ABSTRACT
Approximately one-sixth of the worlds' population is infected with helminths and this class of parasite takes a major toll on domestic livestock. The majority of species of parasitic helminth that infect mammals live in the gut (the only niche for tapeworms) where they contact the hosts' epithelial cells. Here, the helminth-intestinal epithelial interface is reviewed in terms of the impact on, and regulation of epithelial barrier function, both intrinsic (epithelial permeability) and extrinsic (mucin, bacterial peptides, commensal bacteria) elements of the barrier. The data available on direct effects of helminths on epithelial permeability are scant, fragmentary and pales in comparison with knowledge of mobilization of immune reactions and effector cells in response to helminth parasites and how these impact intestinal barrier function. The interaction of helminth-host and helminth-host-bacteria is an important determinant of gut form and function and precisely defining these interactions will radically alter our understanding of normal gut physiology and pathophysiological reactions, revealing new approaches to infection with parasitic helminths, bacterial pathogens and idiopathic auto-inflammatory disease.
KEYWORDS: cestode, enteric microbiota, mucin, nematode, parasitic worm
Introduction
The epithelial lining of the gut is the interface between the body proper (i.e. lamina propria and mucosa) and the gut lumen and is exposed to a myriad of antigens, a vast microbiota, and, more transiently, a variety of protozoan and helminth parasites. Indeed, the gut is a preferred niche for helminth parasites, providing a sheltered environment, a soft mucosal surface that can be readily abraded to gain access to a rich microvasculature for blood-feeders, and a steady stream of host-ingested nutrients.
The barrier function of the gut is the net outcome of the physical character of the epithelial layer, secreted elements (i.e., HCl, mucus, IgA, anti-microbial peptides, electrogenic ion secretion to create a driving force for directed water movement), and the mucosal immune system that would, for example, attack bacteria that enter the mucosa to prevent their systemic dissemination.1,2 The mobilization of mucosal immunity in the context of enteric helminth infection is multi-faceted, complex and intriguing but a comprehensive discussion of such is beyond the scope of this commentary: the reader is referred to excellent recent reviews of this topic.3-8 For the purposes of this review we will use ‘epithelial permeability’ to denote studies that address the physical properties of the epithelial layer and ‘barrier function’ is used as a more encompassing term that refers to the many extrinsic (e.g. mucus, IgA, commensal microbiota) components of the intestinal barrier.
Nematodes can cause significant damage in the small or large intestine of their mammalian hosts that would be a significant breech in the epithelial barrier. Recognizing that physical damage caused to the epithelium by tissue- or blood-feeding nematodes or trematodes can increase epithelial permeability, we will not belabor this point, other than to note that secondary bacterial infection, or sepsis, is not a common clinical feature of infection with gastrointestinal nematodes, likely due to the combination of an effective mucosal immune system and the recuperative power of the epithelium. Here, we briefly discuss helminths as a phylum, the nature of the epithelial barrier and then how infection with helminths can affect this directly or indirectly via host immunity.
Helminth parasites
Helminth parasites are endo-parasites that are classified into 2 major groups: the nematodes (round worms) and platyhelminths (flatworms), with the later subdivided into trematodes (flukes) and cestodes (tapeworms) (Fig. 1A). Parasitic helminths typically exhibit complex life-cycles that involve one or more intermediate hosts for juvenile stages of the worm and a definite host where adults reach sexual maturity (host specificity is the basis of parasitism and while each species of parasite has a preferred definitive host there is promiscuity in the system, with implications for zoonotic disease9). It is also safe to say that for every vertebrate species at least one parasitic helminth has evolved. The successful parasite must: (a) recognize its' preferred host and niche therein; (b) be capable of maintaining itself in the preferred nice and be adapted to the physico-chemical conditions of that environment; (c) obtain nutrition from the host; (d) avoid or counteract the host attempts to eradicate it; and (e), while not essential, it is beneficial if the parasite can synchronize egg production with the hosts' reproductive cycle (Fig. 1B).
Figure 1.
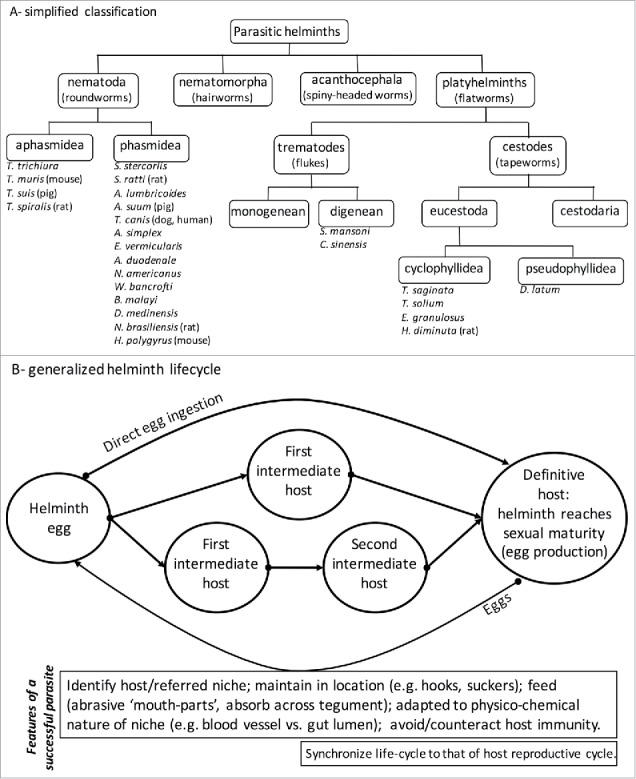
Panel A provides a simplified phylogenic overview of parasitic helminths with typical examples of species that infect humans or are common in laboratory studies (definitive host in parentheses). Detailed classification can be found in “Introduction to Animal Parasitology” by J.D. Smyth, Cambridge University Press, 1994: Anasakis simplex, Ancylostoma duodenale, Ascaris lumbricoides, Ascaris suum, Brugia malayi, Diphyllobothrium latum, Clonorchis sinensis, Dracunculus medinensis, Echinococcus granulosus, Enterobius vermicularis, Heligmosomoides polygyrus, Hymenolepis diminuta, Necator americanus, Nippostrongylus brasiliensis, Schistosoma mansoni, Strongyloides stercorlis, Strongyloides ratti, Taenia saginata, Taenia solium, Toxocara canis, Trichinella spiralis, Trichuris trichiura, Trichuris muris, Trichuris suis, Wuchereria bancrofti). In Panel B the generic complexity of the lifecycle of parasitic helminths is shown along with an inset box presenting essential features of successful parasites (synchronization of the parasite life-cycle with host reproductive cycle is not critical but could be advantageous).
The life-span of a helminth in its definitive host can vary considerably from 2–3 weeks in the case of the nematode Nippostrongylus brasiliensis in the rat to 24 months for the cestode Hymenolepis diminuta in the rat (basically the life-span of the rat). A similar spectrum can be applied to humans where gastrointestinal nematodes (e.g., Ascaris lumbricoides), tapeworms (e.g., Echinococcus granulosus) and trematodes (e.g., Schistosoma mansoni) can co-exist with the host for years. The longevity of the helminth-mammalian host relationship has lead to the suggestion that these parasites have exerted a major evolutionary selection pressure on host immunity.10
Endemic in developing regions of the world, it is estimated that ∼1.5–2.0 billion people suffer from infection with parasitic helminths with effects ranging from loss of nutrition, to anemia, to gastrointestinal upset, to stunted growth, to loss of organ function (e.g., blindness with Onchocerca volvulus (nematode), elephantiasis with Brugia malayi (nematode) and spleno-hepatomegaly with S. mansoni) and fatality (neurocysticercosis from Taenia solium): death from infection with helminth parasites is the extreme and mild/significant morbidity is the norm. A similar situation exists for domestic livestock, a problem in developing and developed regions of the world, where infection with helminth parasites takes a tremendous toll on productivity (e.g., meat yields),11 and resistance to anthelminthics is increasing alarmingly.12 Thus, while helminth biology may be unfamiliar to the reader, helminths are a ubiquitous component of our ecosystem.
The nature of the intestinal barrier
For antigens, microbes or parasites to enter the mucosa several barriers need to be negotiated. If antigens and organisms can run the gauntlet of host-derived stomach acid, proteases, mucus and anti-microbial peptides that are produced throughout the entire gastrointestinal tract, and a washer/sweeper event caused by increased water movement into the lumen of the gut combined with increased peristalsis,13 they are then faced with the physical barrier of the epithelium (and after that an extensive mucosal immune system). In the absence of ulceration and damage to the epithelial layer, soluble/particulate antigen can cross the epithelium via uptake by specialized microfold (M) cells that overlay lymphoid aggregates,14 capture by dendritic cell processes that extend between adjacent epithelial cells into the gut lumen,15 or traversing the epithelium via the paracellular and transcellular pathways.
