Abstract
When producing recombinant proteins, the use of Escherichia coli strain BL21(DE3) in combination with the T7-based pET-expression system is often the method of choice. In a recent study we introduced a mechanistic model describing the correlation of the specific glucose uptake rate (qs,glu) and the corresponding maximum specific lactose uptake rate (qs,lac,max) for a pET-based E. coli BL21(DE3) strain producing a single chain variable fragment (scFv). We showed the effect of qs,lac,max on productivity and product location underlining its importance for recombinant protein production. In the present study we investigated the mechanistic qs,glu/qs,lac,max correlation for four pET-based E. coli BL21(DE3) strains producing different recombinant products and thereby proved the mechanistic model to be platform knowledge for E. coli BL21(DE3). However, we found that the model parameters strongly depended on the recombinant product. Driven by this observation we tested different dynamic bioprocess strategies to allow a faster investigation of this mechanistic correlation. In fact, we succeeded and propose an experimental strategy comprising only one batch cultivation, one fed-batch cultivation as well as one dynamic experiment, to reliably determine the mechanistic model for qs,glu/qs,lac,max and get trustworthy model parameters for pET-based E. coli BL21(DE3) strains which are the basis for bioprocess development.
The bacterium Escherichia coli is one of the most widely used host organisms for recombinant protein production1,2,3,4. It features several advantages including extensive knowledge about its genome coming along with the availability of numerous established methods for genetic modification, multiple engineered strains as well as a dazzling array of expression plasmids2,5. Amongst those, the T7-based pET expression plasmids are frequently employed since the strong T7 promoter6 allows exceptionally high yields of recombinant product5,7. The most common approach for inducing these pET-based E. coli strains is by IPTG7,8. IPTG is not metabolized by bacteria, which is why one-point addition is sufficient to guarantee induction5. However, IPTG is known to put a high metabolic burden on E. coli9,10 and is often associated with the generation of misfolded protein aggregates, called inclusion bodies11,12. In contrast, the alternative inducer lactose has been shown to favour the production of soluble product and trigger enhanced productivity13,14,15,16. Even though lactose metabolism is topic of some recent studies17, lactose is scarcely used in biochemical engineering since induction entails the challenge of continuous supply of the disaccharide as it gets metabolized by E. coli. Furthermore, cultivations have to be conducted at limiting amounts of glucose as otherwise lactose uptake is inhibited due to the well-known phenomenon of carbon catabolite repression (e.g. refs 18, 19 and 20).
To shed more light on the mechanistic correlation between the uptake of glucose and lactose, we recently performed a comprehensive study with a recombinant pET-based E. coli BL21(DE3) strain producing a single chain variable fragment (scFv) against celiac disease16. We succeeded in establishing a mechanistic model of the specific glucose uptake rate (qs,glu) and the corresponding maximum specific lactose uptake rate (qs,lac,max). Furthermore, we showed that qs,lac,max impacted productivity as well as product location and is thus a crucial parameter for recombinant protein production. Finally, we hypothesized that this mechanistic correlation might describe platform knowledge for E. coli BL21(DE3) strains carrying the pET expression system and proposed to conduct at least four bioreactor cultivations (batch and fed-batch experiments) to determine the mechanistic model for any pET-based E. coli BL21(DE3) strain16.
In the present study we put our hypothesis to test and investigated the mechanistic correlation of qs,glu and qs,lac,max for four pET-based E. coli BL21(DE3) strains producing different recombinant proteins. We were able to prove the mechanistic model to be applicable and concluded that this model in fact describes platform knowledge for pET-based E. coli BL21(DE3) strains. However, we found that model parameters were strongly dependent on the recombinant product. This finding argued for the need of physiological characterization of each recombinant pET-based E. coli BL21(DE3) strain in order to optimize recombinant protein production as well as to avoid sugar accumulation and resulting osmotic stress for the cells. Driven by this need we investigated different dynamic strategies to accelerate the establishment of the mechanistic model. In fact, we succeeded and propose a strategy comprising only three cultivations, including a batch, a standard fed-batch and a dynamic fed-batch cultivation, to determine the mechanistic qs,glu/qs,lac,max-model for pET-based E. coli BL21(DE3) strains. We believe that all scientists working with recombinant E. coli BL21(DE3) strains carrying the pET expression system will benefit from this study as we do not only present mechanistic platform knowledge, but also offer a strategy for fast determination of this mechanistic correlation.
Results and Discussion
Does the mechanistic qs,glu/qs,lac,max model represent platform knowledge for E. coli BL21(DE3)?
The main motivation for this study was to test if the previously generated mechanistic model of qs,glu and qs,lac,max (Equation 1) for a recombinant E. coli BL21(DE3) strain producing a scFv with a pET expression system describes platform knowledge for pET-based E. coli BL21(DE3) strains.
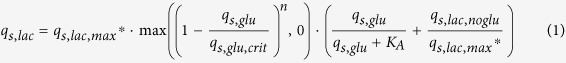 |
qs,lac Specific lactose uptake rate [g/g/h].
qs,lac,max* Maximum specific lactose uptake rate [g/g/h].
qs,glu Specific glucose uptake rate [g/g/h].
qs,glu,crit Critical specific glucose uptake rate up to which lactose is consumed [g/g/h].
qs,lac,noglu Specific lactose uptake rate at qs,glu = 0 [g/g/h].
KA Affinity constant for the specific lactose uptake rate [g/g/h].
n Type of inhibition (noncompetitive, uncompetitive, competitive).
