.
Figure 1.
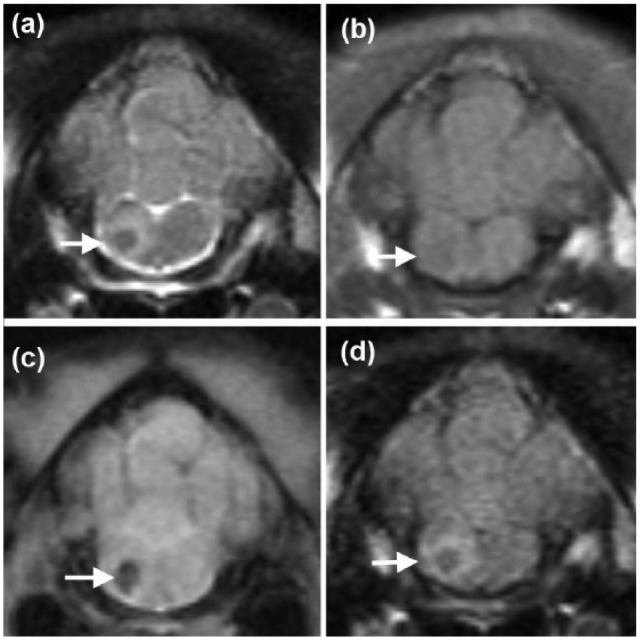
Transverse magnetic resonance images of the head of a 6-month-old female domestic shorthair cat at the level of the myelencephalon, illustrating the well-defined lesion that is hypointense on (a) T2-weighted and iso- to hypo-intense on (b) T1-weighted images, with a signal void on (c) T2*-weighted images and a perilesional rim hyperintensity on (a) T2-weighted and (d) fluid attenuated inversion recovery images (arrows)
Figure 2.
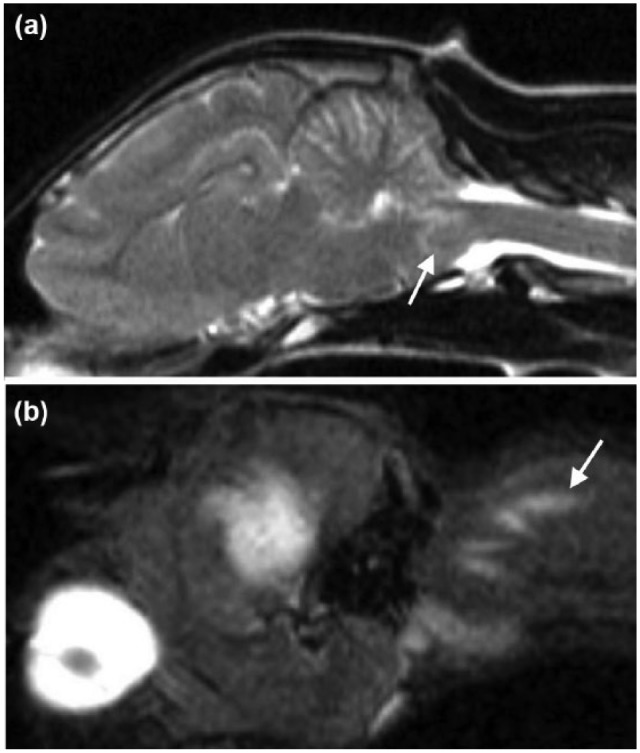
Sagittal magnetic resonance images of the head and neck of a 6-month-old female domestic shorthair cat illustrating the hypointense lesion surrounded by a hyperintense perilesional rim on T2-weighted images (a, white arrow). On the parasagittal view, a caudodorsal to cranioventral linear hyperintense lesion in the muscles of the dorsolateral aspect of the neck and in the direction of the cisterna cerebellomedullaris on short tau inversion recover images was observed (b, white narrow)
The only treatment consisted of fluid therapy, and three consecutive anti-inflammatory doses of dexamethasone to reduce perilesional oedema (0.1 mg/kg IV q24h, Dexadreson; Intervet). Clinical signs gradually improved during hospitalisation and the cat regained ambulation but remained hemiparetic and ataxic by day 4. An intermittent wide base stance was observed during recovery. At the time of discharge, on day 7, the cat remained ambulatory hemiparetic, but postural reactions had improved and withdrawal reflexes and cranial nerve examination had normalised. The cat was re-examined a month and a half after diagnosis. A mild residual right hemiparesis with a mild decrease in right postural reactions were the only abnormalities observed. The owner reported that the cat was able to jump normally on to furniture and a gradual improvement had been observed.
