Abstract
The glutamine transporter ASCT2 has been identified as a promising target to inhibit rapid growth of cancer cells. However, ASCT2 pharmacology is not well established. In this report, we performed a systematic structure activity analysis of a series of substituted benzylproline derivatives. Substitutions on the phenyl ring resulted in compounds with characteristics of ASCT2 inhibitors. Apparent binding affinity increased with increasing hydrophobicity of the side chain. In contrast, interaction of the ASCT2 binding site with specific positions on the phenyl ring was not observed. The most potent compound inhibits the ASCT2 anion conductance with a Ki of 3 μM, which is in the same range as that of more bulky and higher molecular weight inhibitors recently reported by others. The experimental results are consistent with computational analysis based on docking of the inhibitors against an ASCT2 homology model. The benzylproline scaffold provides a valuable tool for further improving binding potency of future ASCT2 inhibitors.
Keywords: Neutral amino acid transporter, ASCT2, inhibitors, glutamine transport, kinetics, patch clamp, pharmacology
Glutamine is transported across mammalian membranes by a variety of transporters from different families, including the alanine cysteine seine transporter, ASCT2 (reviewed in [1–2]). ASCT2 belongs to the solute carrier 1 (SLC1) family of transporters [3]. It transports neutral amino acids, including glutamine, across the plasma membrane in exchange with an intracellular neutral amino acid [4–5]. The process is dependent on Na+, but not driven by the transmembrane concentration gradient of Na+. Net exchange is electroneutral, although it was proposed that the actual translocation process is associated with charge movement [5–6].
Glutamine is an important nutrient, in particular in rapidly-growing cancer cells, in which glutamine serves as a nitrogen source [7]. The increased nitrogen and carbon demand, together with the role of glutamine in the regulation of mammalian target of rapamycin complex 1 (mTORC1) signaling, creates glutamine “dependency” of certain types of cancer cells, resulting in an up-regulation of glutamine transport [8]. Such changes in metabolism of cells once they become cancerous are known as the Warburg effect, which has been shown as increased demand for glucose (glycolysis) as well as glutamine [9].
Due to the increased nitrogen demand, glutamine transporters, in particular ASCT2, show dramatically increased expression levels in cancer cells [10] [11]. For example, ASCT2 up-regulation was demonstrated in prostate cancer and triple negative breast cancer (TNBC) [10]. Furthermore, it was shown that inhibition of ASCT2 expression through antisense RNA methods resulted in cell apoptosis, and even shrinkage of tumors.
Together, these results implicate ASCT2 as an important potential target for inhibiting the growth of cancer cells. However, at present the pharmacology of the ASCT2 substrate binding site and the knowledge of small molecule transport inhibitors are not very well developed. Several amino acid based inhibitors have been identified, including a series of serine derivatives [11], and L-γ-glutamyl-nitroanilide, a commercially available ASCT2 inhibitor with mM affinity, and derivatives [12–13] (Fig. 1A). Based on these anilides, compounds with increased aromatic bulk were developed, which bind to ASCT2 with affinities in the low micromolar range [14].
Figure 1.
(A) Structures of previously identified ASCT2 substrates (alanine) and inhibitors (glutamyl-anilide and serine ester derivatives). (B) Proline and substituted benzylproline derivatives investigated in this study.
Recently, we have utilized an in-silico screening approach to test a compound database for potential hits for ASCT2 binders. Among several other compounds, a proline derivative was identified, γ-2-fluorobenzyl proline, which inhibited ASCT2 with a 87μM affinity [15]. This result was surprising, because proline (Fig. 1B) is not a known substrate/inhibitor of ASCT2 and does not induce any activity in ASCT2 at a concentration up to 1 mM (Fig. 2).
Figure 2. All 4-substituted proline derivatives display inhibitory behavior.
Current responses, Imax, in the presence of saturating concentrations of alanine and proline derivatives relative to a response to saturating [alanine]. Saturating current was calculated from the response at the tested concentration (1 mM) and the known Km value, I/Imax = c/(c+Km) with c = concentration. Outward (positive) current reflects inhibitory behavior due to inhibition of the leak anion conductance.