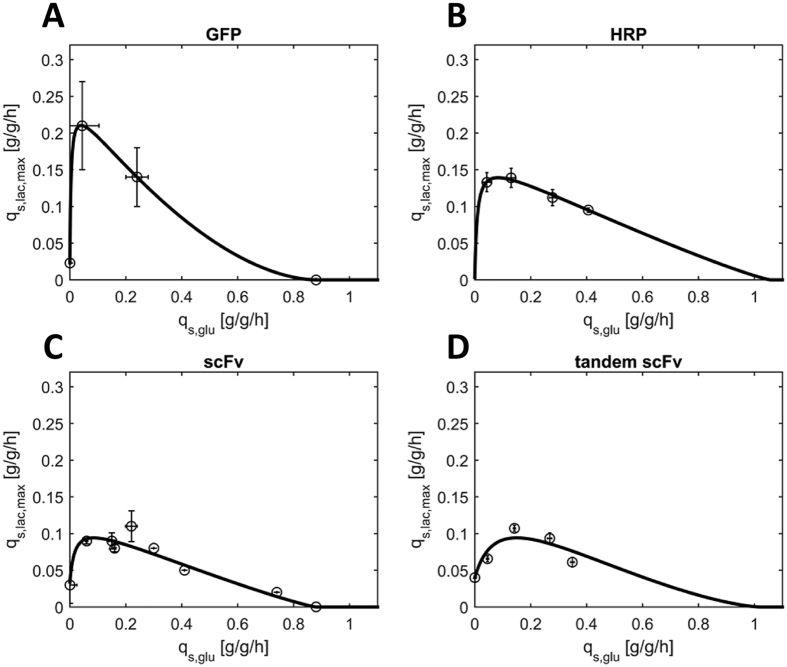
Thus, we investigated this mechanistic correlation for four different pET-based recombinant E. coli BL21(DE3) strains producing either (1) the model protein green fluorescent protein (GFP), (2) the plant enzyme horseradish peroxidase (HRP; e.g. ref. 21), (3) a scFv against celiac disease22 and (4) a novel tandem construct of this scFv. HRP was produced in the periplasm of E. coli, whereas the other three proteins were produced in the cytoplasm. As suggested previously16, we performed at least four bioreactor cultivations, where we adjusted constant process parameters (hereafter referred to as “static experiments”), for each strain to determine the mechanistic correlation of qs,glu and qs,lac,max. Then, we fitted the parameters of the mechanistic model (Equation (1))16 to the data. In fact, we were able to demonstrate that the mechanistic model was applicable for all four strains (Fig. 1). All curves followed the same trend: little lactose uptake at low qs,glu, followed by a steep increase in qs,lac,max, preceded by a comparatively shallow decrease at higher levels of qs,glu. We explain this trend as follows: At low levels of qs,glu little lactose is taken up by the cells, as energy is required for the ATP-related lactose transport into the cell23. Towards higher qs,glu, qs,lac,max increases but then gradually drops again due to the well-studied effects of carbon catabolite repression16,18,19,20,24. Due to the results depicted in Fig. 1, we concluded that the mechanistic model (Equation (1)) in fact describes platform knowledge for pET-based recombinant E. coli BL21(DE3) strains.
Figure 1.
qs,glu/qs,lac,max-correlation for recombinant pET-based E. coli BL21(DE3) strains producing either (A) GFP, (B) HRP, (C) the scFv or (D) the tandem scFv. Data-points were obtained from batch and fed-batch cultivations with constant qs,glu and excess lactose (“static experiments”) and subsequently fitted to the mechanistic model (Equation (1)).
Even though all model parameters were within a physiologically meaningful range, we found striking differences between the four different strains (Table 1).
Table 1. Model parameters for recombinant pET-based E. coli BL21(DE3) strains producing GFP, HRP, the scFv or the tadem scFv.
| rec. product | qs,lac,max* [g/g/h] | KA [g/g/h] | qs,glu,crit [g/g/h] | n [-] | qs,lac,noglu [g/g/h] | NRMSE [%] |
|---|---|---|---|---|---|---|
| GFP | 0.23 | 0.0042 | 0.88 | 1.77 | 0.023 | 0.04 |
| HRP | 0.17 | 0.0092 | 1.06 | 1.15 | 0.0032 | 5.14 |
| scFv | 0.09 | 0.019 | 0.88 | 1.16 | 0.034 | 9.72 |
| tandem scFv | 0.13 | 0.094 | 1.02 | 1.48 | 0.040 | 9.11 |
The mechanistic model is shown in Equation (1).
Thus, we performed an identifiability analysis to verify the model parameters. For the strains producing HRP, IGY and the scFv the analysis revealed identifiable parameters. However, for the strain producing the tandem scFv we had to include general knowledge derived from the other strains to identify the parameters making them slightly more error prone. We believe that for that strain the static experiments were not ideally distributed over the whole qs,glu range (Fig. 1D). However, the normalized root mean square error (NRMSE) of all curves was below 10% attesting a good correlation between experimental data and data points from the fitted curves. Since all these strains were pET-based BL21(DE3) chassis strains, we concluded that mechanistic model parameters mainly depended on the recombinant product. This fact strongly argues for physiological strain characterization of each recombinant E. coli BL21(DE3) strain, not only to optimize recombinant protein production, but also to avoid sugar accumulation and thus osmotic stress for the E. coli cells25. In our previous study we proposed to conduct at least four bioreactor cultivations to determine the mechanistic qs,glu/qs.lac,max-correlation for any pET-based E. coli BL21(DE3) strain. Although this hypothesis obviously held true (Fig. 1), we were eager to find another strategy allowing faster strain characterization and thus faster bioprocess development.
Use of dynamics for strain characterization
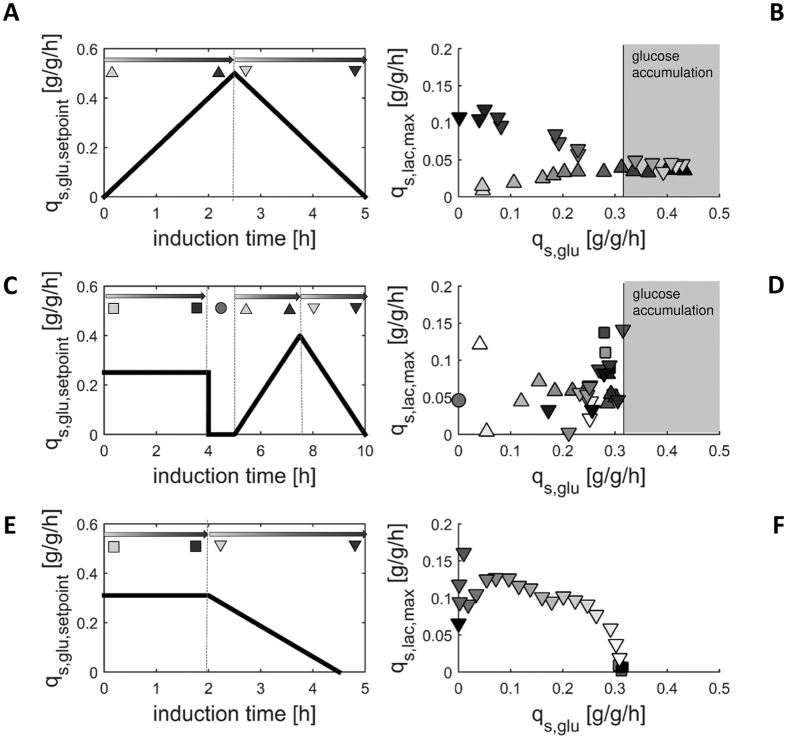
We used the pET-based recombinant E. coli BL21(DE3) strain producing the tandem scFv against celiac disease to test different dynamic methods to possibly allow faster determination of the qs,glu/qs.lac,max-correlation. Applying dynamic process conditions to accelerate bioprocess development is a common approach in our working group26,27,28,29,30. The different dynamic strategies tested are schematically depicted in Figs 2 and 3. In general, all experiments were conducted by employment of qs,glu-ramps and lactose in excess.
