ABSTRACT
Entamoeba histolytica (Eh) is the protozoan parasite responsible for intestinal amebiasis and interacts dynamically with the host intestinal epithelium during disease pathogenesis. A multifaceted pathogenesis profile accounts for why 90% of individuals infected with Eh are largely asymptomatic. For 100 millions individuals that are infected each year, key interactions within the intestinal mucosa dictate disease susceptibility. The ability for Eh to induce amebic colitis and disseminate into extraintestinal organs depends on the parasite competing with indigenous bacteria and overcoming the mucus barrier, binding to host cells inducing their cell death, invasion through the mucosa and outsmarting the immune system. In this review we summarize how Eh interacts with the intestinal epithelium and subverts host defense mechanisms in disease pathogenesis.
Keywords: antimicrobial peptides, cysteine proteases, entamoeba histolytica, epithelium, inflammasome, mucus, tight junction proteins
Introduction
Entamoeba histolyica (Eh) is an enteric human protozoan parasite responsible for amebiasis that is common to developing countries. Accounting for approximately 100 million cases per year, Eh results in amebic dysentery, colitis and if left untreated can develop abscesses in extraintestinal sites, most commonly the liver. In 2013 there were 11,300 global deaths from amebiasis ranking it the fourth leading cause of parasitic diseases.1 Infection occurs through ingestion of contaminated food or water that contains Eh cysts. The vast majority of those infected with Eh are asymptomatic carriers where the parasite stays restricted to the lumen of the colon and finally undergoes encystment for excretion in stool to carry on the lifecycle.2 On average, a carrier will pass 45 million cysts in the stool daily and the infectious dose is greater than 1000 cysts.3 Disease is characterized by acute diarrhea often with the presence of blood and mucus, abdominal cramping and fever. Eh that have invaded the intestinal mucosa often form flask-like ulcers. Infection in children is particularly concerning as this leads to malnourishment and growth stunting which is exacerbated by common reinfection.4 Treatment for invasive amebiasis utilizes nitroimidazoles and often requires multiple interventions for a cure. Although there is no approved vaccine against Eh, vaccination against the major Eh adhesin protein, the Gal/GalNAc lectin, has proved promising in animal models.5 After infection there is resistance to subsequent Eh infections mainly through IFN-γ production and mucosal IgA.2 It still remains to be understood why such a large proportion of colonized individuals resist invasive disease. Owing to the complex pathogenesis profile of Eh, this is likely driven by several factors outlined in this review.
Interactions with the mucus barrier
The first line of innate host defense in the colonic milieu is the mucus barrier that forms a bimodal layer above the single layer of epithelial cells.6 This acts to spatially restrict noxious substances, commensal bacteria and potential pathogens from accessing the epithelial cells while allowing nutrient flux through. The primary component of this mucus barrier is MUC2 mucin, a tremendously large protein of 5179 amino acids that accounts for 80% of its weight through branched glycans and the most prominent member of the mucin family within the intestine. This glycosylation protects the mucin molecule from proteolytic degradation and may also act as a molecular decoy to bacteria or other pathogens that possess adhesins mistaking mucus for a target cell. Undoubtedly a critical aspect in Eh pathogenesis is to overcome the mucus barrier to gain access to the epithelial cells. Indeed, Eh binds to the colonic mucus layer with strong avidity through the Gal/GalNAc lectin, targeting the abundant galactose and N-acetylgalactosamine residues present on the O-linked sugar side chains of mucin.7 The Gal/GalNAc lectin has the highest affinity for multivalent saccharides such as GalNAc39BSA, however has very high affinity for in vivo conjugates such as mucin and fetuin.8 Eh possess a variety of glycosidases that may remove branched polysaccharides from mucin or host cells including sialidase, N-acetylgalactosamidase and N-acetylglucosaminidase.8 Due to the scarcity of free carbohydrates in the colon and competition with the commensal microbiota, Eh may turn on a pathogenicity program for scavenging polysaccharides. Since mucin is the largest source of carbohydrates in the colonic lumen, this would increase the degradation of the mucus barrier and result in Eh encountering epithelial cells. Indeed, Eh glycosidases present in secreted components interact with the polysaccharide side chains of mucin. In a transcriptome analysis of virulent versus non-virulent Eh during colonization, the glycoside hydrolase β-amylase was very strongly associated with invasive trophoziotes.9 Eh lacking this β-amylase was unable to breach the mucus layer and perturb the epithelial barrier. Additionally various other genes related to glycosidase and carbohydrate metabolism were induced in pathogenic Eh following colon invasion. Specifically N-acetylglucosamine modifies these sugar moieties on mucin leading to a loss of the protective functions.10 This occurs in absence of serine or cysteine protease activity however may increase the availability of proteases to interact with the mucin backbone and undergo proteolysis.
Mucin protein degradation occurs via cysteine proteinases present in Eh secreted components and the resulting degradation products are less efficient at preventing Eh adherence to host cells.11 Although EhCP1, EhCP2 and EhCP5 make up more than 90% of the cysteine protease activity in Eh, the degradation of mucin appears to be predominantly from EhCP5 as antisense inhibition drastically reduces the proteolytic activity. Further, Eh deficient in EhCP5 are unable to overcome the mucus barrier of cultured cells that abundantly express and secrete mucin. This leads to lack of cytolysis, however EhCP5-deficient parasites retain their cytopathic effect on cultured cells lacking a mucus barrier.12 EhCP5 specifically targets the C-terminal cysteine rich domain of MUC2 likely due to the lack of glycosylation.13 These regions within MUC2 are responsible for forming disulphide bridges between adjacent mucin molecules resulting in a polymeric sheet of mucus.
In the battle to maintain homeostasis within the host during Eh infection, the host responds to degradation of mucin and presence of a threat by evoking mucus hypersecretion. This acts to repel the invading pathogen from the epithelial surface. This responsibility is executed by colonic goblet cells that produce and secrete MUC2 mucin via regulated exocytosis. The absence of MUC2 in the intestinal epithelium leads to excess gross pathology and serum albumin leakage during Eh infection.14 This is directly coupled to exaggerated pro-inflammatory gene expression and cytokine secretion, particularly TNF-α, IFN-γ and IL-13. Additionally, inhibition of glycosylation of mucin within goblet cells renders the epithelium sensitive to Eh cytopathic effects and monolayer destruction.15 Therefore proper regulation of mucin secretion by goblet cells during Eh pathogenesis is critical. Eh is known to induce massive mucin hypersecretion during infection similar to other known secretagogues such as cholera toxin.16 This leads to the cavitation of goblet cells and mucin depletion, rendering the epithelium sensitive to invasion by Eh. This event is driven primarily through EhCP5 that interacts directly with its cognitive receptor αvβ3 integrin on colonic goblet cells.17 A signal transduction cascade consisting of SRC family kinase, PI3K, and PKCδ ultimately leads to the activation of the mucin vesicle marker myristoylated alanine-rich C-kinase substrate (MARCKS) affording mucin exocytosis (Fig. 1).
Figure 1.
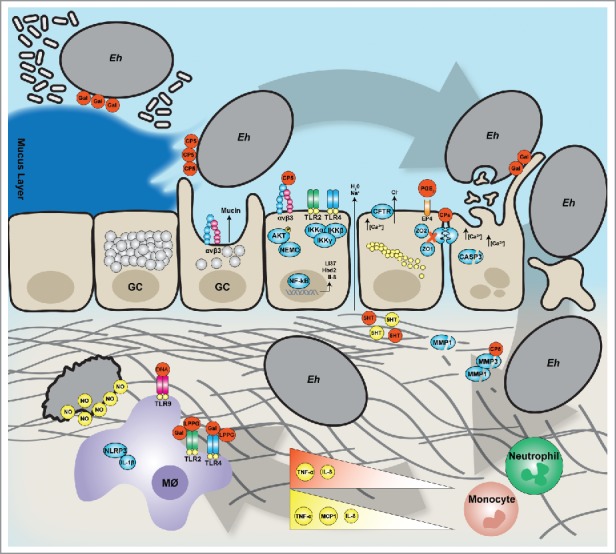
Entamoeba histolytica interactions with the mucosal barriers (clockwise). During Eh invasion the parasite degrades the protective mucus layers and evoke mucus hypersecretion from goblet cells (GC). By interacting with epithelial cells directly, Eh induces a pro-inflammatory responses driven by NF-B and later perturbation of the tight junction proteins to stimulate water and ion secretion. The epithelial barrier is then breached by cytolysis of epithelial cells allowing Eh to migrate in the lamina propria degrading the extracellular matrix (ECM). Here, Eh interacts with the immune compartment specifically macrophages where either Eh death will occur through NO-dependent killing or Eh will establish chronic disease.