Paracellular permeability refers to the flux of material between adjacent epithelial cells, where it must pass the apical junctional complex composed of the tight junction (TJ), the adherens junction and desmosomes: once established the TJ is the rate-limiting step in paracellular permeability.16 The paracellular space is in fact a continuity between the gut lumen and the mucosa and is often considered in terms of a pore pathway, a high capacity, size- and charge-selective route, and a lower capacity leak pathway that allows entry of larger molecules (e.g., 4 kDa dextrans).17 Research over the last 25-years has defined the molecular composition of the TJ, its' dynamic nature, the mechanisms that regulate its' opening and the impact of microbial pathogens (including protozoans) on enteric epithelial paracellular permeability.18
Briefly, the TJ is composed of many transmembrane proteins: TJ-associated MARVEL (MAL and related proteins for vesicle trafficking and membrane) proteins (TAMPs) include occludin, tricellulin (restricted to tri-cellular junctions) and MarvelD3; claudins, a 27-member family, are single-spanning transmembrane proteins that interact homotypically with the extracellular domain of claudins on neighboring cells; and, single transmembrane immunoglobulin-like junction adhesion molecules (JAMs).17 These proteins reside in cholesterol-rich, detergent-insoluble lipid domains of the plasmalemma and are connected to a peri-junctional ring of actin and myosin via the scaffolding proteins zona occludens (ZO)-1, -2 and -3. The function of TJ and ZO proteins is phosphorylation dependent.19,20 Thus, TJ opening and closing (i.e., the permeability characteristics or epithelial permeability coefficient) is dependent on: (1) the molecular composition of the tight junction [occludin was the first TJ protein identified21 but it has emerged that it is dispensable for TJ formation and that claudins are the critical players in TJ permeability22 where increased expression can enhance the barrier or conversely increase TJ permeability via expression of the pore-forming claudin-223 (and claudin 15) (note that interleukin-13 that is mobilized in response to helminths increases claudin-2 expression24)]; (2) phosphorylation status of the TJ proteins; (3) cell membrane fluidity;25 and, (4) contraction/relaxation of the actinomysin ring via control of the F-actin cytoskeleton and the balance of myosin light chain kinase (MLCK) and myosin light chain phosphatase activity (MLCP).26
Intuitively, transcytosis through an enterocyte (i.e., the transcellular pathway) would be a more difficult and hazardous route for lumen-derived material to breech the epithelial barrier. Yet, evidence is accumulating demonstrating that transcellular permeation of antigen and microbes can be increased in epithelial monolayers exposed to metabolic stress, inflammatory cytokines (e.g., interferon-γ) and bacterial pathogens such as Campylobacter jejuni.27-29 While helminths are too big to reside within an epithelial cell this should not deter consideration of the transcellular pathway as a route by which helminth-derived antigen could enter the body.
Why consider epithelial permeability in the context of infection with enteric helminth parasites?
This question needs to be considered from helminth and host perspectives. With respect to tissue- and/or blood-feeding helminths, destruction of the epithelial barrier is essential for survival. Evoking a ‘washer/sweeper’ event would assist the caudal movement of helminth eggs to facilitate dissemination and hence continuation of the life cycle: infection of the intestine with parasitic nematodes (e.g., Trichinella spiralis) is associated with altered neuro-muscular function and electrogenic Cl− flux into the gut lumen.30 At the same time a “washer/sweeper” effect could contribute to expulsion of the worm burden alleviating any detrimental effect of a parasitized gut:13,31 this can be an example of the elegant co-evolution that characterizes the host-parasite relationship – the host wins by removing the parasite and the parasite wins by completing its' life cycle.
A similar argument can be advanced for the passage of helminth-derived antigens or excretory/secretory (E/S) products into the mucosa. With a parasite such as the tapeworm H. diminuta that lacks teeth, hooks and abrasive structures and causes no overt damage to the gut, the host most likely recognizes the presence of the worm by epithelial or immune cell detection of worm products. This can result in the release of alarmins from the enterocyte (e.g., interleukin (IL)-25), the promotion of T helper-2 (TH2) type immunity and upregulation of effector mechanisms (e.g., IL-5 evoked eosinophils) aimed at worm destruction and expulsion.32,33 However, the corollary of this is that helminths are adept at manipulating immunity in their hosts to meet their own needs,34 and do so via the release of molecules that may need to gain access to target cells in the mucosa – a leaky epithelial barrier would facilitate this.
Increased epithelial permeability could have additional host benefits and anti-worm effects, for example allowing increased nutrient uptake into the mucosa could benefit the host to meet the energy requirements needed to defend itself against the parasite. Easing the passage of complement, antibody and putative effector cells (eosinophils, macrophages) into the gut could be invaluable in the attack on lumen-dwelling helminths.
Increased epithelial permeability triggered by infection with helminth parasites
Direct effects on epithelial permeability are typically assessed using cell lines grown as polarized monolayers on filter supports35 or analysis of tissues mounted in Ussing chambers36 (use of isolated loops of intestine for in vivo or ex vivo studies (ex vivo the loops can be inverted) are less frequently used37). Studies with monolayers add the product/drug/agent of interest to either the apical or basal side of the epithelia and then monitor transepithelial resistance (TER) or apical-to-basal flux of marker molecules (e.g., 51Cr-EDTA, FITC-dextrans) over time, typically up to 72-hours post-treatment. Studies with Ussing-chambered tissue use the same approach but are more short-term, seldom extending beyond 4 hours because of issues with tissue viability. Tissue can be retrieved at various time-points post-infection with helminth parasites for assessment in Ussing chambers. Under these circumstances altered epithelial permeability could be due to the host immune response to the helminth and not to direct effects of the helminth or its' E/S products on the epithelium.
Helminths and their products do directly interact with enteric epithelial cells and indeed the enterocytes synthesis of IL-25 and thymic stromal lymphopoietin (TSLP) is important in the initiation of TH2-dominiated immunity that can result in worm expulsion.32,33,38-41 Studies in which helminth E/S products are added directly to epithelial monolayers and permeability subsequently assessed are scarce. The E/S products from the nematodes Haemonchus contortus and Teladorsagia circumcincta when applied to the apical surface of monolayers of the human colon-derived Caco2 epithelial cell line lowered the TER by ∼20% 2 hours post-treatment.42 Caco2 monolayers typically have TER values of 250–300 Ω.cm2 and so in the absence of data on other markers of paracellular permeability it is difficult to determine the functional significance of the E/S-evoked 20% drop in TER. The TER gradually recovered over a 24-hour period but remained lower than that in time-matched naïve monolayers; immunolocalization at 4 hours post-treatment revealed less peri-junctional staining of ZO-1 and occludin, with the latter showing a diffuse cytoplasmic distribution.42
Hiemstra et al. showed that a glycan component of E/S products from the nematode Trichuris suis dose-dependently decreased the electrical impedance (analogous to TER) across monolayers of the mouse cecal CMT93/69 epithelial line and increased the flux of FITC-dextrans (10–100 kDa): this was associated with reduced mRNA for claudin-4 and a claudin-like protein the authors designated epithelial membrane protein-1 (but not claudin-3 or ZO-1) and was not accompanied by increased epithelial cell death.43 This study also showed that the barrier defect permitted T. suis E/S products to cross the epithelial layer. This is noteworthy from 2 contrasting perspectives: first, infection with T. suis has been promoted as a therapy for inflammatory bowel disease44 and here increases in epithelial permeability could exaggerate the disease. In this context, the magnitude of the increase in intestinal permeability in individuals infected with the nematode Anasakis simplex correlated with worse disease.45 Alternatively, the passage of T. suis E/S products across an epithelial layer driven by the presence of the E/S products themselves could be a component of the anti-inflammatory effect of T. suis, as the E/S products would now be positioned to interact with resident immune cells or those recruited to the gut.46
Data on the direct effect of trematodes and cestodes on enteric epithelial permeability are limited. As adults, S. mansoni lodge in mesenteric blood vessels and to complete their lifecycle eggs must ‘breakthrough’ the tissue and enter the gut lumen that may be facilitated, at least in part, by the spine on the egg. This process must disrupt the epithelial barrier but neither the mechanism of the transepithelial passage nor any physiologic/pathophysiological consequences of this are well understood. S. mansoni, and other flukes (e.g., Clonorchis sinensis (human), Fasciola hepatica (cattle)), infect or affect the liver with implications for bile flow and bile salt formation:47 bile acids can directly affect epithelial permeability and electolyte transport,48,49 but we are unaware of data in support of the possibility that infection with these flatworms affect epithelial permeability indirectly via bile salts.
We reported that mice (non-permissive host) infected with 5 cysticercoids of the rat tapeworm, H. diminuta, displayed a small, statistically-significant increase in ionic conductance (the reciprocal or TER) across jejunal tissue mounted in Ussing chambers at 5 d post-infection (dpi.) that returned to control levels by 8 dpi.50 Infection with helminth parasites can result in less severe disease in animal models of colitis.51 The first study in this area used H. diminuta and dextran sodium sulfate (DSS)-induced colitis:52 while improvement in colon function was observed in the infected mice, ion conductance across segments of colon in Ussing chambers was not different between control, H. diminuta-infected (11 dpi.) or DSS ± H. diminuta-treated mice. This is an unusual finding and may, in this instance, reflect on the technique used to assess epithelial barrier – measurements of TER or conductance in Ussing-chambered tissue consider the whole tissue and so increases in epithelial permeability could be off-set by increased tissue thickness due to edema or hyperplasia of the outer muscle layers in inflamed tissue.