Figure 2.
Dynamic bioprocess strategies (A,C,E) and respective resulting qs,glu/qs,lac,max-correlations (B,D,F) for the pET-based recombinant E. coli BL21(DE3) strain producing the tandem scFv against celiac disease. Different feeding phases are marked by different symbols. Time courses within phases go from light grey to dark grey, as indicated on the left. The shape and colour of the symbols of the qs,glu/qs,lac,max data points on the right (B,D,F) correspond to the symbols on the left (A,C,E). Samples were taken every hour during adaptation, every 10 min during qs,glu ramps and every hour after the ramp. For clear data representation error bars were omitted. The error was always between 7% and 15%.
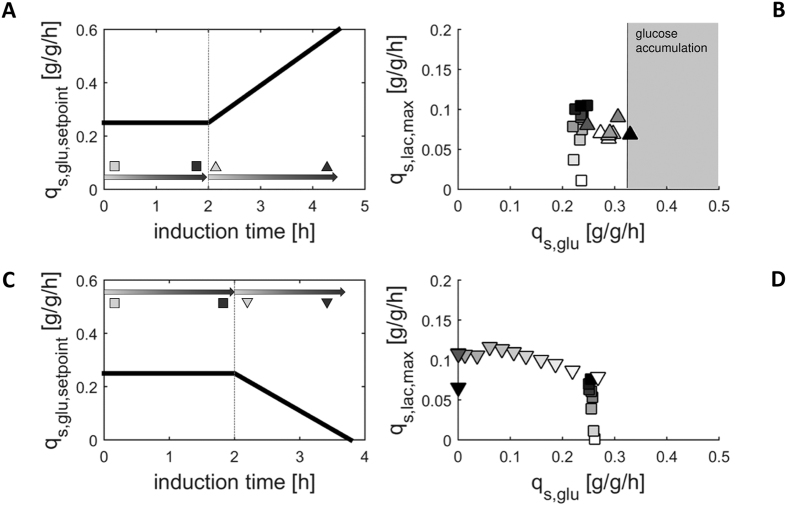
Figure 3.
Optimized adaption followed by two dynamic experiments: ramp up (A,B) and ramp down (C,D). Samples were taken every 10 min during adaptation and every 30 min during qs,glu ramps. In the ramp down experiment another sample was taken after 4 hours at qs,glu = 0 g/g/h (black triangle in Fig. 3D). For clear data representation error bars were omitted. The error was always between 7% and 15%.
Hysteresis of qs,glu
In the first dynamic experiment qs,glu was increased from 0 g/g/h to 0.5 g/g/h and then decreased again to 0 g/g/h within 5 h resulting in a hysteresis of the specific uptake rate of glucose (Fig. 2A). As shown in Fig. 2B, qs,lac,max increased with increasing qs,glu. However, the absolute values were far lower compared to the results in static experiments (Fig. 1D). We hypothesized that E. coli needs time to adapt to lactose since enzymes required for uptake and metabolism of the disaccharide have to be expressed, a phenomenon which has been described for diauxic growth before31,32. Thus, we decided to include an adaptation phase to lactose before the qs,glu ramp.
Adaptation followed by hysteresis of qs,glu
In the second dynamic experiment, we adjusted qs,glu at 0.25 g/g/h for 4 h in the presence of lactose, followed by 1 h without glucose-feeding to investigate lactose uptake in the absence of glucose. Then, we again performed a qs,glu hysteresis experiment (Fig. 2C). However, as shown in Fig. 2D the qs,glu/qs,lac,max-values did not follow the expected trend, but resulted in a quite chaotic cloud of qs,lac,max data points. Viability measurements using FACS revealed fluctuating viability and up to 10% dead E. coli cells during this dynamic cultivation (Supplementary Figure S1). We hypothesized that 1 h without glucose feed and the long overall induction time of 10 h caused cell death and lysis which in turn led to fluctuating qs-values during the experiment. However, we found that qs,lac,max values remained constant after approximately 2.0 h at qs,glu = 0.25 g/g/h indicating that the cells were fully adapted (Supplementary Figure S2). Furthermore, we observed glucose accumulation at qs,glu values higher than 0.32 g/g/h.
Adaptation followed by qs,glu ramp down
Based on our observations that (1) adaption to lactose took approximately 2.0 h at qs,glu 0.25 g/g/h, (2) glucose accumulation was observed for fully adapted cells at qs,glu higher than 0.32 g/g/h, and (3) overall induction time should be kept short to maintain cell fitness (Supplementary Figure S1), we designed the third dynamic experiment as follows: the adaption phase was conducted for 2 h at qs,glu of 0.31 g/g/h, before qs,glu was ramped down to 0 g/g/h within 2.5 h (Fig. 2E). As shown in Fig. 2F, the data followed the expected trend (Fig. 1D) with two exceptions. First, qs,lac,max values at qs,glu of 0.31 g/g/h (light grey triangles in Fig. 2E) were much lower compared to the values obtained in static experiments (Fig. 1D). We concluded that 2 h of lactose presence at qs,glu of 0.31 g/g/h were not sufficient for full adaptation and that adaptation at qs,glu = 0.25 g/g/h was preferred. Furthermore, we observed quite high values for qs,lac,max at low qs,glu values. While in static experiments we determined a qs,lac,max of 0.04 g/g/h at qs,glu = 0 g/g/h (Fig. 1D, Table 1), we found qs,lac,max values higher than 0.1 g/g/h in the dynamic experiment. However, we analyzed qs,lac,max values for a prolonged time and interestingly observed a constant decrease of qs,lac,max over time (Fig. 2F). We believe that the E. coli cells still harboured a great amount of enzymes required for the transport and metabolism of lactose once they had been cultivated in the dynamic ramp experiment and that the presence of these enzymes was only slowly reduced resulting in the initially high qs,lac,max values. In contrast, when performing a static fed-batch experiment at qs,glu of 0 g/g/h, the cells actually did not have the required energy to produce a great amount of these enzymes resulting in a lower qs,lac,max (Fig. 1D). We obviously have to deal with great time effects when working with the pET-based E. coli systems and lactose induction31,32.