Discussion
To our knowledge, this is the first report of an iatrogenic brainstem needle injury in a cat and the first report of a central nervous system (CNS) iatrogenic trauma after a parenteral injection. CNS iatrogenic needle injuries have previously been reported following CSF collection attempts from the CMC and caudal lumbar site in dogs, and following microchip implantation in dogs and cats.5–8 A self-inflicted needle-stick injury and spinal cord infarction has been described in a human being.9 In the present case, we suspect that the needle entered the CNS through the atlanto-occipital junction. The caudodorsal to cranioventral linear hyperintense lesion in the muscles of the dorsal aspect of the neck and the direction towards the CMC supported this theory.
When evaluating the outcome of the four dogs with iatrogenic brainstem injury described in the above literature, one dog died of respiratory arrest, two were euthanased owing to uncertainty of recovery and sudden deterioration 12 days after diagnosis, and one was lost to follow-up after 10 days of hospitalisation and recovery of voluntary urination and ambulation with assistance.5 Overall the outcome within this small cohort of cases is poor. We report a good outcome in this cat.
The MRI signal for intracranial haemorrhage is well documented.10,11 Its appearance varies based on duration and alterations in blood haemoglobin. The cat was imaged <8 h after the traumatic injury. In hyperacute (<24 h) lesions, T2W hyperintense and T1W iso- to hypointense signal intensities are expected in parenchymal haematomas.11 The prompt imaging in this cat may have influenced the absence of a T2W hyperintense lesion signal, but the signal void observed in T2*-weighted images, supported its presence. There was evidence of cytotoxic and/or vasogenic oedema around the suspected haematoma. Similar findings were reported in the dog with haematomyelia after lumbar puncture.6 The absence of coagulopathy supported the iatrogenic origin.
In the absence of trauma, spontaneous intraparenchymal haemorrhage in humans is a common cause of intracranial haemorrhage. The vast majority of cases with primary brainstem haemorrhages occur in the pons and account for 5–10% of intracranial haemorrhages.12 Other causes include ruptured aneurysm, arteriovenous malformations, cavernous haemangioma, moyamoya disease, vasculitis, brain tumours and haemorrhagic infarctions.13 The volume of the brainstem haematoma has been correlated with survival and functional outcome.13 The efficacy of surgery for haematoma evacuation and external ventricular drainage remains a debate and further investigations are required.14 In the present case, we elected for conservative management owing to the non-progressive nature of the cat’s clinical signs since onset.
Spinal shock is defined as a profound temporal depression in segmental spinal reflexes below (ie, caudal to) a lesion, even though the reflex arcs remain physically intact.15,16 It has not only been induced in dogs after experimental thoracic spinal cord transections,17,18 but has also been described in clinical cases with spinal cord injury in the T3–L3 spinal cord segments.19–21 To our knowledge, there is only a single dog described in the veterinary literature with transient decreased segmental spinal reflexes after acute cervical spinal cord injury;20 however, to our knowledge this phenomenon has not been described in either dogs or cats after brainstem injury. Spinal reflexes are thought to be lost because descending facilitatory inputs from two nuclei located in the pons and serotonergic and noradrenergenic fibres arising from the dorsal raphe nucleus and locus coeruleus are lost, causing a reduction in excitability of spinal motor neurons.19,22 The lesion in this cat was located below the level of the pons. The absence of a lesion in the C6–T2 spinal cord segment and the rapid recovery of the withdrawal reflex, supported the presence of spinal shock.
The main limitation of this case report is the absence of histopathological confirmation. However, the acute onset after the neck parenteral injection attempt, the skin lesion, the orientation of the suspected muscle needle tract and the similar imaging findings to the previously described iatrogenic traumatic brainstem and lumbar needle injuries in dogs strongly support our presumptive diagnosis.5,6
Conclusions
This case demonstrates the clinical and imaging appearance of an iatrogenic needle brainstem injury in a cat. It also shows that spinal shock can occur with myelencephalic injuries. Care should be taken with neck parenteral injections in small dogs and cats, especially in the cranial cervical area. Although there is insufficient data in veterinary literature to evaluate properly the outcome of dogs and cats after traumatic brainstem needle injury, the present case suggests that a good outcome is possible in some cats just with supportive treatment.
