Abstract
There are currently four known isoforms of nitric oxide synthase (NOS). Of these, neuronal NOS (nNOS) is known to be present exclusively in neurons, endothelial NOS (eNOS) in vascular endothelium, while the inducible form of NOS (iNOS) is known to be activated in oligodendrocytes, astrocytes and microglia. The fourth isoform, mitochondrial NOS (mtNOS), represents a post translational modification of nNOS. Using western blotting and real time-PCR, we show induction and activation of nNOS following culture of oligodendrocyte progenitor cells (OPC) with lipopolysaccharide (LPS). Activation of nNOS results in accumulation of peroxynitrite and tyrosine nitration of proteins in oligodendrocytes resulting in reduced cell viability. Injection of LPS in vivo into the corpus callosum of rats leads to the development of extensive demyelination of the white matter tracts. Immunostaining of regions close to the injection site shows the presence of nNOS, but not iNOS, in oligodendrocytes. Neither iNOS nor nNOS was seen in astrocytes in areas of demyelination. These studies suggest that activation of nNOS in oligodendrocytes leads to oligodendrocyte injury resulting in demyelination.
Keywords: Oligodendrocyte, LPS, nNOS, iNOS, demyelination
1. INTRODUCTION
Oligodendrocytes are the myelin producing cells of the central nervous system (CNS) (Bradl and Lassmann, 2009a; Bradl and Lassmann, 2009b). Inability of oligodendrocytes to support the integrity of myelin can result from metabolic, infectious and immune causes. Destruction of oligodendrocytes is a central feature of a number of human demyelinating diseases, the most common of which is multiple sclerosis (MS) (Compston and Coles, 2008).
A number of mutually overlapping mechanisms of oligodendrocyte cell death have been proposed in inflammatory demyelinating diseases of the CNS. Demyelination can occur as a consequence of humoral and cell-mediated cytotoxicity or due to activation of apoptotic pathways induced by local cytokines or due to their susceptibility to excitotoxic death. Cytokines such as tumor necrosis factor (TNF) and gamma interferon (IFN-γ) mediate cell death in oligodendrocytes (Akassoglou et al., 1998). The development of demyelinating lesions in transgenic mice that over express interleukin 6 (IL-6), TNF-alpha and interleukin-12 (IL-12) and evidence of oligodendrocyte toxicity following direct injection of inflammatory cytokines support a role for cytokine-mediated demyelination (Campbell, 1998; Cua et al., 1999; Horwitz et al., 1997).
More recently, the free radical NO (nitric oxide) is proposed to be an important mediator of oligodendrocyte death (Ghafourifar and Sen, 2007; Smith et al., 2001; Smith and Lassmann, 2002). NO is produced by the activation of NOS (nitric oxide synthase) and currently four isoforms of NOS are known (Pacher et al., 2007). Neuronal NOS (nNOS) is constitutively present in neurons and endothelial NOS (eNOS) is present in endothelial cells. The inducible form of NOS (iNOS) is only seen in glial cells, including oligodendrocytes, following stimulation with bacterial cell wall products or cytokines (Giulivi, 2003). Recently, a fourth isoform of NOS, mitochondrial mtNOS, has been reported. mtNOS is a post-translational modified product of nNOS that is myrisylated and phosphorylated and is localized to the inner membrane of the mitochondria (Ghafourifar and Cadenas, 2005; Haynes et al., 2004; Kanai et al., 2001; Pearce et al., 2002). At present there are no reagents that will differentiate mtNOS from nNOS and NOS activity in mitochondria is inferred to result from activation of mtNOS. nNOS and eNOS require calcium/calmodulin for activation, while the activity of iNOS is calcium independent. Although by itself nontoxic, NO can be converted to a number of more reactive derivatives, referred to as reactive nitrogen species (RNS) that can impair cellular function. Most importantly, NO can combine with the free radical superoxide, O2 -, to produce peroxynitrite, which can nitrite or oxidize other molecules. Peroxynitrite accumulation has been thought to play a role in a number of inflammatory and degenerative diseases (Pacher and Szabo, 2008; Radi et al., 2002; Wizemann et al., 1994).
In inflammatory demyelinating diseases, the presence of NO derived compounds has been recognized. Increased levels of nitrite and nitrate levels in cerebrospinal fluid (CSF) of MS patients have argued for a role of reactive nitrogen species (RNS) in MS (Giovannoni, 1998; Rejdak et al., 2004a; Rejdak et al., 2004b). Since nNOS and eNOS were thought to be present in neurons and endothelial cells, the source of derivatives of reactive nitrogen species in brain and CSF in inflammatory disorders was thought to result from activation of iNOS (Brown and Bal-Price, 2003). Demyelination and loss of oligodendrocytes are thought to results from collateral damage from production of iNOS in inflammatory cells.
nNOS was initially described to be present exclusively in neurons. Subsequent studies have shown constitutive expression of nNOS in astrocytes, but the presence and activation of nNOS in oligodendrocytes has thus far not been demonstrated (Kugler and Drenckhahn, 1996; Tolias et al., 1999). Although there are reports on the induction of iNOS in immature oligodendrocytes (OC), there are no reports on either the constitutive or inducible activity of nNOS in OC (Baud et al., 2004; Boullerne and Benjamins, 2006). We have recently shown the induction and activation of nNOS in a human hybrid oligodendroglial cell line (Yao et al., 2009). These studies showed that not only do MO3.13 cells express receptors for Toll ligands, but also that MO3.13 cells activate nNOS in respond to LPS that result in mitochondrial injury and cell death.
We chose to examine the activation and expression of nNOS in non transformed rat oligodendrocytes and in the in vivo model of demyelination induced by intracerebral injection of LPS. The pathological features of the demyelination have been shown to resemble one of the types of demyelinating syndromes seen in a sub population of MS patients. In both the in vitro and in vivo systems of LPS-induced stimulation, we show that the activation of nNOS was associated with dysfunction of oligodendrocytes in vitro and correlated with development of demyelination in vivo.
2. MATERIALS AND METHODS
2.1. Reagents
Lipopolysaccharide (O55:B5), nNOS inhibitor 7-nitroindozale (7-NI), iNOS inhibitor L-Canavanin and MTT assay kit were purchased from Sigma (St. Louis, MO). The following antibodies were used in Western blots: anti nNOS, anti iNOS, anti-β actin and peroxidase conjugated secondary antibodies, (Santa Cruz Laboratories, CA) and anti nitrotyrosine antibodies (Millipore, CA); NOS activity assay kit was purchased from Cayman Chemical. [3H]-L-Arginine was purchased from Amersham. For immunostaining, the following antibodies were used: O4 (MAB345), CNPase (MAB326), Olig1 (MAB5540), GFAP (MAB360), nNOS (AB5380), and anti-nitrotyrosine (AB5532) (Millipore, CA); anti-iNOS (sc-650) and Iba1(sc-32725) (Santa Cruz, CA) and Alexa Fluor 488 and 594 conjugated antibodies (Invitrogen, Oregon). HRP conjugated antibody, Peroxidase block and AEC substrate were purchased commercially (Envision System, DAKO, CA). Alkaline phosphatase conjugated antibody was purchased through Sigma, MO and NBT/BCIP substrate from Boehringer-Mannheim. Antigen unmasking solution and Vecta-Shield mounting medium with DAPI were purchased from Vector Laboratories, CA.
2.2. Preparation of rat primary oligodendrocyte progenitor cells (OPC)
Oligodendrocytes were isolated using previously published protocol (Colello and Sato-Bigbee, 2001). Two-day-old Sprague-Dawley rat pups were anesthetized, decapitated, and the brain removed using sterile technique. The neural cells were mechanically dissociated in ice-cold Hank’s balanced salt solution (HBSS) with 25mM HEPES and passed through a 70 μm strainer, and washed and subjected to Percoll gradient centrifugation. The isotonic Percoll solution was prepared and mixed with the cell suspension (5.7:8.5). The cells that were loaded on the Percoll gradient were spun at 19,000 rpm for 30 min. The oligodendrocyte band was harvested, washed and pelleted. The pelleted cells were placed in Petri dishes for 1h to remove contaminated microglia and astrocytes and the non attached cells were predominantly oligodendrocytes. Suspended cells were collected and re-placed in Petri dishes one more time. The cells were cultured in poly-lysine coated dishes in medium consisting of 500 ml DMEM containing 0.1% BSA, 2.5 mg insulin, 25 mg transferrin, 30 nM sodium selenite, 10 nM D-biotin, 10 nM hydrocortisone, 4 mM L-glutamine, 1 mM sodium pyruvate, 5 μg PDGF, 5 μg b-FGF and antibiotics for 5-7 days. After three to five days in culture, the cells were assessed for purity by immunostaining with anti CNPase, GFAP and Iba1 antibody and used in experimental procedures. With the above protocol we were able to achieve 85% purity of oligodendrocytes. The rest of the cell population consisted of 15% astrocytes and rare microglia and neurons. The cells were Olig2+, Olig1+, CNpase + but weakly expressed myelin basic protein suggesting that they were mainly oligodendrocyte progenitor cells (OPC) that had not yet expressed myelin membranes. MO3.13 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal calf serum (Mediatech, Inc USA, # 35-015-CV endotoxin < 10U) and penicillin-streptomycin (100 u/ml, and 100 μg/ml respectively). Purified preparations of astrocytes were a gift from the laboratory of M. Aschner, Vanderbilt Medical Center and cultured in DMEM/10% FBS/ antibiotics.