Optimized adaption followed by two ramp experiments
Based on the conclusions drawn from the first three dynamic experiments, we finally tested a strategy comprising two ramp experiments. To guarantee fast adaptation, we adapted the cells at qs,glu = 0.25 g/g/h for 2.0 h. Once cells were fully adapted to lactose, indicated by a constant qs,lac,max, qs,glu was either ramped up until glucose accumulation was observed (Fig. 3A) or ramped down to qs,glu = 0 g/g/h at a rate of 0.14 g/g/h2 to guarantee a total induction time of less than 3 h (Fig. 3C).
As shown in Fig. 3B, cells were fully adapted after 2.0 h. We observed a decrease of qs,lac, max once we increased qs,glu, which is in line with our previous results (Fig. 1D). At qs,glu = 0.32 g/g/h we again observed glucose accumulation confirming our previous observations. When we decreased qs,glu from 0.25 g/g/h to 0 g/g/h we obtained qs,lac,max values which followed the expected trend, but again were higher compared to the values from static experiments (Fig. 1D). We fitted the data of these two ramp experiments to the model (Equation (1)) and compared the model fit and the parameters to the results from static experiments (Fig. 4; Table 2).
Figure 4.
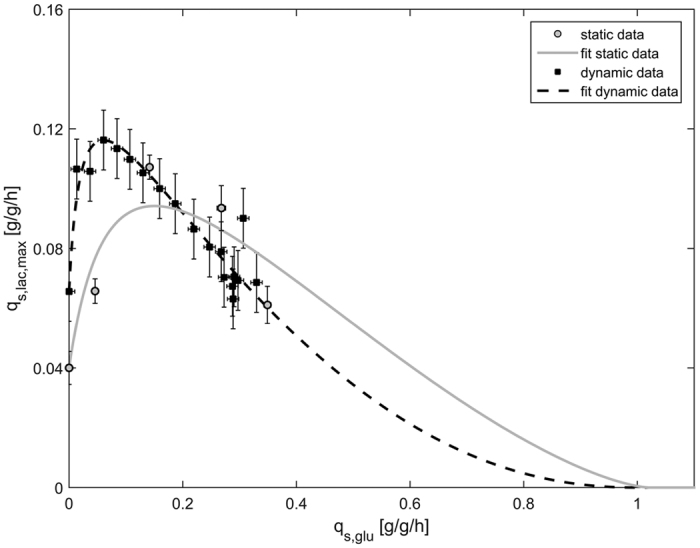
qs,glu/qs,lac,max-correlation derived from data points from static experiments (grey circles, solid line) and from dynamic ramp experiments (black squares, dashed line).
Table 2. Comparison of parameters and NRMSE of models fitted to data from static and dynamic experiments as well as combinations thereof.
| Datasets | qs,lac,max* [g/g/h] | KA [g/g/h] | qs,glu,crit [g/g/h] | n [-] | qs,lac,noglu [g/g/h] | NRMSE [%] |
|---|---|---|---|---|---|---|
| Static data | 0.13 | 0.094 | 1.02 | 1.48 | 0.040 | 9.11 |
| Dynamic data (ramp up and down) | 0.093 | 0.023 | 1.00 | 2.17 | 0.066 | 22.1 |
| Dynamic data (ramp up) | 0.072 | 0.392 | 1.00 | 0.83 | 0.066 | 17.9 |
| Dynamic data (ramp up) & all static data | 0.075 | 0.025 | 1.00 | 1.14 | 0.040 | 12.5 |
| Dynamic data (ramp up) & two static data points (model based) | 0.072 | 0.025 | 1.00 | 1.11 | 0.040 | 12.8 |
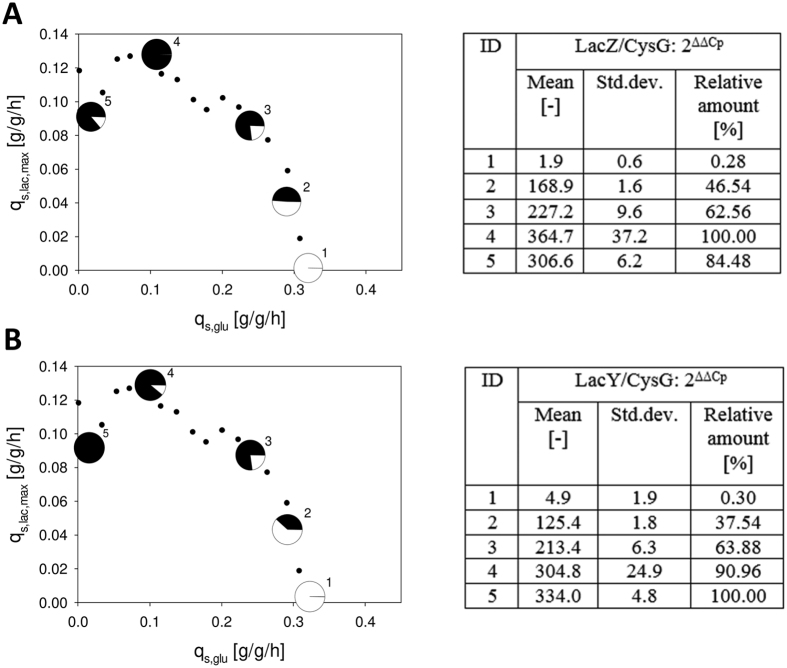
As shown in Fig. 4, the two model fits were very different: the main discrepancy between the two model fits was found at low qs,glu values. However, this also impacted the shape of the curve at high qs,glu values. The NRMSE of the curve derived from ramp experiments compared to the data derived from static experiments was 22.1% (Table 2). Again, we hypothesized that there was still a high amount of enzymes for lactose uptake and metabolism available in the cells distorting the true qs,glu/qs,lac,max-correlation at low qs,glu setpoints. Apparently, the ramp speed was higher than the physiological adaptation of the cells. To put this hypothesis to test and further confirm our observations of the qs,glu/qs,lac,max-correlation by molecular biological data, we performed qPCR-analysis of β-Galactosidase (LacZ) and β-Galactosid-Permease (LacY) (Fig. 5). The transcription of both genes was regulated by the well-studied lac operon which is why abundance of mRNA of either gene depended on the availability of inducer33.
