ABSTRACT
Understanding how intestinal enteropathogens cause acute and chronic alterations has direct animal and human health perspectives. Significant advances have been made on this field by studies focusing on the dynamic crosstalk between the intestinal protozoan parasite model Giardia duodenalis and the host intestinal mucosa. The concept of intestinal barrier function is of the highest importance in the context of many gastrointestinal diseases such as infectious enteritis, inflammatory bowel disease, and post-infectious gastrointestinal disorders. This crucial function relies on 3 biotic and abiotic components, first the commensal microbiota organized as a biofilm, then an overlaying mucus layer, and finally the tightly structured intestinal epithelium. Herein we review multiple strategies used by Giardia parasite to circumvent these 3 components. We will summarize what is known and discuss preliminary observations suggesting how such enteropathogen directly and/ or indirectly impairs commensal microbiota biofilm architecture, disrupts mucus layer and damages host epithelium physiology and survival.
KEYWORDS: commensals, Giardia duodenalis, Giardiasis, host-parasite interactions, intestinal microbiota biofilm, mucus layer, poly-microbial infection
Introduction
The extracellular protozoan parasite Giardia duodenalis (syn. G. lamblia, G. intestinalis) causes giardiasis and diarrheal disease in humans, livestock and companion animals throughout the World. While acute giardiasis may be responsible for diarrhea, intestinal malabsorption, abdominal pain and weight loss, recent evidence also established that the infection can lead to chronic disease and extra-intestinal complications.1,2 As an animal/human health concern, giardiasis joined the “neglected diseases initiative” (established by the World Health Organization) in 2007.3
Like most foodborne enteropathogens, Giardia faces a multitude of hostile factors upon entering the host. In the stomach, excystation of the dormant tetranucleated cysts is initiated by gastric acid and peptidases. Cysts with “weakened” cell walls pass into the duodenum, where each releases a tetranucleated excyzoite, a process that is facilitated by Giardia’s own cysteine proteases.4 Each excyzoite quickly produces 2 binucleated trophozoites, the motile stage of the parasite that divides by binary fission as it colonizes the upper small intestine.5 Freshly released trophozoites adhere to the epithelial cells of the upper small intestine without invading tissues in most of cases. In the gut, trophozoites interact with 3 distinct barriers, namely the intestinal microbiota, the mucus layer, and the epithelial barrier (Fig. 1). Despite important steps toward the comprehension of the pathogenesis of giardiasis in the last decades, the pathophysiology of this disease is still under investigations. Indeed, while the epithelial dysfunctions during acute and chronic infections have been documented,6,7 the pathophysiology of the gastrointestinal symptoms associated with asymptomatic and symptomatic infections remains obscure. In particular, to better characterize host-Giardia interactions, more research needs to consider the gut microbiome, the mucus layer and the epithelium as separate entities forming selective barriers against Giardia. First, trophozoites compete locally with the commensal microbiome for nutrients and ecological niches in the duodenal microenvironment. Resident bacteria play a role in the colonization of G. duodenalis.8 Conversely, recent observations suggest that Giardia also disrupts the microbiota during the acute stage of infection, and the effects of giardiasis on homeostasis may reach the large intestine.1,9,10 To reach the epithelial surface, trophozoites must then cross the mucus gel protecting the surface of the epithelium from microorganisms, proteases and metabolites. Trophozoites finally adhere to epithelial microvilli using a ventral adhesive disk to resist luminal flow while accessing nutrients in the lumen.5
Figure 1.
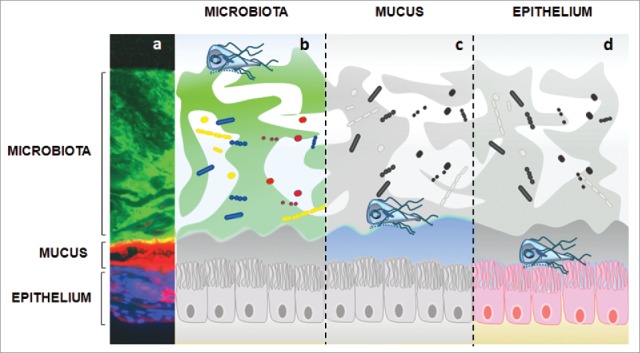
Giardia interactions with gut triple barrier (microbiota, mucus, epithelium). a) characterization of intestinal barriers geographical distribution in mice immunostaining Colonic sections were stained with EUB-388 (bacteria; αDNA), WGA (mucus; Life Technologies) and DAPI (epithelial cells; SIGMA). b) commensals are mostly organized in biofilms throughout the gastrointestinal tract. It has been observed that this resident microbiota plays a role in host susceptibility to Giardia infection. Some commensals (ex: lactic acid bactaria) even exhibit anti-giardial effects. In turn, Giardia has the ability to modulate commensals to pathobionts by inducing virulence factors and also disrupting intestinal biofilms. The resulting shift of gut microbiota may help explain the production of post- infectious symptoms. c) To attach the epithelium, trophozoites must breach the mucus layer, which acts as a biochemical / physical barrier. Little is known regarding the role of mucus during Giardia infection. Ongoing research indicates that Giardia’s proteolytic activity may disrupt MUC2 mucin, the major constituent of intestinal mucus in humans; d) Trophozoites strongly attach to epithelial microvilli, and disrupt the epithelial barrier. Disaccharidase deficiencies, diffuse microvillous shortening, arginine starvation, increased permeability, disruption of tight junctions and enterocyte induced apoptosis have been associated with Giardia infection. Extracellular factors such as cathepsin B-like cysteine proteases contribute to the parasite virulence by degrading CXCL-8 (IL-8) and inducing villin breakdown.
This review provides an update of current advances on the mechanisms regulating this triple barrier in the context of giardiasis. Moreover, the discussion illustrates how G. duodenalis constitutes an excellent model to study the interactions of enteropathogens with their microenvironement.
The gut epithelial barrier in giardia infections
Parasitic and host factors during Giardia-host cell interaction
After excystation, newly formed trophozoites colonize the upper parts of the small intestine by adhering to the surface of the duodenal intestinal epithelial cells, and replicate. In turn, infection leads to a partial or severe disruption of the gut epithelial barrier without provoking an overt inflammatory response. Trophozoites adhere to the apical surface of IEC and take advantage of luminal bio-available nutrients by pinocytosis.5 As a luminal protozoan, Giardia does not invade the epithelial barrier. Recent observations have suggested that under exceptional circumstances, Giardia may invade the sub-epithelial space, between enterocytes or at the base of goblet cells, but the findings warrant further confirmation.11,12 This phenomenon seems to be strain dependent, and is occasionally observed in clinical conditions associated with mucosal injury.13,14
Trophozoites strongly attach to the microvilli through a suction-based mechanism, involving their ventral disk, flagellar movements, and a variety of chemical bonds involving proteins, such as giardins, lectins and variant surface proteins (VSPs).15 VSPs belong to a widely varying cysteine-rich group of proteins, and also contribute to host immune evasion through antigenic variation.16 The antigenic variation of VSPs, occurring by epigenetic mechanisms, may contribute in some cases to the persistence of infection.17 Attachment of G. duodenalis to the epithelial brush border is also mediated by lipid raft membrane microdomains on trophozoites.18
Upon their arrival in the upper small intestine, several cell-surface and extracellular factors contribute to the establishment of trophozoites. Transcriptomic studies revealed that after exposure to intestinal epithelial monolayers, a set of genes coding for surface proteins (VSPs and cysteine-rich proteins), attachment proteins (giardins), cysteine proteases, and proteins involved in the clearance of reactive oxygen species were upregulated in several strains of G. duodenalis (WB, P-1, NF and GS/M).19,20 Likewise, intra- and extracellular enzymes are synthesized by Giardia following its contact with the host intestinal cells.21 In particular, several parasitic extracellular products have been identified by secretome analysis, including metabolobic enzymes involved in arginine uptake and putative enterotoxins.21,22 Giardia cathepsin B-like proteases are upregulated during Giardia-intestinal epithelial cells interaction and are implicated in excystation.23 Cathepsin B-like cysteine proteases also appear to regulate, at least in part, parasite virulence. First, these cathepsins have the ability to cleave the pro-inflammatory chemokine CXCL8 (IL-8) in gut tissues, hence inhibiting neutrophil infiltration.24,25 Second, Giardia cathepsin B-like proteases induce myosin light chain kinase (MLCK)-mediated villin breakdown and contribute to Giardia-induced microbiota toxicity.26,27 Findings from other reports suggest that soluble factors secreted by colonic epithelial cell lines are sufficient to up-regulate trophozoite membranous and secreted proteins, such as cathepsin-B precursors, cystatin and VSPs, independent of direct parasite attachment.20,26 Despite very few virulence factors have been identified and characterized in Giardia to date, an unknown 58-kDa product released by Giardia trophozoites has also been reported to induce physiologic changes at mucosal surface. In particular, this factor activates signal transduction pathways in enterocytes leading to anion hypersecretion and intestinal fluid accumulation.22
Short and long-term exposures to Giardia induce significant changes in the gene expression in host epithelial cells along with a regulation of signaling pathways.28 In the presence of trophozoites, intestinal epithelial cells secrete anti-parasitic factors that contribute to the clearance of Giardia, such as anti-microbial peptides (e.g. α- and β-defensins, cryptdins), lysozyme and chemokines.29 Antimicrobial peptides (AMPs) produced by intestinal epithelial cells (IECs) are critical to intestinal mucosal defense against enteropathogens.30 In addition, intestinal epithelial cells exposed to Giardia produce matrix metalloprotease 7, a mediator known to activate α-defensin.31 The effects of Giardia on anti-microbial peptides, either on its own or during co-infections with other entropathogens, warrant further investigation.
