Abstract
The B-1 B cell population is an important bridge between innate and adaptive immunity primarily because B-1 cells produce natural antibody. Murine B-1 and B-2 cells arise from distinct progenitors. In humans, however, partly because it has been difficult to discriminate between them phenotypically, efforts to pinpoint the developmental origins of human B-1 and B-2 cells have lagged. To characterize progenitors of human B-1 and B-2 cells, we separated cord blood and bone marrow Lin−CD34+ hematopoietic stem cells (HSC) into Lin−CD34+CD38lo and Lin−CD34+CD38hi populations. We found that transplanted Lin−CD34+CD38lo cells but not Lin−CD34+CD38hi cells generated a CD19+ B cell population after transfer into immuno-deficient NOD.Cg-Prkdcscid Il2rgtm1wjl/SxJ neonates. The emergent CD19+ B cell population was found in spleen, bone marrow, and peritoneal cavity of humanized mice, and included distinct populations displaying the B-1 or the B-2 phenotype. Engrafted splenic B-1 cells exhibited a mature phenotype as evidenced by low-to-intermediate CD24 and CD38 expression levels. The engrafted B-1 cell population expressed a VH-DH-JH composition similar to cord blood B-1 cells, including frequent use of VH4-34 (8% versus 10%, respectively). Among patients with hematologic malignancies undergoing HSC transplantation, B-1 cells were found in the circulation as early as 8 weeks post-transplantation. Altogether, our data demonstrate that human B-1 and B-2 cells develop from a Lin−CD34+CD38lo stem cell population, and engrafted B-1 cells in humanized mice exhibit an immunoglobulin usage pattern comparable to B-1 cells in cord blood.
Keywords: Human B cells, B-1 cells, B-2 cells, stem cells, xenotransplantation, hematopoietic stem cell transplantation, NSG, HIS
INTRODUCTION
B-1 cells function as an important bridge between the innate and adaptive immune responses. They provide a rapid response against pathogens during the lag period required for adaptive antibody production by B-2 cells, and they prevent auto-immunity through their capacity to rapidly clear noxious molecules and cellular debris (1–4). Murine B-1 and B-2 cells are derived from two distinct lineages (5, 6). In humans, however, partly because it has been difficult to discriminate between them phenotypically (7–10), efforts to pinpoint the developmental origins of human B-1 and B-2 cells have lagged.
The lineage negative (Lin−), CD34 positive (CD34+) phenotype is a hallmark marker of hematopoietic stem cells (HSCs) in humans (11, 12), and CD34+ enriched cell populations are widely used in human HSC transplantation (13, 14). Whereas, changes in the CD38 expression level indicate reduction in multi-lineage potential of a HSC population (15). In the early 90s, Terstappen et al. used three channel flow cytometric analysis and in vitro blast colony formation culture systems to show that Lin−CD34+ HSCs lost pluripotency as they acquired CD38 expression, suggesting that the increase in CD38 expression indicates differentiation of CD34+ HSCs into a more lineage-committed status (16). In xenogeneic transplant studies, Bhatia et al. and Ishikawa et al. independently showed that only Lin−CD34+CD38lo/− cells gave rise to multi-lineage blood cells, including B cells; whereas, Lin−CD34+CD38+ cells were unable to generate any blood cells after being transplanted into NOD/SCID and NOD/SCID/β2-microglobulin-null (NOD/SCID/BMGnull) mice (17, 18). These data indicate that the Lin−CD34+CD38lo/− population includes B cell progenitors. It is not known if this population contains a single progenitor for all B cell subsets, or contains distinct progenitors for each.
Much progress has been made using different immune-deficient mouse models to study human hematopoiesis. NOD/SCID and NOD/SCID/β2-microglobulin-null mice are the most widely used; however, these immune-deficient models have limitations. The NOD/SCID mouse environment favors human B cell but not T cell engraftment (19). In this respect, the NOD/SCID/β2-microglobulin-null mice, which support the development of a greater variety of blood cells including T cells and B cells, have an advantage over the NOD/SCID model (20). Both NOD/SCID and NOD/SCID/β2-microglobulin-null mice exhibit a shortened lifespan (6–8.5 months) due to thymic lymphomagenesis (20–22). Limited lifespan is not an issue with NOD.Cg-Prkdcscid Il2rgtm1wjl/SxJ (NSG) mice, which have a disease–free lifespan of greater than 16 months (23). NSG mice have been shown to be excellent recipients for engrafting human HSCs. They support the reconstitution of greater numbers of cells and a wider variety of blood cell lineages (24) than the other models (25, 26).