2.3. In Vivo injection of LPS into Corpus Calossum
We established the demyelination model system in the corpus calossum in a manner similar to that seen following injection of LPS into the dorsal columns of rat spinal cord. Two-month old_rats were injected with LPS or saline and were sacrificed on day 2, 7, 14, 21 and 28 respectively (eight rats in each group). Rats were anesthetized and positioned in a small-animal stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) to conform to the brain atlas (Pellagrino, 1979 #278). Microinjection of LPS (Escherichia Coli serotype 055:B5) into the corpus callosum was performed with a 32-gauge needle through a dentist’s burr hole. To perform the injection into the corpus callosum, the following coordinates were used: 1 mm posterior from bregma, 1 mm lateral from the sagittal suture, and 3.3-3.5 mm below the dura mater. LPS-treated rats received 5 μl of PBS containing 5 μg of LPS and injection was done using a microinjection pump over 15 minutes and needle was held for an additional 10 minutes after injection. Saline control received 5 μl of PBS solution.
2.4.1 Immunostaining of paraffin embedded brain tissue and cultured glial cells from neonatal rat brains
For immunostaining of paraffin embedded brain tissue, animals were perfused using 4% paraformaldehyde/4% sucrose in PBS at 2, 7, 14, 21 and 28 days after injection with LPS or saline as injection control. Brains were removed and postfixed in 10% formaldehyde for 24 hours. The injection site was identified by placing the brain in a brain mold and a 2mm thickness of brain which spanned 1 mm anterior and 1 mm posterior to the injection track was cut and embedded in paraffin. Serial coronal sections (8-10 micron thickness) of the brain were cut and subjected to immuno and histochemical (Luxol-Fast blue –Periodic-Acid Schiff) staining.
For immunostaining, sections were de-paraffinized and antigen retrieval was done using antigen retrieval solution (Vector Laboratories) for 20 minutes in a steamer. Sections were washed in water and thereafter in TBS-T (Tris buffered saline with Tween-20). Sections were treated with FBS (fetal bovine serum) diluted 1:30 in Tris Buffered Saline (TBS) containing Triton X-100 (0.2%) for 30 minutes at room temperature. The sections were then incubated with primary antibody diluted in TBS-Triton X buffer (Olig1 1:1000, GFAP 1:500, nNOS 1:500, iNOS 1:500, anti-nitrotyrosine 1:500) overnight at 4°C. Sections were washed five times with TBS-T buffer. Sections that were being used for HRP (Horseradish Peroxidase) detection were treated with peroxidase block for 20 minutes and then washed twice. Secondary alkaline phosphatase (AP) conjugated antibodies were diluted in TBS-T at 1:50 (HRP conjugated antibody from DAKO comes pre-diluted) and incubation was done at room temperature. HRP and AP conjugated antibodies were incubated for one hour, whereas the fluorescently labeled antibodies (Alexa Fluor-488 and 594) were incubated for two hours.
Sections were washed five times with TBS-T. AEC substrate was used with HRP conjugated antibodies and NBT/BCIP substrate was used with AP conjugated antibodies for visualization of staining. Enzymatic double staining was done sequentially and heat treatment using antigen retrieval was done in between the first staining (using AP antibodies) and the second (using HRP antibodies) to prevent non specific staining. Fluorescent double staining was done using simultaneous addition of mouse and rabbit antibodies. The stained sections were viewed under a fluorescence microscope (Olympus microscope Ax70A). Images were captured with a CCD camera (Q color 3, Olympus, USA) and Image J (software provided from the NIH) was used to analyze the fluorescent staining.
For staining of glial cells, purified primary oligodendrocytes or astrocytes obtained from rat brains, were sub-cultured in chamber slides for 7 days. Cells were washed with PBS and fixed for 10 min with 4% paraformaldehyde. The cells were then washed twice with 0.1 M Tris pH 8.0 to neutralize the fixative and then washed with PBS. Detergent (0.5% Triton X-100 in PBS) was added 3 × 5 minutes to permeabilize the cells. Cells were washed again with PBS and blocking solution (10% FBS, 0.3% Triton X in PBS) was added for one hour at room temperature (all steps were at room temperature and on a shaker). Primary antibody, diluted in blocking solution, was added to cells and incubated overnight at 4°C (for CNPase 1:500, for GFAP 1:1000, nNOS 1:1500). Cells were washed with washing buffer (2% FBS, 0.3% Triton X in PBS) four times. Secondary antibodies (Alexa Fluor conjugated antibodies), diluted 1:500 in blocking solution, were added to cells and incubated for two hours. Cells were washed four times and visualized.
2.5. Preparation of whole cell lysates
Purified preparations of oligodendrocytes were plated in 60 mm dish and grown until they reached 80-90% confluence. Cells were harvested from cultures, washed in ice-cold PBS, and treated with lysis buffer (20 mM Tris pH 7.6, 150 mM NaCl, 1% Triton-X 100, 0.2 % SDS, 1 mM EDTA and 1 mM DTT) containing a protease inhibitor cocktail (Sigma) for 30 min at 4°C, and centrifuged at 13,000 rpm at 4°C for 20 min. Supernatants were collected as whole cell lysates and protein concentration was established by a Bio-Rad protein assay (Bio-Rad Laboratories, Inc., USA).
2.6. Western blot
20-30 μg of protein samples were electrophoresed in 7.5 or 15% Tris-glycine, SDS gels and transferred onto PVDF membrane (Millipore, USA). After blocking with 5% milk in Tris-buffered saline-Tween 20 (TBS-T) for 2 hours to prevent non-specific binding, the membrane was incubated overnight with primary antibodies. The blots were then washed four times with TBS-T buffer and incubated with a horseradish peroxidase-conjugated secondary antibody for 2 hours at room temperature. The reactive bands were visualized by an ECL-kit (GE Healthcare USA). The amount of beta-actin was also examined by probing with anti beta-actin antibody and served as a control for equal loading of lanes. Densitometric scanning of the protein bands was performed using the ImageJ software provided by NIH.
2.7. Cell viability assay
To determine cell viability, the MTT assay was performed using a commercially available kit (Sigma, MO.). The MTT assay determines the activation of mitochondrial dehydrogenase. 5×103 primary oligodendrocytes were seeded in 96-well plates. After a pre-treatment with the nNOS inhibitor 7-nitroindozale (7-NI, Sigma, MO.) for 10 minutes, the plated cells received a 24 hour treatment of LPS (5ug/ml). 20 μl (0.1mg) of MTT reagent was added to each well, followed by incubation at 37°C for 4 hours in a CO2 incubator. The supernatants were carefully removed and 200 μl of MTT solubilization solution was added to each well to dissolve the resulting formazan crystals. The absorbance at 550 nm was measured using a microplate reader (Bio-Tek instruments) and expressed as percent of treated cells over cells that were treated with vehicle alone.
2.8. Peroxynitrite mediated oxidation assay
ONOO- mediated oxidation was measured in vitro by the oxidation of dihydro-rhodamine 123 (DHR123; Sigma) to fluorescent rhodamine123 (Kooy et al., 1994). Primary oligodendrocytes were pre-treated with or without the nNOS inhibitor 7-NI in various concentrations for 10 minutes followed by a 5 μg/ml of LPS treatment. After 24 h, 5 mM DHR123 was added to each well. After one hour incubation at 37°C, rhodamine 123 fluorescence was measured in a microplate fluorometer (SpectraMax M5, Molecular Devices Corp) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Results were expressed as ratio of signal in treated cells over cells treated with vehicle alone.