Diffuse shortening of brush border microvilli and malabsorption
One of the significant abnormalities observed in symptomatic giardiasis is a diffuse shortening of brush border microvilli, which reduces mucosal surface area available for water, nutrient, mineral (iron and zinc) and vitamin absorption (i.e. A and B12), electrolyte transport, and digestion.32-40 Villous atrophy and crypt hyperplasia have also been reported, but do not seem to be required for Giardia-induced malabsorption.37,41 Decreased activity of brush border digestive enzymes such as disaccharidases, and Na/D-glucose malabsorption, are commonly associated with disruption of microvilli.36,42-44
Hypersecretion of chloride may also contribute to diarrhea in giardiasis.38 In some cases, Giardia infection may induce the hypersecretion of bile, which, when associated with lipid malabsorption may lead to steatorrhea.45-47
Disaccharidase deficiencies and diffuse microvilli shortening are mediated at least in part by activated CD8+ lymphocytes.36; 37,44 Indeed, in immunodeficient mice unable to produce T cells, the height of microvilli as well as digestive enzyme activities did not vary after Giardia infection when compared with controls. Together, these observations demonstrate that epithelial abnormalities in giardiasis are mediated by parasites as well as host factors.
Intestinal permeability in giardiasis: A combination of tight junctional disruptions, apoptosis, and arginine starvation
During peak trophozoite colonization, giardiasis increases intestinal epithelial permeability in humans as well as in animal model systems.48,49 The alteration of intestinal permeability is the result of Giardia-induced tight junctional protein alterations, epithelial cell apoptosis, and starvation for L-arginine.7,29,50,51
The apical junctional complex (AJC) between enterocytes constitutes a selective barrier protecting subepithelial compartments from the luminal environment. The AJC is mainly composed of tight junctions (TJ), adherent junctions, and desmosomes.52,53 Giardia disrupts the structure of the epithelium by breaking down the AJC, hence increasing paracellular permeability and decreasing transepithelial electrical resistance.38,49,54-56 Giardia is known to alter the integrity and rearrangements of several tight junctional elements, including zonula occludens-1 and 2 proteins (ZO-1; ZO-2).38; 49; 55; 57,58 The rearrangement of ZO-1 is consistent with the elevation of intestinal permeability induced by a broad range of enteropathogens.50 Epithelial occludin and claudin-1 are also disrupted by Giardia.38,57 Disorganization of apical filamentous actin (F-actin) in the cytoskeleton, and rearrangement of α-actinin further contribute to the disruption of the AJC.36; 54; 57; 59,60 Moreover, a change in the spatial distribution of transmembrane proteins like desmocollin has been observed at the level of epithelial desmosomes.54 Mechanistically, the relocalization of F-actin, as well as disruptions of claudin-1 and ZO-1 seem to be mediated by the apoptotic protease caspase-3,56 and rearrangement of F-actin, ZO-1, and villin are myosin light chain kinase (MLCK) dependent.49,61
It is now accepted that the gut barrier disruption following enteropathogens infections is partly mediated by the induction of epithelial apoptosis.50,62 Genes associated with apoptosis are upregulated in epithelial cells exposed to trophozoites.28 Giardia activates caspase-3, caspase-8, caspase-9, and pro-apoptotic Bcl-2-associated X protein (BAX), while decreasing the expression of the anti-apoptotic protein Bcl-2.56,63,64 Poly (ADP-ribose) polymerase (PARP), which is involved in programmed cell death, contributes to Giardia-induced host cell apoptosis.56,63 One of the barrier parameters that may be regulated in apoptosis-dependant fashion is the disruption of cellular ZO-1.56,62 In contrast, cleavage of villin induced by Giardia cathepsin-like protease is independent of caspase-3 activity.26 Therefore, in giardiasis, some of the epithelial pathophysiology is apoptosis-dependent, while some is not.
Other observations obtained in live animals indicate that during the acute phase of infection, Giardia significantly increases permeability and macromolecular uptake through the small intestine, and leads to the delayed recruitment of mucosal and connective tissue mast cells.48 These changes may contribute at least in part to the hypersensitivity reactions associated with giardiasis, such as food allergies, urticaria, and post-infectious irritable bowel syndrome. While important observations have been generated from the use of animal models or studies in human patients, more research is needed to establish the clinical significance of these observations in the living host.38; 49,62
Cell proliferation in response to enteropathogenic infection represents a critical mechanism of epithelial renewal.65 Decreased expression of genes related to cell proliferation has been observed following Giardia exposure to human colonic cell lines.28,66 Furthermore, sodium– glucose transport proteins such as SGLT-1 appear to play a protective role in enterocyte apoptosis during the infection. Indeed, by enhancing epithelial SGLT-1 activity and therefore increasing glucose uptake, enterocytes try to protect against Giardia-induced apoptosis.67
L-arginine is a precursor of nitric oxide (NO), which acts as an antimicrobial compound, and a neurotransmitter and mediator of peristalsis and sphincter actions in the mammalian intestine.68,69 L-arginine is enzymatically converted into NO through the action of 3 different nitric oxide synthases (NOS), nNOS (NOS1), iNOS (NOS2) and eNOS (NOS3) which are respectively neuronal, induced and endothelial types of NOS.70 In enterocytes, NO is produced through iNOS activity and released into the luminal environment, exerting anti-giardial effects. Indeed, in murine models of giardiasis, NO contributes to the clearance of G. duodenalis.71,72 The anti-giardial properties of NO are not completely understood, and studies showed that NO inhibits the proliferation of trophozoites in vitro without killing them.73 However, the cytostatic properties of NO prevent encystation and hence serve to reduce the transmission of Giardia to another host.21,73 During infections, Giardia consumes host arginine, depleting enterocytes and promoting their programmed cell death.73 Indeed, arginine is one of the main sources of energy for Giardia trophozoites for which it is auxotrophic, most likely because this amino acid requires energy for its synthesis.74 Giardia enzymes involved in arginine metabolism, such as arginine deaminase, are upregulated during the infection to allow efficient uptake by the parasite. Moreover, arginine deaminase appears to contribute to antigenic variability by modifying VSPs.75 Taken together, these observations indicate that Giardia-induced host arginine depletion further contributes to innate immune evasion through the prevention of NO production and activation of arginine deaminase-dependent antigen variability. Interestingly, supplementation of arginine or citrulline was suggested to promote the clearance of the parasite, as it restored NO production and proliferation of enterocytes in vitro.76 In addition, arginine depletion has deleterious effects on epithelial cells since it induces apoptosis,77,78 causes villus shortening,79,80 and inhibits epithelial proliferation.76 Further research needs to characterize whether and how Giardia-induced arginine starvation may contribute to the loss of intestinal barrier function during giardiasis.
The role of the mucus barrier during Giardia infection
A thick mucus layer protects the luminal surface of the entire gastrointestinal tract. Mucus acts as both a physical and chemical barrier. Its integrity is critical to prevent foreign materials, commensal bacteria, and enteropathogens from gaining access to the epithelial cells and the underlying tissue, while at the same time allowing for the passage of nutrients and providing lubrication for moving food downstream.81,82 Intestinal mucus is primarily composed of water and the large glycoprotein mucin-2 (MUC2), which is produced by specialized epithelial goblet cells. While the small intestinal mucus has been described as a thin, loosely-attached layer, colonic mucus is organized into a sterile inner layer firmly adherent to the epithelium, and an outer, more loosely-attached layer, which harbours commensal microbial communities.82 In humans, mucus thickness gradually increases from the upper small intestine where it ranges from 150–300 µm, to the colon, where its thickness is estimated at 900 µm.83
To reach and attach to the epithelium, Giardia must first overcome the physical mucus barrier. Trophozoites are highly motile via their flagella, but it has been suggested that Giardia’s flagellar motion on its own may not be sufficient to traverse the mucus barrier.84 Recent findings indicate that the movement through the mucus may be facilitated by Giardia’s proteolytic activity, which may disrupt MUC2 integrity to produce a less viscous physical barrier.9,64 More research is needed to characterize the effects of Giardia on the mucus barrier. Other pathogens, including the protozoan Entamoeba histolytica and the nematode parasite Trichuris muris, have been found to secrete cysteine and serine proteases, respectively, that aid in degradation of the mucus gel.85,86 Interestingly, Helicobacter pylori, an opportunistic pathogen of the stomach, secretes urease in its immediate surroundings to elevate the pH of the mucus and therefore reduce its viscosity.87 Although H. pylori possesses flagella for motility, the added change in viscosity facilitates quick and easy transport through the mucus layers. Additionally, MUC2-deficient mice develop severe and life-threatening disease when exposed to Citrobacter rodentium, and show significantly delayed clearance of T. muris.88,89 Infection with C. rodentium causes goblet cell mucin depletion, leading to a thinner and more porous mucus layer.90 The mechanisms that Giardia may utilize to traverse the mucus barrier are still unclear and require further elucidation. Furthermore, the role of mucus in attenuating or aiding Giardia infection remains under debate. In vitro studies demonstrated that duodenal and jejunal mucus stimulated the growth of trophozoites, while others reported that mucin inhibited trophozoite attachment and proliferation.91,92 Earlier observations proposed that Giardia may in fact adhere to mucus strands.93 Other studies suggested that the mucus layer could itself be protective for trophozoites, by preventing injury from exposure to luminal products.94 The interactions of the mucus layer and mucus-producing cells with Giardia have yet to be fully explored.