Antimicrobial peptides and the microbiota
A key protective mechanism elicited by epithelial cells of the intestine is the production and secretion of antimicrobial peptides. Eh contact with host cells induces the expression of human defensin 2 through TLR2 and TLR4 canonical NF-B signaling. This leads to secretion of the active cationic peptide and ultrastructural alterations in exposed Eh characterized by discontinuous zones of plasma membrane and ruptured areas where cellular material is released.18 Interestingly, Eh was less susceptible to defensin killing compared with bacteria likely due to lipid composition of the plasma membrane and rapid turnover of surface molecules. Eh contains highly charged LPPG glycoconjugates, a component that discriminates between virulent Eh and non-virulent E. dispar.19 Additionally, Eh induces the expression of cathelicidin (LL-37) in both human cultured cells and a mouse model of amebic colitis. The role cathelicidin plays in Eh pathogenesis remains unclear as, unlike many bacterial pathogens, Eh are resistant to cathelicidin killing. Interestingly, Eh can cleave cathelicidins through cysteine proteases, specifically EhCP1. These proteolytic cleavage fragments retain their antimicrobial activity against susceptible bacteria. Despite Eh targeting cathelicidins for degradation, this is not the mechanism of resistance, as inhibition of cysteine proteases did not render Eh sensitive to killing. It would be interesting to decipher if cysteine protease cleavage by Eh processes antimicrobial peptides into smaller active fragments and the relative effect on the microbial communities within the host. With several other pathogens, perturbation of the commensal communities can render the host more susceptible to infection.20 An additional role for antimicrobial peptides in pathogenesis is through the chemoattractant properties they possess for neutrophils, monocytes, T cells and eosinophil's which is mediated through the formyl peptide receptor-like 1 receptor.21,22
The interplay between pathogenic bacteria and Eh has been studied from the observation that co-infection is common in endemic areas. Indeed, co-infection with enteropathogenic bacteria and Eh may enhance invasive disease within the intestine. This can be driven by either Eh ingestion of pathogenic bacteria or the prior alteration of the inflammatory state of host cells by pathogenic bacteria. Eh co-cultured with a commensal E. coli increase the surface expression of Gal/GalNAc lectin and display increased adherence to host cells.23 This is further enhanced if the feeder bacteria are pathogenic such as Enteropathogenic E. coli or Shigella. Interestingly while EPEC is nonviable once ingested, Shigella retains about 70% viability after phagocytosis by Eh, an effect that is limited to Eh and not E. dispar. As one might expect, increased adherence to host cells also leads to increases in the cytopathic effect of Eh induced monolayer destruction however, this was limited to Eh that have ingested pathogenic and not commensal bacteria. Increases in cysteine proteinase activity were also observed in Eh that ingested pathogenic bacteria.
The intestinal microbiota likely plays a role in disease pathogenesis either through direct interaction with Eh or modulation of the host epithelium. Alterations in the host microbiota have been observed in patients infected with Eh including suppression of Clostidium, Bacteroides, Lactobacillus, Campylobacter and Eubacterium with an expansion of Bifdobacterium.24 Interestingly, susceptibility to Eh infection can be predicted to a 79% accuracy based on analysis of microbiota distribution in the host.25 Several taxa that are strongly associated to a facilitative microbiota to allow for Eh infection have been linked to autoimmune disorders and exacerbating a pro-inflammatory state in the intestine including Prevotella copri. There is a direct correlation between Eh burden in infected individuals and abundance of P. copri in endemic areas of amebaisis.26 Specific components of the host microbiota may also educate the intestinal epithelium to subvert Eh infection. In a mouse model of Eh infection, colonization with segmented filimentous bacteria (SFB) has been shown to have a protective effect on Eh infection through inducing IL-23 leading to induction of IL-17 and increases in DC and neutrophil abundance in the cecum.27 The importance of this in human disease however remains to be elucidated, as SFBs in humans is controversial.
Epithelial cell responses and IL-8
As epithelial cells are the first cells too encounter an invading pathogen, they produce various pro-inflammatory cytokines to alert professional immune cells of danger such as IL-1β, IL-8 and TNF-α. Particularly, IL-8 functions as a chemoattractant to neutrophils recruiting them to sites of infection during acute inflammation. Therefore cytokines such as IL-8 likely mediate inflammation and tissue injury during Eh infection. The role of IL-8 during Eh pathogenesis appears to be a deleterious one, as neutrophils are unable of kill Eh at a ratio of 3000:1 and the host may actually be more severely damaged by the presence of neutrophils at the site of infection.28 Specifically, intestinal epithelial cells evoke a pro-inflammatory response characterized by IL-1β and IL-8 that results in neutrophil recruitment and tissue damage.29 Inhibition of NF-B p65 subunit or depletion of neutrophils results in lesser disease and abrogated intestinal permeability. Not surprisingly, Eh can stimulate both IL-8 mRNA and protein secretion from epithelial cells via both a contact dependent and independent event.30 Eh can evoke up regulation of IL-8 from a contact independent mechanism via secreted products however; Eh lysates possess this induction as well. This was not due to cellular damage or paracrine signaling by other cytokines such as IL-1β or TNF-α. Instead, Eh derived PGE2 drives IL-8 mRNA expression via the EP4 receptor.31 This event could be blocked almost entirely with delipidation of Eh secreted products, the broad cyclooxygenase inhibitor aspirin or silencing of the EP4 receptor (Fig. 1). Alternatively, Eh cysteine proteases can increase the expression of IL-8 however, this occurs independent of the protease-activated receptor 2 (PAR2).32
Cysteine proteases have emerged as critical virulence factors in Eh pathogenesis. This stems mainly from observations that attenuation of protease activity with E64 or silencing of EhCP5, the predominant CP that is secreted, leads to reduced gut inflammation, damage to the barrier and Eh that are unable to form liver abscess.33,34 Additionally, EhCP5 is absent in the non-pathogenic E. dispar.35 EhCP5 contains an RGD binding domain that interacts with αvβ3 integrin on colonic cells to trigger NF-B pro-inflammatory gene expression such as IL-8.36 This occurred through integrin-linked kinase mediated phosphorylation of AKT and lead to ubiquitination of NF-B essential modulation (NEMO) and downstream activation of NF-B. Interestingly, this event could be driven by purified EhCP5 and was independent of protease activity indicating a form of pathogen sensing by integrins. By no means is the involvement of NF-B deleterious to the host during Eh pathogenesis as targeted deletion of the p50 subunit of NF-B which abrogates signaling, leads to a worsened outcome.37 However there appears to be a bias toward nonclassical NF-B signaling as epithelial specific IKKβ KO mice manage Eh similarly to WT and are not more susceptible to infection. Alternatively, Eh may control the level of NF-B signaling by inducing epithelial cells to increase the expression of heat shock proteins.38 HSP27 and HSP72 have been shown to dampen NF-B induced pro-inflammatory gene expression by interacting directly with IKKα and IKKβ, ultimately alleviating oxidative and apoptotic injuries.
The chemotactic activity of IL-8 does not fully explain the repertoire of immune cells that are recruited to the site of Eh infection, particularly monocytes and lymphocytes. Accordingly, Eh can also induce epithelial cells to release the monocyte chemotactic protein MCP-1 through non-classical NF-B signaling mediated by PI3K.39 Other chemokines such as GMCSF have proved pivotal in controlling intestinal amebiasis. This can be induced by serum amlyoid A and segmented filamentous bacteria through upregulation of CSF2RA expression and granulocyte monocyte precursors in the bone marrow.40 Consequently Eh responds by producing secreted products that inhibit the chemotaxis and random mobility of monocytes, thus masking this detection mechanism.41 Eh also responds to various cytokines by following the chemotactic gradient toward the source of the chemokine. Specifically, IL-8 is able to bind to a surface receptor on Eh that shares homology with CXCR1 and orchestrate movement of the actin/myosin cytoskeleton to initiate migration. Additionally, Eh contains a BspA-like family protein with high homology to the leucine rich repeats of TNF receptor 1 and toll-like receptors that affords chemotaxis toward TNF-α.42 This occurs via a PI3K dependent mechanism resulting in reorganization of the actin cytoskeleton and up regulation of the Eh adhesin Gal/GalNAc lectin.43 Silencing of this TNFR1 analog, CSP, attenuated the ability of Eh to penetrate the colonic barrier and migrate into the lamina propria of human explants.