2.9. NOS activity assay (citrulline assay)
NOS activity was determined through the conversion of [3H]-L-arginine to [3H]-L-citrulline. Primary oligodendrocytes were incubated in the presence or absence of 5 μg/ml of LPS for 24 hour in a pre-treated condition with/ without 7-NI in different concentrations for 10 minutes. The nNOS activity in samples was measured using a NOS activity assay kit (Cayman Chemical) in the presence of 0.1 μM of calmodulin and 0.6 mM CaCl2 following manufacturer’s instruction. The iNOS activity in samples was measured in the absence of calmodulin (0.1 μM) and CaCl2 (0.6 mM). The NOS activity is expressed as the percent of [3H]-L-citrulline converted from [3H]-L-arginine. Percent conversion = (cpm in final reaction - cpm background)/ total cpm × 100. The results were expressed as percent increase over basal values measured in untreated cells.
2.10. RT-PCR
Total cellular RNA was extracted by TRIzol reagent (Invitrogen, USA). One microgram of total RNA which was digested with RNase-free DNase I (Promega, USA) was reverse transcribed into cDNA using high capacity cDNA reverse transcription kit (Applied Biosystem, USA) following manufacturer’s instruction. RNAs were amplified with Taq polymerase (Roche, USA) in a thermal cycler (MJ Research) using TLR specific primers as shown in table 1. For each primer pair, PCR reactive condition was optimized. PCR mixture was run in a Thermal cycler, at 94°C for 3 min, then 30 cycles of 45 sec at each temperature: 94°C, 60°C and 72°C. PCR products were fractionated by 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized with UV.
Table 1.
Primer Sequences of TLR for RT-PCR.
| Name | Sequences | products size | GeneBank accession no# | |
|---|---|---|---|---|
| Human | ||||
| TLR2 | FW | 5’-TCC GGA GGC TGC ATA TTC CAA AGG-3’ | 295 | NM_004264 |
| RV | 5’-CAG AGT GAG CAA AGT CTC TCC GGT-3’ | |||
| TLR3 | FW | 5’-TCC GTT GAG AAG AAG GTT TTC GGG-3’ | 321 | NM_003265 |
| RV | 5’-ATA TCC TCC AGC CCT CCA AGT GGA-3’ | |||
| TLR4 | FW | 5’-AGG ACT GGG TAA GGA ATG AGC TAG-3’ | 432 | NM_138554 |
| RV | 5’-GTA CCC ACT GGT CCT TCT GGA TTC-3’ | |||
|
| ||||
| Rat | ||||
| TLR2 | FW | 5’-CCA CAG GAC TCA AGA GCA T-3’ | 120 | NM_198769 |
| RV | 5’-AGA ATG GCC TTC CCT TGA-3’ | |||
| TLR3 | FW | 5’-AAC TTG ATT TTC TTG GCA ATT CT-3’ | 137 | NM_198791 |
| RV | 5’- GAG GTT CAG TTG GGC ATT-3’ | |||
| TLR4 | FW | 5’- GGA AAA GCC TTG AAT CCA GA -3’ | 137 | NM_019178 |
| RV | 5’-GCA GAA ACC CAG ATG AAC T-3’ | |||
2.11. Real-Time PCR
Following isolation of mRNA, quantitative PCR’s were carried out by using SYBR Green dye (Roche, USA) in a MYIQ Thermal Cycler (Bio-Rad). Housekeeping genes (β-actin or GAPDH) was used to test the quality of cDNA. nNOS, iNOS and eNOS mRNA were measured by using specific primer sets shown in table 2. The cDNA standards for real-time PCR were prepared from the specific PCR products using a DNA purification kit (Roche, USA). The amount of standard cDNA was determined with a Qubit fluorometer (Invitrogen, USA). Standard concentration ranged from 10 fg/μl to 0.001 fg/μl. Quantification was done in a two step real-time PCR with a denaturation step at 95°C for 3 min and then 40 cycles of 95°C – 30 sec and 60°C -1 min. The primers for the different sets are shown in Table 2.
Table 2.
Primer sequences for Real-Time PCR.
| Name | Sequences | products size | GeneBank accession no# | |
|---|---|---|---|---|
| Rat | ||||
| nNOS | FW | 5’-TCTACGCCACAGAGACAGGCAAAT-3’ | 92 | NM_052799 |
| RV | 5’-CATGGACATTGCCTTGGCATCGAA-3’ | |||
| iNOS | FW | 5’-AGC ATC CCA AGT ACG AGT-3’ | 140 | NM_012611 |
| RV | 5’-AAT CTC GGT GCC CAT GTA-3’ | |||
| eNOS | FW | 5’- CGG AGA ATG GAG AGA GCT TT-3’ | 113 | NM_021838 |
| RV | 5’-GGA GAC ACT GTT GAA TCG GA-3’ | |||
| GAPDH | FW | 5’-ACAAGATGGTGAAGGTCGGTGTGA-3’ | 199 | NM_017008 |
| RV | 5’-AGCTTCCCATTCTCAGCCTTGACT-3’ | |||
2.12. Statistical analysis
All data are presented as mean± SD. Multiple comparisons were made using one-way analysis of variance (ANOVA), followed by the Newman–Keuls Multiple comparison test. Comparisons between treatment-groups versus controls were analyzed by Student’s paired t-test. A p value less than 0.05 was considered as statistically significant. Statistical analyses were performed with Prism3 (GraphPad Software).
3. RESULTS
3.1. Induction of nNOS in oligodendrocyte cultures
Although constitutive expression of nNOS was seen in OPC but not in GFAP+ cells, increased expression on nNOS following culture with LPS was not clearly evident (Figure 1a). We therefore performed western blot studies on OPC as well as purified astrocyte cultures after stimulation with LPS (5 μg/ml) to determine if levels of nNOS or iNOS increased following LPS treatment. Results showed a time dependant increase in nNOS protein (150kd) following stimulation of primary oligodendrocytes with LPS. Densitometric analysis showed a 1.7-fold increase in the expression of nNOS protein in cytosolic extract following 8 hour stimulation with LPS. Re-probing of the membrane with anti iNOS antibody failed to show any iNOS (iNOS focuses at 130kD), suggesting a lack of induction of iNOS in primary oligodendrocytes following stimulation with LPS. To ensure that the induction of nNOS was not due to contaminating astrocytes, we examined the induction of nNOS in astrocytes cultured with LPS. There was no induction of nNOS in astrocyte cultures, but a 5-fold increase in iNOS expression was seen, maximal at 8 hours. These studies demonstrated a restricted and tissue-specific induction of iNOS and nNOS following stimulation with LPS (figure 1b,c).
Figure 1.
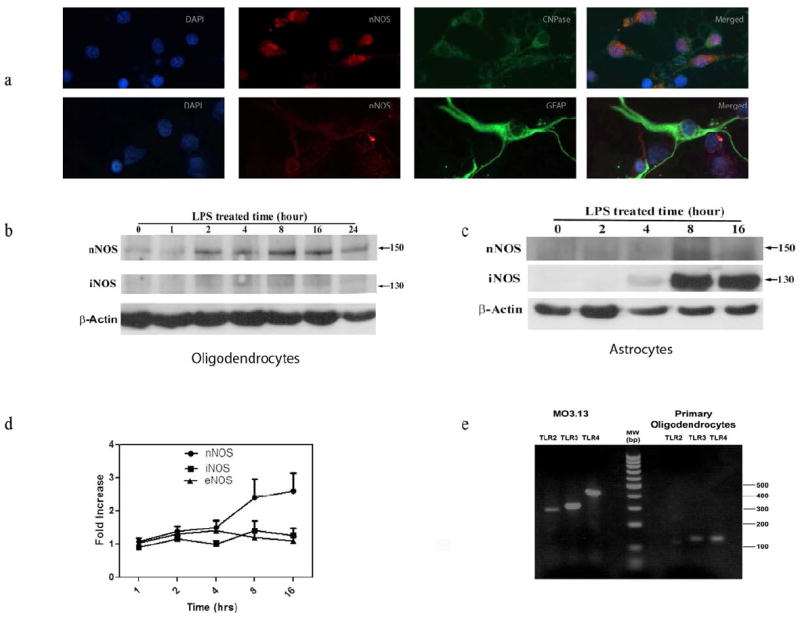
a. Constitutive expression of nNOS in (top panel) primary oligodendrocytes (top panel) but not astrocytes (bottom panel).
b. Western blotting studies on the expression of nNOS and iNOS in oligodendrocyte cells following culture with LPS (5μg/ml).
c. Western blotting studies on the expression of nNOS and iNOS in astrocytes following culture with LPS (5μg/ml).
d. Kinetics of induction of nNOS, iNOS and eNOS mRNA in primary oligodendrocytes following culture with LPS (5μg/ml).
e. RT-PCR analysis of TLR 2, 3 and 4 genes of MO3.13 cells and primary oligodendrocytes using TLR specific primers.