Figure 5.
Time course of transcription of lacZ (A) and lacY (B). qPCR data were referenced to CysG. 2ΔΔCp values were calculated by relating ΔCp to a reference sample which was taken before induction. Pie charts display the percentage of transcription in relation to the highest 2ΔΔCp value obtained.
As shown in Fig. 5, transcription levels of both genes strongly correlated with qs,lac,max. Transcription levels gradually increased with decreasing qs,glu and the consequent increase in qs,lac,max. The transcription level of lacZ was highest at qs,glu = 0.11 g/g/h and then decreased at qs,glu = 0 g/g/h, where also qs,lac,max decreased. However, the transcription level of lacY was highest at qs,glu = 0 g/g/h. We have no explanation for this difference in abundance of lacY and lacZ at this point. However, comparing the transcript levels of both genes at qs,glu = 0 g/g/h and qs,glu = 0.24 g/g/h, where approximately the same qs,lac,max was reached and thus the same amount of inducer was present, we detected large discrepancies (Fig. 5). The amount of mRNA for both genes was 20–40% higher at qs,glu = 0 g/g/h, supporting our hypothesis that the qs,glu ramp was faster than the adaptation capacity of the cells. Thus, we concluded that only static experiments reveal the true qs,glu/qs,lac,max at low qs,glu levels.
Proposed experimental strategy to determine the mechanistic qs,glu/qs,lac,max correlation
In order to find a fast experimental strategy to determine the qs,glu/qs,lac,max-correlation for pET-based recombinant E. coli BL21(DE3) strains, we differently combined static and dynamic experiments and performed sensitivity analyses of the models to investigate the error of fit (Table 2).
As shown in the values of NMRSE (Table 2), data derived from dynamic ramp experiments gave unsatisfactory model fits with respect to static data points (NMRSE = 22.1% and 17.9%, respectively). Combining the data from all static experiments and the ramp up experiment gave a satisfactory fit and a NMRSE of only 12.5% (Table 2). However, since we wanted to develop a strategy comprising less experiments, we conducted a model based experimental design by sensitivity analysis34: we combined the data from the ramp up experiment and from two static experiments at qs,glu = 0 g/g/h and qs,glu = 0.074 g/g/h, respectively, and fitted the model (Fig. 6).
Figure 6.
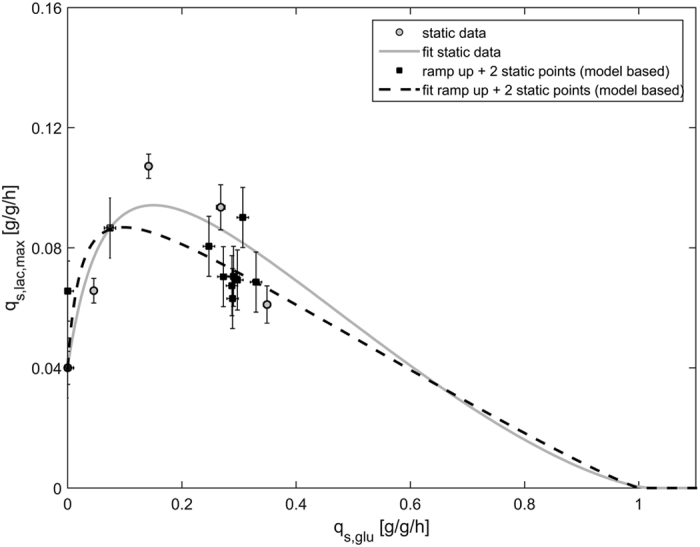
Comparison of the optimal fit for the static experiments and the combination of the dynamic ramp up and two static experiments derived from a model-based experimental design.
In fact, this combination of the dynamic ramp up and two static experiments gave a satisfactory fit and a NMRSE of only 12.8% (Table 2). In terms of the deviating parameters KA and qs,lac,max* (Table 2), we had found that those parameters were hard to identify from static experiments before and were therefore less trustworthy (vide supra). In contrast we were able to identify these parameters from the combination of the dynamic and two static experiments when we performed a practical identifiability analysis. We concluded that the parameters derived from this experimental combination are in fact more trustworthy than the parameters derived from static experiments only. To prove the applicability of the model, we assumed different qs,glu values between 0.1 and 0.8 g/g/h and calculated the respective qs,lac,max values using the model derived from static experiments only as well as from the combination of the dynamic and two static experiments (Table 3).
Table 3. Comparison of qs,lac,max values at different qs,glu setpoints calculated from models derived from static experiments only as well as from the combination of the dynamic and two static experiments.
| qs,glu [g/g/h] | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 |
|---|---|---|---|---|---|---|---|---|
| qs,lac,max [g/g/h] static fit = A | 0.091 | 0.092 | 0.082 | 0.069 | 0.055 | 0.041 | 0.028 | 0.016 |
| qs,lac,max [g/g/h] combination fit = B | 0.087 | 0.081 | 0.072 | 0.061 | 0.050 | 0.039 | 0.029 | 0.018 |
| percentual deviation = (A-B)/A | 4.9% | 12.2% | 13.2% | 11.6% | 8.3% | 3.4% | −3.7% | −13.8% |
As shown in Table 3, the deviation between the calculated values for qs,lac,max for both models were below 15%. Furthermore, a direct comparison of the two model fits gave a NRMSE of only 5.90%, confirming the high similarity thereof. Thus, we propose a strategy comprising only three experiments to determine the mechanistic qs,glu/qs,lac,max correlation for a pET-based recombinant E. coli BL21(DE3) strain. Our strategy can be summarized as:
Perform a batch cultivation on glucose, followed by a lactose pulse to determine the parameter qs,lac,noglu (static experiment No. 1).
Perform a batch cultivation on glucose, followed by a fed-batch cultivation with lactose in excess (>5 g/L) at a low qs,glu value of around 0.1 g/g/h, as sensitivity analysis found data points in this region to be crucial for correct parameter estimation (static experiment No. 2).
Perform a batch cultivation on glucose, followed by a dynamic experiment: Adapt E. coli to lactose at intermediate qs,glu of around 0.25 g/g/h for 2.0 h. However, since adaptation time might differ from strain to strain we recommend at-line HPLC measurements of sugar concentrations and biomass-estimation by OD600 every 30 min to reliably determine full adaptation to lactose (Supplementary Figure S3). After adaption, linearly increase qs,glu at a rate of 0.14 g/g/h2 to guarantee a total induction duration of less than 5 h in order to maintain cell fitness (Supplementary Figure S1).