Tight junction permeability and ion secretion
Loss of integrity of the epithelial tight junction (TJ) barrier precludes cell death, with a decrease in the transepithelial resistance (TER) occurring independent of cell lysis. Instead, Eh appears to modulate the junctional complex to increase paracellular permeability. This event coincides with release and degradation of ZO1 along with the release and dephosphorylation of ZO2.44 The molecular signaling cascades responsible for paracellular permeability remain elusive, as pharmacological inhibition using various inhibitors does not prevent leakage. This event may instead be driven by perturbation of the host cytoskeleton. Ultimately this change in paracellular permeability results in electrolyte and water imbalances resulting in diarrhea. Another mechanism by which Eh may modulate epithelial permeability is through PGE2 production. Following infection of the colonic mucosa, Eh induces a 10-fold increase in PGE2 at the site of inflammation.45 In addition to inducing host cells such as epithelial and mononuclear cells to produce PGE2, Eh possesses the COX-like enzymes to produce the prostanoid itself in the presence of arachidonic acid.31 Indeed, PGE2 regardless of the source is able to perturb the TER of the epithelial barrier by signaling through EP4 to displace claudin-4.46 As a consequence, paracellular leakage of chloride into the lumen drives water culminating in diarrheal disease (Fig. 1).
Specific virulence factors within Eh also home to the tight junctions such as the EhCPADH112 complex. This complex is composed of the EhCP112 cysteine protease and EhADH112 adhesin and can specifically bind to occludin, claudin-1, ZO1 and ZO2 thereby targeting these tight junction proteins for degradation. This leads to the rapid loss of TER and increases paracellular permeability.47 Additionally, Eh contains an occludin-like protein which possibly functions to displace the host TJ occludin resulting in a decrease in TER.48 The expression of TJ proteins has been studied in the context of a mouse colonic loop model using control animals and also mice lacking a bonafide mucus layer. During acute infection with Eh, WT animals increase the expression of occludin whereas mice lacking a mucus barrier increase the expression of the claudin-2 leading to greater paracellular permeability.14 This effect was driven largely by cysteine proteases as pre-treatment of Eh with E64 or antisense-targeting EhCP5 diminished the increase in gene expression of claudin-2 and occludin. Ultimately alterations of TJ proteins lead to a tremendous influx of serum albumin and water into the lumen of the colon. Anion transport, particularly chloride, has been shown to drive diarrheal disease through serotonin that is produced by Eh.49 Characteristic of a neuronal peptide, this effect was specific to the serosal side of the tissue. In addition to detection of serotonin in Eh lysates, neutralization of Eh serotonin by antibodies or desensitization by pre-treatment of bufotenine abolished the effects on ion transport and short circuit current. This occurs through both a calcium dependent mechanism and cAMP activation of CFTR.50 Eh lysates could also exacerbate this effect by inhibiting sodium and chloride absorption.
Invasion into and beyond the epithelial barrier
Eh exerts a cytopathic effect on a variety of different cell types including both immune and epithelial cells as part of its pathogenesis. Cytotoxicity of an activated macrophage will ensure survival whereas direct killing of epithelial cells will allow for passage deeper into the mucosa. It is clear that the cytotoxicity that Eh inflicts on host cells is contact dependent, specifically mediated by the Gal/GalNAc lectin. Addition of exogenous galactose or GalNAc inhibits the cytopathic effect however Gal/GalNAc lectin may induce cytopathic effects in addition to providing adherence.51 A critical virulence mechanism to subvert immune detection of the invading parasite is phagocytosis of apoptotic cells. The importance of phagocytosis in Eh pathogenesis derived from the observation that phagocytosis deficient clones of Eh were less virulent.52 A microarray analysis identified 121 genes that are important to Eh phagocytosis, specifically gene clusters relating to actin binding and cytoskeletal organization.53 Interestingly, pre-exposure to a phagocytosis stimulus enhanced subsequent phagocytosis potential suggesting a feed-forward regulation of genes related to phagocytosis. Apoptosis of host cells precedes phagocytosis and is mediated by exposure of host cell phosphatidylserine.54 Although annexin V masking of phosphatidylserine on erythrocytes can greatly inhibit phagocytosis, this effect is not seen with nucleated cells such as T cells suggesting other apoptotic markers may facilitate this event.55 The Eh receptor that recognizes apoptotic markers on host cells may be part of the large family of Eh trans-membrane kinases (TMK).56,57 Additionally, there is support for the serine rich Eh protein (SREHP) on facilitating Gal/GalNAc lectin independent phagocytosis of apoptotic host cells given the 90% inhibition using a specific monoclonal antibody.58 Opsonization of apoptotic cells by C1q can also occur whereby Eh calreticulin acts as the surface receptor to initiate phagocytosis.59 During engulfment of host cells by Eh, F-actin and myosin 1B localizes to the phagocytic cup to facilitate ingestion. Perturbation of actin polymerization or overexpression of myosin 1B disrupts the phagocytic activity of Eh. Cholesterol also participates in engulfment as seen through an enhancement in phagocytosis, likely through sequestering Gal/GalNAc lectin in lipid raft domains in the plasma membrane at the site of uptake.60,61 Instead of cholesterol loading effecting the abundance of Gal/GalNAc lectin on the surface of Eh, it is likely more a sequestering effect to produce a highly enriched region of receptors that will facilitate a more localized synapse with the host cell.62 In addition to increasing the avidity of binding between Eh and the host cell, this will lead to stronger potentiation of intracellular signal cascades, specifically Rab GTPases.
A recent mechanism of Eh invasion into the epithelium and cell killing has emerged coined Eh trogocytosis, whereby Eh will ingest small fragments of the plasma membrane of host cell.63 Trogocytosis shares similarities to Eh phagocytosis of cells including EhC2PK, a C2 domain containing protein kinase that initiates the phagocytic cup and actin recruitment.64 Not surprisingly, interference with the cytoskeletal and microfilament network within Eh also inhibited trogocytosis. Host cells that were nibbled on also experienced irreversible intracellular calcium increases (Fig. 1). This is in accordance with prior studies that identified inhibitors or chelators of calcium as blocking the cytotoxicity of Eh. The effector for driving this calcium flux may be the Gal/GalNAc lectin as purified lectin and fixed Eh are both able to induce calcium flux in target cells.65 Eh also induces apoptosis of host cells by activating caspase 3 in a contact-dependent manner.66 Mice deficient for caspase 3 resist intestinal colitis by Eh and other caspases such as caspase 8 and 9 are not involved.67 The receptor that induces this fast activation of caspase 3 has not been identified however Fas and TNF-α receptor do not play a role.68 A possible effector for driving caspase 3 activation was identified by an RNAi screen where ion transporters, specifically potassium (K(+)), were found to be involved in Eh cell killing.69 Given low cytosolic K+ concentration can mediate both apoptosis and caspase activation, K+ efflux induced by Eh appears to be critical in cell killing and pathogenesis. Additionally, the adipocytokine leptin appears to regulate Eh-induced cell death in epithelial cells. Overexpression of the leptin receptor or addition of exogenous leptin is protective from Eh induced apoptosis.70 Leptin signaling is dependent on STAT3 and functions through regulation of apoptotic gene expression. In a mouse model of amebiasis, genetic deletion of the leptin receptor in intestinal epithelial cells rendered the host susceptible to Eh infection.71 In humans, a mutation in the leptin receptor (Q223R) increases susceptibility to amebiasis.72 When this polymorphism is expressed in mice a similar susceptibility to Eh intestinal amebiasis is observed resulting in increased caspase 3 activity, and decreased antiapoptotic gene expression.