The gut microbiota: A living barrier
Giardia-microbiota interactions in the gut
The gut microbiota is predominantly composed of 3 major phyla, namely Firmicutes, Bacteroidetes and Actinobacteria, followed by 2 minor phyla, Proteobacteria and Verrucomicrobia.95 Its abundance and composition vary throughout the gastrointestinal tract based on oxygen and pH gradients.96 While in the small intestine the bacterial abundance varies from 101–3 in the duodenum to 107–8 in the ileum, the microbial density reaches 1010–12 in the colon.97
The composition of commensal microbiota in the gut influence the colonization by Giardia.8,41 The mechanisms remain unclear, but research findings indicate that the gut microbiota may modulate both the susceptibility and the severity of giardiasis.44 Animals with the same genetic background, but with distinct microbiota, have varying susceptibility to Giardia infection.8 Susceptibility to infection can be restored by antibiotic treatment in mice that were protected. In co-housing, protected mice confer their resistance against Giardia infection to susceptible mice, suggesting a transfer of “protective” microbiota.8 Duodenal bacterial overgrowth has been reported in subjects with symptomatic giardiasis in some studies, but not in others.47; 98,99 Mucosal Giardia-specific IgA secretion and Giardia specific serum IgM and IgG are increased in conventional mice compare with germ free controls, and these changes were associated with reduced histopathology in germ-free animals.99 These observations are consistent with the fact that germ-free mice have an immature immune system, considering that the gut microbiota plays an important role in shaping host immunity.100 However, it was recently observed that antibiotic treatment may protect against disaccharidase deficiency in giardiasis.44 In that study, the authors suggest that the microbiota may indeed contribute to CD8+ T cell activation and nutrient malabsorption during giardiasis.44 A new model using the nematode Caenorhabditis elegans recently demonstrated that exposure to G. duodenalis makes human gut microbiota toxic to the worm host.27 Human microbiota treated with Giardia, but not the same microbiota without exposure to the parasite, were lethal to the nematode. Exposure of non-invasive E. coli to extracellular metabolites from Giardia was sufficient to make it lethal to C. elegans.27 Exposure to Giardia and/or to C. elegans altered the expression of a broad range of genes in E. coli, including some genes that are involved in bacterial hydrogen sulfide metabolism,27 offering new avenues to investigate the interactions of Giardia with host microbiota. In a recent study comparing the human fecal microbiota of 20 subjects associated with the presence or absence of several intestinal parasites (G. duodenalis, Entamoeba spp., and Blastocystis hominis), Giardia-positive samples were associated with dysbiotic conditions, with an increase of potentially harmful species such as Escherichia coli and Enterococcus spp in the commensal microbiota.101 Previous research had associated colonization by enterobacteria with the development of severe malabsorption during symptomatic giardiasis.102 Collectively, these results illustrate the capacity of G. duodenalis to shape commensal microbial communities.
Anti-giardial effect of commensals
Trophozoites and commensal microbiota compete for the same ecological niches to colonize the small intestinal microenvironement. Lactobacilli (Lactobacillus genus) and more generally lactic acid bacteria are some of the most common bacteria of the human upper small intestine.103 Several studies have explored the inhibitory effects of lactobacilli in giardiasis. For instance, probiotic lactobacilli strains such as L. johnsonii La1 (LjLa1), L. casei MTCC 1423, and L. rhamnosus GG contribute to the clearance of G. duodenalis in vivo by enhancing the host immune response, restoring the integrity of the gut barrier, and reducing cyst shedding and reducing the duration of infection.104-108 The probiotic strain LjLa1 inhibits trophozoite growth both in vitro and in vivo.106,109 A recent study revealed that this inhibitory effect is mediated at least in part by the products of bile salt deconjugation, through LjLa1s bile salt hydrolase enzymatic activity.110 Other microorganisms are known to exhibited anti-giardia properties, including Enterococcus faecium SF68, Saccharomyces boulardii (yeast), and even complex fermented milk products containing lactic acid bacteria (Lactobacillus cremoris, Lactococcus spp, Leuconostoc ssp, etc.) and yeast (Sacharomyces cerevisae, Candida spp).111,112
The mechanisms underlying the strain-specific anti-parasitic effects of lactic acid bacteria on Giardia, and their immuno-modulatory aspects, remain incompletely understood.113,114 In murine models of giardiasis, L. rhamnosus GG stimulates the production of anti-inflammatory cytokines (IL-10) and increases Giardia-specific IgA, helping to restore mucosal integrity, while also helping to eliminate the parasite.104 E. faecium SF68 stimulates specific IgA and IgG secretion, and induces a pro-inflammatory response.111 Other than acting on host immunity, these bacteria compete for binding sites on the epithelial surface by means of a variety of mechanisms. Recently observations indicate that a bacteriocin produced by the probiotic strain L. acidophilus P106 significantly reduces trophozoite's burden in vivo by acting on host enterocyte structure.115 Regarding the anti-microbial properties of lactobacilli, several other factors should be taken into account: (i) adhesion factors such as lipoprotein and the adhesin B (L. acidophilus, L. johnsonii and L. gasseri) which confer a better attachment to the mucosa and a competitive advantage (ii) the ability of lactobacilli to detoxify bile via bile salt hydrolase activity, ensuring persistence in the bile-rich duodenum; this is associated with the accumulation of deconjugated bile in the lumen, which is cytotoxic to trophozoites,110 and (iii) competition for essential nutrients for Giardia growth such as L-arginine, cholesterol, purine nucleobases, and nucleosides.116 Finally, some probiotic strains can influence the attachment of entheropathogens via steric hindrance on enterocyte receptors.117 Taken together, these observations underscore the physiologically significance of the anti-giardial effects that mucosal bacteria may exert on this parasite. More research is required to unravel the mechanisms through which Giardia trophozoites are able to overcome this host protective phenomenon in the gut.
Gut microbiota biofilms and post-infectious complications
Recent findings have established that long after Giardia has been eliminated from the host, post-infectious complications may arise, in the form of failure to thrive, stunting, cognitive deficiencies, allergies, arthritis, Irritable Bowel Syndrome (IBS), and chronic fatigue syndrome.1,118 Long-term gastrointestinal and extra-intestinal complications affect at least 5% of Giardia-infected humans.119 The mechanisms remain obscure.
Intestinal mucosa-associated microbiota communities grow in multi-species biofilms adhering to the mucosal surface, separated from the epithelium by the double mucus layer.120,121 Patients with IBS harbour dysbiotic gut microbiota.122 Recent research revealed how Giardia alters human microbiota biofilm integrity through a cystein-protease dependent mechanism (Fig. 2) (Beatty et al.146). The findings indicate that exposure of human gut microbiota biofilms to Giardia induces the release of planktonic, swimming, bacteria, that in turn may induce epithelial apoptosis, promote bacterial translocation, and increase the production of pro-inflammatory CXCL8 (Interleukin-8).146 These alterations were dependent upon the release of cathepsin-like cysteine proteases by the parasite. Further experiments performed in humanized germ free mice showed that Giardia-modified human microbiota disrupt epithelial barrier integrity, increase TLR-4 expression, and expand mucosal lymphoid aggregates rich in CD45+ B lymphocytes, a B-cell subset known to cause inflammatory flares in patients with inflammatory bowel disease.123-127 Therefore, Giardia has the ability to directly disrupt commensal gut microbiota. Giardia, similarly to other enteropathogens, may activate latent virulence genes in commensal bacteria, disrupt the microbiota biofilm phenotype,27 and promote the release of pathobionts from the commensal biofilm.123,128 These effects may play a key role in the development of post-infectious complications, long after the inciting enteropathogen has been eliminated by the host.
Figure 2.

Disruption of intestinal biofilm following Giardia exposure. Human microbiota biofilms from colonic biopsies were cultured ex vivo in the Calgary Biofilm Device, and exposed to vehicle (Control) or to live Giardia trophozoites (G. duodenalis). G. duodenalis depletes microbiota biofilms of their extracellular matrix coat. Representative Scanning Electron micrographs (13000 x magnification). Modified from Beatty et al.146 © Andre Buret. Reproduced by permission of Andre Buret. Permission to reuse must be obtained from the rightsholder.