To determine the transcriptional levels of NOS genes following stimulation with LPS, real time RT-PCR was done in OPC using primers specific for nNOS, iNOS and eNOS. Primary oligodendrocytes cultured with LPS showed a time dependant 2.5-fold increase in nNOS (*p< 0.05), maximal at 8 hours. There was no substantial increase in iNOS or eNOS observed in LPS-stimulated oligodendrocyte cultures (Fig 1d).
Since activation of LPS is mediated following activation of Toll like receptors, we ensured the expression of TLR genes in oligodendrocytes. As shown in figure 1e, the human MO3.13 cell line expressed the three different TLR genes while in primary rat OPC, the signal for TLR3 and TLR4 was strong but TLR2 was weakly expressed. These studies show the presence of mRNA for key TLR genes that are involved in innate immunity to be present in oligodendrocytes.
3.2. Increase in NOS activity in LPS-treated OPC
Any biological relevance of nNOS can only be inferred if increased expression of nNOS protein coincides with increased enzymatic activity. We therefore determined if increased expression of nNOS in oligodendrocyte progenitor cells was associated with increase in NOS activity. NOS activation results in the conversion of arginine to citrulline and is measured by the accumulation of [3H]-L-citrulline following addition of radiolabelled [3H]-L-arginine in the presence or absence of LPS (5 μg/ml). The specificity of nNOS was determined by the inhibition of the production of [3H]-L-citrulline following addition of 7-NI or the necessity of calcium and calmodulin in the reactions. Our time course studies showed that addition of LPS resulted in a 34% increase in nNOS activity, maximal at 16h. The experiments were repeated following an 8 hour culture with LPS (5 μg/ml) in the presence or absence of 7-NI and the amount of [3H] -L-citrulline was measured. Addition of 7-NI at doses of 0.016 mM or greater resulted in a significant reduction of NOS activity (*p<0.05 as compared to treated with LPS alone). Also, there was no activation of NOS in the absence of calcium, suggesting the majority of the activation of NOS was calcium dependant and therefore unlikely to be due to activation of iNOS (Figure 2a and 2b).
Figure 2.
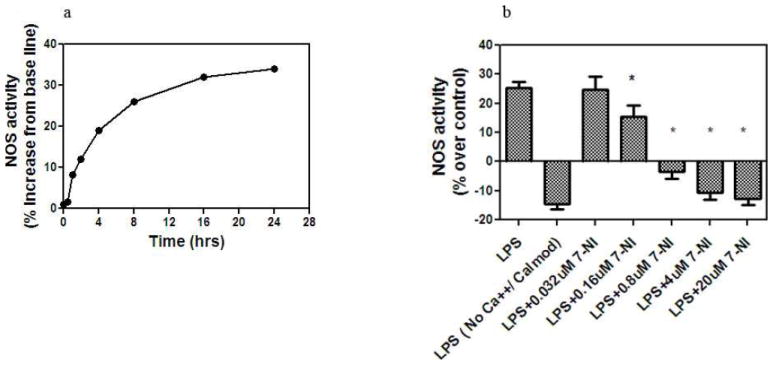
Measurement of NOS activity in oligodendrocyte cultures with LPS (5μg/ml): a) Kinetics of nNOS activity in oligodendrocyte cells cultured with LPS (5μg/ml). Results are expressed as the percent increase in NOS activity over base line. Each value represents mean ±SD of assays done in triplicate; b) Inhibition of NOS activity following culture of oligodendrocytes with LPS for 8 hours, in the absence of calcium and calmodulin and following addition of 7-NI (* p<0.01).
3.3. Induction of nNOS in LPS-stimulated cells results in increased expression of peroxynitrite and decreased cell viability
Since activation of nNOS in the presence of inadequate scavenging of reactive oxygen species leads to the production of peroxynitrite (ONOO-), we examined the induction of ONOO- in OPC treated with LPS (5μg/ml). The oxidation of DHR 123 by peroxynitrite is used to measure the amount of peroxynitrite formation in vitro. Treatment of oligodendrocytes with LPS (5 μg/ml) for 24 hours caused a 67% increase in the amount of rhodamine123 signal, following conversion of DHR 123 to rhodamine 123 (*p< 0.05 compared to baseline). The accumulation of ONOO- was reversed when oligodendrocytes were cultured in the presence of LPS and increasing concentration of 7-NI, suggesting that the increase was due to nNOS activity (Fig. 3a). Since the accumulation of peroxynitrite in the cytosol and in mitochondria can interfere with cellular functions, including cell viability, we examined the viability of the oligodendrocytes cultured with LPS using the MTT assay (3b). There was a 39% decrease in viability in OPC that were cultured for 24 hours in the presence of LPS. Addition of 7-NI to the cultures reversed cell death and restored viability (*p<0.05 as compared with LPS-treated cells).
Figure 3.
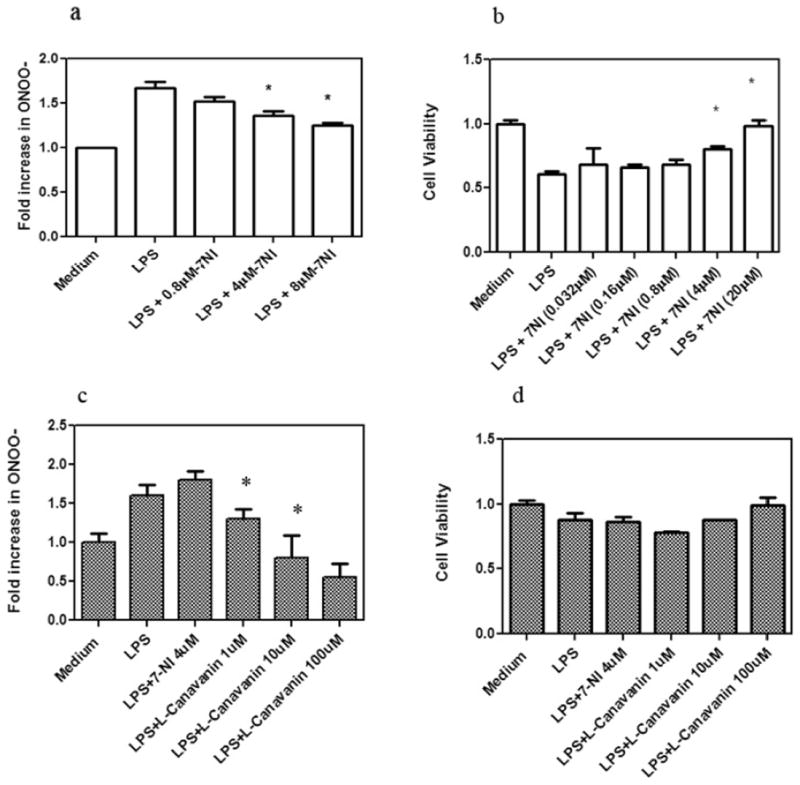
The induction of peroxynitrite and the reduction of cell viability in oligodendrocytes following culture with LPS (5μg/ml).
a) Increase in peroxynitrite in cells cultured with LPS and its reversal with 7- NI. Results are expressed as percent increase, in the amount of rhodamine, over base line. Experiments were done in triplicate and were repeated twice (*p<0.05).
b) MTT assay of oligodendrocyte cells cultured with LPS (5μg/ml), and following addition of 7-NI. Data is expressed as percent decrease of absorbance at 550 nm. Data show the inhibition of the MTT assay in the presence of LPS that is reversed by 7-NI, summary of three experiments (*p<0.05).
c) Increase in peroxynitrite in astrocytes cultured with LPS for 24 h and its reversal with L-canavanin but not 7NI. Results are expressed as percent increase, in the amount of rhodamine, over base line. Experiments were done in triplicate and were repeated twice (*p<0.05).
d) Astrocytes show resistance to LPS induced cell death. MTT assay of astrocytes cells cultured with LPS (5μg/ml), and following addition of L-canavanin. Results are the summary of two experiments (*p<0.05).