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Orpet H, Welsh P. Administration and dispensing of medications. In: Orpet H, Welsh P. (eds). Handbook of veterinary nursing. 1st ed. Oxford: Blackwell Science, 2002, pp 63–64. [Google Scholar]
- 2. Forterre F, Tomek A, Rytz U, et al. Iatrogenic sciatic nerve injury in eighteen dogs and nine cats (1997–2006). Vet Surg 2007; 36: 464–471. [DOI] [PubMed] [Google Scholar]
- 3. Evans HE, de Lahunta A. Spinal nerves. In: Evans HE, de Lahunta A. (eds). Miller’s anatomy of the dog. 4th ed. St Louis, MO: WB Saunders, 2013, pp 650–651. [Google Scholar]
- 4. de Lahunta A, Glass E, Kent M. Lower motor neuron: spinal nerve, general somatic efferent system. In: de Lahunta A, Glass E, Kent M. (eds). Veterinary neuroanatomy and clinical neurology. 4th ed. St Louis, MO: WB Saunders, 2015, pp 110–111. [Google Scholar]
- 5. Luján Feliu-Pascual A, Garosi L, Dennis R, et al. Iatrogenic brainstem injury during cerebellomedullary cistern puncture. Vet Radiol Ultrasound 2008; 49: 467–471. [DOI] [PubMed] [Google Scholar]
- 6. Platt SR, Dennis R, Murphy K, et al. Hematomyelia secondary to lumbar cerebrospinal fluid acquisition in a dog. Vet Radiol Ultrasound 2005; 46: 467–471. [DOI] [PubMed] [Google Scholar]
- 7. Smith TJ, Fitzpatrick N. Surgical removal of a microchip from a puppy’s spinal canal. Vet Comp Orthop Traumatol 2009; 22: 63–65. [DOI] [PubMed] [Google Scholar]
- 8. Platt S, Wieczorek L, Dennis R, et al. Spinal cord injury resulting from incorrect microchip placement in a cat. J Feline Med Surg 2007; 9: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joseph G, Santosh C, Marimuthu R, et al. Spinal cord infarction due to a self-inflicted needle stick injury. Spinal Cord 2004; 42: 655–658. [DOI] [PubMed] [Google Scholar]
- 10. Weingarten K, Zimmerman RD, Deo-Narine V, et al. MR imaging of acute intracranial hemorrhage: findings on sequential spin-echo and gradient-echo images in a dog model. Am J Neuroradiol 1991; 12: 457–467. [PMC free article] [PubMed] [Google Scholar]
- 11. Bagley RS, Gavin PR, Holmes SP. Diagnosis of intracranial disease. In: Bagley RS, Gavin PR. (eds). Practical small animal MRI. 1st ed. Ames, IA: Wiley-Blackwell, 2009, pp 95–102. [Google Scholar]
- 12. Dinsdale HB. Spontaneous hemorrhage in the posterior fossa. A study of primary cerebellar and pontine hemorrhages with observation on their pathogenesis. Arch Neurol 1964; 10: 200–217. [DOI] [PubMed] [Google Scholar]
- 13. Takeuchi S, Suzuki G, Takasato Y, et al. Prognostic factors in patients with primary brainstem hemorrhage. Clin Neurol Neurosurg 2013; 115: 732–735. [DOI] [PubMed] [Google Scholar]
- 14. Hara T, Nagata K, Kawamoto S, et al. Functional outcome of primary pontine hemorrhage: conservative treatment or stereotaxic surgery. No Shinkei Geka 2001; 29: 823–829. [PubMed] [Google Scholar]
- 15. de Lahunta A, Glass E, Kent M. Small animal spinal cord disease. In: de Lahunta A, Glass E, Kent M. (eds). Veterinary neuroanatomy and clinical neurology. 4th ed. St Louis, MO: WB Saunders, 2015, pp 262–263. [Google Scholar]
- 16. Leis AA, Kronenberg MF, Stĕtkárová I, et al. Spinal motoneuron excitability after acute spinal cord injury in humans. Neurology 1996; 47: 231–237. [DOI] [PubMed] [Google Scholar]
- 17. Handa Y, Naito A, Watanabe S, et al. Functional recovery of locomotive behavior in the adult spinal dog. Tohoku J Exp Med 1986; 148: 373–384. [DOI] [PubMed] [Google Scholar]
- 18. Blauch B. Spinal reflex walking in the dog. Vet Med Small Anim Clin 1977; 72: 169–173. [PubMed] [Google Scholar]
- 19. Smith PM, Jeffery ND. Spinal shock-comparative aspects and clinical relevance. J Vet Intern Med 2005; 19: 788–793. [DOI] [PubMed] [Google Scholar]
- 20. Beltran E, Dennis R, Doyle V, et al. Clinical and magnetic resonance imaging features of canine compressive cervical myelopathy with suspected hydrated nucleus pulposus extrusion. J Small Anim Pract 2012; 53: 101–107. [DOI] [PubMed] [Google Scholar]
- 21. De Risio L, Adams V, Dennis R, et al. Association of clinical and magnetic resonance imaging findings with outcome in dogs with presumptive acute non-compressive nucleus pulposus extrusion: 42 cases (2000–2007). J Am Vet Med Assoc 2009; 234: 495–504. [DOI] [PubMed] [Google Scholar]
- 22. Schadt JC, Barnes CD. Motoneuron membrane changes associated with spinal shock and the Schiff-Sherrington phenomenon. Brain Res 1980; 201: 373–383. [DOI] [PubMed] [Google Scholar]