Infection with helminth parasites reduces the severity of colitis in murine model systems,51 and this can be accompanied by a preservation of epithelial barrier function. For example, downregulation of colonic levels of ZO-1 and occludin mRNA and protein and the increase in bacterial translocation to the blood, spleen and mesenteric lymph nodes observed in tri-nitrobenzene sulphonic acid (TNBS)-induced colitis were reduced in mice treated with 10,000 freeze-killed eggs of the trematode Schistosoma japonicum (given by intra-peritoneal injection).53 However, it is unclear whether the worm antigen directly affected the epithelium or enhanced epithelial barrier function occurred via suppression of the inflammation: the latter being the more likely of the 2 possibilities.
Infection with gastrointestinal nematodes increases epithelial permeability,54 which in the acute stages is likely due to the significant damage that species such as the nematodes N. brasiliensis and Trichinella spiralis do to the gut either directly or as a consequence of a cell-mediated immune responses:37 the villus atrophy can resemble that observed in celiac disease. Increases in epithelial permeability after expulsion of the parasite (the post-infectious state) are driven by host cells, principal among these being mast cells.
In the late 1970s it was shown that 10 or 11 dpi. with N. brasiliensis, loops of rat jejunum displayed increased leakiness to lactulose and mannitol (makers of paracellular permeability).37 Subsequently increases in gut permeability as assessed by 51Cr-EDTA and ovalbumin were shown at 4, 10 and 35 dpi., the latter being ∼2 weeks after expulsion of the worm and when the jejunum is characterized by mast cell hyperplasia.55,56 Work by Miller et al. linked helminth-induced mast cell hyperplasia and activation, and the release of proteases with the increase in gut permeability.57-60 Similarly, mast cells are important in the increases in gut permeability that occur as a consequence of anaphylaxis due to challenge of previously infected rats with worm antigen.55,57 cKit+ mast cells have been implicated in the increased intestinal permeability observed 9–10 dpi. with T. spiralis, which was accompanied by reduced expression of occludin.58 This barrier defect was enhanced in T. spiralis-infected IL-9 transgenic mice that have increased numbers of mucosal mast cells58 (increased IL-9 is common following infection with helminth parasites61 and IL-9-knockout mice treated with TNBS displayed increased colonic mRNA and protein levels of claudins 4 and 7, occludin and JAM-A62). In the T. spiralis post-infectious paradigm, the increase in lactulose permeation across the gut correlated with reduced expression of claudin-1 protein in the ileum.63
More recent studies corroborate the participation of mast cells in nematode-evoked increases in epithelial permeability. Thus, 30 dpi. with T. spiralis when there is no histological evidence of damage in the jejunum of rats, there is a ∼2-fold increase in tissue conductance and increased fluxes of 4- and 40-kDa FITC-dextrans across jejunal segments in Ussing chambers.64 This epithelial permeability defect was insensitive to in vitro tetrodotoxin treatment and hence occurred independent of neuronal fast sodium channels. A time-course analysis confirmed increased jejunal permeability at 14 and 30 dpi. with T. spiralis and correlated this with increased numbers of mucosal, but not connective tissue-type, mast cells and increased mRNA expression of the rat mast cell proteases (MCP) 1, 2, 4, 5, 8, 9 and 10 in jejunal mucosa-submucosa at 6 and/or 14 dpi., but not 30 dpi.54 PCR-analysis of the same tissues revealed reduced expression of occludin at 2, 6 (when conductance is not significantly altered), 14 and 30 dpi. A role for mouse MCP1 in the increased permeability observed 8 weeks after infection with the trematode S. mansoni was ruled out using mMCP1−/− mice.65
Given that the 2 most consistent findings observed in humans having irritable bowel syndrome (IBS) are increased gut permeability and mastocytosis,66 one wonders if helminth therapy aimed at treating auto-inflammatory disease34 might predispose an individual to IBS-like symptoms.
The adaptive immune response that follows infection with helminth parasites can also participate in the increase in gut permeability. For instance, infection with the nematode Heligmosomoides polygyrus, a parasite of the mouse duodenum, increases colonic permeability at 7 pdi. characterized by ballooning of the paracellular space, increased transcytosis of the marker protein horse-radish peroxidase (HRP), and loss of the epithelial adherence junction protein, E-cadherin. These findings were not apparent in severe combined immunodeficient mice (SCID), but were recapitulated when SCID mice were re-populated with T cells.67 In addition, mice lacking signal transducer and activator of transcription (STAT)-6, which is critical in IL-4 signaling, did not display the H. polygyrus-induced barrier defect.67 Likewise, the drop in TER observed in muscle-free preparations of jejunum from nematode-infected Balb/c mice was not seen in STAT-6−/− animals.68 The drop in TER in secondary infections with H. polygyrus, was accompanied by a small increase in the epithelial expression of the pore-forming claudin-2, and the barrier defect was absent in IL-13Rα1−/− mice.69 Increased IL-13 production following infection with helminths can be from innate70 or adaptive immune cells,71 and while IL-13 has been shown to directly decrease the barrier function of epithelial monolayers in vitro,24,72 it is unclear if helminth-evoked IL-13 targets the epithelium directly or via other immunoregulatory activities.
With respect to TH2-type cytokines mobilized in response to infection with helminths, many of these have the potential to affect epithelial barrier function. For instance, IL-6 was shown to lower Caco-2 epithelial monolayer TER, increase Na+ (but not macromolecule) permeability and the expression of claudin-2.73 As another example, helminth-evoked IL-5 is critical for eosinophil development and eosinophils can directly increase epithelial permeability,74 or indirectly via mast cells75 (Fig. 2). However, the host-parasite interaction is so exquisitely balanced that the net effect of any given cytokine in vivo is difficult to fully ascertain and will depend on the target cell, temporal kinetics of cytokine production and the microenvironment in which the cytokine operates. Thus, the prototypic TH2 cytokine, IL-4 added to monolayers of human colon-derived epithelial monolayers increased paracellular permeability,76 while reciprocally, one can hypothesize that alternatively activated macrophages induced by IL-4 production after helminth-infection77 could, via their tissue reparatory capacity, enhance epithelial barrier function.
Figure 2.
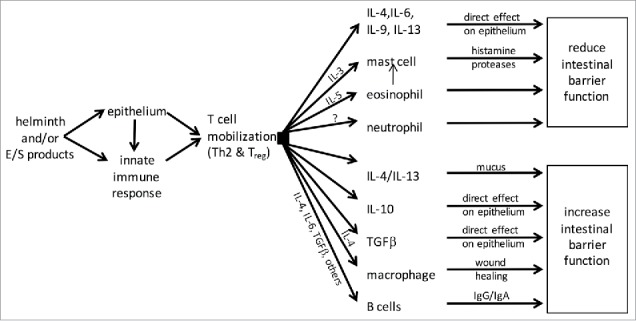
Schema showing the variety of possible mechanisms by which T cell activation following helminth infection could affect epithelial permeability and intestinal barrier function (E/S, helminth-derived excretory/secretory products; IL, interleukin; TGF, transforming growth factor; Th2, T-helper cell type-2; Treg, regulatory T cells).
Collectively, the available evidence suggests that helminths can increase gut permeability by abrading the epithelium directly, that defined helminth-derived products may affect the structure of the tight junction (in general there is a paucity of data here), and that immune activity during or after infection can significantly compromise or enhance epithelial permeability (Fig. 2).
Helminth and host-derived factors impact on intestinal barrier function
Defining the intestinal barrier as mechanisms that prevent material in the lumen entering the circulatory system, there is a vast literature on helminth-evoked changes in intestinal barrier function: goblet/mucus, trefoil factors,78 Paneth cells/defensins/antimicrobial peptides, serotonin, and mucosal immunity are all components of the barrier that can change significantly (cell number and/or function) following infection with parasitic helminths and each is worthy of a focused review.