Polymicrobial infections
Polymicrobial infections have become a topic of great interest in view of their importance on disease outcome during infection, and their known impact on child health in developing countries.129 Polymicrobial enteric infections are common in developing countries with poor sanitation.39,130
Infections with G. duodenalis and other enteropahogens most often arise from the ingestion of contaminated water and food. It is not surprising therefore that giardiasis has been found to occur in concert with other enteric pathogens and opportunistic pathogens, including bacteria (Helicobater pylori, Clostridium difficile, Vibrio cholera, Escherichia coli, Campylobacter sp, Tropheryma whipplei, Salmonella sp, etc.), viral pathogens (norovirus and rotavirus), and other parasites (Hookworm sp, Ascaris sp, Cryptosporidium sp, Cyclospora cayetanensis).131-141 Some reports suggest that in children, polymicrobial infections involving G. duodenalis may be more common than mono-infections.41
Intriguingly, several human studies reveal an association between Giardia infections and a decreased risk for developing acute diarrheal disease.64,142 For instance, children cohorts in Tanzania and in Bengladesh infected with Giardia were found to have reduced incidence of diarrheal disease and fever.131; 143,144 The mechanisms remain incompletely understood. A recent study investigated the role of G. duodenalis cathepsin B-like cysteine proteases in the attenuation of host inflammatory responses induced by other gastrointestinal pathogens.25 Giardia cathepsin-like proteases significantly attenuated the production of pro-inflammatory responses induced by exposure to either pro-inflammatory stimuli, such as interleukin-1β, or to the enteropathogen Salmonella enterica (serovar Typhimurium). Giardia inhibited the Salmonella-induced inflammatory response by degrading intestinal CXCL8 (IL-8).24 Direct inhibition of intestinal inflammation was further demonstrated when inflammatory signaling was triggered by Clostridium difficile toxin, as well as in human biopsy tissues from patients with IBS.25 These data offer strong support to the hypothesis that Giardia may indeed attenuate pathogen-induced pro-inflammatory responses during co-infections, which in turn may explain, at least in part, why Giardia infections can be associated with a reduction of diarrheal disease in countries with poor sanitation.64 Several questions remain, specifically regarding how Giardia may modulate the release of antimicrobial peptides, and whether or not the observed effects are assemblage-specific.145
Conclusion
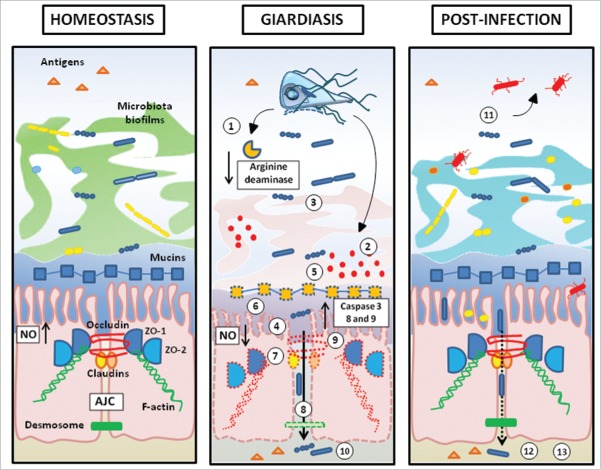
Intestinal parasites target tissues of the GI tract for colonization and persistence within the intestinal milieu. As a consequence, they are constantly battling against the host's immune response, mucosal defenses and commensal microorganisms. Giardia has evolved that capability to evade host immunity and disrupt innate mucosal protective barriers. The release of proteases such as cathepsin-like cysteine proteases allows this parasite to attenuate local inflammatory responses, to deplete mucus, and to disrupt commensal microbiota biofilms (Fig. 3). Giardia’s arginine deiminase contributes to the modulation of the host's intestinal inflammatory responses (by attenuating NO production). Giardia is also able to attenuate disease outcome during polymicrobial infections at least in part by dampening host CXCL8 signaling.64
Figure 3.
Barrier disruption in giardiasis and post-infection consequences. (1) Consumption of arginine via high arginine deaminase activity. Arginine starvation leads to an impaired secretion of anti-giardial Nitric Oxide (NO); (2) Giardia’s proteolytic activity (cathepsin-B-like cysteine proteases) lead to (3) An impairment of commensal microbiota biofilms, (4) the cleavage of pro-inflamatory chemokines (CXCL-8), (5) The disruption of MUC2 mucin integrity; (6) Diffuse shortening of brush border microvilli; (7) Disruption and/or rearrangement of the apical junction complex (AJC) (ZO-1, ZO-2, claudin-1, claudin-4, occludin), filamentous actin (F-actin and α-actinin), and at desmosomal level (desmocollin); (8) Bacterial and antigen translocation in the lamina propria; (9) Induction of pro-apoptotic factors caspase-3, 8 and 9, BAX, PARP, and impairment of anti-apoptotic protein Bcl-2; (10) Immune response in giardiasis is reviewed in Einarsson et al. 2016; (11) Toxic effects of pathobionts released by dysbiotic microbiota; (12) Paracellular translocation (13) Activation of pathogenic endocrine and immunological signals.2
More research is needed to characterize the mechanisms whereby Giardia modifies microbiota and mucus barriers during acute and chronic infections. Likewise, the putative anti-giardial effects of mucin, and the biologic factors by which resident bacteria could influence Giardia infection warrant further investigation. Recent epidemiological evidences have shown that Giardia may lead to chronic post-infectious gastrointestinal and extraintestinal disorders, even after complete clearance of the parasite (Fig. 3).1,2 Clinical manifestations of these complications may occur several years following the infection, and can either last for a few days or become chronic. The post-infectious gastrointestinal disorders reported following giardiasis share many similarities with those associated with bacterial (E. coli, Campylobacter jejuni, Salmonella sp, etc.) or viral (i.e., norovirus) enteropathogens. Further research into the mechanisms responsible for the intestinal barrier disruptions caused by Giardia, be it at the level of the microbiota, the mucus, or the epithelium, will shed new light toward novel clinical intervention strategies.
Acknowledgments
We gratefully acknowledge Troy Feener for his contribution to this review.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research findings discussed in this review were generated through funding from the Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery program, a NSERC CREATE grant, and the Crohn's Colitis Canada Grant in Aid program.
References
- [1].Halliez MC, Buret AG. Extra-intestinal and long term consequences of giardia duodenalis infections. World J Gastroenterol 2013; 19(47):8974-85; PMID:24379622; http://dx.doi.org/ 10.3748/wjg.v19.i47.8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Halliez MC, Motta JP, Feener TD, Guerin G, LeGoff L, Francois A, Colasse E, Favennec L, Gargala G, Lapointe TK et al.. Giardia duodenalis induces paracellular bacterial translocation and causes postinfectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2016; 310(8):G574-585; PMID:26744469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Savioli L, Smith H, Thompson A. Giardia and cryptosporidium join the ‘neglected diseases initiative’. Trends Parasitol 2006; 22(5):203-208; PMID:16545611; http://dx.doi.org/ 10.1016/j.pt.2006.02.015 [DOI] [PubMed] [Google Scholar]
- [4].Slavin I, Saura A, Carranza PG, Touz MC, Nores MJ, Lujan HD. Dephosphorylation of cyst wall proteins by a secreted lysosomal acid phosphatase is essential for excystation of giardia lamblia. Mol Biochem Parasitol 2002; 122(1):95-8; PMID:12076774; http://dx.doi.org/ 10.1016/S0166-6851(02)00065-8 [DOI] [PubMed] [Google Scholar]
- [5].Ankarklev J, Jerlstrom-Hultqvist J, Ringqvist E, Troell K, Svard SG. Behind the smile: Cell biology and disease mechanisms of giardia species. Nat Rev Microbiol 2010; 8(6):413-22; PMID:20400969. [DOI] [PubMed] [Google Scholar]
- [6].Buret AG. Mechanisms of epithelial dysfunction in giardiasis. Gut 2007; 56(3):316-7; PMID:17339241; http://dx.doi.org/ 10.1136/gut.2006.107771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Einarsson E, Ma'ayeh S, Svard SG. An up-date on giardia and giardiasis. Curr Opin Microbiol 2016; 34:47-52; PMID:27501461; http://dx.doi.org/ 10.1016/j.mib.2016.07.019 [DOI] [PubMed] [Google Scholar]
- [8].Singer SM, Nash TE. The role of normal flora in giardia lamblia infections in mice. J Infect Dis 2000; 181(4):1510-2; PMID:10751141; http://dx.doi.org/ 10.1086/315409 [DOI] [PubMed] [Google Scholar]
- [9].Amat CB, Motta J-P, Chadee K, Buret AG. Giardia duodenalis directly depletes mucins in intestinal goblet cells. FASEB J 2016; 30(1 Supplement):162.161. [Google Scholar]
- [10].Beatty J, Akierman S, Rioux K, Beck P, McKnight W, Feener T, Wallace J, Buret A. Gut microbiota biofilm disruptions by giardia: Pathology in human enterocytes and germ-free mice. FASEB J 2013; 27(1 Supplement):131.131. [Google Scholar]
- [11].Martinez-Gordillo MN, Gonzalez-Maciel A, Reynoso-Robles R, Montijo-Barrios E, Ponce-Macotela M. Intraepithelial giardia intestinalis: a case report and literature review. Medicine 2014; 93(29):e277; PMID:25546671; http://dx.doi.org/ 10.1097/MD.0000000000000277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reynoso-Robles R, Ponce-Macotela M, Rosas-Lopez LE, Ramos-Morales A, Martinez-Gordillo MN, Gonzalez-Maciel A. The invasive potential of giardia intestinalis in an in vivo model. Scientific Rep 2015; 5:15168; PMID:26470844; http://dx.doi.org/ 10.1038/srep15168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saha TK, Ghosh TK. Invasion of small intestinal mucosa by giardia lamblia in man. Gastroenterology 1977; 72(3):402-5; PMID:832787. [PubMed] [Google Scholar]