In preparation of astrocytic cultures, we also noted the increased expression of ONOO- following stimulation with LPS (Figure 3c). However, in contrast to OPC, the accumulation of ONOO- was reversed by the iNOS specific inhibitor L-canavanin but not by 7-NI. Although the fold increase in ONOO- was similar in astrocytes to that seen in oligodendrocytes, there was less effects of the accumulation of ONOO- on the viability of astrocytes (Figure 3d)
3.4. Induction of primary demyelination following intra cerebral injection of LPS into body of corpus callosum
Stereotactic injection of LPS into the corpus callosum resulted in development of demyelinating lesions that was first noted 7 days after injection and was restricted initially to the region in the immediate vicinity of the injection site area. Staining with LFB and anti myelin basic protein antibodies confirmed the loss of myelin. Fourteen days following injection, the demyelinating lesions had expanded to involve the body of the corpus callosum and by day 21 had crossed to the callosal tracts of the opposite, uninjected hemisphere. The demyelination did not involve white matter tracts other than the corpus callosum (4a and 4b).
Figure 4.
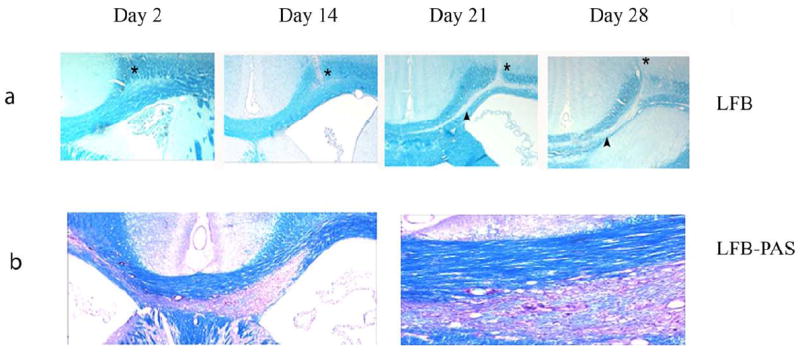
Immunohistochemical and histological study of LPS-induced demyelination. Upper panel shows coronal section of rat brains injected with LPS obtained on days 2, 14, 21 and 28 days after injection. Paraffin embedded sections were stained with LFB. * represents the injection site and the arrow heads the region of demyelination. Lower panel (left 10x and right panel 20x) shows the extension of demyelination into the trunk and to the white matter tracts of the opposite hemisphere.
3.5. Identification of nNOS staining of glial cells in corpus callosum regions following injection of LPS
Immunohistochemical studies were done to examine the expression of nNOS and iNOS in regions of demyelination. The expression of nNOS and iNOS were examined in the corpus callosum on days 2, 7, 14, 21 and 28 days. In saline injected rats, nNOS was not seen in normal appearing white matter tracts, but nNOS was seen in some of the neurons, in grey matter and deep nuclei. Following injection of LPS, expression of nNOS was seen 14 days after LPS injection. At this time, demyelination in the regions close to injection site was evident. When staining of contiguous regions showing early demyelination was done, nNOS positive cells were seen in the cytoplasm of cells that had the morphology of oligodendrocytes. These cells appeared as a linear array of hemotoxylin positive stained cells that had the features of interfascicular oligodendrocytes and appeared as a row of box cars. Double staining of the cells with anti olig1 or olig2 antibody and nNOS antibody confirmed that the interfascicular staining nuclei were oligodendrocytes. Double staining of the sections with anti-GFAP antibody and anti-nNOS antibody failed to demonstrate the co-localization of nNOS to astrocytes (figure 5 a, b and c).
Figure 5.
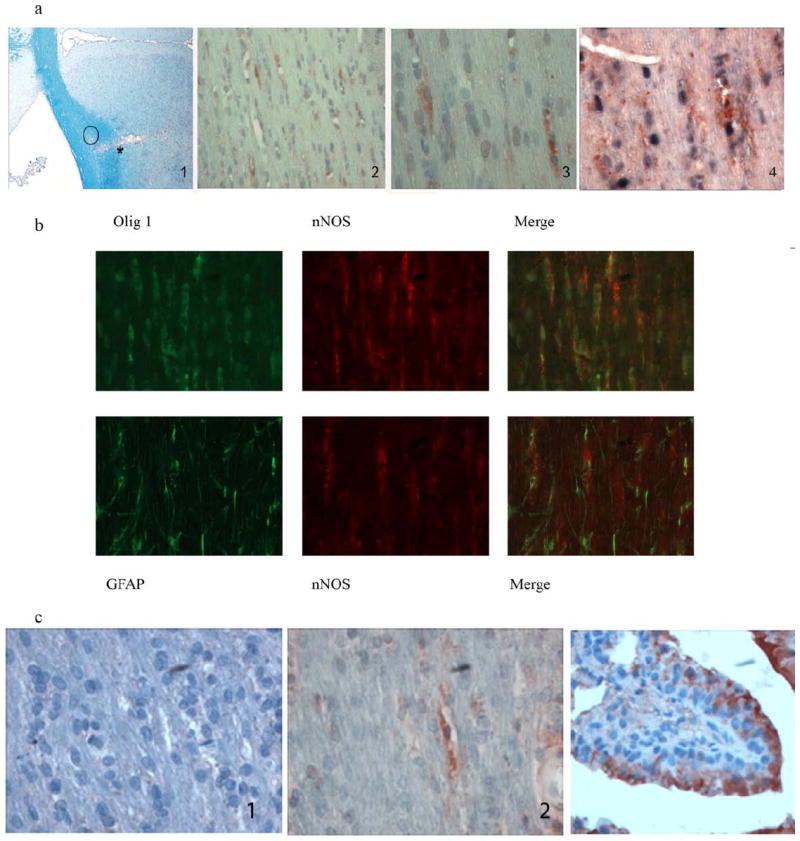
Expression of nNOS and iNOS in LPS injected regions of corpus callosum.
a) Detection of nNOS in areas of demyelination following injection of LPS into corpus callosum. Immunostaining for nNOS in regions in close proximity to demyelinated area. From left to right, 1, shows the injection site on day 14 with early loss of myelin; *, injection site, closed circle, denotes the regions of section stained in panels 2-4 from contiguous paraffin sections; panel 2, immunohistochemical staining for nNOS (10x), panel 3, same area as panel 2, 20x, counterstained with hemotoxylin. Panel 2 and 3 show the hemotoxylin stained interfascicular nuclei arranged as a row of box cars that are typical of oligodendrocytes. These show the presence of signal for nNOS. In panel 4, sections were stained with olig2 antibody followed by alkaline phosphatase detection using NBT/BCIP (black) as substrate, then anti nNOS antibody followed by horseradish peroxidase conjugated secondary antibody and detected with AEC substrate (red) (40X). Note the staining for nNOS in cytoplasm of oligodendrocytes.
b) Co-localization of nNOS with oligodendrocytes but not with astrocytes. Double staining of oligodendrocytes and astrocytes with nNOS in demyelinated areas of rats at day 28 was done by simultaneously staining for Olig1 or GFAP with nNOS and using Alexa Fluor conjugated secondary antibodies for visualization. Upper panel shows co-localization of nNOS with oligodendrocytes, lower panel does not show nNOS to co-localize to astrocytes.
c) Lack of iNOS in demyelinated area.
Left panel shows lack of iNOS in region of demyelination, center panel shows immunostaining for nNOS, right panel shows iNOS detection in mouse lung used as positive control.
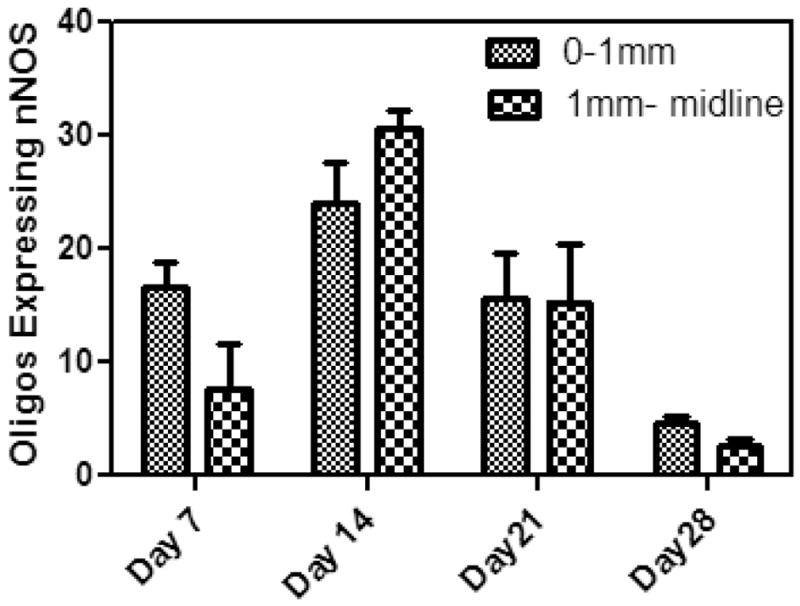
d) Expression of nNOS in Oligodendrocytes. Graphic representation of the development of nNOS+/Olig 1 positive cells in the corpus calossum of rats injected with LPS. Cells were counted from two regions: a) site of injection to 1mm medial to injected site, b) 1mm from injection site to midline. Each cell count mean number of cells two animals from each time point.