Enhanced epithelial cell turnover may contribute to the hosts' anti-helminth defenses79-81 and this has the concomitant benefit of clearing any microbe-infected enterocytes. Stimulated electrogenic ion transport creates a driving force for water movement that can lubricate the epithelial surface and may assist in the expulsion of intestinal helminths:82 water is important in the physical properties of mucus.83,84
Intestinal goblet cell hyperplasia is perhaps the most prominent gut characteristic of infection with gastrointestinal parasitic helminths3,85 and increases in mucin production, type (e.g., Muc5a) and glycosylation can be critical components of the epithelial barrier that aids the expulsion of helminths, such as the nematode N. brasiliensis86-89 and the trematode, Echinostoma caproni;90 indeed, helminths in their own defense may release proteases to degrade mucus.91 The advent of mucin-gene knockout mice positions the field to unequivocally test the role of mucus in the expulsion of a range of parasitic helminths. The goblet cell/mucus response can be driven by the helminth-evoked TH2 response, principally IL-4 and IL-1392,93 and IL-2594 (Fig. 3) and potentially by contact with the worms or their E/S products: the latter needs to be explored on a species-by-species basis. The potential of helminth-evoked changes in the enteric nervous system95 and the regulation of mucus production by acetylcholine should also be pursued.96 Finally, recent work has shown that goblet cells can sense their microenvironment and pass this information to dendritic cells;97 the implications of this for barrier function and host-parasite interactions are poorly understood.
Figure 3.
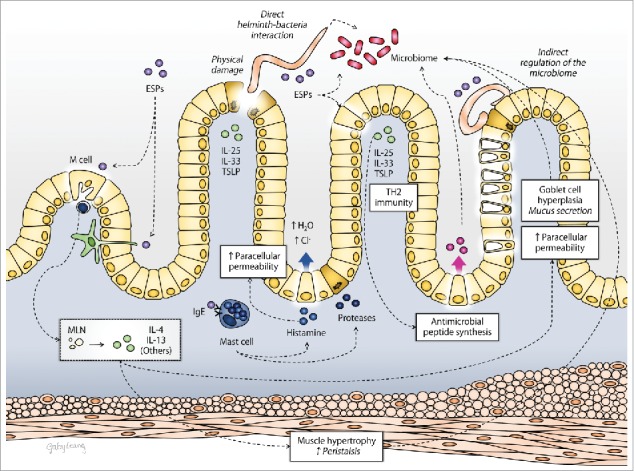
Schematic overview of the mechanisms by which infection with intestinal parasitic helminths (worm or their excretory/secretory products (ESP)) can directly or indirectly impact the barrier function of the gut (IL, interleukin; MLN, mesenteric lymph nodes; TH2, T-helper cells type 2; TSLP, thymic stromal lymphopoietin).
Paneth cells are a significant source of anti-microbial peptides in the mammalian small intestine and infection with nematodes and S. mansoni was shown to induce Paneth cell hyperplasia,98 while Toxocara canis (nematode) infection increased the number of secretory granules in Paneth cells.99 Helminth-regulation of anti-microbial peptides has implications for the overall barrier function of the gut as this will affect the composition and structure of the commensal microbiota. For instance, N. brasiliensis infection has been associated with reduced expression of the mRNA of the anti-microbial peptides, lysozyme-1 and RegIIIγ.89 In contrast, expulsion of the nematode Trichuris muris from mice was accompanied by increased expression of the anti-microbial peptide, angiogenin-4,84 with goblet cells identified as a source of angiogenin-4 in the colon of infected mice.100 Reduced numbers of segmented filamentous bacteria (SFB) in the gut of N. brasiliensis-infected mice have been reported (Table 1):89 SFB are important stimuli of TH17 cells and should this be a general outcome of infection with helminths, the question arises are there any short or long-term consequences to the host to a reduced TH17-TH1 axis in terms of gut homeostasis or vulnerability to microbial pathogens. For example, mice infected with H. polygyrus have an impaired response to concurrent infection with the bacterial pathogen, Citrobacter rodentium that the investigators related to mobilization of alternatively activated macrophages in the co-infected mice.101,102
Table 1.
Changes in the gut microbiota associated with infection with helminth parasites.
| Species | Host | Location | Diversity | class level change in relative abundance | Ref. |
|---|---|---|---|---|---|
| Nematodes | |||||
| Necator americanus | Human | Small intestine | NC | NC | 115 |
| ↑ species richness | NC | 116 | |||
| Trichuris trichuria | Human | Small intestine | NC | ↓ Clostridia | 117 |
| Trichuris trichuria | Macaques | Small intestine | ↑ α diversity | ↑ Bacteroidia | 118 |
| ↑ α and β diversity | ↑ Chlorophyta, ↓ Bacteroidia | 119 | |||
| Trichuris suis | Pig | Proximal colon | NA | ↑ Fibrobacter & Clostridia | 120 |
| NC | ↑ Deferribacteres | 121 | |||
| Heligmosomoides polygyrus | Mouse | Small intestine | NA | ↑ Bacilli (Lactobacillus) | 122 |
| Trichinella spiralis | Mouse | NA | ↑ Bacilli (Lactobacillus) | 123 | |
| Small intestine | NC | ↑ Clostridia (Lachnospiraceae) | 112 | ||
| increased (?) | ↑ Gammaproteobacteria | 124 | |||
| NA | ↑ Bacilli (Lactobacillus) & | 106 | |||
| Clostridia (Clostridiales) | |||||
| ↓ Bacilli (Turicibacteraceae) | |||||
| Nippostrongylus brasiliensis | Mouse | Ileum | NC α diversity | ↓ Fimicutes (Lactobacillaceae) | 89 |
| (↓ segmented filamentous bacteria) | |||||
| ↑ Bacteroides & Actinobacteria | |||||
| Trichuris muris | Mouse | Large intestine | ↓ α and β diversity | ↑ Bacilli (Lactobacillus) | 125 |
| ↓ α diversity | ↓ Bacteriodia | 126 | |||
| NA | ↑ Clostridia (Lachnospiraceae) | 127 | |||
| ↓ Bacteriodia | |||||
| Cestodes | |||||
| Hymenolepis diminuta | Rat | Cecum | NC | ↑ Clostridia, ↓ Bacilli | 128 |
| Rat | Small intestine | ↓aerobic bacteria | NA | 92 | |
Note. NC, no statistical change reported; NA, no data available; ↑ increased abundance; ↓ decreased abundance
Entrochromaffin cells (ECs) within the enteric epithelium are the body's major source of serotonin.103 Increases104 and decreases103 in intestinal ECs and serotonin have been demonstrated after infection with helminths, with early studies linking serotonin to worm expulsion,105 possibly via its ability to elicit electrogenic Cl− secretion into the gut to create a driving force for directed water movement.30
This sampling of studies aptly illustrates the magnitude of changes in the amount and function of factors that comprise the extrinsic component of the intestinal barrier (noting we have not addressed changes in antibody or complement) that can accompany, or follow, infection with parasitic helminths. The challenge is to understand the consequences of these changes for gut barrier function in a host-parasite specific manner, and the putative implications for host interaction with microbes and concomitant inflammatory disease.
Helminth-microbial interactions at the epithelial barrier
The mammalian intestine is home to trillions of bacteria from a diverse array of species. Layer on top of this virus and fungi, protozoan and helminth parasites and a complex ecosystem of microbiota and macrobiota emerges. Intuitively, one can accept that the interplay between these species and the host is critically important in controlling gut function: yet, knowledge of inter-species communication or cross-kingdom interactions in the regulation of gut function is rudimentary.106
A normal commensal microbiota is considered an extrinsic component of the intestinal barrier. The commensal bacteria can produce bacteriostatic factors to influence other bacteria in the gut and may prevent pathogen colonization by niche-exclusion via competition for nutrients and space. The release of bacterial molecules (e.g., PAMPS: pathogen-associated molecular patterns), may keep the gut primed to respond to invasion by pathogens.
Recent studies have begun to catalog changes in the gut bacteria that occur following infection with helminth parasites, typically those that seek to establish in the intestine (Table 1).107,108 It is unclear if helminth-regulation of the enteric microbiota is a direct effect, since they are in the same location, or indirect via the host anti-worm immune response; for example, IL-25 mobilized in response to helminths could suppress synthesis of IL-22 (and vice versa109) which is an important regulator of the epithelial response to bacteria, promoting mucin and anti-bacterial peptide synthesis,110,111 and under certain circumstances may aid worm expulsion from the gut.88
Functional consequences of perturbation of the enteric microbiome are largely unknown, with a notable exception being that the suppression of airways inflammation observed in H. polygyrus-infected mice was dependent on bacteria-derived short-chain fatty acids.112 Intriguingly, the decreased barrier function reported following infection and rejection of T. spiralis was ameliorated by treatment with probiotics:63 the probiotic treatment also resulted in lower expression of pro-inflammatory cytokines, returning us to the conundrum of whether the probiotic effect was by interaction at the level of the enterocyte or the immune system. The possibility that infection with helminths that do not localize to the gut could affect the composition of the enteric microbiota should be assessed: we are unaware of any data in this area.
