ABSTRACT
Intrahepatic lesions of hepatocellular carcinoma (HCC) have been controlled by significant advances in treatment using loco-regional therapies, including, surgery, ablative therapy, catheter-based chemotherapy, and embolization. Consequently, the number of patients with extrahepatic metastatic lesions has increased. Their prognosis remains poor with approximately <1 y of survival from the time of diagnosis. A molecularly targeted drug, sorafenib, have been used to treat extrahepatic lesions and shown the prolonged survival time. However, the therapeutic benefit for the brain metastasis remains unclear, since it causes intratumor bleeding leading to the severe brain damage. No guidelines for the brain metastasis of HCC have been developed to date due to the shortage of the experiences and evidences. Therefore, the development of standard therapy for brain metastasis following the early diagnosis is essential by accumulating the information of clinical courses and evidences. For this purpose, we reviewed cases of HCC brain metastasis reported to date and analyzed additional 8 cases from our hospital, reviewing 592 advanced HCC cases to estimate the possible metastatic lesions in the brain. With careful review of cases and literature, we suggest that the cases with lung metastasis with increase tendency of tumor markers within recent 3–6 months have higher risks of brain metastasis. Therefore, they should be carefully followed by imaging modalities. In addition, the loco-regional treatment, including surgical resection and radiation therapy should be performed for better prognosis by preventing re-bleeding from the tumors.
KEYWORDS: Hepatocellular carcinoma, brain metastasis, surgery, radiation, tumor marker, AFP, L3
Introduction
Brain metastasis in patients with hepatocellular carcinoma (HCC) is relatively rare1-3 with an approximate rate of 1–6%.4-7 The prognosis of these patients is poor with survival period of a few weeks.4,5,7-9 Therefore, brain metastasis is considered as the terminal state of patients with HCC. In addition, various neurologic symptoms including headache, vomiting, hemiparesis, diplopia, hemianopsia, dysarthria, gait disturbance, and seizure affect their quality of life (QOL), and re-bleeding from the tumors cause a worse prognosis.2,10-16 Recent developments in loco-regional therapies, including, surgery, ablative therapy, catheter-based transarterial chemoembolization (TACE), and chemotherapy have demonstrated improved controllability of intrahepatic HCCs. In addition, a molecularly targeted drug, sorafenib (SFN), a multikinase inhibitor, has been reported to delay the disease progression and prolong the overall survival (OS) period. Therefore, the incidence of complications occurring in the later period of disease, such as extrahepatic metastases, might increase.17,18 SFN exhibits anti-tumor effects against extrahepatic lesions; however, due to the risk of intratumor bleeding in the brain, the therapeutic benefit of administrating SFN for patients who have brain metastases remains unclear. Because HCC has a lower incidence of brain metastasis than other malignant tumors, no standard therapeutic strategy has been reported to date. Loco-regional treatment, including surgical resection, radiation therapy (e.g., whole brain radiation therapy (WBRT), stereotactic radiation therapy (SRT)), showed promising results in a few case studies.4,7,17,19-22 The efficacy of these treatment modalities is based on the control of tumor progression in the local area, improvement of neurologic symptoms, and maintenance of QOL. It is also noteworthy that controlling the intrahepatic lesions and providing supportive therapy for hepatic reserve function are essential in treating the patients with extrahepatic lesions.18 Therefore, it is highly important to understand the clinical course of the cases and learn the purpose and goal of each treatment.
Herein, we present 8 cases from our institute and show that other extrahepatic metastases, especially lung, with increase tendency of tumor markers within recent 3–6 months have higher risks of brain metastasis. Therefore they should be carefully followed by imaging modalities. In addition, the loco-regional therapeutic approaches for brain tumor can control disease progression and prevent tumor bleeding for the better survivorship.