Despite controversy (27–35), recently human B-1 cells are defined as (CD20+CD27+CD43+CD38lo/int) with clinically relevant potential (36, 37). This population exhibits repertoire skewing toward expression of the immunoglobulin (Ig) VH4-34 gene (37), which encodes autoreactive antibody (38, 39), and produces natural antibodies (36), characteristics of mouse B-1 cells. In this study, we report that human Lin−CD34+CD38lo cells from cord blood, and bone marrow, give rise to both B-1 and B-2 cells; whereas, Lin−CD34+CD38hi cells do not give rise to B cells. In patients with hematologic malignancies undergoing autologous and allogeneic transplantation of mobilized HSCs (CD34+ enriched mononuclear cells) both B-1 and B-2 cells were reconstituted. Thus, our data demonstrate that in humans both B-1 and B-2 B cell populations can be generated from Lin−CD34+CD38lo stem cells derived from cord blood or bone marrow.
MATERIALS AND METHODS
Human samples
Umbilical cord blood samples (n=44) were obtained from healthy neonate cords immediately following uncomplicated delivery. Bone marrow tissues (n=12) were obtained from otherwise healthy adults undergoing hip surgery, and peripheral blood samples were obtained from patients undergoing hematopoietic stem cell transplantation (HSCT) for treatment of hematologic malignancies. All human materials were obtained in accordance with protocols approved by the Northwell Health Institutional Review Board.
Mice
NOD.Cg-Prkdcscid Il2rgtm1wjl/SxJ (NSG) mice were obtained from the Jackson Laboratory, and were bred and maintained in ventilated cages with irradiated chow and sterile acid water (pH 3.2). All mice were cared for and handled in accordance with Institutional Animal Care and Use Committee guidelines at the Feinstein Institute for Medical Research.
Cell isolation
Cells from human tissues
Mononuclear cells (MC) were obtained from cord blood and bone marrow by density gradient separation using lymphocyte separation medium (Cellgro). Mononuclear cells were washed (2 mM EDTA in PBS) and re-suspended in cell isolation/sort buffer (0.5% BSA in PBS). Mononuclear cells were then subjected to lineage cell depletion using a Lineage Cell Depletion Kit (Miltenyi), and lineage negative (Lin−) cells were stored short-term at −80°C in Liquid Nitrogen in freezing medium (10% DMSO in FBS) until use.
Cells from xenotransplanted NSG mouse tissues
Bone marrow, spleen, peritoneal cells and serum were collected from xenotransplanted NSG mice 10 to 17 weeks post transplantation. Isolated cells were stained with fluorophore-conjugated antibodies as described below. Stained cells were sort purified and/or analyzed using a BD Influx cell sorter or a Beckman Coulter Gallios flow cytometer.
Cell sorting and flow cytometry
Before transplantation, thawed Lin− cells were treated with normal mouse serum, and stained with a lineage-specific antibody cocktail (containing antibodies distinct from those used in the lineage depletion kit), hematopoietic stem cell markers (CD34 and CD38), and Aqua LiveDead dye in cell sorting buffer. After washing, cells were re-suspended in cell sorting buffer with 10 U/ml DNase. Stained cells were then subjected to sort purification using a BD Influx cell sorter.
For analysis, single-cell suspensions (1–2×106 cells/sample) of spleen cells from xenotransplanted NSG mice and PBMC from patients undergoing HSCT were stained with predetermined optimal concentrations of fluorophore-conjugated mAbs. The following mouse anti-human antibodies were used: anti-CD19-AF700 or PE-Cy7, anti-CD20-Pacific Blue or APC-Cy7, anti-CD3-ECD, anti-CD4-ECD, anti-CD7-ECD, anti-CD27-APC, anti-CD38-PerCP-Cy5.5 or PE-Cy7, anti-CD43-APC-AF750 or FITC, CD22-APC-AF700, CD5-PE-Cy7, CD24-APC-AF750, Aqua LiveDead dye, and MitroTracker Green (MTG) dye (Life Techonologies). Events (3×105 – 1.5×106) were collected on a Gallios (Beckman Coulter) flow cytometer and/or a BD Influx cell sorter. Data were analyzed using Flowjo software v. 9.7.6 (Tree Star, San Carlos, CA). Antibodies were purchased from Beckman Coulter, BD Biosciences or Biolegend. Cells were stained and run in FACS buffer (2.5% FBS, 1 mM EDTA, 0.02% NaN3). B cell subsets were gated as described in Quach et al. 2016 (37), unless otherwise is noted.
Xenotransplantation
The transplantation protocol was adapted from Vuyyuru et al (40). Sort purified Lin−CD34+CD38lo or Lin−CD34+CD38hi stem cells were transplanted into NSG neonates (24–48 hours after birth) by intrahepatic injection of 0.7–1×105 cells in 25 µl PBS using a 30-gauge needle; 57 neonates received Lin−CD34+CD38lo cord blood cells, 9 received Lin−CD34+CD38hi cord blood cells, 20 received Lin−CD34+CD38lo bone marrow cells, and 5 received Lin−CD34+CD38hi bone marrow cells. Mice were weaned at 3 weeks of age and randomly distributed among different experimental groups. Hereafter, NSG mice transplanted with human stem cells are designated human immune system (HIS) mice, and cells obtained from HIS mice are so designated, e.g., HIS B-1 cells.