Thus, the stage is set to delve into analyses of the functional outcome of helminth-induced changes in microbiota content and diversity for the host, the bacteria and the helminth. How important is the commensal bacteria to intestinal barrier function following infection with helminth parasites? What happens to the bacterial metabolome? Is the energy status of the host impacted by helminth regulation of the microbiota? Is the host more vulnerable to opportunistic bacterial infections because of the helminth? Does the altered microbiome impact the course of the helminth-infection113 and any associated pathology? How important is the microbiota to helminth-modulation of disease? These questions and many more await rigorous research efforts. Understanding the interplay between the triumvirate of host epithelium (or immune system), helminth and bacteria (or virus or fungi) is a new frontier in enteric biology that will revolutionize awareness of the control of gut form and function, and how we manage digestive disease.106
Concluding comments
The epithelial surface of the gut is the largest contact site with the outside world and is recognized as a dynamic regulated barrier composed of 6 cell phenotypes (goblet, tuft, enteroendocrine, M-cell, Paneth and transporting enterocyte), the activation of which directs mucosal immunity. To date the there has been little commentary on the barrier defect due to the physical damage caused by infection with abrasive helminths, with more information on the mechanism(s) of host immunopathology following primary or secondary infections with parasitic helminths, and anaphylactic reactions due to exposure to worm antigen in previously infected animals (Fig. 3). Data on the direct effect of helminths or their E/S products on epithelial permeability is lacking. Given the contribution of the epithelium to innate immunity, we suggest that focused efforts to define how helminths affect epithelial permeability will reveal novel aspects of host-parasite interactions and new ways to combat infection with gastrointestinal parasites. Finally, we underscore that the gut is an ecosystem and that the myriad of cells that reside there or are recruited in response to infection form an integrated circuit: while challenging, we contend that the holistic approach of integrated neuroimmunophysiology114 needs to be applied if we are to understand the complex regulation of intestinal barrier function (or indeed any aspect of gut function) and the consequences of perturbed barrier function for gut homeostasis and disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. G. Leung for the artwork in Fig. 3.
Funding
D.M. McKay holds a Canada Research Chair (Tier 1) in Intestinal Immunophysiology in Health and Disease and whose research is supported by grants from the Canadian Institutes of Health (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC) and Crohn's Colitis Canada (CCC). A. Shute is supported by a Beverly Philips Studentship through the Snyder Institute for Chronic Disease at the Univ. Calgary and a stipend from the NSERC CREATE Host-Parasite Interactions (HPI) program at Univ. Calgary. F. Lopes holds post-doctoral fellowships from Alberta Innovates-Health Solutions (AI-HS) and CIHR/Canadian Association of Gastroenterology (CAG)/Allergan NCE and NSERC CREATE HPI.
References
- [1].Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9(11):799-809; PMID:19855405; http://dx.doi.org/ 10.1038/nri2653 [DOI] [PubMed] [Google Scholar]
- [2].Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther 2016; 13:19; PMID:27073405; http://dx.doi.org/ 10.1186/s12981-016-0103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grencis RK. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Ann Rev Immunol 2015; 33:201-25; http://dx.doi.org/ 10.1146/annurev-immunol-032713-120218 [DOI] [PubMed] [Google Scholar]
- [4].Faz-Lopez B, Morales-Montor J, Terrazas LI. Role of macrophages in the repair process during the tissue migrating and resident helminth infections. BioMed Res Internat 2016; 2016:8634603; http://dx.doi.org/ 10.1155/2016/8634603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016; 16(10):639-49; http://dx.doi.org/ 10.1038/nri.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015; 43(1):29-40; PMID:26200011; http://dx.doi.org/ 10.1016/j.immuni.2015.07.007 [DOI] [PubMed] [Google Scholar]
- [7].Huang L, Appleton JA. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol 2016; 32(10):798-807; PMID:27262918; http://dx.doi.org/ 10.1016/j.pt.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vukman KV, Lalor R, Aldridge A, O'Neill SM. Mast cells: new therapeutic target in helminth immune modulation. Parasite Immunol 2016; 38(1):45-52; PMID:26577605; http://dx.doi.org/ 10.1111/pim.12295 [DOI] [PubMed] [Google Scholar]
- [9].Schurer JM, Ndao M, Skinner S, Irvine J, Elmore SA, Epp T, Jenkins EJ. Parasitic zoonoses: one health surveillance in northern Saskatchewan. PLoS Negl Trop Dis 2013; 7(3):e2141; PMID:23556025; http://dx.doi.org/ 10.1371/journal.pntd.0002141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, Bresolin N, Sironi M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med 2009; 206(6):1395-408; PMID:19468064; http://dx.doi.org/ 10.1084/jem.20082779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stromberg BE, Gasbarre LC, Ballweber LR, Dargatz DA, Rodriguez JM, Kopral CA, Zarlenga DS. Prevalence of internal parasites in beef cows in the United States: Results of the National Animal Health Monitoring System's (NAHMS) beef study, 2007-2008. Can J Vet Res 2015; 79(4):290-5; PMID:26424909 [PMC free article] [PubMed] [Google Scholar]
- [12].Holsback L, Luppi PA, Silva CS, Negrao GK, Conde G, Gabriel HV, Balestrieri JV, Tomazella L. Anthelmintic efficiency of doramectin, fenbendazole, and nitroxynil, in combination or individually, in sheep worm control. Braz J Vet Parasitol 2016; 25(3):353-8; http://dx.doi.org/ 10.1590/S1984-29612016025 [DOI] [PubMed] [Google Scholar]
- [13].Wood JD. Neuro-immunophysiology of colon function. Pharmacology 1993; 47Suppl 1:7-13; PMID:8234444; http://dx.doi.org/ 10.1159/000139836 [DOI] [PubMed] [Google Scholar]
- [14].Ohno H. Intestinal M cells. J Biochem 2016; 159(2):151-60; PMID:26634447; http://dx.doi.org/ 10.1093/jb/mvv121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009; 206(13):3101-14; PMID:20008524; http://dx.doi.org/ 10.1084/jem.20091925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Capaldo CT, Nusrat A. Claudin switching: physiological plasticity of the tight junction. Sem Cell Devp Biol 2015; 42:22-9; http://dx.doi.org/ 10.1016/j.semcdb.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [17].Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 2016; 14(1):9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Sem Cell Devp Biol 2014; 36:166-76; http://dx.doi.org/ 10.1016/j.semcdb.2014.09.002 [DOI] [PubMed] [Google Scholar]
- [19].Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem 2009; 284(31):21036-46; PMID:19478092; http://dx.doi.org/ 10.1074/jbc.M109.016766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, et al.. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 2011; 193(3):565-82; PMID:21536752; http://dx.doi.org/ 10.1083/jcb.201010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123(6):1777-88; PMID:8276896; http://dx.doi.org/ 10.1083/jcb.123.6.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001; 120(2):411-22; PMID:11159882; http://dx.doi.org/ 10.1053/gast.2001.21736 [DOI] [PubMed] [Google Scholar]
- [23].Weber CR, Liang GH, Wang Y, Das S, Shen L, Yu AS, Nelson DJ, Turner JR. Claudin-2-dependent paracellular channels are dynamically gated. Elife 2015; 4:e09906; PMID:26568313; http://dx.doi.org/ 10.7554/eLife.09906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, et al.. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005; 129(2):550-64; PMID:16083712; http://dx.doi.org/ 10.1016/j.gastro.2005.05.002 [DOI] [PubMed] [Google Scholar]
- [25].Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, et al.. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 2010; 189(1):111-26; PMID:20351069; http://dx.doi.org/ 10.1083/jcb.200902153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Samak G, Gangwar R, Crosby LM, Desai LP, Wilhelm K, Waters CM, Rao R. Cyclic stretch disrupts apical junctional complexes in Caco-2 cell monolayers by a JNK-2-, c-Src-, and MLCK-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2014; 306(11):G947-58; PMID:24722904; http://dx.doi.org/ 10.1152/ajpgi.00396.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nazli A, Wang A, Steen O, Prescott D, Lu J, Perdue MH, Söderholm JD, Sherman PM, McKay DM. Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect Immun 2006; 74(1):192-201; http://dx.doi.org/ 10.1128/IAI.74.1.192-201.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon-γ induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 2005; 128(5):1258-67; PMID:15887109; http://dx.doi.org/ 10.1053/j.gastro.2005.01.046 [DOI] [PubMed] [Google Scholar]