In the present work, we aimed at using benzylproline as a scaffold for systematic structure activity analysis (Fig. 1B), altering substituents on the benzyl ring. We tested a number of benzylproline derivatives with substitutions at the 2, 3, and 4 positions of the benzyl ring (Fig. 1B). Through this approach, we identified a new ASCT2 inhibitor, which binds to the apo (unbound) transporter with a 3 μM apparent affinity. Interestingly, the position of the substituent on the phenyl ring had only a minor effect on inhibitory potency. In contrast, the ability of the substituent to affect hydrophobicity played a major role. Our new results add to the understanding of the molecular parameters that govern inhibitor interaction with the ASCT2 binding site.
The first strategy was to test whether the nature of the substituent on the 2-position of the phenyl ring affected binding potency. To test this question, we determined kinetic parameters for ligand interaction with ASCT2 for six γ-benzylproline derivatives with varying 2-substituents, ranging from hydrogen to halogens and the methyl group (structures shown in Fig. 1B, R1). Only the (R) enantiomers were experimentally tested. To determine kinetic parameters of binding, we recorded currents in response to compound application to ASCT2-expressing cells. Rat ASCT2 was transiently expressed in HEK293T cells, as was shown previously [5, 11, 16]. HEK293T cells do not express detectable levels of ASCT2 before transfection with ASCT2 cDNA-containing plasmids. All six compounds showed characteristics of ASCT2 inhibitors, because they blocked the permanent ASCT2 leak anion current (Fig. 2). In the presence of intracellular anion (SCN−), this leak anion current is inward directed (SCN− outflow). Therefore, application of blockers reduces the inward leak anion current, generating apparent outward current (Fig. 3A middle panels and right panel), as reported previously for other ASCT2 blockers [11, 15–16]. In contrast, transported substrates, such as alanine, activate a substrate-dependent anion current, which in the presence of intracellular anion (SCN−) is inward directed [5]. Thus, alanine and other transported substrates induce inward current (SCN− outflow) under these conditions (Fig. 3A, left panel). This characteristic behavior of ASCT2 substrates/inhibitors has been demonstrated in several reports, and is caused by the kinetic relationship between substrate transport and the visitation of anion conducting states along the transport pathway [5, 11, 15–16].
Figure 3. Benzyl-proline derivatives substituted in the 2 position of the phenyl ring inhibit ASCT2 activity.
(A) Typical whole-cell current recording traces from ASCT2-transfected HEK293T cells in the presence of 1 mM alanine (left panel) and 1 mM of 2-Br, 2-Cl, and 2-nitro-benzylproline. Timing of substrate/inhibitor application is indicated by the gray bars. (B) Dose response relationships for the three inhibitors shown in (A). All responses were normalized to the response at 1 mM of each compound. Experiments were performed at 0 mV transmembrane potential in the presence of 140 mM external NaCl, 135 mM internal NaSCN, and 10 mM internal alanine.
The apparent affinity of ASCT2 for the 2-substituted benzylproline derivatives, which was determined by measuring the dose response relationships of the outward currents (Fig. 3B), varied over almost 2 orders of magnitude, with γ-benzylproline (H-substituent) having the lowest affinity (highest Ki, 2.0 ± 1.5 mM), whereas the 2-bromo derivative displayed the lowest Ki of 25 ± 15 μM (highest affinity, Fig. 3B). The apparent affinity data for these compounds are summarized in table 1. These results show that halogen substituents, as well as a methyl group in 2 position result in a significant increase in apparent affinity over the H-substituted compound.
Table 1.
Ki and Imax values for the benzylproline derivatives (Imax are relative to the current at saturating alanine concentration). Errors represent ± S.D. All compounds were the hydrochloride salts.