- [14].Brandborg LL, Tankersley CB, Gottieb S, Barancik M, Sartor VE. Histological demonstration of mucosal invasion by giardia lamblia in man. Gastroenterology 1967; 52(2):143-50; PMID:4164028. [PubMed] [Google Scholar]
- [15].Woessner DJ, Dawson SC. The giardia median body protein is a ventral disc protein that is critical for maintaining a domed disc conformation during attachment. Eukaryotic cell 2012; 11(3):292-301; PMID:22247266; http://dx.doi.org/ 10.1128/EC.05262-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gargantini PR, Serradell Mdel C, Rios DN, Tenaglia AH, Lujan HD. Antigenic variation in the intestinal parasite giardia lamblia. Curr Opin Microbiol 2016; 32:52-8; PMID:27177351; http://dx.doi.org/ 10.1016/j.mib.2016.04.017 [DOI] [PubMed] [Google Scholar]
- [17].Adam RD, Nigam A, Seshadri V, Martens CA, Farneth GA, Morrison HG, Nash TE, Porcella SF, Patel R. The giardia lamblia vsp gene repertoire: Characteristics, genomic organization, and evolution. BMC genomics 2010; 11:424; PMID:20618957; http://dx.doi.org/ 10.1186/1471-2164-11-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carranza PG, Gargantini PR, Prucca CG, Torri A, Saura A, Svard S, Lujan HD. Specific histone modifications play critical roles in the control of encystation and antigenic variation in the early-branching eukaryote giardia lamblia. Int J Biochem Cell Biol 2016; 81(Pt A):32-43; PMID:27771437; http://dx.doi.org/ 10.1016/j.biocel.2016.10.010 [DOI] [PubMed] [Google Scholar]
- [19].Ma'ayeh SY, Brook-Carter PT. Representational difference analysis identifies specific genes in the interaction of giardia duodenalis with the murine intestinal epithelial cell line, iec-6. Int J Parasitol 2012; 42(5):501-9; PMID:22561399; http://dx.doi.org/ 10.1016/j.ijpara.2012.04.004 [DOI] [PubMed] [Google Scholar]
- [20].Emery SJ, Mirzaei M, Vuong D, Pascovici D, Chick JM, Lacey E, Haynes PA. Induction of virulence factors in giardia duodenalis independent of host attachment. Scientific Rep 2016; 6:20765; PMID:26867958; http://dx.doi.org/ 10.1038/srep20765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ringqvist E, Palm JE, Skarin H, Hehl AB, Weiland M, Davids BJ, Reiner DS, Griffiths WJ, Eckmann L, Gillin FD et al.. Release of metabolic enzymes by giardia in response to interaction with intestinal epithelial cells. Mol Biochem Parasitol 2008; 159(2):85-91; PMID:18359106; http://dx.doi.org/ 10.1016/j.molbiopara.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shant J, Ghosh S, Bhattacharyya S, Ganguly NK, Majumdar S. Mode of action of a potentially important excretory–secretory product from giardia lamblia in mice enterocytes. Parasitology 2005; 131(Pt 1):57-69; PMID:16038397; http://dx.doi.org/ 10.1017/S0031182005007262 [DOI] [PubMed] [Google Scholar]
- [23].DuBois KN, Abodeely M, Sajid M, Engel JC, McKerrow JH. Giardia lamblia cysteine proteases. Parasitol Res 2006; 99(4):313-6; PMID:16598471; http://dx.doi.org/ 10.1007/s00436-006-0149-4 [DOI] [PubMed] [Google Scholar]
- [24].Cotton JA, Bhargava A, Ferraz JG, Yates RM, Beck PL, Buret AG. Giardia duodenalis cathepsin b proteases degrade intestinal epithelial interleukin-8 and attenuate interleukin-8-induced neutrophil chemotaxis. Infect Immun 2014; 82(7):2772-87; PMID:24733096; http://dx.doi.org/ 10.1128/IAI.01771-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cotton JA, Motta JP, Schenck LP, Hirota SA, Beck PL, Buret AG. Giardia duodenalis infection reduces granulocyte infiltration in an in vivo model of bacterial toxin-induced colitis and attenuates inflammation in human intestinal tissue. PloS one 2014; 9(10):e109087; PMID:25289678; http://dx.doi.org/ 10.1371/journal.pone.0109087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bhargava A, Cotton JA, Dixon BR, Gedamu L, Yates RM, Buret AG. Giardia duodenalis surface cysteine proteases induce cleavage of the intestinal epithelial cytoskeletal protein villin via myosin light chain kinase. PloS one 2015; 10(9):e0136102; PMID:26334299; http://dx.doi.org/ 10.1371/journal.pone.0136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gerbaba TK, Gupta P, Rioux K, Hansen D, Buret AG. Giardia duodenalis-induced alterations of commensal bacteria kill caenorhabditis elegans: a new model to study microbial-microbial interactions in the gut. Am J Physiol Gastrointest Liver Physiol 2015; 308(6):G550-561; PMID:25573177; http://dx.doi.org/ 10.1152/ajpgi.00335.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roxstrom-Lindquist K, Ringqvist E, Palm D, Svard S. Giardia lamblia-induced changes in gene expression in differentiated caco-2 human intestinal epithelial cells. Infect Immun 2005; 73(12):8204-8; PMID:16299316; http://dx.doi.org/ 10.1128/IAI.73.12.8204-8208.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eckmann L. Mucosal defences against giardia. Parasite immunology 2003; 25(5):259-70; PMID:12969444; http://dx.doi.org/ 10.1046/j.1365-3024.2003.00634.x [DOI] [PubMed] [Google Scholar]
- [30].Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9(5):356-68; PMID:21423246; http://dx.doi.org/ 10.1038/nrmicro2546 [DOI] [PubMed] [Google Scholar]
- [31].Tako EA, Hassimi MF, Li E, Singer SM. Transcriptomic analysis of the host response to giardia duodenalis infection reveals redundant mechanisms for parasite control. mBio 2013; 4(6):e00660-00613; PMID:24194537; http://dx.doi.org/ 10.1128/mBio.00660-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oberhuber G, Stolte M. Symptoms in patients with giardiasis undergoing upper gastrointestinal endoscopy. Endoscopy 1997; 29(8):716-20; PMID:9427489; http://dx.doi.org/ 10.1055/s-2007-1004295 [DOI] [PubMed] [Google Scholar]
- [33].Buret A, Gall DG, Nation PN, Olson ME. Intestinal protozoa and epithelial cell kinetics, structure and function. Parasitol Today 1990; 6(12):375-80; PMID:15463275; http://dx.doi.org/ 10.1016/0169-4758(90)90145-T [DOI] [PubMed] [Google Scholar]
- [34].Buret A, Gall DG, Olson ME. Effects of murine giardiasis on growth, intestinal morphology, and disaccharidase activity. J Parasitol 1990; 76(3):403-9; PMID:2191103; http://dx.doi.org/ 10.2307/3282675 [DOI] [PubMed] [Google Scholar]
- [35].Buret A, Hardin JA, Olson ME, Gall DG. Pathophysiology of small intestinal malabsorption in gerbils infected with giardia lamblia. Gastroenterology 1992; 103(2):506-13; PMID:1634068; http://dx.doi.org/ 10.1016/0016-5085(92)90840-U [DOI] [PubMed] [Google Scholar]
- [36].Scott KG, Logan MR, Klammer GM, Teoh DA, Buret AG. Jejunal brush border microvillous alterations in giardia muris-infected mice: role of t lymphocytes and interleukin-6. Infect Immun 2000; 68(6):3412-8; PMID:10816492; http://dx.doi.org/ 10.1128/IAI.68.6.3412-3418.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Scott KG, Yu LC, Buret AG. Role of cd8+ and cd4+ t lymphocytes in jejunal mucosal injury during murine giardiasis. Infect Immun 2004; 72(6):3536-42; PMID:15155662; http://dx.doi.org/ 10.1128/IAI.72.6.3536-3542.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, Jelinek T, Zeitz M, Fromm M, Schulzke JD. Effect of chronic giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 2007; 56(3):328-35; PMID:16935925; http://dx.doi.org/ 10.1136/gut.2006.100198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Muhsen K, Levine MM. A systematic review and meta-analysis of the association between giardia lamblia and endemic pediatric diarrhea in developing countries. Clin Infect Dis 2012; 55 Suppl 4:S271-293; http://dx.doi.org/ 10.1093/cid/cis762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Behera B, Mirdha BR, Makharia GK, Bhatnagar S, Dattagupta S, Samantaray JC. Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig Dis Sci 2008; 53(3):672-9; PMID:17763958; http://dx.doi.org/ 10.1007/s10620-007-9927-9 [DOI] [PubMed] [Google Scholar]
- [41].Bartelt LA, Sartor RB. Advances in understanding giardia: determinants and mechanisms of chronic sequelae. F1000prime reports 2015; 7:62; PMID:26097735; http://dx.doi.org/ 10.12703/P7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nain CK, Dutt P, Vinayak VK. Alterations in enzymatic activities of the intestinal mucosa during the course of giardia lamblia infection in mice. Ann Tropical Med Parasitol 1991; 85(5):515-22; PMID:1667077; http://dx.doi.org/ 10.1080/00034983.1991.11812602 [DOI] [PubMed] [Google Scholar]
- [43].Rana SV, Bhasin DK, Vinayak VK. Lactose hydrogen breath test in giardia lamblia-positive patients. Dig Dis Sci 2005; 50(2):259-61; PMID:15745082; http://dx.doi.org/ 10.1007/s10620-005-1592-2 [DOI] [PubMed] [Google Scholar]