Case presentation
We reviewed the medical records of 592 advanced HCC cases treated in our hospital from January 2005 to January 2015 and found 8 cases (1.4%) of HCC with brain metastasis. The case information is summarized in Table 1. All 8 patients were male with a median age of 70.5 y (range, 41–84). All were diagnosed with HCC and liver cirrhosis (LC). The etiologies of LC were HBV infection (n = 3), HCV infection (n = 2), and alcohol abuse (n = 3). The Child–Pugh classes were A and B in 7 and one cases, respectively. Our case series showed lung metastasis in 6 and lymph node metastasis in the other 2 cases; 7 had been treated with systemic chemotherapy. The median period until brain metastases from diagnosis of HCC was 28.5 months (range, 12–96), and the period from diagnosis of extrahepatic metastases was 23.0 months (range, 8–60). Patients with brain metastases showed various symptoms, including headache, vomiting, hemianopsia, disorientation, aphasia, hemiparesis, ataxia, and mental deterioration, which significantly affected their QOL. Importantly, their AFP, AFP-L3, and DCP levels showed averaged increase of 131%, 121%, and 242%, respectively, compared with levels taken 3–6 months before the diagnosis of brain metastasis, although the systemic chemotherapy showed disease control in other organs.
Table 1.
Characteristics of patients.
| Case No. | age | Gender | Etiology | Intrahepatic Lesions (Liver Segment) | Extrahepatic Metastases | Systemic Chemotherapy | Period from Diagnosis of HCC (months) | Period from Extrahepatic Metastasis (months) | Area of Brain | Symptoms | Child-Pugh Score | Treatment | re-bleeding | Survival after the Diagnosis of Brain Metastasis | Quality of Life | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | HBV | S6, S7 | Lung | Yes | 15 | 11 | Lt. frontal | aphasia, hemiparesis, vomiting | 6 | None | + | 4 days | N/A | Bleeding |
| 2 | 59 | M | Alcohol | S3, S6–7, S8 | Lung, Rt. Atrium | Yes | 12 | 8 | Lt. parietal | aphasia, hemiparesis | 5 | Resection | − | 6 months | Improvement of symptoms, Discharge | HCC |
| 3 | 78 | M | HCV | S8 | LN | Yes | 36 | 28 | Multiple | aphasia, disorientation, heiparesis | 5 | WBRT | − | 5 months | Improvement of symptoms, Discharge | Recurrence of Brain Metastasis |
| 4 | 84 | M | Alcohol | S2, S3 | Lung | No | 84 | 36 | Lt. frontal | hemiparesis | 5 | Resection+WBRT | − | 21 months | Improvement of symptoms, Discharge | HCC |
| 5 | 41 | M | HBV | S6, S7 | Lung, IVC | Yes | 16 | 15 | Bil. Occipital | headache, vomiting | 6 | None | + | 2 days | N/A | Bleeding |
| 6 | 69 | M | HBV | S6, S7, S8 | Lung | Yes | 72 | 60 | Rt. Parietal & Occipital | hemianopsia, ataxia, mental deterioration | 6 | Resection+WBRT | − | 1 year | Improvement of symptoms, Discharge | HCC |
| 7 | 72 | M | HCV | S6 | Lung | No | 96 | 48 | Rt. Frontal | hemianopsia, hemiparesis | 6 | Resection | − | 4 months | Improvement of symptoms, Discharge | HCC |
| 8 | 77 | M | Alcohol | S2, S3 | LN | Yes | 21 | 18 | Lt. parietal | aphasia, hemiparesis | 7 | SRT | − | 1 month | Improvement of symptoms | HCC |
HBV, hepatitis B virus; HCV, hepatitis C virus; IVC, inferior vena cava; LN, lymph node; WBRT, whole brain radiation therapy; SRT, stereotactic radiation therapy; HCC, hepatocellular carcinoma