Sequence analysis
Single cell sorting, cDNA synthesis, PCR, and VH usage analysis were performed as described (37). Briefly, following initial sorting, single cells of each subset were re-sorted at one cell per well into 96-well PCR plates containing 20 µl lysis buffer, immediately frozen at −20°C, and stored at −80°C. cDNA was synthesized, and cDNA products were subjected to 2 rounds of PCR to amplify immunoglobulin heavy chains. All PCR products were checked for concentration and efficiency using QIAxcel Advanced (QIAGEN), sequenced (Genewiz), quality checked (4peaks software) and analyzed (IMGT database). Identical sequences from different wells were counted as a single clone.
Statistical analysis
All Statistical analysis was done using Graphpad Prism and R (http://www.r-project.org/)
Online Supplementary Materials
Supplementary figures provide results of: 1) Frequency of cells coexpressing CD19 and CD43 increase as CD34+ cells lose CD34 and gain CD38 expression, 2) Surface antigen expression of B cell subsets from HIS mice as compared to those from cord blood and adult peripheral blood, 3) HIS B-1 and CD20+CD38hi preplasmablast phenotype cells spontaneously secrete antibodies, and memory B cells do not, 4) The pattern of immunoglobulin variable region usage by splenic HIS B-2 cell subsets.
RESULTS
Xenotransplanted cord blood Lin−CD34+CD38lo hematopoietic stem cells give rise to human CD19+ B cells
Engraftment of xenotransplanted human Lin−CD34+ cells into immunodeficient mouse systems has been shown to generate a functional human immune system (23, 25). In vitro and in vivo studies that fractionated Lin−CD34+ cells into Lin−CD34+CD38−/low and Lin−CD34+CD38+/high populations showed that the Lin−CD34+CD38−/low population includes hematopoietic stem cells with multi-lineage potential (18, 25, 41–44). On the other hand, although in vivo xenotransplant study models showed that Lin−CD34+CD38+/high cells do not generate any blood cells in vivo (45), a study from Galy et al. showed that Lin−CD34+CD38+/high cells are able to generate B cells during in vitro culture in the presence of cytokines (IL-3, IL-6, and Leukemia inhibitory factor) (46). To determine whether NSG mice support hematopoiesis by Lin−CD34+CD38−/low and Lin−CD34+CD38+/high cells (hereafter, termed Lin−CD34+CD38lo and Lin−CD34+CD38hi cells, respectively) Lin−CD34+ HSCs were sort purified and intrahepatically injected into 1–2 day old NSG neonates (Fig. 1A–B). We found that transplanted Lin−CD34+CD38lo cells (52 of 57 transplanted mice, 91%) led to human B cell (CD19+, mean ± SD, 14.5% ± 13.6 of total live spleen cells) development, whereas Lin−CD34+CD38hi cells did not lead to CD19+ B cell development (0 of 9 transplanted mice) (Fig. 1C). Similar to previous reports (16, 18, 47), these data suggest that Lin−CD34+CD38hi cells are lineage-committed progenitor cells and have limited engraftment capability in our xenotransplantation model.
Figure 1. Xenotransplanted cord blood Lin−CD34+CD38lo hematopoietic stem cells give rise to human CD19+ B cells.
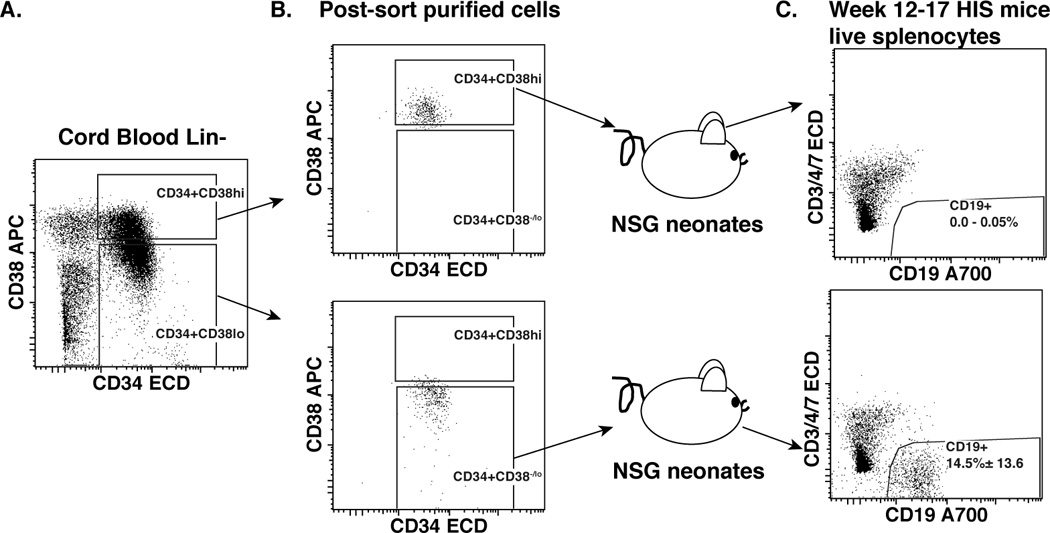
Lineage negative (Lin−) cells were isolated from cord blood mononuclear cells using a Miltenyi Lineage depletion kit, and were stained with a lineage antibody cocktail from eBioscience and hematopoietic stem cell markers (CD34 and CD38) for flow cytometry. A. Dot plot represents CD34 versus CD38 expression of Lin− gated cells. B. Dot plots show purity of sort purified Lin−CD34+CD38hi (top panel), and Lin−CD34+CD38lo (lower panel) cells prior to transfer into NSG neonates to generate HIS mice. C. Dot plots show reconstituted human B (CD19+) cells from spleens of HIS mice 12–17 weeks after transplant with sort purified human cord blood Lin−CD34+CD38hi (top panel, number displays the range of events in the CD19+ gate), or Lin−CD34+CD38lo (lower panel, number displays the frequency means of events ± SD in the CD19+ gate) cells.