Plot the qs,glu/qs,lac,max values and fit them to the mechanistic equation to be able to determine the model parameters and thus the physiological limits of the respective E. coli BL21(DE3) strain.
Conclusions
In this study we were able to show that our previously generated mechanistic qs,glu/qs,lac,max model in fact describes platform knowledge for pET-based recombinant E. coli BL21(DE3) strains. We found that model parameters were greatly affected by the recombinant product, pushing for physiological strain characterization of each E. coli strain to allow efficient recombinant protein production and to avoid sugar accumulation and the resulting osmotic stress for the cells. We compared data from different dynamic strategies to the data obtained from static experiments. Finally, we propose a strategy comprising only one batch cultivation, one fed-batch cultivation as well as one dynamic experiment, to reliably determine the mechanistic model for qs,glu/qs,lac,max and get trustworthy model parameters for pET-based recombinant E. coli BL21(DE3) strains.
Material and Methods
Strains
All cultivations were conducted with the E. coli BL21(DE3) strain (Life technologies, Carlsbad, CA, USA). Green fluorescent protein (GFP) was expressed using a pET21a(+) plasmid. Periplasmic horseradish peroxidase (HRP) was expressed from a pET39(+) plasmid. For expression of both the recombinant scFv and the tandem-scFv a pET28a(+) plasmid was used.
Bioreactor cultivations
Media
All fermentations were carried out in defined minimal medium according to DeLisa et al.35. Depending on the antibiotic resistance genes on the plasmid the medium was either supplemented with 0.1 g/L ampicillin or 0.02 g/L kanamycin. Feeds contained 250 g/L Glucose or 200 g/L Lactose, respectively.
Pre-culture
Pre-cultures were conducted by inoculating 500 mL of sterile DeLisa pre-culture medium in a 2500 mL High-Yield shake flask with frozen stocks (1.5 mL, −80 °C) and subsequent incubation in an Infors HR Multitron shaker (Infors, Bottmingen, Switzerland) at 37 °C and 230 rpm for 20 h. Bioreactors were inoculated using a tenth of the final batch volume.
Overall cultivation strategy
All cultivations comprised three phases (batch, non-induced fed-batch, induced fed-batch) including dynamic experiments during induction. Induction was performed by lactose which was applied by a pulse to reach concentrations of 20–25 g/L and then always kept higher than 5 g/L. For that purpose at-line lactose measurements by HPLC were performed.
Static experiments
Experiments for static strain characterisation were performed in DASbox Mini Bioreactors (Eppendorf, Hamburg, Germany) with a working volume of 250 mL. The reactors were supplied with 2 vvm of a mixture of pressurized air and oxygen, the ratio was adjusted in a way to keep dissolved oxygen (dO) above 40% during cultivation. dO was monitored using a fluorescence dissolved oxygen electrode Visiferm DO120 (Hamilton, Reno, NV, USA). The reactors were stirred constantly at 2,000 rpm. pH was monitored by a pH-Sensor EasyFerm Plus (Hamilton, Reno, NV, USA), and kept at 7.2. If necessary, it was adjusted by addition of NH4OH (12.5%). Base uptake was monitored via flowrates with the DASbox MP8 Multipumpmodul. A DASGIP GA gas analyzer (Eppendorf, Hamburg, Germany) was used for monitoring CO2 and O2 concentrations in the offgas. All process parameters were logged and controlled by DASware control.
The batch phase was carried out at 35 °C and yielded a biomass concentration of 8–9 g dry cell weight (DCW) per liter. When the CO2 off-gas signal dropped, indicating the end of the batch phase or glucose depletion respectively, a fed-batch to generate biomass was conducted. Fed-batch phases were conducted at a qs,glu of 0.25 g/g/h. When the DCW reached 25 g/L, the temperature was set to 30 °C and cultures were induced by a lactose pulse to reach a lactose concentration of 25 g/L in the bioreactor. The feed rate was adjusted to control qs,glu (Equation (2)). DCW in the bioreactor was estimated by using a Soft-sensor-tool36.
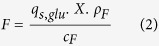 |
F feedrate [g/h].
qs,glu specific glucose uptake rate [g/g/h].
X absolute biomass [g].
ρF feed density [g/L].
cF feed concentration [g/L].
Dynamic experiments
For development of the dynamic strategy, batch and fed-batch cultivations were done in a stainless steel Sartorius Biostat Cplus bioreactor (Sartorius, Göttingen, Germany) with a working volume of 10 L. The reactor was stirred at 1,400 rpm. pH was monitored with an EasyFerm electrode (Hamilton, Reno, NV, USA) and was kept at 7.2 by addition of NH4OH (12.5%) or HCl (18.75%), respectively. Base and acid consumption were determined gravimetrically. For monitoring O2 and CO2 concentrations in the offgas a DASGIP GA gas analyzer (Eppendorf, Hamburg, Germany) was used. Aeration was performed with a mixture of pressurized air and pure oxygen at 1.5 vvm, varying the ratio of pressurized air to pure oxygen in a way that dissolved oxygen (dO) was kept above 40% throughout all cultivations. dO was monitored with a fluorescence dissolved oxygen electrode Visiferm DO120 (Hamilton, Reno, NV, USA). Process parameters were logged and controlled by the process information management system Lucullus (Biospectra, Schlieren, Switzerland).
The batch phase was conducted at 35 °C with an initial glucose concentration of 20 g/L and led to biomass concentrations of 8–9 g DCW per litre. After the end of the batch phase, a fed-batch to generate biomass was carried out.
We fed at a constant specific glucose uptake rate (qs,glu) of 0.25 g/g/h. When the DCW reached 25 g/L, the temperature was lowered to 30 °C and the culture was induced by lactose. During non-induced fed-batch as well as during induction with lactose, DCW was calculated assuming a constant biomass yield (YX/S = 0.37 g/g, own unpublished data; Equation (3)). The feed rate was adjusted to control qs,glu and was calculated according to Equation (2).
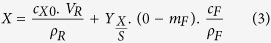 |
cX0 initial biomass concentration [g/L].
VR reactor volume [L].
ρR density of fermentation broth [g/L].
YX/S biomass-yield [g/g].
mF balance signal of feed balance [g].
ρF feed density [g/L].
cF feed concentration [g/L].