Case 1
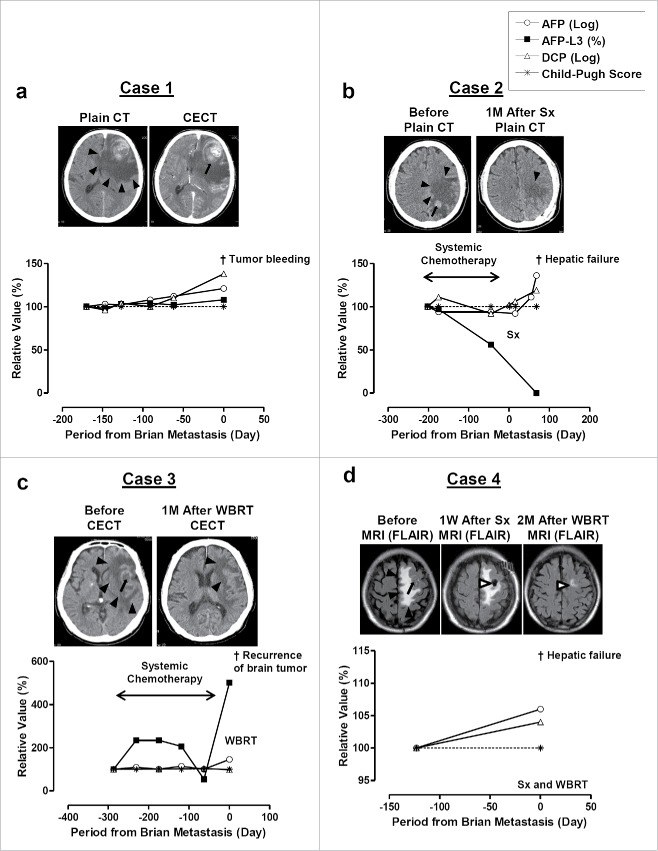
A 64-year-old Japanese man was diagnosed with HCC and liver cirrhosis (LC) due to chronic hepatitis B (CH-B) in 2006. The intrahepatic tumor was resected; however, recurrence was found in the chest 1 month after the surgery and systemic chemotherapy was performed using S1-tegafur-oxonate combination until when he showed neurologic symptoms of aphasia, hemiparesis, and vomiting, 11 months after the diagnosis of lung metastasis. The tumor markers of α-fetoprotein (AFP), LCA-reactive α-fetoprotein isoform (AFP-L3), and des-gamma-carboxy prothrombin (DCP) significantly increased from 17,581 ng/ml, 47.5%, and 3,460 mAU/ml to 135,050 ng/ml, 65.7%, and 75,000 mAU/ml, respectively within 170 d (Fig. 1a). His brain computed tomography (CT) revealed a tumor in the left frontal lobe (Fig. 1a) with significant swelling of the lesion and contrast-enhanced CT (CECT) revealed intratumor bleeding (Fig. 1a). His Child-Pugh score (CPS) was 6 points upon the admission, therefore, the surgical resection or whole brain radiation therapy (WBRT) were considered, however, the rapid and progressive clinical course did not allow us to perform those treatments of brain metastatic lesions (Table 1).
Figure 1.
CT and/or MRI images of brain tumor and clinical courses of Case 1 (a), Case 2 (b), Case 3 (c), and Case 4 (d). CECT, contrast enhanced CT; Sx, surgical resection; WBRT, whole brain radiation therapy. Black arrowheads indicate area of swelling. Black arrows indicate tumors and bleeding from the tumor. White arrowheads indicate the change after Sx and WBRT. AFP and DCP are monitored its increment ratio using their log value and comparing with the initial value available in our hospital. AFP-L3 (%) and Child-Pugh score were monitored for its changes from initial value.
Case 2
A 59-year-old man was diagnosed with HCC with LC due to alcohol abuse in 2009. He underwent TACE for intrahepatic tumor; however, he was diagnosed with lung and right atrium metastases due to HCC. He underwent radiation therapy for the right atrium in 2010 followed by the systemic chemotherapy using S1-tegafur-oxonate combination until he was admitted to our hospital with neurologic symptoms including aphasia and hemiparesis 8 months after radiation therapy (Table 1). He was diagnosed with brain metastasis in the left parietal lobe; his CT revealed bleeding from the tumor (Fig. 1b). AFP and DCP showed no significant increase from 28.7 ng/ml and 698 mAU/ml to 23.3 ng/ml and 416 mAU/ml until 1 month before his admission, however, DCP increased to 817 upon the admission probably due to the brain lesion. Based on the relatively good hepatic reserved function, 5 points of CPS, a surgical resection (Sx) of the lesion was performed after a primary treatment with prednisolone administration to reduce swelling in the brain. His CT showed mild swelling (Fig. 1b) that remained for 1 month after the resection; however, his neurologic symptoms improved remarkably and his QOL maintained for the next 6 months. He died of hepatic failure due to the intrahepatic lesions.