Of interest, in mice, CD43 is expressed at early lineage commitment stages, and then the expression is lost as pre-B cells develop (48). In humans, it has been shown that CD43 is expressed on some transitional B cells (9), and is highly expressed on terminally differentiated plasmablast/plasma cells. Recently, we have shown that CD43 is a hallmark marker for human B-1 cells in addition to CD27, CD20, and CD38 (37). Interestingly, although CD43 is expressed on both Lin−CD34+CD38lo and Lin−CD34+CD38hi cells, it has been shown that only Lin−CD34+CD38loCD43+ cells have multi-lineage potential and self-renewal capacity (43). To investigate the expression of CD43 in the context of the B cell lineage, we examined the expression of CD19 and CD43 on CD34+ cells, and found that the frequency of cells co-expressing CD19 and CD43 increased as CD34+ cells lost CD34 and gained CD38 expression in cord blood, unlike cells in bone marrow. More importantly, CD43 expression was reduced as these cells matured into CD34−CD38loCD19+ B cells in both cord blood and bone marrow (Supplementary Figure S1). These data suggest that CD43 expression is characteristic of human B cell development, although our data do not speak to the functional significance of CD43 expression in the development of human B-1 and B-2 cells.
Human B-1 cells arising in the NSG model display a mature phenotype, according to CD38 versus CD24 expression pattern, and are widely distributed in tissues of HIS mice
We evaluated B cell subsets within the engrafted CD19+ B cell compartment in mice that were transplanted with human cord blood Lin−CD34+CD38lo cells (hereafter, data presented are from transplanted cord blood Lin−CD34+CD38lo cells unless otherwise indicated). Splenocytes from 12–17 week old HIS mice were examined for surface marker expression. We found that similar to the B cell compartment in human peripheral blood samples (37), the engrafted CD19+ B cell compartment consisted of B-1 (CD20+CD27+CD43+CD38lo/int) and B-2 cells, including memory (CD20+CD27+CD43−) and pre-plasmablast (CD20+CD38hi) cells (Fig. 2A).
Figure 2. Human B-1 cells arising in the NSG model display a mature phenotype, according to CD38 versus CD24 expression pattern, and are widely distributed in tissues of HIS mice.