- [44].Keselman A, Li E, Maloney J, Singer SM. The microbiota contributes to cd8+ t cell activation and nutrient malabsorption following intestinal infection with giardia duodenalis. Infect Immun 2016; 84(10):2853-60; PMID:27456829; http://dx.doi.org/ 10.1128/IAI.00348-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gillin FD, Cooper RW, Reiner DS, Das S. Secretory defenses against giardia lamblia. Adv Exp Med Biol 1991; 310:227-33; PMID:1809002; http://dx.doi.org/ 10.1007/978-1-4615-3838-7_30 [DOI] [PubMed] [Google Scholar]
- [46].Morken MH, Lind RA, Valeur J, Wilhelmsen I, Berstad A. Subjective health complaints and quality of life in patients with irritable bowel syndrome following giardia lamblia infection: a case control study. Scand J Gastroenterol 2009; 44(3):308-13; PMID:19031266; http://dx.doi.org/ 10.1080/00365520802588091 [DOI] [PubMed] [Google Scholar]
- [47].Morken MH, Nysaeter G, Strand EA, Hausken T, Berstad A. Lactulose breath test results in patients with persistent abdominal symptoms following giardia lamblia infection. Scand J Gastroenterol 2008; 43(2):141-5; PMID:17943632; http://dx.doi.org/ 10.1080/00365520701673960 [DOI] [PubMed] [Google Scholar]
- [48].Hardin JA, Buret AG, Olson ME, Kimm MH, Gall DG. Mast cell hyperplasia and increased macromolecular uptake in an animal model of giardiasis. J Parasitol 1997; 83(5):908-912; PMID:9379297; http://dx.doi.org/ 10.2307/3284287 [DOI] [PubMed] [Google Scholar]
- [49].Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with giardia spp. Reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology 2002; 123(4):1179-90. [DOI] [PubMed] [Google Scholar]
- [50].O'Hara JR, Buret AG. Mechanisms of intestinal tight junctional disruption during infection. Front Biosci 2008; 13:7008-21; PMID:18508712. [DOI] [PubMed] [Google Scholar]
- [51].Koh WH, Geurden T, Paget T, O'Handley R, Steuart RF, Thompson RC, Buret AG. Giardia duodenalis assemblage-specific induction of apoptosis and tight junction disruption in human intestinal epithelial cells: effects of mixed infections. J Parasitol 2013; 99(2):353-8; PMID:22924932; http://dx.doi.org/ 10.1645/GE-3021.1 [DOI] [PubMed] [Google Scholar]
- [52].Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 2016; 17(9):564-80; PMID:27353478; http://dx.doi.org/ 10.1038/nrm.2016.80 [DOI] [PubMed] [Google Scholar]
- [53].Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2(4):285-93; PMID:11283726; http://dx.doi.org/ 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- [54].Maia-Brigagao C, Morgado-Diaz JA, De Souza W. Giardia disrupts the arrangement of tight, adherens and desmosomal junction proteins of intestinal cells. Parasitol Int 2012; 61(2):280-7; PMID:22146155; http://dx.doi.org/ 10.1016/j.parint.2011.11.002 [DOI] [PubMed] [Google Scholar]
- [55].Buret AG, Mitchell K, Muench DG, Scott KG. Giardia lamblia disrupts tight junctional zo-1 and increases permeability in non-transformed human small intestinal epithelial monolayers: effects of epidermal growth factor. Parasitology 2002; 125(Pt 1):11-9; PMID:12166516. [DOI] [PubMed] [Google Scholar]
- [56].Chin AC, Teoh DA, Scott KG, Meddings JB, Macnaughton WK, Buret AG. Strain-dependent induction of enterocyte apoptosis by giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immun 2002; 70(7):3673-80; PMID:12065509; http://dx.doi.org/ 10.1128/IAI.70.7.3673-3680.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Humen MA, Perez PF, Lievin-Le Moal V. Lipid raft-dependent adhesion of giardia intestinalis trophozoites to a cultured human enterocyte-like caco-2/tc7 cell monolayer leads to cytoskeleton-dependent functional injuries. Cell Microbiol 2011; 13(11):1683-702; PMID:21790940; http://dx.doi.org/ 10.1111/j.1462-5822.2011.01647.x [DOI] [PubMed] [Google Scholar]
- [58].Di Genova BM, Tonelli RR. Infection strategies of intestinal parasite pathogens and host cell responses. Front Microbiol 2016; 7:256; PMID:26973630; http://dx.doi.org/ 10.3389/fmicb.2016.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Teoh DA, Kamieniecki D, Pang G, Buret AG. Giardia lamblia rearranges f-actin and alpha-actinin in human colonic and duodenal monolayers and reduces transepithelial electrical resistance. J Parasitol 2000; 86(4):800-6; PMID:10958459. [DOI] [PubMed] [Google Scholar]
- [60].Lievin-Le Moal V. Dysfunctions at human intestinal barrier by water-borne protozoan parasites: Lessons from cultured human fully differentiated colon cancer cell lines. Cell Microbiol 2013; 15(6):860-9; PMID:23437821; http://dx.doi.org/ 10.1111/cmi.12126 [DOI] [PubMed] [Google Scholar]
- [61].Buret AG, Bhargava A. Modulatory mechanisms of enterocyte apoptosis by viral, bacterial and parasitic pathogens. Critical Rev Microbiol 2014; 40(1):1-17; PMID:23297858; http://dx.doi.org/ 10.3109/1040841X.2012.746952 [DOI] [PubMed] [Google Scholar]
- [62].Chin AC, Vergnolle N, MacNaughton WK, Wallace JL, Hollenberg MD, Buret AG. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc Natl Acad Sci U S A 2003; 100(19):11104-9; PMID:12960392; http://dx.doi.org/ 10.1073/pnas.1831452100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Panaro MA, Cianciulli A, Mitolo V, Mitolo CI, Acquafredda A, Brandonisio O, Cavallo P. Caspase-dependent apoptosis of the hct-8 epithelial cell line induced by the parasite giardia intestinalis. FEMS Immunol Med Microbiol 2007; 51(2):302-9; PMID:17714487; http://dx.doi.org/ 10.1111/j.1574-695X.2007.00304.x [DOI] [PubMed] [Google Scholar]
- [64].Cotton JA, Amat CB, Buret AG. Disruptions of host immunity and inflammation by giardia duodenalis: potential consequences for co-infections in the gastro-intestinal tract. Pathogens 2015; 4(4):764-92; PMID:26569316; http://dx.doi.org/ 10.3390/pathogens4040764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C. Bacterial interactions with the host epithelium. Cell Host Microbe 2010; 8(1):20-35; PMID:20638639; http://dx.doi.org/ 10.1016/j.chom.2010.06.006 [DOI] [PubMed] [Google Scholar]
- [66].Banik S, Renner Viveros P, Seeber F, Klotz C, Ignatius R, Aebischer T. Giardia duodenalis arginine deiminase modulates the phenotype and cytokine secretion of human dendritic cells by depletion of arginine and formation of ammonia. Infect Immun 2013; 81(7):2309-17; PMID:23589577; http://dx.doi.org/ 10.1128/IAI.00004-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yu LC, Huang CY, Kuo WT, Sayer H, Turner JR, Buret AG. Sglt-1-mediated glucose uptake protects human intestinal epithelial cells against giardia duodenalis-induced apoptosis. Int J Parasitol 2008; 38(8-9):923-34; PMID:18281046; http://dx.doi.org/ 10.1016/j.ijpara.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig 1997; 99(12):2818-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Salzman AL. Nitric oxide in the gut. New horizons 1995; 3(1):33-45; PMID:7704593. [PubMed] [Google Scholar]
- [70].Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discovery 2008; 7(2):156-67; PMID:18167491; http://dx.doi.org/ 10.1038/nrd2466 [DOI] [PubMed] [Google Scholar]
- [71].Li E, Zhou P, Singer SM. Neuronal nitric oxide synthase is necessary for elimination of giardia lamblia infections in mice. J Immunol 2006; 176(1):516-21; http://dx.doi.org/ 10.4049/jimmunol.176.1.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Andersen YS, Gillin FD, Eckmann L. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against giardia spp. Infect Immun 2006; 74(4):2473-76; PMID:16552082; http://dx.doi.org/ 10.1128/IAI.74.4.2473-2476.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Eckmann L, Laurent F, Langford TD, Hetsko ML, Smith JR, Kagnoff MF, Gillin FD. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen giardia lamblia. J Immunol 2000; 164(3):1478-87; http://dx.doi.org/ 10.4049/jimmunol.164.3.1478 [DOI] [PubMed] [Google Scholar]
- [74].Schofield PJ, Costello M, Edwards MR, O'Sullivan WJ. The arginine dihydrolase pathway is present in giardia intestinalis. Int J Parasitol 1990; 20(5):697-9; PMID:2228433; http://dx.doi.org/ 10.1016/0020-7519(90)90133-8 [DOI] [PubMed] [Google Scholar]
- [75].Touz MC, Ropolo AS, Rivero MR, Vranych CV, Conrad JT, Svard SG, Nash TE. Arginine deiminase has multiple regulatory roles in the biology of giardia lamblia. J Cell Sci 2008; 121(Pt 17):2930-8; PMID:18697833; http://dx.doi.org/ 10.1242/jcs.026963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Stadelmann B, Merino MC, Persson L, Svard SG. Arginine consumption by the intestinal parasite giardia intestinalis reduces proliferation of intestinal epithelial cells. PloS one 2012; 7(9):e45325; PMID:23028934; http://dx.doi.org/ 10.1371/journal.pone.0045325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Potoka DA, Upperman JS, Zhang XR, Kaplan JR, Corey SJ, Grishin A, Zamora R, Ford HR. Peroxynitrite inhibits enterocyte proliferation and modulates src kinase activity in vitro. Am J Physiol Gastrointest Liver Physiol 2003; 285(5):G861-869; PMID:12842830; http://dx.doi.org/ 10.1152/ajpgi.00412.2002 [DOI] [PubMed] [Google Scholar]