To examine the kinetics of expression of nNOS in oligodendrocytes, coronal sections of rat brains were examined by immunohistochemistry. The distribution of cells expressing nNOS were counted from regions that encompassed from the injection site to 1mm medially, and from a point 1 mm from the injection site to mid line. Cells which stained for nNOS and olig2 protein were counted in the body of the corpus calossum, 7 days, 14 days, 21 days and 28 days after injection with LPS. As shown in Figure 5d, nNOS positive cells were seen at the injection site by day 7 and reached a peak by day 14. By day 28, the number of nNOS+/Olig2+ cells had reduced to 3% in areas of demyelination.
3.6. Presence of tyrosine nitration in oligodendrocytes in vivo and in vitro following stimulation with LPS
To show the presence of NO derivatives in the areas of demyelination, we examined for the presence of nitrotyrosine (NT) in situ in LPS injected rats. Double immuno-staining was done with anti-nitrotyrosine and anti Olig1 antibodies. As shown in figure 6a, signal for nitrotyrosine co-localized to cells staining with anti Olig1 antibody. NT was not seen in GFAP staining cells. We also performed western blots of primary oligodendrocyte cells that were cultured with LPS and probed with anti nitrotyrosine antibodies. There was a time dependant increase in the signal of nitrated proteins that was seen as four protein bands that focused at molecular weights of 62kd, 52 kd, 45 kd and 29 kd. The increase in the tyrosine nitration was seen as early as 30 minutes after culture with LPS (Figure 6b).
Figure 6.
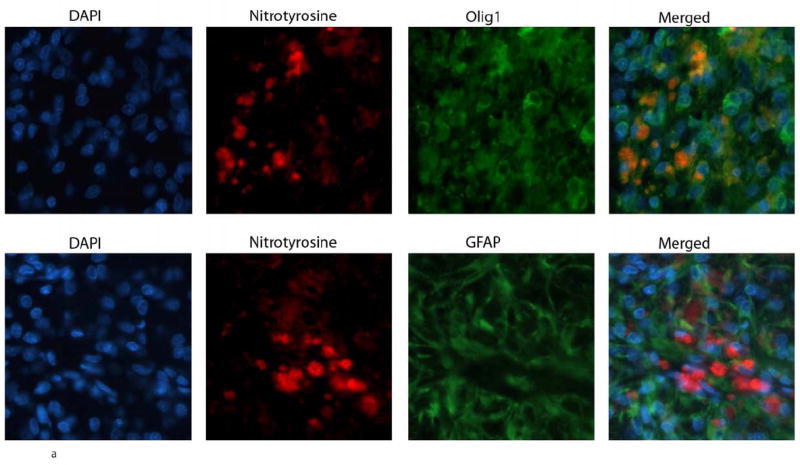
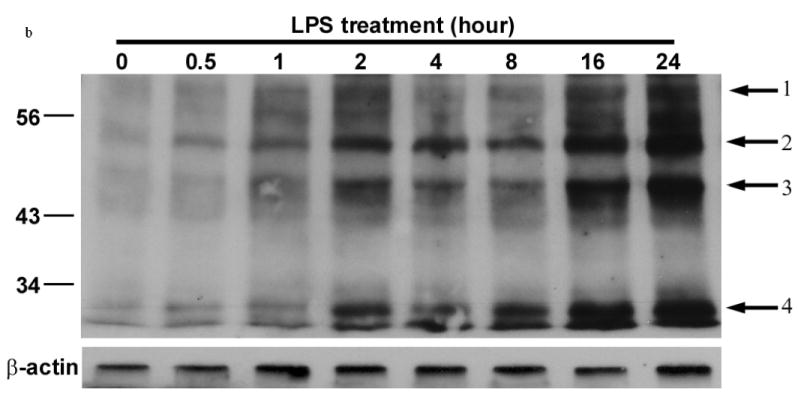
Tyrosine nitration of proteins in oligodendrocyte cultures stimulated with LPS.
a) Double immuno-staining was done in regions of demyelination induced by the injection of LPS for 21 days in rats, with anti-nitrotyrosine and Olig 1 antibody or GFAP antibodies. NT is seen in oligodendrocytes but not astrocytes.
b) Western blot of whole cell lysates from primary oligodendrocytes cultured with LPS and probed with anti nitro-tyrosine antibodies. Arrows show the appearance of nitrated proteins.
4. DISCUSSION
Our study demonstrates the activation and induction of nNOS but not iNOS in OPC following stimulation with LPS. In contrast, iNOS but not nNOS was induced in astrocytes upon culture with LPS and the effect of iNOS on production of ONOO- was reversed by inhibitor of iNOS but not nNOS. LPS induced activation of nNOS and its inhibition by 7-NI was similar in magnitude to that seen in other studies (Boulouard et al., 2007; Cordelier et al., 2006; Perez-Rodriguez et al., 2009; Yuan et al., 2004b). The possibility of contaminating cells is unlikely since neither astrocytes or microglia are known to induce or activate nNOS (Brannan and Roberts, 2004). Constitutive expression of nNOS in astrocytes has been reported but activation and induction of nNOS by LPS has not been described (Tolias et al., 1999; Yuan et al., 2004a). There have been no reports of nNOS induction in microglial cells treated with LPS, hence, the activation of nNOS appear to be predominantly in oligodendrocytes.
We believe that the reversal of enzymatic activity of NOS with 7-NI suggests that the NOS activity was due to activation of nNOS and not iNOS. These results are similar to our results in MO3.13 cells that showed the induction and activation of nNOS but not iNOS following culture with LPS. Although the induction of nNOS protein by LPS was modest (2.6± 0.66-fold increase by RT-PCR and 1.7-fold increase in nNOS protein), we noted an increase in nNOS activity and also increase in accumulation of ONOO- which reduced cell viability.
In contrast to the rapid induction of nNOS in vitro, the kinetic of expression of nNOS in vivo was delayed. We detected nNOS in the corpus calossum 7 days following injection of LPS and the reason for the delayed response in vivo when compared to that seen in vitro is not clear. Furthermore, the spread of the demyelinating lesions from the injection site both medially and laterally suggests that the glial injury was unlikely to be neurotoxic and might involve the activation of signaling pathways that ultimately resulted in loss of myelin. The presence of nNOS however, coincided with the appearance of demyelination. nNOS was seen in areas of normal appearing white matter that was in close proximity to areas undergoing demyelination, suggesting that induction of nNOS may be an early event in the demyelinating process.
iNOS is present in a number of inflammatory disorders of the CNS including models of experimental demyelination and is induced rapidly in monocytes and macrophages following culture with LPS (Merrill and Matsushima, 1988; Merrill et al., 1997). However, both in vitro and in vivo, LPS failed to induce iNOS in OPC, but induced a robust response in purified preparation of astrocytes. Other investigators using similar culture conditions have shown the induction of iNOS in primary oligodendrocyte precursor cells (Bhat et al., 1999; Boullerne and Benjamins, 2006; Molina-Holgado et al., 2001). However, others have questioned as to whether the induction of iNOS in these experiments were due to contaminating microglial cells (Hewett et al., 1999). Although we did not examine the activation of NOS in microglia, other investigators have shown the induction of iNOS, but not its activation, in microglia following culture with LPS in vitro (Ding et al., 1997; Jack et al., 2005; Li et al., 2005).
The ability of LPS to induce and activate nNOS following stimulation with ligands of the toll like receptor family has not been reported before. Our results on the expression of toll like receptors in oligodendrocyte precursor cells and their activation by LPS are similar to those previously described by us using the MO3.13 cell line (Yao et al., 2009?). The expression of TLR genes in both immature and mature oligodendrocytes suggest that oligodendrocytes cannot be completely excluded from being a player in innate immunity (Bsibsi et al., 2002; van Noort and Bsibsi, 2009). Our study does not identify if the induction of nNOS is seen in both mature and OPC. Double staining of LPS injected CNS tissue showed nNOS in cells that stained strongly for Olig2+, suggesting that the different stages of oligodendrocyte development may influence nNOS activation.