The extracellular matrix (ECM) provides a scaffold for the intestinal epithelia and is composed primarily of collagen. The ECM is divided into 2 compartments, a basement membrane comprising a tight meshwork that underlay the epithelial layer and a looser 3-dimensional interstitial collagen fiber network underneath that supports the lamina propria. After breaching the epithelial layer, Eh must navigate through this ECM as a precursor for amebic ulcer formation within the mucosa/submucosa and dissemination into extra intestinal sites. Eh is able to degrade the collagen network through cysteine proteinase activity and non-virulent strains of Eh lack collagenolytic activity.73 However, it is more likely that instead of completely degrading the collagen network within the ECM, Eh remodels the collagen fibers to increase the porosity of the matrix in a cysteine proteinase manner. This would suggest that if amoeboid movement through the matrix is too restrictive due to the large size of Eh or tightness of the matrix, Eh might deploy cysteine proteinases to facilitate pore formation. Interestingly, human factors appear to play a role in EhCP5 dependent collagen remodelling, specifically human matrix metalloproteinases (Fig. 1; MMPs). These MMPs are transcriptionally regulated by pro-inflammatory cytokines that have been characterized during Eh infection such as TNF-α and IL-1β and are also overexpressed in patients with amebiasis.74 These MMPs are secreted as inactive proform molecules whose activation state depends on other MMPs or host proteases. EhCP5 can directly cleave latent MMP-3 into the active form, resulting in downstream cleavage of MMP-1.75 In a vicious feedback loop, Eh can evoke further MMP expression through EhCP5-NF-B pro-inflammatory gene expression and readily activate MMPs leading to ECM remodelling and tissue invasion. Interestingly, once Eh has penetrated into the lamina propria where the ECM network is more porous, it does not need protease dependent migration and may turn off this virulence mechanism to avoid detection by immune cells. This environment would favor an amoeboid or bleb-like migration that is controlled by instability of intracellular pressure and executed by the actomyosin contractile machinery.76 Specifically, myosin II has been implicated in Eh motility and also has roles in inducing cytotoxicity.77,78
Another form of Eh migration has been postulated from the observation that following fibronectin treatment Eh sequesters actin in a dot-like compartment analogous to invadosomes.79 Invadosomes are found in transformed cells that exert migration through the ECM by degradation. This process is highly influenced by growth factors and ECM cues that are largely sensed by host integrins. Eh has been implicated in possessing a fibronectin receptor that is antigenically similar to human β1 integrin.80 This fibronectin receptor participates in the formation of actin rich dots within Eh in a Rab21 GTPase dependent manner.81 This event was positively regulated by fibronectin, inducing membrane protrusions and negatively regulated by collagen type I resulting in smooth Eh. Accordingly, Eh can bind to various ECM components such as fibronectin and collagen via lipid rafts.82 Inhibition of Gal/GalNAc lectin binding to collagen suppresses actin dot formation however this was not observed with fibronectin. This constitutes a model where biogenesis of invadosomes is induced by fibronectin with Gal/GalNAc lectin as a co-stimulatory molecule. This model has not been validated in an in vivo model of Eh invasion however. In support of invadosome mediated migration with extensive ECM degradation, Eh possesses 86 genes encoding proteases including 22 metalloproteases.83 The role these Eh metalloproteases play in pathogenesis has not been explored yet.
Eh virulence factors and interactions with immune cells
Perhaps the best-studied virulence factor within Eh is the Gal/GalNAc lectin that mediates attachment to host cells. Early studies demonstrated that Eh contact with host cells, immune cells and intestinal mucus could be inhibited by exogenous galactose and GalNAc while other carbohydrates had no effect. This adherence was mediated by a disulfide bridged dimeric lectin consisting of a heavy (170kDa) and light subunits (35kDa) that was heavily cysteine rich in the carbohydrate binding region (CRD).84,85 The active site of this CRD was mapped using monoclonal antibodies to resides 596–1082 of the heavy chain.86 The 170kDa subunit of Gal/GalNAc lectin is highly targeted by the humoral response in patients previously infected with Eh with more than 90% having immune sera against this antigen.87 Despite this, acquired immunity from Eh infection is likely more skewed to a cell-mediated mechanism than humoral. Accordingly, reinfection with Eh is extremely low in endemic areas possibly due to anti-Gal/GalNAc lectin antibodies and thus this antigen presents as a suitable target for vaccine development. In a gerbil model of amebic liver abscess, fusion proteins derived from Gal/GalNAc lectin as a vaccine conferred up to 81% protection.88 The causation of this is largely unknown however interestingly Gal/GalNAc lectin appears to have a mitogenic effect stimulating lymphocytes from previously immunized animals or infected humans to proliferate and produce IL-2 and IFN-γ.89
Soluble Gal/GalNAc lectin has the ability to stimulate TNF-α production from naive macrophages and can be inhibited with specific monoclonal antibodies against the CRD of the Gal/GalNAc lectin.86 TNF-α is primarily released from activated macrophages and can induce both cell proliferation and apoptosis of target cells, act as a chemoattractant and upregulate other pro-inflammatory cytokines. In IFN-γ primed macrophages, native Gal/GalNAc lectin simulated both TNF-α and iNOS mRNA expression. This coincided with an increase in TNF-α secretion and NO production that lead to macrophage killing of Eh trophozoites.90 TNF-α can also act directly on Eh by inhibited the growth rate without inducing any cytotoxic effects.91 Secretion of TNF-α is not purely beneficial however, as this cytokine has been shown to exacerbate tissue damage during Eh infection and increase permeability of the barrier.92 Intriguingly there is an direct correlation to TNF-α levels in patients previously infected with Eh that developed diarrheal disease compared with those that were asymptomatic.74 Isolated PBMCs that produced the highest levels of TNF-α correlated with patients with increased risk of first and recurrent Eh diarrheal episodes.
Gal/GalNAc lectin may also contribute to induction of inflammatory responses through upregulating PRR expression, specifically TLR2 on macrophages. Purified Gal/GalNAc lectin was found to act through p38 MAPK to activate NF-B signaling and increase TLR2 mRNA and surface expression.93 Upregulation of TLR2 and TLR4 by Gal/GalNAc lectin was later shown to occur in epithelial cells through the classical MyD88 signaling cascade culminating in NF-B induction.23 This is analogous to pathogenic bacteria signaling though PRR to increase the expression of TLR2/4 and co-infection may render the epithelium more responsive to such PAMPs. Interestingly, the CRD of the Gal/GalNAc lectin was shown to directly interact with TLR4, likely due to the N-linked glycosylation of the receptor.94 During infection when Eh Gal/GalNAc lectin upregulated TLR expression on the cell surface, Eh possesses a greater adherence potential. This occurred in naive macrophages suggesting that this up regulation of specific TLRs could skew the inflammatory response to a protective Th1 response. Further, by altering the expression of TLR2 on either epithelial or immune cells, Gal/GalNAc lectin may change the way the host responds to stimuli from commensal or pathogenic bacteria.