- [29].Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog 2009; 1(1):2; PMID:19338680; http://dx.doi.org/ 10.1186/1757-4749-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harari Y, Russell DA, Castro GA. Anaphylaxis-mediated epithelial Cl− secretion and parasite rejection in rat intestine. J Immunol 1987; 138(4):1250-5; PMID:3805721 [PubMed] [Google Scholar]
- [31].Russell DA, Castro GA. Immunological regulation of colonic ion transport. Am J Physiol Gastrointestinal Liver Physiol 1989; 256(2):G396-403 [DOI] [PubMed] [Google Scholar]
- [32].Lopes F, Reyes JL, Wang A, Leung G, McKay DM. Enteric epithelial cells support growth of Hymenolepis diminuta in vitro and trigger TH2-promoting events in a species-specific manner. Internat J Parasitol 2015; 45(11):691-6; http://dx.doi.org/ 10.1016/j.ijpara.2015.05.004 [DOI] [PubMed] [Google Scholar]
- [33].Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al.. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016; 351(6279):1329-33; PMID:26847546; http://dx.doi.org/ 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016; 138(3):666-75; PMID:27476889; http://dx.doi.org/ 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang A, Keita AV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, et al.. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol 2014; 184(9):2516-27; PMID:25034594; http://dx.doi.org/ 10.1016/j.ajpath.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Soderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, Sherman PM, Croitoru K, Perdue MH. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor-alpha. Gut 2004; 53(12):1817-24; PMID:15542521; http://dx.doi.org/ 10.1136/gut.2004.041426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cobden I, Rothwell J, Axon AT. Intestinal permeability in rats infected by Nippostrongylus brasiliensis. Gut 1979; 20(8):716-21; PMID:488766; http://dx.doi.org/ 10.1136/gut.20.8.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang SW, Wang ZQ, Cui J. Protein change of intestinal epithelial cells induced in vitro by Trichinella spiralis infective larvae. Parasitol Res 2011; 108(3):593-9; PMID:20922411; http://dx.doi.org/ 10.1007/s00436-010-2102-9 [DOI] [PubMed] [Google Scholar]
- [39].Andronicos NM, McNally J, Kotze AC, Hunt PW, Ingham A. Trichostrongylus colubriformis larvae induce necrosis and release of IL33 from intestinal epithelial cells in vitro: implications for gastrointestinal nematode vaccine design. Internat J Parasitol 2012; 42(3):295-304; http://dx.doi.org/ 10.1016/j.ijpara.2012.01.007 [DOI] [PubMed] [Google Scholar]
- [40].Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 2009; 206(3):655-67; PMID:19273626; http://dx.doi.org/ 10.1084/jem.20081499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stadnyk AW, Kearsey JA. Pattern of proinflammatory cytokine mRNA expression during Trichinella spiralis infection of the rat. Infect Immun 1996; 64(12):5138-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rehman ZU, Deng Q, Umair S, Savoian MS, Knight JS, Pernthaner A, Simpson HV. Excretory/secretory products of adult Haemonchus contortus and Teladorsagia circumcincta which increase the permeability of Caco-2 cell monolayers are neutralised by antibodies from immune hosts. Vet Parasitol 2016; 221:104-10; PMID:27084480; http://dx.doi.org/ 10.1016/j.vetpar.2016.03.017 [DOI] [PubMed] [Google Scholar]
- [43].Hiemstra IH, Klaver EJ, Vrijland K, Kringel H, Andreasen A, Bouma G, Kraal G, van Die I, den Haan JM. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol Immunol 2014; 60(1):1-7; PMID:24705296; http://dx.doi.org/ 10.1016/j.molimm.2014.03.003 [DOI] [PubMed] [Google Scholar]
- [44].Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry-Wheeler A, Silver N, Harnett MD, Hanauer SB. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Aliment Pharmacol Therapeut 2013; 38(3):255-63; http://dx.doi.org/ 10.1111/apt.12366 [DOI] [PubMed] [Google Scholar]
- [45].Polimeno L, Loiacono M, Pesetti B, Mallamaci R, Mastrodonato M, Azzarone A, Annoscia E, Gatti F, Amoruso A, Ventura MT. Anisakiasis, an underestimated infection: effect on intestinal permeability of Anisakis simplex-sensitized patients. Foodborne Pathog Dis 2010; 7(7):809-14; PMID:20367330; http://dx.doi.org/ 10.1089/fpd.2009.0484 [DOI] [PubMed] [Google Scholar]
- [46].Maizels RM. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin Microbiol Infect 2016; 22(6):481-6; PMID:27172808; http://dx.doi.org/ 10.1016/j.cmi.2016.04.024 [DOI] [PubMed] [Google Scholar]
- [47].Symonds HW, Mather DL, Mallinson CB, Hughes DL. Bile flow, bile salt secretion and the excretion of iron, copper, zinc and manganese in the bile of calves infected with Fasciola hepatica. Res Vet Sci 1983; 35(1):69-74; PMID:6622848 [PubMed] [Google Scholar]
- [48].Stenman LK, Holma R, Eggert A, Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 2013; 304(3):G227-34; PMID:23203158; http://dx.doi.org/ 10.1152/ajpgi.00267.2012 [DOI] [PubMed] [Google Scholar]
- [49].Kelly OB, Mroz MS, Ward JB, Colliva C, Scharl M, Pellicciari R, Gilmer JF, Fallon PG, Hofmann AF, Roda A, et al.. Ursodeoxycholic acid attenuates colonic epithelial secretory function. J Physiol 2013; 591(9):2307-18; PMID:23507881; http://dx.doi.org/ 10.1113/jphysiol.2013.252544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Persaud R, Wang A, Reardon C, McKay DM. Characterization of the immuno-regulatory response to the tapeworm Hymenolepis diminuta in the non-permissive mouse host. Internat J Parasitol 2007; 37(3-4):393-403; http://dx.doi.org/ 10.1016/j.ijpara.2006.09.012 [DOI] [PubMed] [Google Scholar]
- [51].Lopes F, Matisz C, Reyes JL, Jijon H, Al-Darmaki A, Kaplan GG, McKay DM. Helminth regulation of immunity: a three-pronged approach to treat colitis. Inflamm Bowel Dis 2016; 22(10):2499-512; PMID:27575495; http://dx.doi.org/ 10.1097/MIB.0000000000000889 [DOI] [PubMed] [Google Scholar]
- [52].Reardon C, Sanchez A, Hogaboam CM, McKay DM. Tapeworm infection reduces epithelial ion transport abnormalities in murine dextran sulfate sodium-induced colitis. Infect Immun 2001; 69(7):4417-23; http://dx.doi.org/ 10.1128/IAI.69.7.4417-4423.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xia CM, Zhao Y, Jiang L, Jiang J, Zhang SC. Schistosoma japonicum ova maintains epithelial barrier function during experimental colitis. World J Gastroenterol 2011; 17(43):4810-6; PMID:22147983; http://dx.doi.org/ 10.3748/wjg.v17.i43.4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fernandez-Blanco JA, Estevez J, Shea-Donohue T, Martinez V, Vergara P. Changes in epithelial barrier function in response to parasitic infection: implications for IBD pathogenesis. J Crohn's Colitis 2015; 9(6):463-76; http://dx.doi.org/ 10.1093/ecco-jcc/jjv056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ramage JK, Stanisz A, Scicchitano R, Hunt RH, Perdue MH. Effect of immunologic reactions on rat intestinal epithelium. Correlation of increased permeability to chromium 51-labeled ethylenediaminetetraacetic acid and ovalbumin during acute inflammation and anaphylaxis. Gastroenterology 1988; 94(6):1368-75; PMID:3129330; http://dx.doi.org/ 10.1016/0016-5085(88)90675-0 [DOI] [PubMed] [Google Scholar]
- [56].Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Nat Acad Sci (USA) 1987; 84(9):2975-9; http://dx.doi.org/ 10.1073/pnas.84.9.2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].King SJ, Miller HR. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology 1984; 51(4):653-60; PMID:6368371 [PMC free article] [PubMed] [Google Scholar]
- [58].McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Nati Acad Sci (USA) 2003; 100(13):7761-6; http://dx.doi.org/ 10.1073/pnas.1231488100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Scudamore CL, Pennington AM, Thornton E, McMillan L, Newlands GF, Miller HR. Basal secretion and anaphylactic release of rat mast cell protease-II (RMCP-II) from ex vivo perfused rat jejunum: translocation of RMCP-II into the gut lumen and its relation to mucosal histology. Gut 1995; 37(2):235-41; PMID:7557574; http://dx.doi.org/ 10.1136/gut.37.2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Scudamore CL, Thornton EM, McMillan L, Newlands GF, Miller HR. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med 1995; 182(6):1871-81; PMID:7500033; http://dx.doi.org/ 10.1084/jem.182.6.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Licona-Limon P, Arias-Rojas A, Olguin-Martinez E. IL-9 and Th9 in parasite immunity. Sem Immunopathol 2017; 49(1): 29-38; PMID: 2790045024595218 [DOI] [PubMed] [Google Scholar]