Currently there are three models of primary oligodendrogliopathies. Injection of Theiler’s virus induces the death of oligodendrocytes and is followed by wide spread demyelination (Brahic et al., 2005; Lipton et al., 2007). Cuprizone, lysolecithin and ethidium bromide are known to be gliotoxic and induce demyelination (Birgbauer et al., 2004; Matsushima and Morell, 2001). Of these, the cuprizone model has been extensively studied because of the ease of induction of demyelination following oral treatment with cuprizone. The mechanism of demyelination caused by cuprizone is not clear, but is thought to be mediated by chelating effects of cuprizone on cytochrome oxidase C (copper containing oxidase), resulting in oligodendrocyte death. Interestingly, nNOS-/- mice, but not eNOS-/- or iNOS-/- knock-out mice, were protected from the cuprizone mediated demyelination (Linares et al., 2006). The protection of the large white matter tracts from demyelination in nNOS-/- mice was associated with increased survival of oligodendrocytes and decreased apoptosis arguing for an important role of nNOS in the cuprizone model of CNS demyelination. It therefore appears that in at least two models of primary demyelination (LPS mediated and cuprizone-induced); nNOS may play a role in oligodendrocyte death.
The LPS-induced model of demyelination reflects in part the pathological picture seen in type III oligodendrogliopathy (Felts et al., 2005; Marik et al., 2007). In the initial description of this model of CNS demyelination, injection of 100 ng of LPS into the dorsal spinal cord of rats caused an initial influx of polymorphonuclear cells and a few T lymphocytes. After 5-7 days and extending up to 21 days, following disappearance of T cells, there was an increase in the size of the area of myelin loss and a well defined area of demyelination that appeared in the absence of an overt presence of T lymphocytes. Our findings in the corpus callosum are similar to those reported by Felts et al. A number of indirect observations suggested that the LPS model might represent the type III oligodendrogliopathy that is seen in a subset of MS patients. These include: (a) lack of inflammatory cytokines, including iNOS, at the height of demyelination, (b) loss of myelin associated glycoproteins (MAG) similar to that seen in type III lesions and (c) failure of treatment with dexamethasone to protect rats from demyelination while attenuating the initial inflammatory signal. Recent studies have speculated on the activation of TLR ligands in neurodegenerative disorders (Owens, 2009). Many neurodegenerative diseases have shown to have inflammatory components in the CNS, although the tie in between inflammation and degeneration has remained elusive. We propose that in a TLR mediated injury of demyelination, the presence of nNOS and evidence of nitro-tyrosine derivatives in oligodendrocytes suggest that nitrosative injury to myelin could be a key player along with other cytokines in demyelination and could underlie the neurodegenerative process.
Evidence for the role of nNOS in human demyelinating diseases such as MS comes from immunohistochemical and micro-array studies. Derivatives of NO are seen in both regions of inflammation and demyelination in MS and are present in the cerebrospinal fluid of MS patients (Bitsch et al., 2000; Brundin et al., 1999; Calabrese et al., 2002; Giovannoni, 1998; Rejdak et al., 2004a; Rejdak et al., 2004b; Rejdak et al., 2008; Smith and Lassmann, 2002). Microarray studies and RT-PCR analysis of normal appearing white matter from MS patients showed increased expression of nNOS but not iNOS (Zeis et al., 2008a). In a case study of a patient with rapidly progressive MS due to type III oligodendrogliopathy, a 20-fold increase in the mRNA levels of nNOS was seen at the leading edge of demyelination (Zeis et al., 2008a; Zeis et al., 2008b). These areas also showed increased accumulation of nitrated products in oligodendrocytes. In contrast, only a 2.2-fold increase in the expression of iNOS was seen in the same regions suggesting that the accumulation of nitrated proteins were due to increased activation of nNOS.
The currently held opinion is that NO-mediated injury in inflammatory demyelination is most likely due to the activation of iNOS from infiltrating immune cells and activated glial cells. In this model, free-radical damage from the excessive production of NO intermediates is responsible for oligodendrocyte death. However, a clear and well defined role for iNOS in demyelination is not certain since although iNOS is seen in areas of demyelination, there is very little correlation between expression of iNOS and extent and severity of demyelination in MS (Jack et al., 2007; Jack et al., 2005). Also, addition of NO donor to oligodendrocyte cultures did not produce an increase in peroxynitrite formation or cell death. In addition, there are conflicting results on the protective effects of inhibitors of iNOS in experimental allergic encephalomyelitis (EAE), and iNOS-/- mice are not protected from EAE (Dalton and Wittmer, 2005; Fenyk-Melody et al., 1998).
Activation of nNOS by a specific signal(s) that can irreversibly injure oligodendrocytes, preferentially over other glial cells, provides a basis for the specificity of the lesions to oligodendrocytes in inflammatory disorders such as MS. Also, since mtNOS is a post translational modified product of nNOS and is embedded in the mitochondrial membrane, activation of nNOS and mtNOS can lead to NO mediated injury to mitochondria which can lead to cell death. At present, the agonists that lead to the activation of nNOS in MS are not known. However, if these studies are replicated, nNOS might serve as a target for drug development in MS.
Some of the unanswered questions are factors that dictate the specificity of the lesions to MS. Since there is nothing unusual or peculiar to the inflammatory infiltrates in MS, as compared to other neuro-inflammatory disorders, the development of the demyelinating plaque is unlikely to be due to a particular cytokine or chemical signature. However, the induction of nNOS and mtNOS in oligodendrocytes, by an as yet undefined agonist, might lead to a preponderant loss of oligodendrocytes and demyelination. Other glial cells either do not express the receptors for the agonist or lack the inducibility of nNOS or have mechanisms to resist NO mediated tissue injury. Targeting nNOS might provide a novel avenue for protection of oligodendrocytes from injury.