In addition to the Gal/GalNAc lectin, Eh can stimulate TLR2/4 signaling through lipopeptidophosphoglycan (LPPG). This cell surface molecule is predominately composed of carbohydrates and is immunogenic as most patients previously infected with Eh have anti-LPPG immunoglobulins in their sera.95 The structural composition of LPPG likely contributes to pathogenesis as non-virulent strains vary in their polysaccharide compared with virulent counterparts.96 This molecule is transferred to enteric cell layers during pathogenesis following adhesion but before alterations in the tight junction.97 LPPG appears to have both anti-inflammatory and pro-inflammatory effects on macrophages and monocytes. Exposure of monocytes to LPPG initially results in secretion of TNF-α followed later by IL-12p40 and IL-8.98 Macrophages appear to be more sensitive to LPPG as a greater secretion was observed compared with monocytes. Regardless, LPPG can signal through both TLR2 and 4 by canonical NF-B signaling and ablation of these receptors leads to attenuated TNF-α and IL-6 release. The anti-inflammatory functions of LPPG are to dampen TLR2 mRNA expression and induce IL-10 from monocytes.99 This balance between induction of a pro-inflammatory response characterized by TNF-α mediated NO killing by macrophages, IL-8 chemotaxis of neutrophils, IL-12p40 skewing to Th1 T cell responses and immunosuppression by IL-10 likely reflects the complexity of responses to Eh infection. Indeed, minor alterations in how the host responds to Eh through this cytokine concert could begin to explain the variation in disease onset by individuals in a population. During infection with Eh and while the host fights to maintain homeostasis by eliciting amebicidal activity, Eh lysis is bound to occur. This will indefinitely liberate Eh genomic DNA which is unmethylated analogous to various other pathogens and bacteria. This Eh DNA can signal similarly to CpG DNA through TLR9 to activate NF-B and initiate an inflammatory signaling cascade culminating to TNF-α and iNOS production.100
Inflammasome activation
The innate immune system is tasked with sensing countless microbial products within the gut and responding with appropriate action to deal with the threat while minimizing damage to the host. The detection mechanisms discussed prior focused on sensing specific molecular patterns within Eh products to activate a pro-inflammatory response however, does not discriminate between live and dead Eh. Specifically, Eh that have invaded into the colonic barrier present the highest threat and require a vigorous immune response to prevent further dissemination to extraintestinal sites. Macrophages are likely first responders in the innate immune compartment as they reside in the lamina propria and orchestrate a strong pro-inflammatory response characterized by activation of the inflammasome and IL-1β secretion.101 Interestingly, activation of the NLRP3 inflammasome in macrophages requires direct contact by Eh through binding of Gal/GalNAc lectin. Patients with invasive amebiasis typically have a strong antibody response against Gal/GalNAc lectin and immune sera are sufficient to inhibit contact and inflammasome mediated IL-1β secretion. Soluble Eh components have no effect on eliciting IL-1β secretions however, are sufficient to prime macrophages and evoke non-inflammasome mediated cytokine secretion such as TNF-α. The putative virulence factor that evokes inflammasome activation after contact is EhCP5 by binding to the α5β1 integrin on macrophages.102 Activation of α5β1 integrin results in pannexin-1 mediated ATP release that then signals back on the macrophage through P2×7 receptors to deliver the co-stimulatory signal necessary for NLRP3 activation. This intimate contact event mediated by Gal/GalNAc lectin forms an immune cell synapse that potentiates signal transduction through the actions of EhCP5. Inflammasome activation is also dependent on potassium efflux as inhibition of K+ channel activity blocks caspase 1 activation, IL-1β secretion and pyroptotic death in macrophages.69 Surprisingly, inflammasome activation by Eh may coordinate other inflammatory cytokine secretion as NLRP3 and ASC deficient mice failed to elicit IL-10, IL12p70 and MIP-1 secretion. It is likely that pathogen detection by inflammasome activation within macrophages is a precursor for driving effector responses. While calculated immune responses are critical to controlling infection, Eh tampers with this balance to subvert the host into mounting a more aggressive response. Specifically, Eh cysteine proteinases possess IL-1 converting enzyme (ICE) activity that can process inactive pro-IL-1β that is released from dead cells into active IL-1β. This Eh cysteine proteinase processed IL-1β mimics the endogenously processed IL-1β by caspase-1 and is able to induce nitrite production. Not all caspase-1 cleavage products are processed similarly by Eh however, as pro-IL-18 is proteolytically cleaved into inactive fragments by EhCP5.103
Conclusion
Upon colonizing the colon of the infected host, Eh likely changes its relationship within the host from a non-pathogen to pathogen. This is likely driven by interaction with the microbial communities and could be driven by nutrient availability. Eh then targets the mucus barrier for degradation using the glycans as a food source and evoking mucin hypersecretion from goblet cells. Upon mucin depletion, Eh contacts the epithelial cells via the Gal/GalNAc lectin inducing robust pro-inflammatory gene expression, release of chemotaxic factors and antimicrobial peptides. The subsequent cytolysis of the epithelial cells leads to a barrier breach where Eh migrates into the mucosa. Once reaching the lamina propria Eh induces macrophage activation, resists neutrophil killing and induces massive pro-inflammatory cytokine release. The ability for Eh to establish in this niche will ultimately decide if infection persists or if the host successfully clears the parasite (Fig. 1).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Dr. Chadee is a Canada Research Chair in Gastrointestinal Inflammation and his research cited in this chapter were supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Canadian Institutes of Health Research and Crohn's and Colitis Canada. SC is the recipient of an NSERC Alexander Graham Bell studentship.
References
- [1].GBD 2013 Mortality and Causes of Death Collaborators, I. for H. M. and et al.. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2015; 385:117-71; PMID:25530442; http://dx.doi.org/ 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mortimer L, Chadee K. The immunopathogenesis of Entamoeba histolytica. Exp Parasitol 2010; 126:366-80; http://dx.doi.org/ 10.1016/j.exppara.2010.03.005 [DOI] [PubMed] [Google Scholar]
- [3].Ryan KJ, Kenneth J, Ray CG, Sherris JC. Sherris medical microbiology: an introduction to infectious diseases. (McGraw-Hill, 2004). [Google Scholar]
- [4].Mondal D, Petri WA Jr, Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg 2006; 100:1032-8. [DOI] [PubMed] [Google Scholar]
- [5].Quach J, St-Pierre J, Chadee K. The future for vaccine development against Entamoeba histolytica. Hum Vaccin Immunother 2014; 10:1514-21; http://dx.doi.org/ 10.4161/hv.27796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008; 105:15064-9; PMID:18806221; http://dx.doi.org/ 10.1073/pnas.0803124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chadee K, Petri WA Jr, Innes DJ, Ravdin JI, Ravdin JI. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest 1987; 80:1245-54; http://dx.doi.org/ 10.1172/JCI113199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Frederick JR, Petri WA Jr. Roles for the galactose-/N-acetylgalactosamine-binding lectin of Entamoeba in parasite virulence and differentiation. Glycobiology 2005; 15:53R-59R; PMID:16037494; http://dx.doi.org/ 10.1093/glycob/cwj007 [DOI] [PubMed] [Google Scholar]
- [9].Thibeaux R, Weber C, Hon CC, Dillies MA, Avé P, Coppée JY, Labruyère E, Guillén N. Guillén N Identification of the virulence landscape essential for Entamoeba histolytica invasion of the human colon. PLoS Pathog 2013; 9:e1003824; PMID:24385905; http://dx.doi.org/ 10.1371/journal.ppat.1003824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moncada D, Keller K, Chadee K. Entamoeba histolytica-secreted products degrade colonic mucin oligosaccharides. Infect Immun 2005; 73:3790-3; PMID:15908414; http://dx.doi.org/ 10.1128/IAI.73.6.3790-3793.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moncada D, Keller K, Chadee K. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infect Immun 2003; 71:838-44; PMID:12540564; http://dx.doi.org/ 10.1128/IAI.71.2.838-844.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moncada D, Keller K, Ankri S, Mirelman D, Chadee K. Antisense inhibition of Entamoeba histolytica cysteine proteases inhibits colonic mucus degradation. Gastroenterology 2006; 130:721-730; PMID:16530514; http://dx.doi.org/ 10.1053/j.gastro.2005.11.012 [DOI] [PubMed] [Google Scholar]