Case 3
A 78-year-old man was diagnosed with HCC with LC due to chronic hepatitis C (CH-C) in 2009. TACE and radiofrequency ablation (RFA) were performed, resulting in complete response in the intrahepatic lesion. However, a metastatic lesion in his neck lymph node was found in the same year using needle biopsy. The administration of SFN controlled tumor growth and no progression was seen in the neck and other organs until he developed dysarthria, aphasia, disorientation, and right hemiparesis 28 months later (Table 1). A significant increase of AFP-L3 from 1.2% to 5.4% was seen with mild increase of AFP from 11.1 ng/ml to 32.3 ng/ml in 6 months before the admission (Fig. 1c). His CT showed multiple brain tumors, mild bleeding with brain edema, and mass effect in the brain (Fig. 1c). Based on the reserved hepatic function, WBRT was performed, which significantly reduced the tumor size, mass effect, and edema (Fig. 1c), resulting in improvement of his neurologic symptoms. He stayed at his home until he showed recurrence of brain tumor and died 3 months later.
Case 4
An 84-year-old man was diagnosed with HCC with LC due to alcohol abuse. A surgical resection was performed in 2007 and he was tumor-free until an extrahepatic lesion of lung metastasis was found in 2011. Oral chemotherapy showed control of tumor progression until he developed right hemiparesis in 2014. His MRI revealed a 22 mm mass lesion with edema in the left frontal lobe of his brain (Fig. 1d). Surgical resection followed by WBRT was performed, and the tumor was successfully removed. His neurologic symptoms fully recovered and his QOL was maintained.
The detail information for cases 5–8 is summarized in supplementary information and Fig. S1.
Discussion
The prognosis of extrahepatic lesions of HCC is poor, with approximately <1 y of survival time.4,19,23 Extrahepatic metastatic lesions include lung, bone, lymph node, adrenal grand, peritoneum, diaphragm, pancreas, skin, brain, muscle, and spinal code.6,23 Our case series showed lung metastasis in 6 and lymph node metastasis in the other 2 cases (Table 1). Among the abovementioned organs, brain metastasis is considered rare with an approximate incidence rate of 1–6%.1-3 Intrahepatic lesions of HCC have been controlled by significant advances in loco-regional therapies. Recently, sorafenib (SFN) has been used to treat extrahepatic metastatic lesions and has been shown to prolong survival,17,18 however the indication of SFN for brain metastases remains unclear due to its ability to cause intratumor bleeding. Because the prognosis of these patients is poor, with a survival period of only a few weeks,4,5,14 and its adverse effects on the patient's quality of life (QOL) due to uncontrolled tumor growth and severe neurologic symptoms, the development of a standard therapeutic strategy to overcome brain metastasis is essential.
In the literature, the time period from intrahepatic lesion until the diagnosis of brain metastasis has been reported to be approximately 20 months,5,8 whereas our case series showed a median of 30 months (Table 1) and their prognosis is poor, and the survival period after the diagnosis of brain metastasis is a few weeks when no therapy is administered (Table 2).4,5,7-9 Consistent with previous reports, our case series showed a median survival period of 3 d in the non-treated group although it depends on the timing of the diagnosis. In our cases, Case 1, 2, 5, and 8 showed rapid progression of brain metastases after the diagnosis of extrahepatic metastatic lesions (median of 13.5 months) in lung or lymphnodes. In these cases, tumor markers started to increase from 3–6 months before the diagnosis of brain metastasis, probably due to the progression of tumor and occurrence of extrahepatic metastatic lesions. Therefore, it might be recommended to check up the brain lesion when the tumor markers show rapid increase (Table 1).