Sampling and Analysis
Samples were taken at the beginning and end of the batch and the non-induced fed-batch. During induction, sampling was performed every 30 min to analyze DCW and OD600. In addition, every 10 min supernatant for sugar analysis was collected via an in-line ceramic 0.2 μm filtration probe (IBA, Heiligenstadt, Germany). DCW was determined by centrifuging (4500 g, 4 °C, 10 min) 1 mL cultivation broth, washing the obtained cell pellet with a 0.9% NaCl solution and subsequent drying at 105 °C for 72 h. Optical density at 600 nm (OD600) was determined using a Genesys 20 photometer (Thermo Scientific,Waltham, MA, USA). For staying within the linear range of the photometer (OD600 0.1–0.8) samples were diluted with 0.9% NaCl solution. A calibration correlation OD600 to DCW was established. Sugar concentrations were analysed via HPLC (Thermo Scientific, Waltham, MA, USA) on a Supelcogel column (Supelco Inc., Bellefonte, PA, USA) with 0.1% H3PO4 as eluent at a constant flow of 0.5 ml/min. The method for sugar analysis lasted 15 min. Analysis of the chromatograms was performed using Chromeleon Software (Dionex, Sunnyvale, CA, USA). In order to examine cell-death during bioreactor cultivations, fluorescence-activated cell sorting (FACS) was conducted via Cube 8 (Sysmex Partec, Görlitz, Germany) according to Langemann et al.37.
Data-Analysis
Fitting the data to the mechanistic qs,glu/qs,lac,max-model was carried out according to our previous study16. In short, unknown parameters of Equation (1) were identified using the Nelder-Mead simplex method in MATLAB R2014b to minimize the objective function with respect to physiologically meaningful boundaries (Equation (4)).
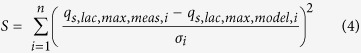 |
S objective function.
qs,lac,max,meas,i ith measurement of qs,lac,max.
qs,lac,max,model,i predicted qs,lac,max at timepoint of ith measurement.
σi standard deviation of the ith data point.
For calculating qs,lac,max-values biomass concentrations as well as lactose amounts were interpolated using a Savitzky-Golay-filter in MATLAB R2014b. A practical parameter identifiability analysis was performed by a method similar to Raue38: for each parameter a physiologically meaningful range [pmin, pmax] was defined and the parameter was held fix at various values inside this range. The objective function S (Equation (4)) was then iteratively minimized for each parameter value with respect to the other parameters resulting in a trajectory Sp. If Sp has a minimum the parameter can be interpreted to be identifiable.
To suggest a model based experimental design to optimally estimate the mechanistic model parameters a local sensitivity analysis of the model parameters was conducted similar to Franceschini et al.34: the parameters were disturbed by ±10%. Those qs,glu values where the deviation of the qs,lac values is maximal with respect to the original parameter values were assumed to contain maximal information to estimate the parameter.
qPCR-Analysis
qPCR-Analysis was done as a commercial service offered by Microsynth AG (Balgach, Switzerland). Reference gene CysG was used. 2ΔΔCp-values were calculated and normalized to the highest value found.
Additional Information
How to cite this article: Wurm, D. J. et al. Mechanistic platform knowledge of concomitant sugar uptake in Escherichia coli BL21(DE3) strains. Sci. Rep. 7, 45072; doi: 10.1038/srep45072 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors thank SCIOTEC Diagnostic Technologies GmbH (Tulln, Austria) for providing the scFv and tandem scFv strains and for fruitful collaboration.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.J.W. and J.H. performed the experiments. S.U. assisted in data evaluation and model fitting. C.H. gave valuable scientific input. O.S. initiated and supervised the study. D.J.W., J.H. and O.S. wrote the manuscript.
References
- Huang C. J., Lin H. & Yang X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J. Ind. Microbiol. Biotechnol. 39, 383–399, doi: 10.1007/s10295-011-1082-9 (2012). [DOI] [PubMed] [Google Scholar]
- Jia B. & Jeon C. O. High-throughput recombinant protein expression in Escherichia coli: current status and future perspectives. Open Biol. 6, 160196, doi: 10.1098/rsob.160196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. et al. Metabolic engineering of Escherichia coli to improve recombinant protein production. Appl. Microbiol. Biotechnol. 99, 10367–10377, doi: 10.1007/s00253-015-6955-9 (2015). [DOI] [PubMed] [Google Scholar]
- Gupta S. K. & Shukla P. Microbial platform technology for recombinant antibody fragment production: A review. Crit. Rev. Microbiol. 4, 31–42, doi: 10.3109/1040841X.2016.1150959 (2017). [DOI] [PubMed] [Google Scholar]
- Rosano G. L. & Ceccarelli E. A. Recombinant protein expression in Escherichia coli: advances and challenges. Front. Microbiol. 5, 172, doi: 10.3389/fmicb.2014.00172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegel H., Ottosson J. & Hober S. Enhancing the protein production levels in Escherichia coli with a strong promoter. FEBS J. 278, 729–739, doi: 10.1111/j.1742-4658.2010.07991.x (2011). [DOI] [PubMed] [Google Scholar]
- Durani V., Sullivan B. J. & Magliery T. J. Simplifying protein expression with ligation-free, traceless and tag-switching plasmids. Protein Expr. Purif. 85, 9–17, doi: 10.1016/j.pep.2012.06.007 (2012). [DOI] [PubMed] [Google Scholar]
- Marbach A. & Bettenbrock K. lac operon induction in Escherichia coli: Systematic comparison of IPTG and TMG induction and influence of the transacetylase LacA. J. Biotechnol. 157, 82–88, doi: 10.1016/j.jbiotec.2011.10.009 (2012). [DOI] [PubMed] [Google Scholar]
- Dvorak P. et al. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microb. Cell Fact. 14, 201, doi: 10.1186/s12934-015-0393-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadin F. T. & Harcum S. W. Transcriptome profiles for high-cell-density recombinant and wild-type Escherichia coli. Biotechnol. Bioeng. 90, 127–153, doi: 10.1002/bit.20340 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang Z. et al. High-level production of membrane proteins in E. coli BL21(DE3) by omitting the inducer IPTG. Microb. Cell Fact. 14, 142, doi: 10.1186/s12934-015-0328-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sina M., Farajzadeh D. & Dastmalchi S. Effects of Environmental Factors on Soluble Expression of a Humanized Anti-TNF-α scFv Antibody in Escherichia coli. Adv. Pharm. Bull. 5, 455–461, doi: 10.15171/apb.2015.062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir H. et al. Simple procedure applying lactose induction and one-step purification for high-yield production of rhCIFN. Biotechnol. Appl. Biochem. 63, 708–714, doi: 10.1002/bab.1426 (2016). [DOI] [PubMed] [Google Scholar]