- [62].Gerlach K, McKenzie AN, Neurath MF, Weigmann B. IL-9 regulates intestinal barrier function in experimental T cell-mediated colitis. Tiss Barriers 2015; 3(1-2):e983777; http://dx.doi.org/ 10.4161/21688370.2014.983777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, Qian W, Hou X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PloS One 2014; 9(3):e90153; PMID:24595218; http://dx.doi.org/ 10.1371/journal.pone.0090153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fernandez-Blanco JA, Barbosa S, Sanchez de Medina F, Martinez V, Vergara P. Persistent epithelial barrier alterations in a rat model of postinfectious gut dysfunction. Neurogastroenterol Motility 2011; 23(11):e523-33; ; http://dx.doi.org/ 10.1111/j.1365-2982.2011.01777.x [DOI] [PubMed] [Google Scholar]
- [65].Rychter JW, Van Nassauw L, Brown JK, Van Marck E, Knight PA, Miller HR, Kroese AB, Timmermans JP. Impairment of intestinal barrier and secretory function as well as egg excretion during intestinal schistosomiasis occur independently of mouse mast cell protease-1. Parasite Immunol 2010; 32(4):221-31; PMID:20398222; http://dx.doi.org/ 10.1111/j.1365-3024.2009.01182.x [DOI] [PubMed] [Google Scholar]
- [66].Gonzalez-Castro AM, Martinez C, Salvo-Romero E, Fortea M, Pardo-Camacho C, Perez-Berezo T, Alonso-Cotoner C, Santos J, Vicario M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J Gastroenterol Hepatol 2017; 32(1):53-63; PMID:27087165; http://dx.doi.org/ 10.1111/jgh.13417 [DOI] [PubMed] [Google Scholar]
- [67].Su CW, Cao Y, Kaplan J, Zhang M, Li W, Conroy M, Walker WA, Shi HN. Duodenal helminth infection alters barrier function of the colonic epithelium via adaptive immune activation. Infect Immun 2011; 79(6):2285-94; http://dx.doi.org/ 10.1128/IAI.01123-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol 2002; 169(8):4417-22; PMID:12370375; http://dx.doi.org/ 10.4049/jimmunol.169.8.4417 [DOI] [PubMed] [Google Scholar]
- [69].Sun R, Urban JF Jr, Notari L, Vanuytsel T, Madden KB, Bohl JA, Ramalingam TR, Wynn TA, Zhao A, Shea-Donohue T. Interleukin-13 receptor-α1-dependent responses in the intestine are critical to parasite clearance. Infect Immun 2016; 84(4):1032-44; http://dx.doi.org/ 10.10.1007/500281-016-0609-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol 2005; 175(5):3207-13; PMID:16116211; http://dx.doi.org/ 10.4049/jimmunol.175.5.3207 [DOI] [PubMed] [Google Scholar]
- [71].Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun 2003; 71(5):2430-8; http://dx.doi.org/ 10.1128/IAI.71.5.2430-2438.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Heller F, Fromm A, Gitter AH, Mankertz J, Schulzke JD. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol 2008; 1 Suppl 1:S58-61; PMID:19079233; http://dx.doi.org/ 10.1038/mi.2008.46 [DOI] [PubMed] [Google Scholar]
- [73].Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 2011; 286(36):31263-71; PMID:21771795; http://dx.doi.org/ 10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Resnick MB, Colgan SP, Parkos CA, Delp-Archer C, McGuirk D, Weller PF, Madara JL. Human eosinophils migrate across an intestinal epithelium in response to platelet-activating factor. Gastroenterology 1995; 108(2):409-16; PMID:7835581; http://dx.doi.org/ 10.1016/0016-5085(95)90067-5 [DOI] [PubMed] [Google Scholar]
- [75].Wallon C, Persborn M, Jonsson M, Wang A, Phan V, Lampinen M, Vicario M, Santos J, Sherman PM, Carlson M, et al.. Eosinophils express muscarinic receptors and corticotropin-releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology 2011; 140(5):1597-607; PMID:21277851; http://dx.doi.org/ 10.1053/j.gastro.2011.01.042 [DOI] [PubMed] [Google Scholar]
- [76].Ceponis PJ, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. Lack of evidence for STAT 6 involvement. J Biol Chem 2000; 275(37):29132-7; PMID:10871612; http://dx.doi.org/ 10.1074/jbc.M003516200 [DOI] [PubMed] [Google Scholar]
- [77].Anthony RM, Urban JF Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 2006; 12(8):955-60; PMID:16892038; http://dx.doi.org/ 10.1038/nm1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dehlawi MS, Mahida YR, Hughes K, Wakelin D. Effects of Trichinella spiralis infection on intestinal pathology in mice lacking interleukin-4 (IL-4) or intestinal trefoil factor (ITF/TFF3). Parasitol Internat 2006; 55(3):207-11; http://dx.doi.org/ 10.1016/j.parint.2006.05.002 [DOI] [PubMed] [Google Scholar]
- [79].Cortes A, Munoz-Antoli C, Martin-Grau C, Esteban JG, Grencis RK, Toledo R. Differential alterations in the small intestine epithelial cell turnover during acute and chronic infection with Echinostoma caproni (Trematoda). Parasites Vect 2015; 8:334; http://dx.doi.org/ 10.1186/s13071-015-0948-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 2005; 308(5727):1463-5; PMID:15933199; http://dx.doi.org/ 10.1126/science.1108661 [DOI] [PubMed] [Google Scholar]
- [81].Knight PA, Pemberton AD, Robertson KA, Roy DJ, Wright SH, Miller HR. Expression profiling reveals novel innate and inflammatory responses in the jejunal epithelial compartment during infection with Trichinella spiralis. Infect Immun 2004; 72(10):6076-86; http://dx.doi.org/ 10.1128/IAI.72.10.6076-6086.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol 2004; 172(9):5616-21; PMID:15100305; http://dx.doi.org/ 10.4049/jimmunol.172.9.5616 [DOI] [PubMed] [Google Scholar]
- [83].Mullaly SC, Oudhoff MJ, Min PH, Burrows K, Antignano F, Rattray DG, Chenery A, McNagny KM, Ziltener HJ, Zaph C. Requirement for core 2 O-glycans for optimal resistance to helminth infection. PloS One 2013; 8(3):e60124; PMID:23555902; http://dx.doi.org/ 10.1371/journal.pone.0060124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].D'Elia R, DeSchoolmeester ML, Zeef LA, Wright SH, Pemberton AD, Else KJ. Expulsion of Trichuris muris is associated with increased expression of angiogenin 4 in the gut and increased acidity of mucins within the goblet cell. BMC Genomics 2009; 10:492; PMID:19852835; http://dx.doi.org/ 10.1186/1471-2164-10-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].McKay DM, Halton DW, McCaigue MD, Johnston CF, Fairweather I, Shaw C. Hymenolepis diminuta: intestinal goblet cell response to infection in male C57 mice. Exp Parasitol 1990; 71(1):9-20; PMID:2354717; http://dx.doi.org/ 10.1016/0014-4894(90)90003-U [DOI] [PubMed] [Google Scholar]
- [86].Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, et al.. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 2011; 208(5):893-900; PMID:21502330; http://dx.doi.org/ 10.1084/jem.20102057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, Grencis RK, Thornton DJ, Khan WI. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 2010; 138(5):1763-71; PMID:20138044; http://dx.doi.org/ 10.1053/j.gastro.2010.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Turner JE, Stockinger B, Helmby H. IL-22 mediates goblet cell hyperplasia and worm expulsion in intestinal helminth infection. PLoS Pathog 2013; 9(10):e1003698; PMID:24130494; http://dx.doi.org/ 10.1371/journal.ppat.1003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fricke WF, Song Y, Wang AJ, Smith A, Grinchuk V, Mongodin E, Pei C, Ma B, Lu N, Urban JF Jr, et al.. Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis. Microbiome 2015; 3:40; PMID:26377648; http://dx.doi.org/ 10.1186/s40168-015-0103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cortes A, Munoz-Antoli C, Sotillo J, Fried B, Esteban JG, Toledo R. Echinostoma caproni (Trematoda): differential in vivo mucin expression and glycosylation in high- and low-compatible hosts. Parasite Immunol 2015; 37(1):32-42; PMID:25382212; http://dx.doi.org/ 10.1111/pim.12159 [DOI] [PubMed] [Google Scholar]
- [91].Hasnain SZ, McGuckin MA, Grencis RK, Thornton DJ. Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. PLoS Negl Trop Dis 2012; 6(10):e1856; PMID:23071854; http://dx.doi.org/ 10.1371/journal.pntd.0001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Takeda K, Hashimoto K, Uchikawa R, Tegoshi T, Yamada M, Arizono N. Direct effects of IL-4/IL-13 and the nematode Nippostrongylus brasiliensis on intestinal epithelial cells in vitro. Parasite Immunol 2010; 32(6):420-9; PMID:20500673; http://dx.doi.org/ 10.1111/j.1365-3024.2010.01200.x [DOI] [PubMed] [Google Scholar]
- [93].Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol 2015; 8(3):672-82; PMID:25336167; http://dx.doi.org/ 10.1038/mi.2014.101 [DOI] [PubMed] [Google Scholar]
- [94].Munoz-Antoli C, Cortes A, Santano R, Sotillo J, Esteban JG, Toledo R. Interleukin-25 induces resistance against intestinal trematodes. Sci Rep 2016; 6:34142; PMID:27658962; http://dx.doi.org/ 10.1038/srep34142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].McKay DM, Fairweather I. A role for the enteric nervous system in the response to helminth infections. Parasitol Today 1997; 13(2):63-9; PMID:15275125; http://dx.doi.org/ 10.1016/S0169-4758(96)10079-X [DOI] [PubMed] [Google Scholar]