Table 2.
Summary of literatures.
| Reference | Number of Cases | Overall Prognosis of Cases with Brain Metastasis |
|---|---|---|
| 8 | 20 | 8 weeks after diagnosis |
| 5 | 62 | 6.8 weeks after diagnosis. Including cases treated by Sx, WBRT, Steroid |
| 4 | 33 | Sx or Sx and WBRT, 25.3 weeks; GKS, WBRT, or GKS and WBRT, 10.4 weeks; Steroid, 1 week |
| 7 | 11 | 4.6 months after diagnosis. Including surgically treated cases |
| 16 | 15 | Radiation therapy, 22.4 weeks; No treatment, 2.24 weeks |
| 9 | 118 | 6.1 weeks after diagnosis |
Sx, Surgical resection; WBRT, whole brain radiation therapy; GKS, gamma knife surgery;
To date, loco-regional treatment, including surgical resection and radiation therapy.4,5,16,17,21,22,25; WBRT, SRT (gamma-knife, and cyber-knife) is recommended, even in advanced stages, to improve the general condition and neurologic symptoms due to the brain lesions19 These treatments prevent re-bleeding from the tumors, which is one of the key prognostic factors.14 Several reports showed that the longest survival period was approximately 20–30 weeks after surgical resection followed by WBRT, 10–20 weeks with radiation monotherapy, and 1–2 weeks with steroid infusion or no treatment.4 Han et al. reported that patients undergoing resection and resection followed by WBRT had a 25.3-week median survival period, whereas patients undergoing radiation monotherapy had a 10.4-week median survival period, and patients not undergoing any therapy or steroid infusion had 1-week survival period.4 Similar results have recently been reported by Yamanaka et al., who reported 22.4 weeks of survival in the radiation-treated group, whereas the non-treated group had 2.24 weeks of survival.16 In addition, they reported that no re-bleeding was seen in the radiation-treated group.16
Our cases series showed that 6 cases treated with resection and/or radiation therapy had a better prognosis, with a median survival of 24 weeks after the diagnosis of brain metastasis. In addition, they showed significant improvement in neurologic symptoms and maintained QOL, allowing them to go home after undergoing treatment. On the other hand, 2 patients for whom no treatment was performed due to a delay in diagnosis. With unfavorable general conditions, they experienced re-bleeding and expired 4 and 2 d after hospital admission. To control the disease progression, it may be advisable to perform surgical resection of brain metastases in patients who can tolerate the invasiveness of surgery,4,18 as confirmed in our cases. However, in cases with poor hepatic reserve, less invasive strategies, such as SRT, might improve their prognosis, like one case in our study who had a Child–Pugh score of 7, showed significant improvement in neurologic symptoms, and maintained a good QOL until he died of hepatic failure 1 month later. Based on our case series and literature review, it is clear that early therapeutic intervention can help cases; therefore, the scheduled follow-up of extrahepatic lesions is recommended. Cases with lung metastases showing an increase in tumor marker levels should be carefully monitored for brain metastasis.23 Loco-regional treatment is important for patients with brain metastasis, even if complications exist in other organs with metastasis or uncontrollable intrahepatic lesions.14 The strategies might be chosen based on the hepatic reserve function and patient's condition.