- Fruchtl M., Sakon J. & Beitle R. Expression of a collagen-binding domain fusion protein: effect of amino acid supplementation, inducer type, and culture conditions. Biotechnol. Prog. 31, 503–509, doi: 10.1002/btpr.2048 (2015). [DOI] [PubMed] [Google Scholar]
- Ma X., Su E., Zhu Y., Deng S. & Wei D. High-level expression of glutaryl-7-aminocephalosporanic acid acylase from Pseudomonas diminuta NK703 in Escherichia coli by combined optimization strategies. J. Biotechnol. 168, 607–615, doi: 10.1016/j.jbiotec.2013.08.024 (2013). [DOI] [PubMed] [Google Scholar]
- Wurm D. J. et al. The E. coli pET expression system revisited-mechanistic correlation between glucose and lactose uptake. Appl. Microbiol. Biotechnol. 100, 8721–8729, doi: 10.1007/s00253-016-7620-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Y. W., Kim J. Y., Lee W. H. & Seo J. H. Enhanced production of 2′-fucosyllactose in engineered Escherichia coli BL21star(DE3) by modulation of lactose metabolism and fucosyltransferase. J. Biotechnol. 210, 107–115, doi: 10.1016/j.jbiotec.2015.06.431 (2015). [DOI] [PubMed] [Google Scholar]
- Kremling A., Geiselmann J., Ropers D. & de Jong H. Understanding carbon catabolite repression in Escherichia coli using quantitative models. Trends Microbiol. 23, 99–109, doi: 10.1016/j.tim.2014.11.002 (2015). [DOI] [PubMed] [Google Scholar]
- Brückner R. & Titgemeyer F. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209, 141–148, doi: 10.1111/j.1574-6968.2002.tb11123.x (2002). [DOI] [PubMed] [Google Scholar]
- Warner J. B. & Lolkema J. S. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67, 475–490, doi: 10.1128/MMBR.67.4.475-490.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadiut O. & Herwig C. Production and purification of the multifunctional enzyme horseradish peroxidase. Pharm. Bioprocess. 1, 283–295, doi: 10.4155/pbp.13.23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlmann V. et al. Novel avian single-chain fragment variable (scFv) targets dietary gluten and related natural grain prolamins, toxic entities of celiac disease. BMC Biotechnol. 15, 109, doi: 10.1186/s12896-015-0223-z (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Zhang T. & Wu H. The transport and mediation mechanisms of the common sugars in Escherichia coli. Biotechnol. Adv. 32, 905–919, doi: 10.1016/j.biotechadv.2014.04.009 (2014). [DOI] [PubMed] [Google Scholar]
- Bettenbrock K. et al. A quantitative approach to catabolite repression in Escherichia coli. J. Biol. Chem. 281, 2578–2584, doi: 10.1074/jbc.M508090200 (2006). [DOI] [PubMed] [Google Scholar]
- Shiloach J. & Fass R. Growing E. coli to high cell density—a historical perspective on method development. Biotechnol. Adv. 23, 345–357, doi: 10.1016/j.biotechadv.2005.04.004 (2005). [DOI] [PubMed] [Google Scholar]
- Dietzsch C., Spadiut O. & Herwig C. A fast approach to determine a fed batch feeding profile for recombinant Pichia pastoris strains. Microb. Cell Fact. 10, 85, doi: 10.1186/1475-2859-10-85 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalai D., Dietzsch C., Herwig C. & Spadiut O. A dynamic fed batch strategy for a Pichia pastoris mixed feed system to increase process understanding. Biotechnol. Prog. 28, 878–886, doi: 10.1002/btpr.1551 (2012). [DOI] [PubMed] [Google Scholar]
- Spadiut O., Rittmann S., Dietzsch C. & Herwig C. Dynamic process conditions in bioprocess development. Eng. Life Sci. 13, 88–101, doi: 10.1002/elsc.201200026 (2013). [DOI] [Google Scholar]
- Jazini M. & Herwig C. Effect of post-induction substrate oscillation on recombinant alkaline phosphatase production expressed in Escherichia coli. J. Biosci. Bioeng. 112, 606–610, doi: 10.1016/j.jbiosc.2011.08.013 (2011). [DOI] [PubMed] [Google Scholar]
- Dietzsch C., Spadiut O. & Herwig C. A dynamic method based on the specific substrate uptake rate to set up a feeding strategy for Pichia pastoris. Microb. Cell Fact. 10, 14, doi: 10.1186/1475-2859-10-14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulineau S. et al. Single-cell dynamics reveals sustained growth during diauxic shifts. PLOS ONE 8, e61686, doi: 10.1371/journal.pone.0061686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F. Jr. & Magasanik B. Glucose-lactose diauxie in Escherichia coli. J. Bacteriol. 93, 1397–1401 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcrand G. et al. DNA supercoiling, a critical signal regulating the basal expression of the lac operon in Escherichia coli. Sci. Rep. 6, 19243, doi: 10.1038/srep19243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini G. & Macchietto S. Model-based design of experiments for parameter precision: State of the art. Chem. Eng. Sci. 63, 4846–4872, doi: 10.1016/j.ces.2007.11.034 (2008). [DOI] [Google Scholar]
- DeLisa M. P., Li J., Rao G., Weigand W. A. & Bentley W. E. Monitoring GFP-operon fusion protein expression during high cell density cultivation of Escherichia coli using an on-line optical sensor. Biotechnol. Bioeng. 65, 54–64, doi: 10.1002/(Sici)1097-0290 (1999). [DOI] [PubMed] [Google Scholar]
- Wechselberger P., Sagmeister P. & Herwig C. Real-time estimation of biomass and specific growth rate in physiologically variable recombinant fed-batch processes. Bioprocess. Biosyst. Eng. 36, 1205–1218, doi: 10.1007/s00449-012-0848-4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langemann T., Mayr U. B., Meitz A., Lubitz W. & Herwig C. Multi-parameter flow cytometry as a process analytical technology (PAT) approach for the assessment of bacterial ghost production. Appl. Microbiol. Biotechnol. 100, 409–418, doi: 10.1007/s00253-015-7089-9 (2016). [DOI] [PubMed] [Google Scholar]
- Raue A. et al. Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics 25, 1923–1929, doi: 10.1093/bioinformatics/btp358 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.