| Compound | Ki (μM) | Relative Imax |
|---|---|---|
| (R)-γ-benzyl-L-proline hydrochloride | 2000 ± 1500 | 0.52 ± 0.21 |
| (R)-γ-(4-fluoro-benzyl)-L-proline | 190 ± 110 | 0.57 ± 0.03 |
| (R)-γ-(3-fluoro-benzyl)-L-proline | 177 ± 31 | 0.67 ± 0.04 |
| (R)-γ-(2-fluoro-benzyl)-L-proline | 83 ± 20 | 0.61 ± 0.09 |
| (R)-γ-(2-nitro-benzyl)-L-proline | 373 ± 65 | 0.61 ± 0.03 |
| (R)-γ-(2-chloro-benzyl)-L-proline | 195 ± 15 | 0.46 ± 0.03 |
| (R)-γ-(2-bromo-benzyl)-L-proline | 25 ± 15 | 0.55 ± 0.02 |
| (R)-γ-(2-methyl-benzyl)-L-proline | 38 ± 25 | 0.59 ± 0.12 |
| (R)-γ-(3,4-difluoro-benzyl)-L-proline | 73 ± 55 | 0.51 ± 0.20 |
| (R)-γ-(2,4-dichloro-benzyl)-L-proline | 30 ± 14 | 0.56 ± 0.01 |
| (R)-γ-(2-trifluoromethyl-benzyl)-L-proline | 220 ± 36 | 0.49 ± 0.06 |
| (R)-γ-(3-trifluoromethyl-benzyl)-L-proline | 77 ± 36 | 0.43 ± 0.03 |
| (R)-γ-(4-trifluoromethyl-benzyl)-L-proline | 360 ± 60 | 0.47 ± 0.01 |
| (R)-γ-(4-biphenylmethyl)-L-proline | 3 ± 2 | 0.57 ± 0.08 |
While no significant correlation of Ki value with substituent size (as quantified through Taft steric parameter [17]) or electron withdrawing/donating properties of the substituent (Hammett substituent constant [18]) were observed, Ki decreased with increasing hydrophobicity of the benzylproline side chain (Fig. 4, quantified by log(P), with P = octanol/water partition coefficient of the side chain).
Figure 4. Inhibitor affinity correlates with the hydrophobicity of the substituent.
The log(Ki) is plotted as a function of log(P) of the side chain. log(P) was calculated according to [22]. R2 for the linear regression (solid line) is 0.73. Pearson’s r value is -0.85, indicating good correlation.
Next, we tested the effect of the position of a fluorine substituent on the benzyl ring. All three tested derivatives (2-fluoro, 3-fluoro, and 4-fluoro γ-benzylproline) showed inhibitory behavior (Fig. 2). As illustrated in Suppl. Fig. 1 and table 1, the position of the fluorine substituent did not have a large effect on apparent affinity, although binding potency was most favorable with the fluorine atom in the 2 position on the benzyl ring. This result indicates that the main effect of the fluorine substituent on inhibitor potency is the general increase of hydrophobicity, rather than localized and specific molecular interactions of the fluorine atoms with the ASCT2 ligand binding site.
Two di-substituted chloro and fluoro derivatives were also included in the analysis (table 1). While both compounds showed inhibitory activity, their Ki value of the 3,4-difluoro derivative was not dramatically improved over the mono-substituted analog. This finding was not unexpected, since the two side chains have very similar hydrophobicity. In contrast, adding a second Cl substituent in the 4 position (2,4 dicholoro-γ-benzylproline) resulted in a 6-fold increase in apparent affinity, presumably because the di-substituted side chain has a significantly higher log(P) value than the mono-substituted compound.
To further test the correlation of side chain hydrophobicity with inhibitor potency, we tested two compounds with substituents that increase the log(P) value, phenyl and trifluoromethyl groups. While the trifluoromethyl substituted compounds showed no improvement in Ki over the other mono-substituted benzylproline derivatives (table 1), the 4-phenyl-substitued derivative was found to have an apparent inhibition constant of 3 ± 2 μM (Supplementary Fig. 2, table 1), an about 8-fold higher affinity compared to any other ASCT2 inhibitor we have tested so far [11, 15].