- [13].Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci 2006; 103:9298-9303; http://dx.doi.org/ 10.1073/pnas.0600623103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(-/-) mice. Am J Pathol 2013; 182:852-65; PMID:23357502; http://dx.doi.org/ 10.1016/j.ajpath.2012.11.035 [DOI] [PubMed] [Google Scholar]
- [15].Belley A, Keller K, Grove J, Chadee K. Interaction of LS174T human colon cancer cell mucins with Entamoeba histolytica: an in vitro model for colonic disease. Gastroenterology 1996; 111:1484-92; PMID:8942726; http://dx.doi.org/ 10.1016/S0016-5085(96)70009-4 [DOI] [PubMed] [Google Scholar]
- [16].Chadee K, Keller K, Forstner J, Innes DJ, Ravdin JI. Mucin and nonmucin secretagogue activity of Entamoeba histolytica and cholera toxin in rat colon. Gastroenterology 1991; 100:986-97; PMID:2001836 [DOI] [PubMed] [Google Scholar]
- [17].Cornick S, Moreau F, Chadee K. Entamoeba histolytica cysteine proteinase 5 evokes mucin exocytosis from colonic goblet cells via αvβ3 Integrin. PLoS Pathog 2016; 12:e1005579; PMID:27073869; http://dx.doi.org/ 10.1371/journal.ppat.1005579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ayala-Sumuano JT, Téllez-López VM, Domínguez-Robles Mdel C, Shibayama-Salas M, Meza I. Toll-like receptor signaling activation by Entamoeba histolytica induces beta defensin 2 in human colonic epithelial cells: its possible role as an element of the innate immune response. PLoS Negl Trop Dis 2013; 7:e2083; PMID:23469306; http://dx.doi.org/ 10.1371/journal.pntd.0002083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moody S, Becker S, Nuchamowitz Y, Mirelman D. Virulent and avirulent Entamoeba histolytica and E. dispar differ in their cell surface phosphorylated glycolipids. Parasitology 1997; 114:95-104; PMID:9051918; http://dx.doi.org/ 10.1017/S0031182096008396 [DOI] [PubMed] [Google Scholar]
- [20].Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 2003; 71:2839-58; PMID:12704158; http://dx.doi.org/ 10.1128/IAI.71.5.2839-2858.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol 2006; 140:103-112; PMID:16557028; http://dx.doi.org/ 10.1159/000092305 [DOI] [PubMed] [Google Scholar]
- [22].De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. Chertov O LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 2000; 192:1069-74; PMID:11015447; http://dx.doi.org/ 10.1084/jem.192.7.1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Galván-Moroyoqui JM, del Carmen Domínguez-Robles M, Meza I. Pathogenic bacteria prime the induction of Toll-like receptor signalling in human colonic cells by the Gal/GalNAc lectin Carbohydrate Recognition Domain of Entamoeba histolytica. Int J Parasitol 2011; 41:1101-1112; http://dx.doi.org/ 10.1016/j.ijpara.2011.06.003 [DOI] [PubMed] [Google Scholar]
- [24].Verma AK, Verma R, Ahuja V, Paul J. Real-time analysis of gut flora in Entamoeba histolytica infected patients of Northern India. BMC Microbiol 2012; 12:183; PMID:22913622; http://dx.doi.org/ 10.1186/1471-2180-12-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morton ER, Lynch J, Froment A, Lafosse S, Heyer E, Przeworski M, Blekhman R, Ségurel L. Ségurel L Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and subsistence. PLoS Genet 2015; 11:e1005658; PMID:26619199; http://dx.doi.org/ 10.1371/journal.pgen.1005658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gilchrist CA, Petri SE, Schneider BN, Reichman DJ, Jiang N, Begum S, Watanabe K, Jansen CS, Elliott KP, Burgess SL, et al.. Role of the gut microbiota of children in diarrhea due to the protozoan parasite Entamoeba histolytica. J Infect Dis 2016; 213:1579-85; PMID:26712950; http://dx.doi.org/ 10.1093/infdis/jiv772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burgess SL, Buonomo E, Carey M, Cowardin C, Naylor C, Noor Z, Wills-Karp M, Petri WA Jr. Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. MBio 2014; 5:e01817-14; PMID:25370489; http://dx.doi.org/ 10.1128/mBio.01817-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Espinosa-Cantellano M, Martínez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev 2000; 13:318-31; PMID:10756002; http://dx.doi.org/ 10.1128/CMR.13.2.318-331.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seydel KB, Li E, Zhang Z, Stanley SL. Epithelial cell-initiated inflammation plays a crucial role in early tissue damage in amebic infection of human intestine. Gastroenterology 1998; 115:1446-53; PMID:9834272; http://dx.doi.org/ 10.1016/S0016-5085(98)70023-X [DOI] [PubMed] [Google Scholar]
- [30].Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 1997; 112:1536-47; PMID:9136832; http://dx.doi.org/ 10.1016/S0016-5085(97)70035-0 [DOI] [PubMed] [Google Scholar]
- [31].Dey I, Keller K, Belley A, Chadee K. Identification and characterization of a cyclooxygenase-like enzyme from Entamoeba histolytica. Proc Natl Acad Sci U S A 2003; 100:13561-6; PMID:14585927; http://dx.doi.org/ 10.1073/pnas.1835863100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee YA, Nam YH, Min A, Kim KA, Nozaki T, Saito-Nakano Y, Mirelman D, Shin MH. Entamoeba histolytica-secreted cysteine proteases induce IL-8 production in human mast cells via a PAR2-independent mechanism. Parasite 2014; 21:1; PMID:24502918; http://dx.doi.org/ 10.1051/parasite/2014001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ankri S, Stolarsky T, Bracha R, Padilla-Vaca F, Mirelman D. Antisense inhibition of expression of cysteine proteinases affects Entamoeba histolytica-induced formation of liver abscess in hamsters. Infect Immun 1999; 67:421-2; PMID:9864246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ankri S, Stolarsky T, Mirelman D. Antisense inhibition of expression of cysteine proteinases does not affect Entamoeba histolytica cytopathic or haemolytic activity but inhibits phagocytosis. Mol Microbiol 2002; 28:777-85; http://dx.doi.org/ 10.1046/j.1365-2958.1998.00837.x [DOI] [PubMed] [Google Scholar]
- [35].Jacobs T, Bruchhaus I, Dandekar T, Tannich E, Leippe M. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol Microbiol 1998; 27:269-76; PMID:9484883; http://dx.doi.org/ 10.1046/j.1365-2958.1998.00662.x [DOI] [PubMed] [Google Scholar]
- [36].Hou Y, Mortimer L, Chadee K. Entamoeba histolytica cysteine proteinase 5 binds integrin on colonic cells and stimulates NFB-mediated pro-inflammatory responses. J Biol Chem 2010; 285:35497-35504; PMID:20837477; http://dx.doi.org/ 10.1074/jbc.M109.066035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cho KN, Becker SM, Houpt ER. The NFB p50 subunit is protective during intestinal Entamoeba histolytica infection of 129 and C57BL/6 mice. Infect Immun 2010; 78:1475-81; PMID:20086086; http://dx.doi.org/ 10.1128/IAI.00669-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kammanadiminti SJ. Suppression of NF-B activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J Biol Chem 2006; 281:26112-20; PMID:16840786; http://dx.doi.org/ 10.1074/jbc.M601988200 [DOI] [PubMed] [Google Scholar]
- [39].Kammanadiminti SJ, Dey I, Chadee K. Induction of monocyte chemotactic protein 1 in colonic epithelial cells by Entamoeba histolytica is mediated via the phosphatidylinositol 3-kinase/p65 pathway. Infect Immun 2007; 75:1765-70; PMID:17283105; http://dx.doi.org/ 10.1128/IAI.01442-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burgess SL, Saleh M, Cowardin CA, Buonomo E, Noor Z, Watanabe K, Abhyankar M, Lajoie S, Wills-Karp M, Petri WA Jr. Role of serum amyloid A, granulocyte-macrophage colony-stimulating factor, and bone marrow granulocyte-monocyte precursor expansion in segmented filamentous bacterium-mediated protection from Entamoeba histolytica. Infect Immun 2016; 84:2824-32; http://dx.doi.org/ 10.1128/IAI.00316-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kretschmer R, Collado ML, Pacheco MG, Salinas MC, López-Osuna M, Lecuona M, Castro EM, Arellano J. Inhibition of human monocyte locomotion by products of axenically grown E. histolytica. Parasite Immunol 1985; 7:527-43; http://dx.doi.org/ 10.1111/j.1365-3024.1985.tb00097.x [DOI] [PubMed] [Google Scholar]