Although this is a case series, the increasing tendency of tumor markers within recent 3–6 months with other extrahepatic metastases, especially lung, has high risk of brain metastasis. For such cases, the close follow up is necessary to treat with loco-regional therapeutic approaches. We believe that these minute information reported here will help physicians to treat patients with HCC with brain metastasis in the earlier stage, thereby improving their prognosis and QOL for better survivorship.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Del Ben M, Caporale A, Feole K, Alessandri C, Angelico F. Intracranial hemorrage due to brain metastases in an Italian HCV patient with hepatocellular carcinoma. J Exp Clin Cancer Res 2003; 22:641-4; PMID:15053309 [PubMed] [Google Scholar]
- 2.Peres MF, Forones NM, Malheiros SM, Ferraz HB, Stavale JN, Gabbai AA. Hemorrhagic cerebral metastasis as a first manifestation of a hepatocellular carcinoma. Case report. Arq Neuropsiquiatr 1998; 56:658-60; PMID:9850766; http://dx.doi.org/ 10.1590/S0004-282X1998000400023 [DOI] [PubMed] [Google Scholar]
- 3.Seinfeld J, Wagner AS, Kleinschmidt-DeMasters BK. Brain metastases from hepatocellular carcinoma in US patients. J Neurooncol 2006; 76:93-8; PMID:16402279; http://dx.doi.org/ 10.1007/s11060-005-4175-3 [DOI] [PubMed] [Google Scholar]
- 4.Han MS, Moon KS, Lee KH, Cho SB, Lim SH, Jang WY, Kim IY, Jung S. Brain metastasis from hepatocellular carcinoma: the role of surgery as a prognostic factor. BMC Cancer 2013; 13:567; PMID:24289477; http://dx.doi.org/ 10.1186/1471-2407-13-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi HJ, Cho BC, Sohn JH, Shin SJ, Kim SH, Kim JH, Yoo NC. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol 2009; 91:307-13; PMID:18949445; http://dx.doi.org/ 10.1007/s11060-008-9713-3 [DOI] [PubMed] [Google Scholar]
- 6.Terada T, Maruo H. Unusual extrahepatic metastatic sites from hepatocellular carcinoma. Int J Clin Exp Pathol 2013; 15:816-20; PMID:2363821221212425 [PMC free article] [PubMed] [Google Scholar]
- 7.Shao YY, Lu LC, AL Cheng, Hsu CH. Increasing incidence of brain metastasis in patients with advanced hepatocellular carcinoma in the era of antiangiogenic targeted therapy. Oncologist 2011; 16:82-6; PMID:21212425; http://dx.doi.org/ 10.1634/theoncologist.2010-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han JH, Kim DG, Park JC, Chung HT, Paek SH, Chung YS. Little response of cerebral metastasis from hepatocellular carcinoma to any treatments. J Korean Neurosurg Soc 2010; 47:325-31; PMID:20539790; http://dx.doi.org/ 10.3340/jkns.2010.47.5.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim S, Lee S, Lim JY, Park JS, Seong JS, Chang WS, Han KH, Choi HJ. Hepatocellular carcinoma specific graded prognostic assessment can predict outcomes for patients with brain metastases from hepatocellular carcinoma. J Neurooncol 2014; 120:199-207; PMID:25062667; http://dx.doi.org/ 10.1007/s11060-014-1546-7 [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Chen YL, Kao MC. Intracranial metastasis of hepatocellular carcinoma: review of 45 cases. Surg Neurol 2004; 62:172-7; PMID:15261518; http://dx.doi.org/ 10.1016/j.surneu.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Yano M, Fukuda S, Fukuda T, Nakahara H, Katayama K, Itamoto T, Dohi K, Nakanishi T, Kitamoto M, et al.. Brain metastasis from hepatocellular carcinoma after radical hepatectomy. Hiroshima J Med Sci 1999; 48:91-4; PMID:10598411 [PubMed] [Google Scholar]
- 12.Kuga Y, Waga S, Itoh H. Intracranial hemorrhage due to brain metastasis from hepatocellular carcinoma–case report. Neurol Med Chir 1990; 30:768-71; PMID:1708453; http://dx.doi.org/7533269 10.2176/nmc.30.768 [DOI] [PubMed] [Google Scholar]