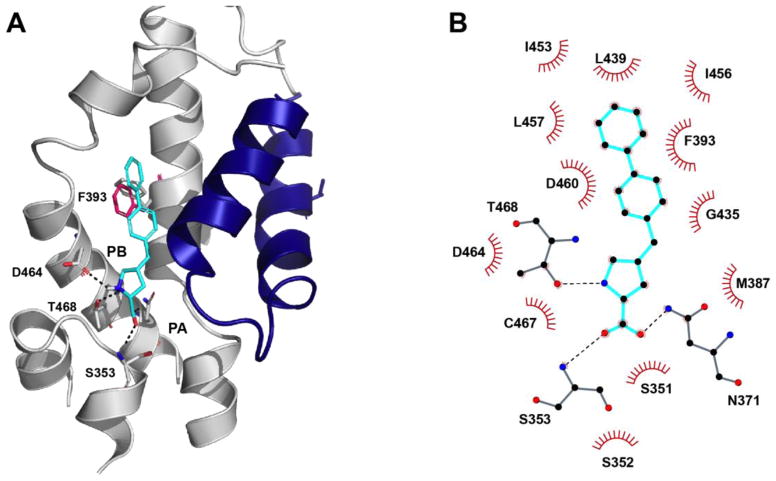
To visualize the binding mode of the most potent newly identified ASCT2 inhibitor γ-(4-biphenylmethyl)-L-proline, we conducted in-silico Induced Fit Docking (IFD, [19]) of this compound against an ASCT2 homology model (Fig. 5) [16]. This ASCT2 model was built based on the outward open conformation of GltPh [20], in which hairpin loop 2 (HP2) is propped open by the bound, bulky inhibitor TBOA, thus preventing translocation of the C-terminal transport domain across the membrane. The outward-open ASCT2 model reveals two hydrophobic pockets PA and PB, which can be targeted with small molecule inhibitors (Fig 5) [21]. The hydrophobic side chains of the original inhibitor γ-2-fluorobenzylproline as well as the newly discovered ligands are predicted to interact with PB. Interestingly, during IFD, in which the flexibility of the binding site is introduced, Phe393 is reoriented, thereby making additional accessible volume in PB and facilitating the binding of the hydrophobic bulk of the biphenylmethyl substituent. The carboxy and pyrrolidine groups of the newly discovered ligands are predicted to form polar interactions with key binding site residues, including Ser353, Asn371, and Thr468, which are also predicted to make similar polar interactions with known ligands (Fig. 5) [15].
Figure 5.
Predicted binding pose of γ-(4-biphenylmethyl)-L-proline in the homology model of the human ASCT2. (A) The coordinates of γ-(4-biphenylmethyl)-L-proline are visualized in cyan sticks. The ASCT2 binding site is shown in gray cartoons, with the open HP2 loop in dark blue. The residues forming hydrogen bonds with the ligand are shown in sticks. The regions of each pocket PA and PB are labeled. Two orientations of Phe393 are displayed: in gray, the phenyl side chain of Phe393 is perpendicular to the vertical axis of the binding site; in pink, Phe393 is flipped parallel to the vertical axis, as a result of the IFD. (B) The docking pose of γ-(4-biphenylmethyl)-L-proline is shown in a two-dimensional representation generated by ligplot, where the residues forming hydrogen bonds are colored in gray, and the residues establishing hydrophobic interactions with the ligand are shown as red spoked arcs.
In summary, our work highlights the usefulness of substituted benzylprolines as a scaffold for the development of ASCT2 inhibitors with improved binding potency. The newly-identified biphenylmethyl derivative interacts with rat ASCT2 with a low micromolar apparent affinity. To our knowledge only one compound has been reported with higher affinity (1.3 μM, [14]), based on a substituted diaminobutanoic acid. However, this compound has substantially higher molecular weight, as well as hydrophobicity. If the γ-(4-biphenylmethyl)-L-proline scaffold can be used to further improve affinity, this would provide further advance in the ongoing work to generate ASCT2 inhibitors with sub-micromolar binding affinity.
Our results also provide a quantitative basis for our understanding of the molecular parameters that govern interaction of the inhibitors with the ASCT2 binding site. They suggest that specific atomic interactions between substituents of the hydrophobic moiety and the ASCT2 binding site are less important than the overall hydrophobicity of the side chain (Fig. 4). Future structure function analysis can exploit this finding by increasing the hydrophobicity of the side chain further, as well as by integrating a specific side chain hydrogen bonding interactions, as proposed in a previous report [12]. This hydrogen bond interaction is missing in the proline derivatives reported here, and, if additive with the hydrophobic effect, may further strengthen ASCT2 interaction with the bound ligand. Therefore, the prospects of developing ASCT2 inhibitors with sub-micromolar affinity are encouraging.