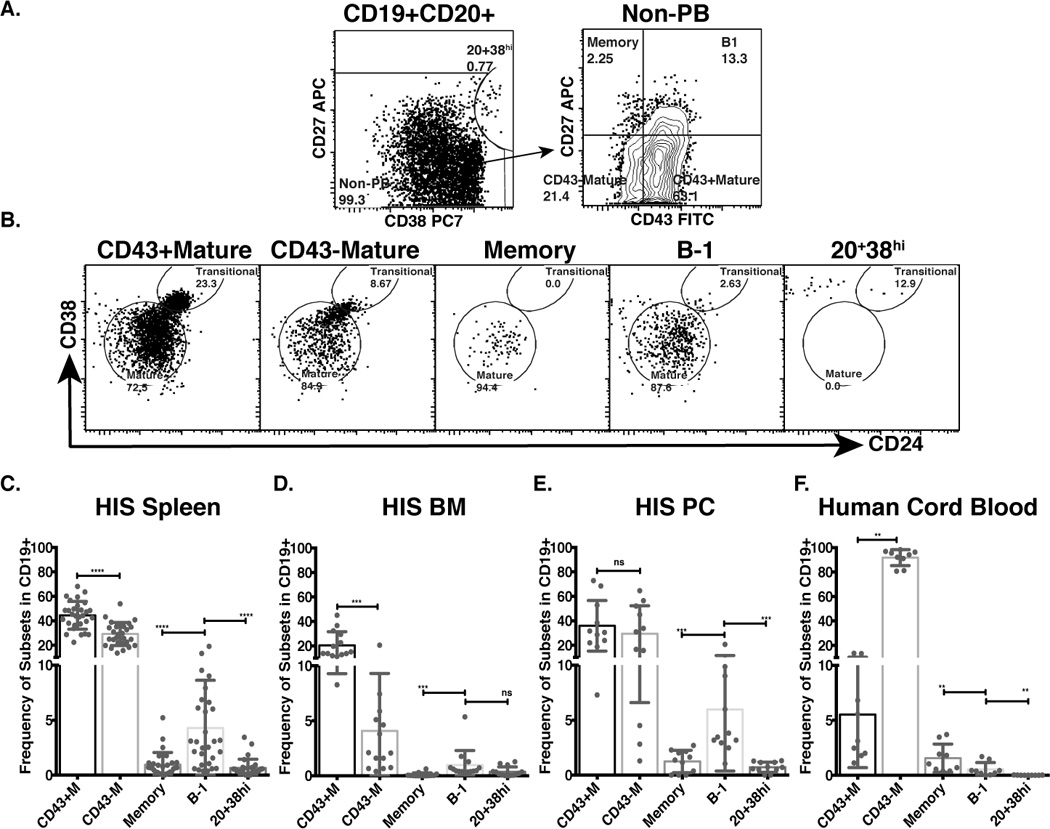
Splenic, bone marrow, and peritoneal cavity cells from HIS mice 10–17 weeks after transplant with cord blood Lin−CD34+CD38lo cells were isolated and stained for human B cell surface makers. A. Dot plots show expression of CD38 and CD27 by live CD3/4/7−CD19+CD20+ gated splenic B cells from HIS mice (left panel) separating pre-plasmablasts (CD20+CD38hi cells) from other B cells (non-PB), and CD43 and CD27 expression (right panel) by non-PB separating B-1 cells from other B-2 cells (Memory, CD43− mature, and CD43+ mature B cells). B. Dot plots show expression of CD24 and CD38 on selected B cell subsets. C–E. Bar graphs show frequency of selected subsets in HIS spleen (C), bone marrow (HIS BM) (D) and peritoneal cavity (HIS PC) (E). F. Bar graph shows the frequencies of selected B cell subsets in human cord blood mononuclear cells. (nsp>0.05, *p≤0.05, **p≤0.01 ***p≤0.001, ****p≤0.0001, Wilcoxon matched-pairs signed rank test).
To determine the maturation status of B-1 and B-2 cell subsets, human B cell developmental markers (49), including CD24 and CD38, were evaluated. We found that engrafted B-1 and memory B cells exhibited mature phenotypes as evidenced by low-to-intermediate CD24 and CD38 expression levels, similar to CD43− mature cells; whereas, CD43+ mature cells exhibited a partial transitional phenotype on the basis of high CD24 and CD38 expression levels (Fig. 2B). Pre-plasmablasts exhibited a differentiated phenotype, having lost CD24 expression and expressing very high CD38 levels (Fig. 2B). Supplementary figure S2A shows representative CD24 versus CD38 expression levels of examined B cell subsets from cord blood and adult peripheral blood. To further explore the similarities and differences between human B-1 cells developing in murine system versus human system, we investigated CD5 expression, which is a hallmark marker of murine B cells, yet is expressed on transitional and activated human B cells. In Supplementary figure 2B–D, we found that more than 50% of B cells from HIS mice express CD5, similar to that of B cells in cord blood, but very different from peripheral blood. It is possible that the high frequency of CD5 expression found in B cells from HIS mice and native human cord blood could indicate that 50% of B cells from these two sources are in either an activated or a less mature state than that of adult peripheral blood, and it also raises the question about whether using CD5 alone is enough to measure maturity/activation status of human B cells. Additionally, another yet to be explained phenomenon was observed. In the human system, CD22, an inhibitory co-receptor of the BCR (50), is expressed on most B cells (Supplementary figure 2F), except plasmablasts and preblastmablasts (37); however, as shown in Supplementary figure 2E, less than 50% of cells from each B cell subset in HIS mice express CD22. The data suggests other extrinsic developmental factor(s) is the local environment could be important in regulating the expression of surface CD22 on human B cells.