- [42].Silvestre A, et al.. In Entamoeba histolytica, a BspA family protein is required for chemotaxis toward tumour necrosis factor. Microb Cell 2015; 2:235-46; http://dx.doi.org/ 10.15698/mic2015.07.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Blazquez S, Guigon G, Weber C, Syan S, Sismeiro O, Coppée JY, Labruyère E, Guillén N. Chemotaxis of Entamoeba histolytica towards the pro-inflammatory cytokine TNF is based on PI3K signalling, cytoskeleton reorganization and the GalactoseN-acetylgalactosamine lectin activity. Cell Microbiol 2008; 10:1676-86; PMID:18419774; http://dx.doi.org/ 10.1111/j.1462-5822.2008.01158.x [DOI] [PubMed] [Google Scholar]
- [44].Leroy A, Lauwaet T, De Bruyne G, Cornelissen M, Mareel M. Entamoeba histolytica disturbs the tight junction complex in human enteric T84 cell layers. FASEB J 2000; 14:1139-46; PMID:10834936. [DOI] [PubMed] [Google Scholar]
- [45].Stenson WF, Zhang Z, Riehl T, Stanley SL. Amebic infection in the human colon induces cyclooxygenase-2. Infect Immun 2001; 69:3382-8; PMID:11292761; http://dx.doi.org/ 10.1128/IAI.69.5.3382-3388.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lejeune M, Moreau F, Chadee K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am J Pathol 2011; 179:807-18; PMID:21683675; http://dx.doi.org/ 10.1016/j.ajpath.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Betanzos A, Javier-Reyna R, García-Rivera G, Bañuelos C, González-Mariscal L, Schnoor M, Orozco E. The EhCPADH112 complex of Entamoeba histolytica interacts with tight junction proteins occludin and claudin-1 to produce epithelial damage. PLoS One 2013; 8:e65100; PMID:23762290; http://dx.doi.org/ 10.1371/journal.pone.0065100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Goplen M, Lejeune M, Cornick S, Moreau F, Chadee K. Entamoeba histolytica contains an occludin-like protein that can alter colonic epithelial barrier function. PLoS One 2013; 8:e73339; PMID:24058468; http://dx.doi.org/ 10.1371/journal.pone.0073339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McGowan K, Kane A, Asarkof N, Wicks J, Guerina V, Kellum J, Baron S, Gintzler AR, Donowitz M. Entamoeba histolytica causes intestinal secretion: role of serotonin. Science 1983; 221:762-4; PMID:6308760; http://dx.doi.org/ 10.1126/science.6308760 [DOI] [PubMed] [Google Scholar]
- [50].McGowan K, Piver G, Stoff JS, Donowitz M. Role of prostaglandins and calcium in the effects of Entamoeba histolytica on colonic electrolyte transport. Gastroenterology 1990; 98:873-80; PMID:2155844; http://dx.doi.org/ 10.1016/0016-5085(90)90010-X [DOI] [PubMed] [Google Scholar]
- [51].Saffer LD, Petri WA Jr. Role of the galactose lectin of Entamoeba histolytica in adherence-dependent killing of mammalian cells. InfectImmun 1991; 59:4681-3; PMID:1937828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Orozco E, Guarneros G, Martinez-Palomo A, Sánchez T. Entamoeba histolytica. phagocytosis as a virulence factor. J Exp Med 1983:158:1511-21; PMID:6313842; http://dx.doi.org/ 10.1084/jem.158.5.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sateriale A, Vaithilingam A, Donnelly L, Miller P, Huston CD. Feed-forward regulation of phagocytosis by Entamoeba histolytica. Infect Immun 2012; 80:4456-62; PMID:23045476; http://dx.doi.org/ 10.1128/IAI.00671-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huston CD, Boettner DR, Miller-Sims V, Petri WA Jr. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun 2003; 71:964-72; PMID:12540579; http://dx.doi.org/ 10.1128/IAI.71.2.964-972.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Boettner DR, Huston CD, Sullivan JA, Petri WA Jr. Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect Immun 2005; 73:3422-30; PMID:15908370; http://dx.doi.org/ 10.1128/IAI.73.6.3422-3430.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Buss SN, Hamano S, Vidrich A, Evans C, Zhang Y, Crasta OR, Sobral BW, Gilchrist CA, Petri WA Jr. Members of the Entamoeba histolytica transmembrane kinase family play non-redundant roles in growth and phagocytosis. Int J Parasitol 2010; 40:833-843; PMID:20083116; http://dx.doi.org/ 10.1016/j.ijpara.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sateriale A, Huston CD. A Sequential model of host cell killing and phagocytosis by Entamoeba histolytica. J Parasitol Res 2011; 2011:926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stanley SL, Becker A, Kunz-Jenkins C, Foster L, Li E. Cloning and expression of a membrane antigen of Entamoeba histolytica possessing multiple tandem repeats. Proc Natl Acad Sci U S A 1990; 87:4976-80; PMID:1695007; http://dx.doi.org/ 10.1073/pnas.87.13.4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vaithilingam A, Teixeira JE, Miller PJ, Heron BT, Huston CD. Entamoeba histolytica cell surface calreticulin binds human c1q and functions in amebic phagocytosis of host cells. Infect Immun 2012; 80:2008-18; PMID:22473608; http://dx.doi.org/ 10.1128/IAI.06287-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Katiyar SK, Prasad AK, Ghoshal S, Das SR, Sagar P. Cholesterol induced changes in glucose-6-phosphate generating enzymes, concanavalin A agglutinability and haemolytic activity of axenic Entamoeba histolytica. Ann Trop Med Parasitol 1987; 81:201-5; http://dx.doi.org/ 10.1080/00034983.1987.11812113 [DOI] [PubMed] [Google Scholar]
- [61].Welter BH, Goldston AM, Temesvari LA. Localisation to lipid rafts correlates with increased function of the Gal/GalNAc lectin in the human protozoan parasite, Entamoeba histolytica. Int J Parasitol 2011; 41:1409-19; PMID:22085647; http://dx.doi.org/ 10.1016/j.ijpara.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Laughlin RC, McGugan GC, Powell RR, Welter BH, Temesvari LA. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect Immun 2004; 72:5349-57; PMID:15322032; http://dx.doi.org/ 10.1128/IAI.72.9.5349-5357.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ralston KS, Solga MD, Mackey-Lawrence NM, SomlataBhattacharya A, Petri WA Jr. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 2014; 508:526-30; PMID:24717428; http://dx.doi.org/ 10.1038/nature13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Somlata, Bhattacharya S, Bhattacharya A. A C2 domain protein kinase initiates phagocytosis in the protozoan parasite Entamoeba histolytica. Nat Commun 2011; 2:230; PMID:21407196; http://dx.doi.org/ 10.1038/ncomms1199 [DOI] [PubMed] [Google Scholar]
- [65].Ravdin JI, Moreau F, Sullivan JA, Petri WA Jr, Mandell GL. Relationship of free intracellular calcium to the cytolytic activity of Entamoeba histolytica. Infect Immun 1988; 56:1505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Huston CD, Houpt ER, Mann BJ, Hahn CS, Petri WA Jr. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol 2000; 2:617-25; http://dx.doi.org/ 10.1046/j.1462-5822.2000.00085.x [DOI] [PubMed] [Google Scholar]
- [67].Becker SM, Cho KN, Guo X, Fendig K, Oosman MN, Whitehead R, Cohn SM, Houpt ER. Epithelial cell apoptosis facilitates Entamoeba histolytica infection in the gut. Am J Pathol 2010; 176:1316-1322; PMID:20093500; http://dx.doi.org/ 10.2353/ajpath.2010.090740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Seydel KB, Stanley SL. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect Immun 1998; 66:2980-3; PMID:9596776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Marie C, Verkerke HP, Theodorescu D, Petri WA Jr. A whole-genome RNAi screen uncovers a novel role for human potassium channels in cell killing by the parasite Entamoeba histolytica. Sci Rep 2015; 5:13613; http://dx.doi.org/ 10.1038/srep13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Marie CS, Verkerke HP, Paul SN, Mackey AJ, Petri WA Jr. Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect Immun 2012; 80:1934-43; PMID:22331430; http://dx.doi.org/ 10.1128/IAI.06140-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC Jr, Myers MG Jr, Duggal P, Houpt ER, Petri WA Jr. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol 2011; 4:294-303; PMID:21124310; http://dx.doi.org/ 10.1038/mi.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, et al.. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest 2011; 121:1191-8; PMID:21393862; http://dx.doi.org/ 10.1172/JCI45294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Thibeaux R, Dufour A, Roux P, Bernier M, Baglin AC, Frileux P, Olivo-Marin JC, Guillén N, Labruyère E. Newly visualized fibrillar collagen scaffolds dictate Entamoeba histolytica invasion route in the human colon. Cell Microbiol 2012; 14:609-621; PMID:22233454; http://dx.doi.org/ 10.1111/j.1462-5822.2012.01752.x [DOI] [PubMed] [Google Scholar]