Supplementary Material
Acknowledgments
This study was supported by a grant from the National Institutes of Health (http://www.nih.gov) (R01 GM108911) to AS, CC and CG.
References
- 1.Bode BP. Recent Molecular Advances in Mammalian Glutamine Transport. J Nutr. 2001;131:2475S–2485. doi: 10.1093/jn/131.9.2475S. [DOI] [PubMed] [Google Scholar]
- 2.Zander CB, Zhang Z, Albers T, Grewer C. Amino Acid Transporters and Glutamine. In: Rajendram R, Preedy VR, Patel VB, editors. Glutamine in Clinical Nutrition. Springer; 2015. [Google Scholar]
- 3.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Archiv - European Journal of Physiology. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 4.Broer A, Wagner C, Lang F, Broer S. Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochemical Journal. 2000;346:705–710. [PMC free article] [PubMed] [Google Scholar]
- 5.Zander CB, Albers T, Grewer C. Voltage-dependent processes in the electroneutral amino acid exchanger ASCT2. J Gen Physiol. 2013;141:659–672. doi: 10.1085/jgp.201210948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussolati O, Laris PC, Rotoli BM, Dall’Asta V, Gazzola GC. Transport system ASC for neutral amino acids. An electroneutral sodium/amino acid cotransport sensitive to the membrane potential. J Biol Chem. 1992;267:8330–8335. [PubMed] [Google Scholar]
- 7.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, Ritchie W, Feng Y, Bailey CG, Deng N, Harvey K, Beith JM, Selinger CI, O’Toole SA, Rasko JE, Holst J. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–3208. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers T, Marsiglia W, Thomas T, Gameiro A, Grewer C. Defining Substrate and Blocker Activity of Alanine Serine Cysteine Transporter 2 (ASCT2) Ligands with Novel Serine Analogs. Mol Pharmacol. 2011 doi: 10.1124/mol.111.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esslinger CS, Cybulski KA, Rhoderick JF. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem. 2005;13:1111–1118. doi: 10.1016/j.bmc.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Schulte ML, Dawson ES, Saleh SA, Cuthbertson ML, Manning HC. 2-Substituted Ngamma-glutamylanilides as novel probes of ASCT2 with improved potency. Bioorg Med Chem Lett. 2015;25:113–116. doi: 10.1016/j.bmcl.2014.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulte ML, Khodadadi AB, Cuthbertson ML, Smith JA, Manning HC. 2-Amino-4-bis(aryloxybenzyl)aminobutanoic acids: A novel scaffold for inhibition of ASCT2-mediated glutamine transport. Bioorg Med Chem Lett. 2016;26:1044–1047. doi: 10.1016/j.bmcl.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colas C, Grewer C, Otte NJ, Gameiro A, Albers T, Singh K, Shere H, Bonomi M, Holst J, Schlessinger A. Ligand Discovery for the Alanine-Serine-Cysteine Transporter (ASCT2, SLC1A5) from Homology Modeling and Virtual Screening. PLoS Comput Biol. 2015;11:e1004477. doi: 10.1371/journal.pcbi.1004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grewer C, Grabsch E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J Physiol. 2004;557:747–759. doi: 10.1113/jphysiol.2004.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taft RW. Linear Free Energy Relationships from Rates of Esterification and Hydrolysis of Aliphatic and Ortho-substituted Benzoate Esters. J Am Chem Soc. 1952;74:2729–2732. [Google Scholar]
- 18.Hammett LP. The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. J Am Chem Soc. 1937;59:96–103. [Google Scholar]
- 19.Sherman W, Beard HS, Farid R. Use of an induced fit receptor structure in virtual screening. Chem Biol Drug Des. 2006;67:83–84. doi: 10.1111/j.1747-0285.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 20.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 21.Colas C, Ung PMU, Schlessinger A. SLC transporters: structure, function, and drug discovery. Med Chem Commun. 2016:1069–1081. doi: 10.1039/C6MD00005C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrauskas A, Kolovanov E. ACD/Log P Method Description. Persp in Drug Design. 2000;19:1–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.