In mice, B-1 cells of fetal origin reside in the spleen, peritoneal cavity, and bone marrow (6, 51). We determined the distribution of cord blood-derived, engrafted B cells in spleen, bone marrow, and peritoneal cavity of HIS mice. We found that, like murine B-1 cells, engrafted human B-1 cells were present in the spleen, bone marrow and peritoneal cavity (Fig. 2C–E), and were present at greater frequencies in spleen and peritoneal cavity than are found in cord blood (Fig. 2F). Altogether, our data show that engrafted human B cells from Lin-CD34+CD38lo cells found in different HIS mouse tissues can be characterized phenotypically as either B-1 or B-2 cells, and although the majority of CD43+ B-2 cells displayed transitional phenotypes, most engrafted B-1 and other B-2 cells displayed a more mature B cell phenotype by expressing low CD38 and CD24.
To examine the ability of HIS B-1 cells to spontaneously secrete antibody as seen with B-1 cells from adult peripheral blood (37), we sort purified B-1, memory and CD20+CD38hi preplasmablast cells from HIS mice and tested for IgM antibody secretion using ELISPOT. As shown in supplementary figure S3A and B, similar to previously published data (37), on average 0.3% HIS B-1 cells secreted antibody, compared to 0% HIS memory cells, and 7.9% CD20+CD38hi preplasmablast cells. These data suggest that HIS B-1 and CD20+CD38hi phenotype preplasmablast cells behave like adult peripheral blood B-1 and CD20+CD38hi preplasmablast cells.
Splenic HIS B-1 cells express immunoglobulin with similar diversity to native B-1 cells from cord blood
High frequency VH4-34 usage represents a hallmark feature distinguishing human B-1 cells from memory B cells and pre-plasmablasts (37). To determine whether HIS B-1 cells display VH usage similar to that of cord blood B-1 cells, engrafted B-1 cells from HIS mouse spleens and B-1 cells directly isolated from cord blood were single cell sort purified, and their IgM heavy chains were amplified and sequenced (Fig. 3). We found that HIS B-1 cells and cord blood B-1 cells expressed a largely similar distribution of Ig VH, DH, and JH family use (Fig. 3A). Detailed analysis of VH gene usage indicated a similar diversity between HIS B-1 cells and cord blood B-1 cells (Fig. 3B–C). The analysis also demonstrated that HIS B-1 cells and cord blood B-1 cells exhibited comparable high frequency VH4-34 use (Fig. 3B–C). On average, 8% of analyzed sequences from HIS B-1 cells utilized VH4-34 similar to that found in cord blood (Fig. 3D) and adult peripheral blood B-1 cells (37), and quite different from HIS pre-plasmablasts (Fig. 3D) and adult peripheral blood pre-plasmablasts, memory B cells, and plasmablasts (37). In keeping with the checkpoint paradigm (52), HIS transitional (Trans, CD19+CD20+CD27−MTG+) B cells express relatively frequent utilization of VH4-34 and naïve (CD19+CD20+CD27−CD43−MTG−) B cells less so (37, 53, 54), consistent with the autoreactive nature of VH4-34. Detailed VH subfamily usage of HIS transitional, naïve and pre-plasmablast B cells is shown in supplementary figure S4.
Figure 3. Splenic HIS B-1 cells express immunoglobulin with similar diversity to native B-1 cells from cord blood.
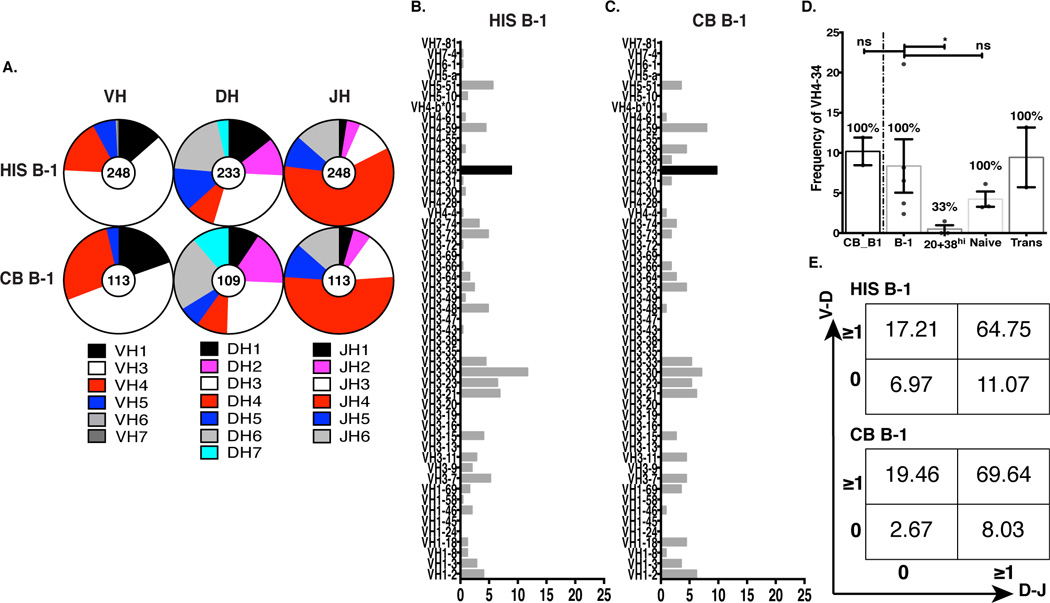
Sort purified B-1 cells were dispensed by single cell sorting into 96 well plates, and expressed VH regions were individually amplified for sequence analysis. A. Graphic representation of the distribution of expressed VH, DH, and JH families among HIS B-1 cells (sequences, n=248; mice, n=5) and CB B-1 cells (sequences, n=113; donors, n=2) using IMGT®. B–C. Percentages of individual VH genes expressed by HIS B-1 cells and CB B-1 cells. D. VH4-34 usage among cord blood B-1 cells and HIS B cells, including HIS pre-plasmablast (CD20+CD28hi), HIS naïve (CD19+CD20+CD27−CD43−MTG−) and HIS Transitional (Trans, CD19+CD20+CD27−MTG+) cells. Numbers on top of each bar show the frequency of samples expressing VH4-34 (nsp>0.05, *p≤0.05, Unpaired Mann-Whitney t test). E. Numbers in each quadrant indicate the percentages of genes with N-additions at V-D (y-axis) and D-J (x-axis) junctions among VH4-34 genes expressed by HIS B-1 cells (top) and CB B-1 cells (bottom).