- [96].Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol 2015; 8(1):198-210; PMID:25005358; http://dx.doi.org/ 10.1038/mi.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012; 483(7389):345-9; PMID:22422267; http://dx.doi.org/ 10.1038/nature10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kamal M, Dehlawi MS, Brunet LR, Wakelin D. Paneth and intermediate cell hyperplasia induced in mice by helminth infections. Parasitology 2002; 125(3):275-81; PMID:12358424; http://dx.doi.org/ 10.1017/S0031182002002068 [DOI] [PubMed] [Google Scholar]
- [99].Fan CK, Hung CC, Lin YH, Li MH, Su KE. Enhanced expression of transforming growth factor-β1 in inflammatory cells and secretory granules in Paneth cells in the small intestine of mice infected with Toxocara canis. Parasitol Res 2004; 94(6):397-404; PMID:15490236; http://dx.doi.org/ 10.1007/s00436-004-1233-2 [DOI] [PubMed] [Google Scholar]
- [100].Forman RA, deSchoolmeester ML, Hurst RJ, Wright SH, Pemberton AD, Else KJ. The goblet cell is the cellular source of the anti-microbial angiogenin 4 in the large intestine post Trichuris muris infection. PloS One 2012; 7(9):e42248; PMID:22970115; http://dx.doi.org/ 10.1371/journal.pone.0042248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chen CC, Louie S, McCormick B, Walker WA, Shi HN. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect Immun 2005; 73(9):5468-81; http://dx.doi.org/ 10.1128/IAI.73.9.5468-5481.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Weng M, Huntley D, Huang IF, Foye-Jackson O, Wang L, Sarkissian A, Zhou Q, Walker WA, Cherayil BJ, Shi HN. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol 2007; 179(7):4721-31; PMID:17878371; http://dx.doi.org/ 10.4049/jimmunol.179.7.4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Manocha M, Shajib MS, Rahman MM, Wang H, Rengasamy P, Bogunovic M, Jordana M, Mayer L, Khan WI. IL-13-mediated immunological control of enterochromaffin cell hyperplasia and serotonin production in the gut. Mucosal Immunol 2013; 6(1):146-55; PMID:22763407; http://dx.doi.org/ 10.1038/mi.2012.58 [DOI] [PubMed] [Google Scholar]
- [104].McKay DM, Halton DW, Johnston CF, Fairweather I, Shaw C. Hymenolepis diminuta: changes in intestinal morphology and the enterochromaffin cell population associated with infection in male C57 mice. Parasitology 1990; 101(1):107-13; PMID:2235067; http://dx.doi.org/ 10.1017/S0031182000079816 [DOI] [PubMed] [Google Scholar]
- [105].Murray M, Smith WD, Waddell AH, Jarrett WF. Nippostrongylus brasiliensis: histamine and 5-hydroxytryptamine inhibition and worm expulsion. Exp Parasitol 1971; 30(1):58-63; PMID:4400480; http://dx.doi.org/ 10.1016/0014-4894(71)90070-1 [DOI] [PubMed] [Google Scholar]
- [106].Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, Tomov VT, Kobuley D, Tran SV, Bittinger K, et al.. Coinfection. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. Science 2014; 345(6196):578-82; PMID:25082704; http://dx.doi.org/ 10.1126/science.1256942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Reynolds LA, Finlay BB, Maizels RM. Cohabitation in the intestine: interactions among helminth parasites, bacterial microbiota, and host immunity. J Immunol 2015; 195(9):4059-66; PMID:; PMID:26477048; http://dx.doi.org/ 10.4049/jimmunol.1501432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Loke P, Lim YA. Can helminth infection reverse microbial dysbiosis? Trends Parasitol 2015; 31(11):534-5; http://dx.doi.org/ 10.1016/j.pt.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Reyes JL, Fernando MR, Lopes F, Leung G, Mancini NL, Matisz CE, Wang A, McKay DM. IL-22 restrains tapeworm-mediated protection against experimental colitis via regulation of IL-25 expression. PLoS Pathog 2016; 12(4):e1005481; PMID:27055194; http://dx.doi.org/ 10.1371/journal.ppat.1005481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bergstrom KS, Morampudi V, Chan JM, Bhinder G, Lau J, Yang H, Ma C, Huang T, Ryz N, Sham HP, et al.. Goblet cell derived RELM-β recruits CD4+ T cells during infectious colitis to promote protective intestinal epithelial cell proliferation. PLoS Pathog 2015; 11(8):e1005108; PMID:26285214; http://dx.doi.org/ 10.1371/journal.ppat.1005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Parks OB, Pociask DA, Hodzic Z, Kolls JK, Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front Cell Devp Biol 2015; 3:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zaiss MM, Rapin A, Lebon L, Dubey LK, Mosconi I, Sarter K, Piersigilli A, Menin L, Walker AW, Rougemont J, et al.. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 2015; 43(5):998-1010; PMID:; PMID:26522986; http://dx.doi.org/ 10.1016/j.immuni.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hayes KS, Bancroft AJ, Goldrick M, Portsmouth C, Roberts IS, Grencis RK. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 2010; 328(5984):1391-4; PMID:20538949; http://dx.doi.org/ 10.1126/science.1187703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Perdue MH, McKay DM. Integrative immunophysiology in the intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 1994; 267(2):G151-65 [DOI] [PubMed] [Google Scholar]
- [115].Cantacessi C, Giacomin P, Croese J, Zakrzewski M, Sotillo J, McCann L, Nolan MJ, Mitreva M, Krause L, Loukas A. Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis 2014; 210(9):1431-4; PMID:24795483; http://dx.doi.org/ 10.1093/infdis/jiu256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Giacomin P, Zakrzewski M, Croese J, Su X, Sotillo J, McCann L, Navarro S, Mitreva M, Krause L, Loukas A, et al.. Experimental hookworm infection and escalating gluten challenges are associated with increased microbial richness in celiac subjects. Sci Rep 2015; 5:13797; PMID:26381211; http://dx.doi.org/ 10.1038/srep13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cooper P, Walker AW, Reyes J, Chico M, Salter SJ, Vaca M, Parkhill J. Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS One 2013; 8(10):e76573; PMID:24124574; http://dx.doi.org/ 10.1371/journal.pone.0076573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lee SC, Tang MS, Lim YA, Choy SH, Kurtz ZD, Cox LM, Gundra UM, Cho I, Bonneau R, Blaser MJ, et al.. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl Trop Dis 2014; 8(5):e2880; PMID:24851867; http://dx.doi.org/ 10.1371/journal.pntd.0002880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, et al.. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog 2012; 8(11):e1003000; PMID:23166490; http://dx.doi.org/ 10.1371/journal.ppat.1003000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wu S, Li RW, Li W, Beshah E, Dawson HD, Urban JF Jr. Worm burden-dependent disruption of the porcine colon microbiota by Trichuris suis infection. PLoS One 2012; 7(4):e35470; PMID:22532855; http://dx.doi.org/ 10.1371/journal.pone.0035470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Li RW, Wu S, Li W, Navarro K, Couch RD, Hill D, Urban JF Jr. Alterations in the porcine colon microbiota induced by the gastrointestinal nematode Trichuris suis. Infect Immun 2012; 80(6):2150-7; http://dx.doi.org/ 10.1128/IAI.00141-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm Bowel Dis 2010; 16(11):1841-9; PMID:20848461; http://dx.doi.org/ 10.1002/ibd.21299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Reynolds LA, Smith KA, Filbey KJ, Harcus Y, Hewitson JP, Redpath SA, Valdez Y, Yebra MJ, Finlay BB, Maizels RM. Commensal-pathogen interactions in the intestinal tract: lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 2014; 5(4):522-32; PMID:25144609; http://dx.doi.org/ 10.4161/gmic.32155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Rausch S, Held J, Fischer A, Heimesaat MM, Kuhl AA, Bereswill S, Hartmann S. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS One 2013; 8(9):e74026; PMID:24040152; http://dx.doi.org/ 10.1371/journal.pone.0074026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Holm JB, Sorobetea D, Kiilerich P, Ramayo-Caldas Y, Estelle J, Ma T, Madsen L, Kristiansen K, Svensson-Frej M. Chronic Trichuris muris infection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of Lactobacilli. PLoS One 2015; 10(5):e0125495; PMID:25942314; http://dx.doi.org/ 10.1371/journal.pone.0125495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Houlden A, Hayes KS, Bancroft AJ, Worthington JJ, Wang P, Grencis RK, Roberts IS. Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: effects reversed by pathogen clearance. PLoS One 2015; 10(5):e0125945; PMID:25938477; http://dx.doi.org/ 10.1371/journal.pone.0125945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, et al.. Helminth infection promotes colonization resistance via type 2 immunity. Science 2016; 352(6285):608-12; PMID:27080105; http://dx.doi.org/ 10.1126/science.aaf3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].McKenney EA, Williamson L, Yoder AD, Rawls JF, Bilbo SD, Parker W. Alteration of the rat cecal microbiome during colonization with the helminth Hymenolepis diminuta. Gut Microbes 2015; 6(3):182-93; PMID:25942385; http://dx.doi.org/ 10.1080/19490976.2015.1047128 [DOI] [PMC free article] [PubMed] [Google Scholar]