- [78].Aloisio F, Filippini G, Antenucci P, Lepri E, Pezzotti G, Caccio SM, Pozio E. Severe weight loss in lambs infected with giardia duodenalis assemblage b. Veterinary parasitology 2006; 142(1-2):154-8; PMID:16891057; http://dx.doi.org/ 10.1016/j.vetpar.2006.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, Hoffman P, Warren C, Singer S, Guerrant R. Persistent g. Lamblia impairs growth in a murine malnutrition model. J Clin Investig 2013; 123(6):2672-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ventura LL, Oliveira DR, Viana JC, Santos JF, Caliari MV, Gomes MA. Impact of protein malnutrition on histological parameters of experimentally infected animals with giardia lamblia. Exp Parasitol 2013; 133(4):391-5; PMID:23337825; http://dx.doi.org/ 10.1016/j.exppara.2013.01.007 [DOI] [PubMed] [Google Scholar]
- [81].Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 2012; 15(1):57-62; PMID:22177113; http://dx.doi.org/ 10.1016/j.mib.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen-Larsson JM, Thomsson KA, Bergstrom JH et al.. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 2011; 68(22):3635-41; PMID:21947475; http://dx.doi.org/ 10.1007/s00018-011-0822-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 2001; 280(5):G922-929; PMID:11292601. [DOI] [PubMed] [Google Scholar]
- [84].Paget TA, James SL. The mucolytic activity of polyamines and mucosal invasion. Biochem Soc Trans 1994; 22(4):394S; PMID:7698417; http://dx.doi.org/ 10.1042/bst022394s [DOI] [PubMed] [Google Scholar]
- [85].Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the muc2 mucin in its c-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci U S A 2006; 103(24):9298-303; PMID:16754877; http://dx.doi.org/ 10.1073/pnas.0600623103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hasnain SZ, McGuckin MA, Grencis RK, Thornton DJ. Serine protease(s) secreted by the nematode trichuris muris degrade the mucus barrier. PLoS Negl Trop Dis 2012; 6(10):e1856; PMID:23071854; http://dx.doi.org/ 10.1371/journal.pntd.0001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S et al.. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci U S A 2009; 106(34):14321-6; PMID:19706518; http://dx.doi.org/ 10.1073/pnas.0903438106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB et al.. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS pathogens 2010; 6(5):e1000902; PMID:20485566; http://dx.doi.org/ 10.1371/journal.ppat.1000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hasnain SZ, Thornton DJ, Grencis RK. Changes in the mucosal barrier during acute and chronic trichuris muris infection. Parasite Immunol 2011; 33(1):45-55; PMID:21155842; http://dx.doi.org/ 10.1111/j.1365-3024.2010.01258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bergstrom KS, Guttman JA, Rumi M, Ma C, Bouzari S, Khan MA, Gibson DL, Vogl AW, Vallance BA. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect Immun 2008; 76(2):796-811; PMID:17984203; http://dx.doi.org/ 10.1128/IAI.00093-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gault MJ, Gillin FD, Zenian AJ. Giardia lamblia: stimulation of growth by human intestinal mucus and epithelial cells in serumfree medium. Exp Parasitol 1987; 64(1):29-37; PMID:3609228; http://dx.doi.org/ 10.1016/0014-4894(87)90005-1 [DOI] [PubMed] [Google Scholar]
- [92].Roskens H, Erlandsen SL. Inhibition of in vitro attachment of giardia trophozoites by mucin. J Parasitol 2002; 88(5):869-73; PMID:12435122; http://dx.doi.org/ 10.1645/0022-3395(2002)088%5b0869:IOIVAO%5d2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- [93].Da Silva JR, Coutinho SG, Dias LB, Defiguieredo N. Histopathologic findings in giardiasis: a biopsy study. Am J Digestive Dis 1964; 9:355-65; PMID:14157562; http://dx.doi.org/ 10.1007/BF02232578 [DOI] [PubMed] [Google Scholar]
- [94].Zinneman HH, Kaplan AP. The association of giardiasis with reduced intestinal secretory immunoglobulin a. Am J Dig Dis 1972; 17(9):793-7; PMID:5056860; http://dx.doi.org/ 10.1007/BF02231148 [DOI] [PubMed] [Google Scholar]
- [95].Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2016; 14(1):20-32; PMID:26499895; http://dx.doi.org/ 10.1038/nrmicro3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol 2015; 21(29):8787-803; PMID:26269668; http://dx.doi.org/ 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends Ecol Evol 2006; 21(9):517-23; PMID:16820245; http://dx.doi.org/ 10.1016/j.tree.2006.06.013 [DOI] [PubMed] [Google Scholar]
- [98].Chen TL, Chen S, Wu HW, Lee TC, Lu YZ, Wu LL, Ni YH, Sun CH, Yu WH, Buret AG et al.. Persistent gut barrier damage and commensal bacterial influx following eradication of giardia infection in mice. Gut pathogens 2013; 5(1):26; PMID:23991642; http://dx.doi.org/ 10.1186/1757-4749-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Torres MF, Uetanabaro AP, Costa AF, Alves CA, Farias LM, Bambirra EA, Penna FJ, Vieira EC, Nicoli JR. Influence of bacteria from the duodenal microbiota of patients with symptomatic giardiasis on the pathogenicity of giardia duodenalis in gnotoxenic mice. J Med Microbiol 2000; 49(3):209-15; PMID:10707940; http://dx.doi.org/ 10.1099/0022-1317-49-3-209 [DOI] [PubMed] [Google Scholar]
- [100].Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term ige levels. Cell Host Microbe 2013; 14(5):559-70; PMID:24237701; http://dx.doi.org/ 10.1016/j.chom.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Iebba V, Santangelo F, Totino V, Pantanella F, Monsia A, Di Cristanziano V, Di Cave D, Schippa S, Berrilli F, D'Alfonso R. Gut microbiota related to giardia duodenalis, entamoeba spp. And blastocystis hominis infections in humans from cote d'ivoire. J Infect Dev Countries 2016; 10(9):1035-41. [DOI] [PubMed] [Google Scholar]
- [102].Tomkins AM, Wright SG, Drasar BS, James WP. Bacterial colonization of jejunal mucosa in giardiasis. Trans R Soc Trop Med Hyg 1978; 72(1):33-36; PMID:635972; http://dx.doi.org/ 10.1016/0035-9203(78)90294-8 [DOI] [PubMed] [Google Scholar]
- [103].Bakhtiar SM, LeBlanc JG, Salvucci E, Ali A, Martin R, Langella P, Chatel JM, Miyoshi A, Bermudez-Humaran LG, Azevedo V. Implications of the human microbiome in inflammatory bowel diseases. FEMS Microbiol Lett 2013; 342(1):10-7; PMID:23431991; http://dx.doi.org/ 10.1111/1574-6968.12111 [DOI] [PubMed] [Google Scholar]
- [104].Shukla G, Sidhu RK. Lactobacillus casei as a probiotic in malnourished giardia lamblia-infected mice: a biochemical and histopathological study. Can J Microbiol 2011; 57(2):127-35; PMID:21326354; http://dx.doi.org/ 10.1139/W10-110 [DOI] [PubMed] [Google Scholar]
- [105].Goyal N, Rishi P, Shukla G. Lactobacillus rhamnosus gg antagonizes giardia intestinalis induced oxidative stress and intestinal disaccharidases: an experimental study. World J Microbiol Biotechnol 2013; 29(6):1049-57; PMID:23361971; http://dx.doi.org/ 10.1007/s11274-013-1268-6 [DOI] [PubMed] [Google Scholar]
- [106].Humen MA, De Antoni GL, Benyacoub J, Costas ME, Cardozo MI, Kozubsky L, Saudan KY, Boenzli-Bruand A, Blum S, Schiffrin EJ et al.. Lactobacillus johnsonii la1 antagonizes giardia intestinalis in vivo. Infect Immun 2005; 73(2):1265-9; PMID:15664978; http://dx.doi.org/ 10.1128/IAI.73.2.1265-1269.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shukla G, Singh S, Verma A. Oral administration of the probiotic lactobacillus casei ameliorates gut morphology and physiology in malnourished-giardia intestinalis-infected balb/c mice. ISRN Parasitol 2013; 2013:762638; PMID:27335861; http://dx.doi.org/ 10.5402/2013/762638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shukla G, Sidhu RK, Verma A. Restoration of anthropometric, biochemical and histopathological alterations by lactobacillus casei supplementation in giardia intestinalis infected renourished balb/c mice. Antonie van Leeuwenhoek 2012; 102(1):61-72; PMID:22382675; http://dx.doi.org/ 10.1007/s10482-012-9713-3 [DOI] [PubMed] [Google Scholar]
- [109].Perez PF, Minnaard J, Rouvet M, Knabenhans C, Brassart D, De Antoni GL, Schiffrin EJ. Inhibition of giardia intestinalis by extracellular factors from lactobacilli: an in vitro study. App Environ Microbiol 2001; 67(11):5037-42; PMID:11679323; http://dx.doi.org/ 10.1128/AEM.67.11.5037-5042.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Travers MA, Sow C, Zirah S, Deregnaucourt C, Chaouch S, Queiroz RM, Charneau S, Allain T, Florent I, Grellier P. Deconjugated bile salts produced by extracellular bile-salt hydrolase-like activities from the probiotic lactobacillus johnsonii la1 inhibit giardia duodenalis in vitro growth. Front Microbiol 2016; 7:1453; PMID:27729900; http://dx.doi.org/ 10.3389/fmicb.2016.01453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Benyacoub J, Perez PF, Rochat F, Saudan KY, Reuteler G, Antille N, Humen M, De Antoni GL, Cavadini C, Blum S et al.. Enterococcus faecium sf68 enhances the immune response to giardia intestinalis in mice. J Nutrition 2005; 135(5):1171-6; PMID:15867299. [DOI] [PubMed] [Google Scholar]
- [112].Franco MC, Golowczyc MA, De Antoni GL, Perez PF, Humen M, Serradell Mde L. Administration of kefir-fermented milk protects mice against giardia intestinalis infection. J Med Microbiol 2013; 62(Pt 12):1815-22; PMID:24072759; http://dx.doi.org/ 10.1099/jmm.0.068064-0 [DOI] [PubMed] [Google Scholar]