N-region addition is an important mechanism of generating Ig diversity, which along with combinatorial variation and somatic mutation results in numerous Ig specificities (55–57). In mice, B-1, in particular B-1a, cell-derived IgM antibodies are characterized by low numbers of N-additions (58), which are added by the enzyme terminal deoxynucleotidyl transferase (TdT) (59). TdT expression is restricted to adult life in mice (60), after the development of fetal derived B-1a cells (58, 61). Therefore, in mice, fetal derived B-1a cells for the most part lack N-additions (61); whereas, adult bone marrow derived B-1a cells display a high level of N-additions. (62–65). In contrast, TdT is expressed during both fetal and adult life in humans (66), and as a result, both fetal and adult derived B cells, including B-1 cells, express Ig with numerous N-additions (37, 67). It has been shown that the repertoires of human and mouse fetal immunoglobulins exhibit both similarities and differences (68). For example, it has been demonstrated in humans that B cells from pre-term and term infants exhibit fewer N-nucleotide additions and shorter CDR-H3 length than adults (69). To determine whether N-additions at VH-DH and DH-JH junctions in human B-1 cells are affected by development in the murine environment, we compared N-additions at VH-DH and DH-JH junctions of HIS B-1 cells and cord blood B-1 cells. We found that similar to cord blood B-1 cell sequences, HIS B-1 cell Ig sequences contained N-additions at both junctions (Fig. 3E). We found N-additions at one or more junctions in 93.0% of HIS B-1 cells and in 97.3% of cord blood B-1 cells. Overall, these data show comparable VH-DH-JH composition between HIS and cord blood B-1 cells.
Xenotransplanted adult bone marrow Lin−CD34+CD38lo hematopoietic stem cells give rise to human CD19+ B cells, including B-1 cells
After birth, bone marrow is the major habitat for HSCs, Lin−CD34+CD38lo cells, and Lin−CD34+CD38hi cells. We evaluated whether transplantation of bone marrow Lin−CD34+CD38lo or Lin−CD34+CD38hi cells into NSG neonates resulted in human B cell engraftment, and more specifically, B-1 cell engraftment. Adult bone marrow Lin−CD34+CD38lo and Lin−CD34+CD38hi cells were sort purified and intrahepatically transplanted into 1–2 day old NSG neonates (Fig. 4A–B). We found that transplantation of Lin−CD34+CD38lo cells (7 of 24 injected mice) led to human B cell (CD19+) engraftment, whereas, transplantation of Lin−CD34+CD38hi cells did not (0 of 5 injected mice) (Fig. 4C). Similar to the transplanted cord blood Lin−CD34+CD38lo cell findings, the bone marrow-derived B cell compartment contained cells of both B-1 and B-2 phenotypes. B-1 cells were found in both bone marrow and spleen of HIS mice (Fig. 4 D–E), and were present at a higher frequency (1.6%, and 1.8% of total B cells respectively) than in native adult bone marrow (0.7%) (Fig. 4F). Together these data demonstrate that Lin−CD34+CD38lo stem cells from human adult bone marrow are also capable of reconstituting both B-1 and B-2 cell subsets.
Figure 4. Xenotransplanted adult bone marrow Lin-CD34+CD38lo hematopoietic stem cells give rise to human CD19+ B cells, including B-1 cells.