- 13.Tanabe H, Kondo A, Kinuta Y, Matsuura N, Hasegawa K, Chin M, Saiki M. Unusual presentation of brain metastasis from hepatocellular carcinoma–two case reports. Neurol Med Chir 1994; 34:748-53; PMID:7533269; http://dx.doi.org/ 10.2176/nmc.34.748 [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MJ, Lu CH, Tsai NW, Lui CC, Chuang YC, Huang CR, Chen SF, Chang CC, Chang HW, Chang WN. Prediction, clinical characteristics and prognosis of intracerebral hemorrhage in hepatocellular carcinoma patients with intracerebral metastasis. J Clin Neurosci 2009; 16:394-8; PMID:19147364; http://dx.doi.org/ 10.1016/j.jocn.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 15.Jang SY, Kim CH, Cheong JH, Kim JM. Concomitant subdural hemorrhage and intracerebral hemorrhage due to brain metastasis of the hepatocellular carcinoma. Brain Tumor Res Treat 2015; 3:48-51; PMID:25977908; http://dx.doi.org/ 10.14791/btrt.2015.3.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamakawa Y, Moriguchi M, Aramaki T, Mitsuya K, Asakura K, Sawada A, Endo M, Nakasu Y. Brain metastasis from hepatocellular carcinoma: The impact of radiotherapy on control of intracranial hemorrhage. Hepatol Res 2015; 45:1071-5; PMID:25470452; http://dx.doi.org/ 10.1111/hepr.12457 [DOI] [PubMed] [Google Scholar]
- 17.Park Y, Kim KS, Kim K, Chie EK, Kim JH, Kim JS, Kim TH, Kim DY, Jang WI, Kim MS, et al.. Nomogram prediction of survival in patients with brain metastases from hepatocellular carcinoma treated with whole-brain radiotherapy: a multicenter retrospective study. J Neurooncol 2015; 125:377-83; PMID:26342711; http://dx.doi.org/ 10.1007/s11060-015-1926-7 [DOI] [PubMed] [Google Scholar]
- 18.Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 2011; 117:4475-83; PMID:21437884; http://dx.doi.org/ 10.1002/cncr.25960 [DOI] [PubMed] [Google Scholar]
- 19.Imamura I. Prognostic efficacy of treatment for extrahepatic metastasis after surgical treatment of hepatocellular carcinoma. Kurume Med J 2003; 50:41-8; PMID:12971262; http://dx.doi.org/ 10.2739/kurumemedj.50.41 [DOI] [PubMed] [Google Scholar]
- 20.Moriya H, Ohtani Y, Tsukui M, Tanaka Y, Tajima T, Makuuchi H, Tanaka Y, Itou K. A case report: tumorectomy for brain metastasis of hepatocellular carcinoma. Tokai J Exp Clin Med 1999; 24:105-10; PMID:10733157 [PubMed] [Google Scholar]
- 21.Hiraoka A, Horiike N, Koizumi Y, Tazuya N, Ichiryu M, Nakahara H, Ochi H, Tanabe A, Doi H, Kodama A, et al.. Brain metastasis from hepatocellular carcinoma treated with a cyber-knife. Intern Med 2008; 47:1993-6; PMID:19015615; http://dx.doi.org/ 10.2169/internalmedicine.47.1373 [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Wu P, Feng Y, Ye K, Tong Y, Zhou Y. Gamma knife surgery for brain metastasis from hepatocellular carcinoma. PLoS One 2014; 9:e88317; PMID:24516635; http://dx.doi.org/ 10.1371/journal.pone.0088317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 2005; 20:1781-7; PMID:16246200; http://dx.doi.org/ 10.1111/j.1440-1746.2005.03919.x [DOI] [PubMed] [Google Scholar]
- 24.Hsiao SY, Chen SF, Chang CC, Lin CH, Chang WN, Lu CH, Chuang YC, Tsai NW. Central nervous system involvement in hepatocellular carcinoma: clinical characteristics and comparison of intracranial and spinal metastatic groups. J Clin Neurosci 2011; 18:364-8; PMID:21247770; http://dx.doi.org/ 10.1016/j.jocn.2010.04.037 [DOI] [PubMed] [Google Scholar]
- 25.Park TY, Na YC, Lee WH, Kim JH, Chang WS, Jung HH, Chang JH, Chang JW, Park YG. Treatment options of metastatic brain tumors from hepatocellular carcinoma: Surgical resection vs. Gamma knife radiosurgery vs. Whole brain radiation therapy. Brain Tumor Res Treat 2013; 2:78-84; PMID:24904896; http://dx.doi.org/ 10.14791/btrt.2013.1.2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.