Acknowledgments
We thank Dr. Michael Aschner for providing of astrocytes, Dr. Chris Kao for assistance with sterotactic surgery and Pooja Pandey for technical assistance in the completion of the study. This study was supported in part by grants from the NIH R21 NS058787 and by generous gift from the William Weaver fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud O, Li J, Zhang Y, Neve RL, Volpe JJ, Rosenberg PA. Nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. Eur J Neurosci. 2004;20:1713–1726. doi: 10.1111/j.1460-9568.2004.03616.x. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Bhat AN. Cytokine induction of inducible nitric oxide synthase in an oligodendrocyte cell line: role of p38 mitogen-activated protein kinase activation. J Neurochem. 1999;72:472–478. doi: 10.1046/j.1471-4159.1999.0720472.x. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res. 2004;78:157–166. doi: 10.1002/jnr.20248. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123(Pt 6):1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Boullerne AI, Benjamins JA. Nitric oxide synthase expression and nitric oxide toxicity in oligodendrocytes. Antioxid Redox Signal. 2006;8:967–980. doi: 10.1089/ars.2006.8.967. [DOI] [PubMed] [Google Scholar]
- Boulouard M, Schumann-Bard P, Butt-Gueulle S, Lohou E, Stiebing S, Collot V, Rault S. 4-substituted indazoles as new inhibitors of neuronal nitric oxide synthase. Bioorg Med Chem Lett. 2007;17:3177–3180. doi: 10.1016/j.bmcl.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2009;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Progressive multiple sclerosis. Semin Immunopathol. 2009;31:455–465. doi: 10.1007/s00281-009-0182-3. [DOI] [PubMed] [Google Scholar]
- Brahic M, Bureau JF, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler’s virus. Annu Rev Microbiol. 2005;59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- Brannan CA, Roberts MR. Resident microglia from adult mice are refractory to nitric oxide-inducing stimuli due to impaired NOS2 gene expression. Glia. 2004;48:120–131. doi: 10.1002/glia.20066. [DOI] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Brundin L, Morcos E, Olsson T, Wiklund NP, Andersson M. Increased intrathecal nitric oxide formation in multiple sclerosis; cerebrospinal fluid nitrite as activity marker. Eur J Neurol. 1999;6:585–590. doi: 10.1046/j.1468-1331.1999.650585.x. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system:The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Bella R, Foresti R, Bates TE, Giuffrida Stella AM, Pennisi G. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70:580–587. doi: 10.1002/jnr.10408. [DOI] [PubMed] [Google Scholar]
- Campbell IL. Structural and functional impact of the transgenic expression of cytokines in the CNS. Ann N Y Acad Sci. 1998;840:83–96. doi: 10.1111/j.1749-6632.1998.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Colello RJ, Sato-Bigbee C. Purification of oligodendrocytes and their progenitors using immunomagnetic separation and Percoll gradient centrifugation. Curr Protoc Neurosci. 2001;3:3–12. doi: 10.1002/0471142301.ns0312s03. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cordelier P, Esteve JP, Najib S, Moroder L, Vaysse N, Pradayrol L, Susini C, Buscail L. Regulation of neuronal nitric-oxide synthase activity by somatostatin analogs following SST5 somatostatin receptor activation. J Biol Chem. 2006;281:19156–19171. doi: 10.1074/jbc.M602024200. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DK, Wittmer S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice. J Neuroimmunol. 2005;160:110–121. doi: 10.1016/j.jneuroim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ding M, St Pierre BA, Parkinson JF, Medberry P, Wong JL, Rogers NE, Ignarro LJ, Merrill JE. Inducible nitric-oxide synthase and nitric oxide production in human fetal astrocytes and microglia. A kinetic analysis. J Biol Chem. 1997;272:11327–11335. doi: 10.1074/jbc.272.17.11327. [DOI] [PubMed] [Google Scholar]
- Felts PA, Woolston A-M, Fernando HB, Asquith S, Gregson NA, Mizzi OJ, Smith KJ. Inflammation and primary demyelination induced by the intraspinal injection of lipopolysaccharide. Brain. 2005;128:1649–1666. doi: 10.1093/brain/awh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26:190–195. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Sen CK. Mitochondrial nitric oxide synthase. Front Biosci. 2007;12:1072–1078. doi: 10.2741/2127. [DOI] [PubMed] [Google Scholar]
- Giovannoni G. Cerebrospinal fluid and serum nitric oxide metabolites in patients with multiple sclerosis. Mult Scler. 1998;4:27–30. doi: 10.1177/135245859800400107. [DOI] [PubMed] [Google Scholar]
- Giulivi C. Characterization and function of mitochondrial nitric-oxide synthase. Free Radic Biol Med. 2003;34:397–408. doi: 10.1016/s0891-5849(02)01298-4. [DOI] [PubMed] [Google Scholar]
- Haynes V, Elfering S, Traaseth N, Giulivi C. Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. J Bioenerg Biomembr. 2004;36:341–346. doi: 10.1023/B:JOBB.0000041765.27145.08. [DOI] [PubMed] [Google Scholar]
- Hewett JA, Hewett SJ, Winkler S, Pfeiffer SE. Inducible nitric oxide synthase expression in cultures enriched for mature oligodendrocytes is due to microglia. J Neurosci Res. 1999;56:189–198. doi: 10.1002/(sici)1097-4547(19990415)56:2<189::aid-jnr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Horwitz MS, Evans CF, McGavern DB, Rodriguez M, Oldstone MB. Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C, Antel J, Bruck W, Kuhlmann T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia. 2007;55:926–934. doi: 10.1002/glia.20514. [DOI] [PubMed] [Google Scholar]
- Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. J Neurosci Res. 2005;81:363–373. doi: 10.1002/jnr.20482. [DOI] [PubMed] [Google Scholar]
- Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P, Drenckhahn D. Astrocytes and Bergmann glia as an important site of nitric oxide synthase I. Glia. 1996;16:165–173. doi: 10.1002/(SICI)1098-1136(199602)16:2<165::AID-GLIA8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares D, Taconis M, Mana P, Correcha M, Fordham S, Staykova M, Willenborg DO. Neuronal nitric oxide synthase plays a key role in CNS demyelination. J Neurosci. 2006;26:12672–12681. doi: 10.1523/JNEUROSCI.0294-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Gu Z, Nakamura T. Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders. Int Rev Neurobiol. 2007;82:1–27. doi: 10.1016/S0074-7742(07)82001-0. [DOI] [PubMed] [Google Scholar]
- Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–2815. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Matsushima K. Production of and response to interleukin 1 by cloned human oligodendroglioma cell lines. J Biol Regul Homeost Agents. 1988;2:77–86. [PubMed] [Google Scholar]
- Merrill JE, Murphy SP, Mitrovic B, Mackenzie-Graham A, Dopp JC, Ding M, Griscavage J, Ignarro LJ, Lowenstein CJ. Inducible nitric oxide synthase and nitric oxide production by oligodendrocytes. J Neurosci Res. 1997;48:372–384. [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Guaza C. LPS/IFN-gamma cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. Eur J Neurosci. 2001;13:493–502. doi: 10.1046/j.0953-816x.2000.01412.x. [DOI] [PubMed] [Google Scholar]
- Owens T. Toll-like receptors in neurodegeneration. Curr Top Microbiol Immunol. 2009;336:105–120. doi: 10.1007/978-3-642-00549-7_6. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LL, Kanai AJ, Birder LA, Pitt BR, Peterson J. The catabolic fate of nitric oxide: the nitric oxide oxidase and peroxynitrite reductase activities of cytochrome oxidase. J Biol Chem. 2002;277:13556–13562. doi: 10.1074/jbc.M109838200. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez R, Roncero C, Olivan AM, Gonzalez MP, Oset-Gasque MJ. Signaling mechanisms of interferon gamma induced apoptosis in chromaffin cells: involvement of nNOS, iNOS, and NFkappaB. J Neurochem. 2009;108:1083–1096. doi: 10.1111/j.1471-4159.2008.05862.x. [DOI] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- Rejdak K, Eikelenboom MJ, Petzold A, Thompson EJ, Stelmasiak Z, Lazeron RH, Barkhof F, Polman CH, Uitdehaag BM, Giovannoni G. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology. 2004a;63:1439–1445. doi: 10.1212/01.wnl.0000142043.32578.5d. [DOI] [PubMed] [Google Scholar]
- Rejdak K, Petzold A, Sharpe MA, Kay AD, Kerr M, Keir G, Thompson EJ, Giovannoni G. Cerebrospinal fluid nitrite/nitrate correlated with oxyhemoglobin and outcome in patients with subarachnoid hemorrhage. J Neurol Sci. 2004b;219:71–76. doi: 10.1016/j.jns.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Rejdak K, Petzold A, Stelmasiak Z, Giovannoni G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult Scler. 2008;14:59–66. doi: 10.1177/1352458507082061. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Hall SM, Davies M. Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol. 2001;49:470–476. [PubMed] [Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Tolias CM, McNeil CJ, Kazlauskaite J, Hillhouse EW. Superoxide generation from constitutive nitric oxide synthase in astrocytes in vitro regulates extracellular nitric oxide availability. Free Radic Biol Med. 1999;26:99–106. doi: 10.1016/s0891-5849(98)00146-4. [DOI] [PubMed] [Google Scholar]
- van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–148. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- Wizemann TM, Gardner CR, Laskin JD, Quinones S, Durham SK, Goller NL, Ohnishi ST, Laskin DL. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J Leukoc Biol. 1994;56:759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- Yao S, Pandey P, Rose A, Sriram S. LPS mediated injury to oligodendrocytes is mediated by the activation of nNOS: Relevance to human demyelinating disease. Nitric Oxide. 2009 doi: 10.1016/j.niox.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Liu B, Yuan L, Zhang Y, Dong X, Lu J. Evidence of nuclear localization of neuronal nitric oxide synthase in cultured astrocytes of rats. Life Sci. 2004a;74:3199–3209. doi: 10.1016/j.lfs.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Yuan ZR, Liu B, Zhang Y, Yuan L, Muteliefu G, Lu J. Upregulated expression of neuronal nitric oxide synthase by insulin in both neurons and astrocytes. Brain Res. 2004b;1008:1–10. doi: 10.1016/j.brainres.2004.01.076. [DOI] [PubMed] [Google Scholar]
- Zeis T, Graumann U, Reynolds R, Schaeren-Wiemers N. Normal-appearing white matter in multiple sclerosis is in a subtle balance between inflammation and neuroprotection. Brain. 2008a;131:288–303. doi: 10.1093/brain/awm291. [DOI] [PubMed] [Google Scholar]
- Zeis T, Probst A, Steck AJ, Stadelmann C, Bruck W, Schaeren-Wiemers N. Molecular Changes in White Matter Adjacent to an Active Demyelinating Lesion in Early Multiple Sclerosis. Brain Pathol. 2008b doi: 10.1111/j.1750-3639.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