- [74].Peterson KM, Guo X, Elkahloun AG, Mondal D, Bardhan PK, Sugawara A, Duggal P, Haque R, Petri WA Jr. The expression of REG 1A and REG 1B is increased during acute amebic colitis. Parasitol Int 2011; 60:296-300; PMID:21586335; http://dx.doi.org/ 10.1016/j.parint.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Thibeaux R, Avé P, Bernier M, Morcelet M, Frileux P, Guillén N, Labruyère E. The parasite Entamoeba histolytica exploits the activities of human matrix metalloproteinases to invade colonic tissue. Nat Commun 2014; 5:5142; PMID:25291063; http://dx.doi.org/ 10.1038/ncomms6142 [DOI] [PubMed] [Google Scholar]
- [76].Maugis B, Brugués J, Nassoy P, Guillen N, Sens P, Amblard F. Dynamic instability of the intracellular pressure drives bleb-based motility. J Cell Sci 2010; 123:3884-92; PMID:20980385; http://dx.doi.org/ 10.1242/jcs.065672 [DOI] [PubMed] [Google Scholar]
- [77].Arhets P, Olivo JC, Gounon P, Sansonetti P, Guillén N. Virulence and functions of myosin II are inhibited by overexpression of light meromyosin in Entamoeba histolytica. Mol Biol Cell 1998; 9:1537-47; http://dx.doi.org/ 10.1091/mbc.9.6.1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Coudrier E, Amblard F, Zimmer C, Roux P, Olivo-Marin JC, Rigothier MC, Guillén N. Myosin II and the Gal-GalNAc lectin play a crucial role in tissue invasion by Entamoeba histolytica. Cell Microbiol 2004; 7:19-27; http://dx.doi.org/ 10.1111/j.1462-5822.2004.00426.x [DOI] [PubMed] [Google Scholar]
- [79].RIOS A, Hernández-Ramírez VI, Moguel M, Zárate Bahena AI, Rosales-Encina JL, Vargas MA, Talamás-Rohana P. Participation of Rho, ROCK-2, and GAP activities during actin microfilament rearrangements in Entamoeba histolytica induced by fibronectin signaling. Cell Biol Int 2008; 32:984-1000; PMID:18501645; http://dx.doi.org/ 10.1016/j.cellbi.2008.04.016 [DOI] [PubMed] [Google Scholar]
- [80].Talamás-Rohana P, Hernández-Ramirez VI, Perez-García JN, Ventura-Juárez J. Entamoeba histolytica contains a beta 1 integrin-like molecule similar to fibronectin receptors from eukaryotic cells. J Eukaryot Microbiol 1998; 45:356-360; PMID:9627997; http://dx.doi.org/ 10.1111/j.1550-7408.1998.tb04549.x [DOI] [PubMed] [Google Scholar]
- [81].Emmanuel M, Nakano YS, Nozaki T, Datta S. Small GTPase Rab21 mediates fibronectin induced actin reorganization in Entamoeba histolytica: implications in pathogen invasion. PLOS Pathog 2015; 11:e1004666; PMID:25730114; http://dx.doi.org/ 10.1371/journal.ppat.1004666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mittal K, Welter BH, Temesvari LA. Entamoeba histolytica: lipid rafts are involved in adhesion of trophozoites to host extracellular matrix components. Exp. Parasitol 2008; 120:127-134; PMID:18588878; http://dx.doi.org/ 10.1016/j.exppara.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tillack M, Biller L, Irmer H, Freitas M, Gomes MA, Tannich E, Bruchhaus I. The Entamoeba histolytica genome: primary structure and expression of proteolytic enzymes. BMC Genomics 2007; 8:170; PMID:17567921; http://dx.doi.org/ 10.1186/1471-2164-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Petri WA Jr, Smith RD, Schlesinger PH, Murphy CF, Ravdin JI. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin Invest 1987; 80:1238-1244; http://dx.doi.org/ 10.1172/JCI113198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pillai DR, Wan PS, Yau YC, Ravdin JI, Kain KC. The cysteine-rich region of the Entamoeba histolytica adherence lectin (170-kgdalton subunit) is sufficient for high-affinity Gal/GalNAc-specific binding in vitro. Infect Immun 1999; 67:3836-41; PMID:10417146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Séguin R, Mann BJ, Keller K, Chadee K. Identification of the galactose-adherence lectin epitopes of Entamoeba histolytica that stimulate tumor necrosis factor-alpha production by macrophages. Proc Natl Acad Sci U S A 1995; 92:12175-9; PMID:8618866; http://dx.doi.org/ 10.1073/pnas.92.26.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Petri WA Jr, Joyce MP, Broman J, Smith RD, Murphy CF, Ravdin JI. Recognition of the galactose- or N-acetylgalactosamine-binding lectin of Entamoeba histolytica by human immune sera. Infect Immun 1987; 55:2327-31; PMID:2888730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Soong CJ, Torian BE, Abd-Alla MD, Jackson TF, Gatharim V, Ravdin JI. Protection of gerbils from amebic liver abscess by immunization with recombinant Entamoeba histolytica 29-kgdalton antigen. Infect Immun 1995; 63:472-7; PMID:7822012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Schain DC, Salata RA, Ravdin JI. Human T-lymphocyte proliferation, lymphokine production, and amebicidal activity elicited by the galactose-inhibitable adherence protein of Entamoeba histolytica. Infect Immun 1992; 60:2143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Séguin R, Mann BJ, Keller K, Chadee K. The tumor necrosis factor alpha-stimulating region of galactose-inhibitable lectin of Entamoeba histolytica activates gamma interferon-primed macrophages for amebicidal activity mediated by nitric oxide. Infect Immun 1997; 65:2522-7; PMID:9199414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ghadirian E. In vitro effect of human recombinant tumor necrosis factor on Entamoeba histolytica trophozoites. Immunobiology 1990; 180:339-350; PMID:2397931; http://dx.doi.org/ 10.1016/S0171-2985(11)80297-4 [DOI] [PubMed] [Google Scholar]
- [92].Zhang Z, Mahajan S, Zhang X, Stanley SL. Tumor necrosis factor alpha is a key mediator of gut inflammation seen in amebic colitis in human intestine in the SCID mouse-human intestinal xenograft model of disease. Infect Immun 2003; 71:5355-9; PMID:12933883; http://dx.doi.org/ 10.1128/IAI.71.9.5355-5359.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kammanadiminti SJ, Mann BJ, Dutil L, Chadee K. Regulation of Toll-like receptor-2 expression by the Gal-lectin of Entamoeba histolytica. FASEB J 2003; 18(1):155-7. [DOI] [PubMed] [Google Scholar]
- [94].da Silva Correia J, Ulevitch RJ. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 2002; 277:1845-1854; PMID:11706042; http://dx.doi.org/ 10.1074/jbc.M109910200 [DOI] [PubMed] [Google Scholar]
- [95].Campos-Rodríguez R, Barranco-Tovar C, Isibasi-Araujo A, Kumate-Rodríguez J. Anti-amebic plasma cells in peripheral blood of patients with amebic liver abscess. Arch Invest Med (Mex) 1986; 17 Suppl 1:303-6; PMID:3592896 [PubMed] [Google Scholar]
- [96].Isibasi A, Blanco F, Arreguín C, Martínez G, Pelayo R, Orozco E, Kumate J. Immunochemical differences in the surface polysaccharides obtained from Entamoeba histolytica strain HM1:IMSS and its virulent (C-A) and non-virulent (L-6) clones. Arch Invest Med (Mex) 1990; 21 Suppl 1:175-81; PMID:2136483 [PubMed] [Google Scholar]
- [97].Lauwaet T, Oliveira MJ, De Bruyne G, Bruchhaus I, Duchêne M, Mareel M, Leroy A. Entamoeba histolytica trophozoites transfer lipophosphopeptidoglycans to enteric cell layers. Int J Parasitol 2004; 34:549-556; PMID:15064119; http://dx.doi.org/ 10.1016/j.ijpara.2003.11.013 [DOI] [PubMed] [Google Scholar]
- [98].Maldonado-Bernal C, Kirschning CJ, Rosenstein Y, Rocha LM, Rios-Sarabia N, Espinosa-Cantellano M, Becker I, Estrada I, Salazar-González RM, López-Macías C, et al.. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol 2005; 27:127-137; PMID:15910421; http://dx.doi.org/ 10.1111/j.1365-3024.2005.00754.x [DOI] [PubMed] [Google Scholar]
- [99].Maldonado C, Trejo W, Ramírez A, Carrera M, Sánchez J, López-Macías C, Isibasi A. Lipophosphopeptidoglycan of Entamoeba histolytica induces an anti inflammatory innate immune response and downregulation of toll-like receptor 2 (TLR-2) gene expression in human monocytes. Arch Med Res 31:S71-3; PMID:11070229; http://dx.doi.org/ 10.1016/S0188-4409(00)00199-5 [DOI] [PubMed] [Google Scholar]
- [100].Ivory CP, Prystajecky M, Jobin C, Chadee K. Toll-like receptor 9-dependent macrophage activation by Entamoeba histolytica DNA. Infect Immun 2008; 76:289-97; PMID:17984204; http://dx.doi.org/ 10.1128/IAI.01217-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mortimer L, Moreau F, Cornick S, Chadee K. Gal-lectin-dependent contact activates the inflammasome by invasive Entamoeba histolytica. Mucosal Immunol 2014; 7:829-841; PMID:24253103; http://dx.doi.org/ 10.1038/mi.2013.100 [DOI] [PubMed] [Google Scholar]
- [102].Mortimer L, Moreau F, Cornick S, Chadee K. The NLRP3 Inflammasome is a pathogen sensor for invasive Entamoeba histolytica via activation of α5β1 integrin at the macrophage-amebae intercellular junction. PLoS Pathog 2015; 11:e1004887; PMID: 25955828; http://dx.doi.org/ 10.1371/journal.ppat.1004887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Que X, Kim SH, Sajid M, Eckmann L, Dinarello CA, McKerrow JH. Reed SL A surface amebic cysteine proteinase inactivates interleukin-18. Infect Immun 2003; 71:1274-80; PMID: 12595442; http://dx.doi.org/ 10.1128/IAI.71.3.1274-1280.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]