- [113].Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes & nutrition 2011; 6(3):261-74; PMID:21499799; http://dx.doi.org/ 10.1007/s12263-011-0218-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Travers MA, Florent I, Kohl L, Grellier P. Probiotics for the control of parasites: An overview. J Parasitol Res 2011; 2011:610769; PMID:21966589; http://dx.doi.org/ 10.1155/2011/610769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Amer EI, Mossallam SF, Mahrous H. Therapeutic enhancement of newly derived bacteriocins against giardia lamblia. Exp Parasitol 2014; 146:52-63; PMID:25300763; http://dx.doi.org/ 10.1016/j.exppara.2014.09.005 [DOI] [PubMed] [Google Scholar]
- [116].Baum KF, Berens RL, Marr JJ. Purine nucleoside and nucleobase cell membrane transport in giardia lamblia. J Eukaryot Microbiol 1993; 40(5):643-9; PMID:8401476; http://dx.doi.org/ 10.1111/j.1550-7408.1993.tb06122.x [DOI] [PubMed] [Google Scholar]
- [117].Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamma Bowel Dis 2009; 15(2):300-10; PMID:18626975; http://dx.doi.org/ 10.1002/ibd.20602 [DOI] [PubMed] [Google Scholar]
- [118].Hanevik K, Wensaas KA, Rortveit G, Eide GE, Morch K, Langeland N. Irritable bowel syndrome and chronic fatigue 6 years after giardia infection: a controlled prospective cohort study. Clin Infect Dis 2014; 59(10):1394-400; PMID:25115874; http://dx.doi.org/ 10.1093/cid/ciu629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Naess H, Nyland M, Hausken T, Follestad I, Nyland HI. Chronic fatigue syndrome after giardia enteritis: clinical characteristics, disability and long-term sickness absence. BMC Gastroenterol 2012; 12:13; PMID:22316329; http://dx.doi.org/ 10.1186/1471-230X-12-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Motta JP, Flannigan KL, Agbor TA, Beatty JK, Blackler RW, Workentine ML, Da Silva GJ, Wang R, Buret AG, Wallace JL. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm Bowel Dis 2015; 21(5):1006-17; PMID:25738373; http://dx.doi.org/ 10.1097/MIB.0000000000000345 [DOI] [PubMed] [Google Scholar]
- [121].Macfarlane S, Bahrami B, Macfarlane GT. Mucosal biofilm communities in the human intestinal tract. Adv Appl Microbiol 2011; 75:111-43; PMID:21807247; http://dx.doi.org/ 10.1016/B978-0-12-387046-9.00005-0 [DOI] [PubMed] [Google Scholar]
- [122].Ringel Y, Ringel-Kulka T. The intestinal microbiota and irritable bowel syndrome. J Clin Gastroenterol 2015; 49 Suppl 1:S56-59; http://dx.doi.org/ 10.1097/MCG.0000000000000418 [DOI] [PubMed] [Google Scholar]
- [123].Buret A. Enteropathogen-induced microbiota biofilm disruptions and post-infectious intestinal inflammatory disorders. Curr Trop Med Rep 2016; 3 (3):94-101; http://dx.doi.org/ 10.1007/s40475-016-0079-x [DOI] [Google Scholar]
- [124].Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002; 122(7):1778-83; PMID:12055584; http://dx.doi.org/ 10.1053/gast.2002.33579 [DOI] [PubMed] [Google Scholar]
- [125].Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol 2009; 104(2):392-400; PMID:19174797; http://dx.doi.org/ 10.1038/ajg.2008.94 [DOI] [PubMed] [Google Scholar]
- [126].Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated b cells in human inflammatory bowel disease. J Leukoc Biol 2009; 86(4):1007-16; PMID:19589946; http://dx.doi.org/ 10.1189/jlb.0309203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjovall H, Simren M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol 2009; 104(5):1205-12; PMID:19367268; http://dx.doi.org/ 10.1038/ajg.2009.116 [DOI] [PubMed] [Google Scholar]
- [128].Gupta S. Infectious disease: something in the water. Nature 2016; 533(7603):S114-115; PMID:27191491; http://dx.doi.org/ 10.1038/533S114a [DOI] [PubMed] [Google Scholar]
- [129].Rall G, Knoll LJ. Development of complex models to study co- and polymicrobial infections and diseases. PLoS pathogens 2016; 12(9):e1005858; PMID:27607188; http://dx.doi.org/ 10.1371/journal.ppat.1005858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ryan U, Caccio SM. Zoonotic potential of giardia. Int J Parasitol 2013; 43(12-13):943-56; PMID:23856595; http://dx.doi.org/ 10.1016/j.ijpara.2013.06.001 [DOI] [PubMed] [Google Scholar]
- [131].Bilenko N, Levy A, Dagan R, Deckelbaum RJ, El-On Y, Fraser D. Does co-infection with giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol 2004; 19(9):877-83. [DOI] [PubMed] [Google Scholar]
- [132].Ankarklev J, Hestvik E, Lebbad M, Lindh J, Kaddu-Mulindwa DH, Andersson JO, Tylleskar T, Tumwine JK, Svard SG. Common coinfections of giardia intestinalis and helicobacter pylori in non-symptomatic ugandan children. PLoS Negl Trop Dis 2012; 6(8):e1780; PMID:22953010; http://dx.doi.org/ 10.1371/journal.pntd.0001780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vasco K, Graham JP, Trueba G. Detection of zoonotic enteropathogens in children and domestic animals in a semirural community in ecuador. App Environ Microbiol 2016; 82(14):4218-24; PMID:27208122; http://dx.doi.org/ 10.1128/AEM.00795-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang L, Xiao L, Duan L, Ye J, Guo Y, Guo M, Liu L, Feng Y. Concurrent infections of giardia duodenalis, enterocytozoon bieneusi, and clostridium difficile in children during a cryptosporidiosis outbreak in a pediatric hospital in china. PLoS Negl Trop Dis 2013; 7(9):e2437; PMID:24069491; http://dx.doi.org/ 10.1371/journal.pntd.0002437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Eldash HH, Bekhit OE, Algameel AA. Impact of helicobacter pylori-giardiasis coinfection on children with recurrent abdominal pain. J Egypt Soc Parasitol 2013; 43(2):509-16; PMID:24260829; http://dx.doi.org/ 10.12816/0006407 [DOI] [PubMed] [Google Scholar]
- [136].Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between 2 intestinal parasites in humans: The importance of co-infection for infection risk and recovery dynamics. Proc Biol Sci 2013; 280(1769):20131671; PMID:23986108; http://dx.doi.org/ 10.1098/rspb.2013.1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Koru O, Araz E, Inci A, Tanyuksel M. Co-infection of giardia intestinalis and cyclospora cayetanensis in an immunocompetent patient with prolonged diarrhea: case report. J Microbiol 2006; 44(3):360-2. [PubMed] [Google Scholar]
- [138].Grazioli B, Matera G, Laratta C, Schipani G, Guarnieri G, Spiniello E, Imeneo M, Amorosi A, Foca A, Luzza F. Giardia lamblia infection in patients with irritable bowel syndrome and dyspepsia: a prospective study. World J Gastroenterol 2006; 12(12):1941-4; PMID:16610003; http://dx.doi.org/ 10.3748/wjg.v12.i12.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Mukherjee AK, Chowdhury P, Rajendran K, Nozaki T, Ganguly S. Association between giardia duodenalis and coinfection with other diarrhea-causing pathogens in india. BioMed Res Int 2014; 2014:786480; PMID:25009820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hagel I, Cabrera M, Puccio F, Santaella C, Buvat E, Infante B, Zabala M, Cordero R, Di Prisco MC. Co-infection with ascaris lumbricoides modulates protective immune responses against giardia duodenalis in school venezuelan rural children. Acta tropica 2011; 117(3):189-95; PMID:21172297; http://dx.doi.org/ 10.1016/j.actatropica.2010.12.001 [DOI] [PubMed] [Google Scholar]
- [141].Fenollar F, Lepidi H, Gerolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis 2003; 188(6):828-34; PMID:12964113; http://dx.doi.org/ 10.1086/378093 [DOI] [PubMed] [Google Scholar]
- [142].Muhsen K, Cohen D, Levine MM. Can giardia lamblia infection lower the risk of acute diarrhea among preschool children? Journal of tropical pediatrics 2014; 60(2):99-103. [DOI] [PubMed] [Google Scholar]
- [143].Veenemans J, Mank T, Ottenhof M, Baidjoe A, Mbugi EV, Demir AY, Wielders JP, Savelkoul HF, Verhoef H. Protection against diarrhea associated with giardia intestinalis is lost with multi-nutrient supplementation: a study in tanzanian children. PLoS Negl Trop Dis 2011; 5(6):e1158; PMID:21666789; http://dx.doi.org/ 10.1371/journal.pntd.0001158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fraser D, Bilenko N, Deckelbaum RJ, Dagan R, El-On J, Naggan L. Giardia lamblia carriage in israeli bedouin infants: risk factors and consequences. Clin Infect Dis 2000; 30(3):419-24; PMID:10722422; http://dx.doi.org/ 10.1086/313722 [DOI] [PubMed] [Google Scholar]
- [145].Haque R, Mondal D, Karim A, Molla IH, Rahim A, Faruque AS, Ahmad N, Kirkpatrick BD, Houpt E, Snider C et al.. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in bangladesh. Clin Infect Dis 2009; 48(9):1191-7; PMID:19323634; http://dx.doi.org/ 10.1086/597580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Beatty JK, Akierman SV, Motta JP, Muise S, Workentine ML, Harrison JJ, Bhargava A, Beck PL, Rioux KP, McKnight GW et al.. Giardia duodenalis induces dysbiosis of human intestinal microbiota biofilms. Int J Parasitol 2017. [in press] [DOI] [PubMed] [Google Scholar]