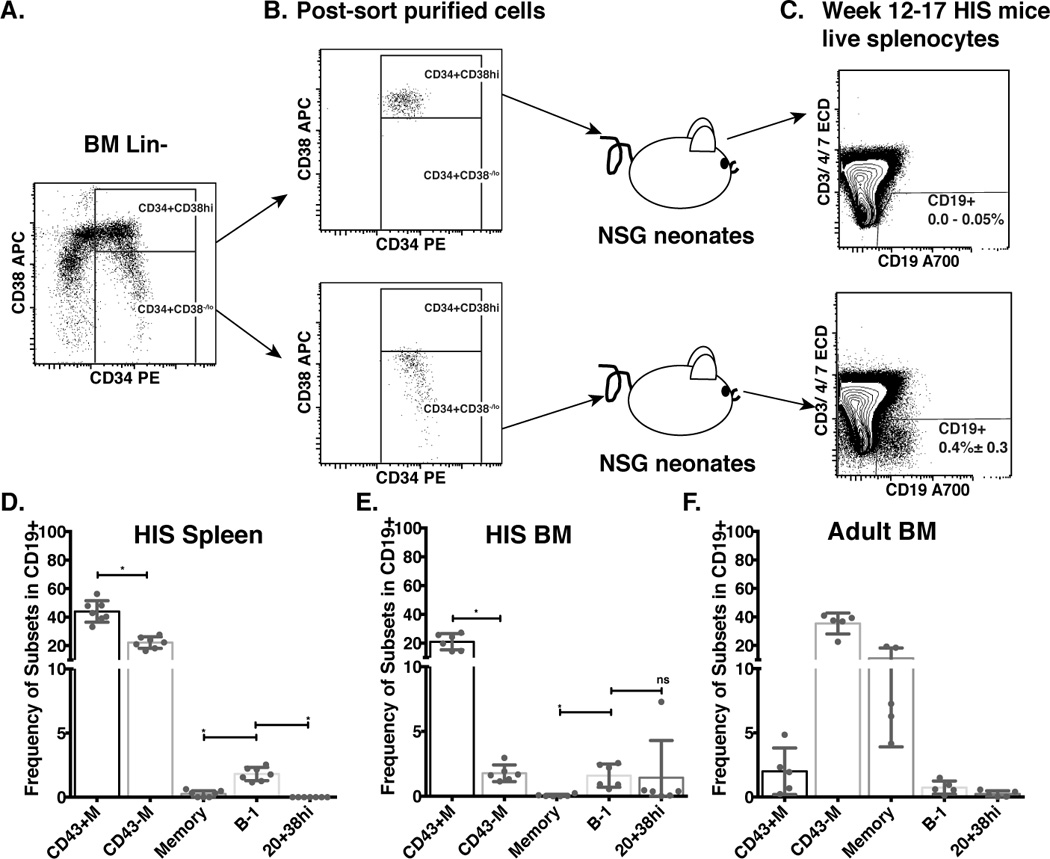
Lineage negative (Lin−) cells were isolated from bone marrow mononuclear cells and stained as described in Figure 1. A. Dot plot representation of CD34 versus CD38 expression of Lin− gated cells. B. Dot plots show purity of sort purified Lin−CD34+CD38hi (top panel) and Lin−CD34+CD38lo (lower panel) cells prior to transfer into NSG neonates to generate HIS mice. C. Dot plots show reconstituted human B (CD19+, CD3/4/7−) cells from spleens of HIS mice 12–17 weeks after transplant with sort purified human bone marrow CD34+CD38hi (top panel, number displays the range of events in the CD19+ gate), or Lin−CD34+CD38lo (lower panel, number displays the frequency means of events ± SD in the CD19+ gate) cells). D–E. Spleen (D) and Bone Marrow (BM, E) mononuclear cells from 12–17 week HIS mice were isolated and stained for B cell subset markers as in Figure 2A. Bar graphs show the frequencies of selected B cell subsets in individual mice (n=6) (nsp>0.05, *p≤0.05, Wilcoxon matched-pairs signed rank test). F. Bar graph shows the frequencies of selected B cell subsets in human adult bone marrow mononuclear cells (8 bone marrow samples were analyzed).
Of interest, we also observed that in HIS mice, overall, the efficiency of B cell engraftment after transplantation with stem cells from bone marrow (29%) was significantly lower than the efficiency of B cell engraftment after transplantation with stem cells from cord blood (91%,), and of total live HIS splenocytes, the frequency of engrafted B cells was much lower in mice transplanted with bone marrow versus cord blood, (0.4 ± 0.3% versus 14.5 ± 13.6%, respectively (Fig. 1C and 4C). However, this result is consistent with a previous report that cord blood is a better stem cell source than bone marrow for human CD45+ cell engraftment in a xenotransplantation model (NOD/SCID) (70).
Reconstitution of B-1 and B-2 cell compartments in patients undergoing HSC transplantation for treatment of hematologic malignancy
Recently, it has been shown that following clinical transplantation of allogeneic CD34+ enriched stem cells, recipients reconstituted B cells, some of which may be B-1 cells (71). Although this report lacked functional analysis and did not fully discriminate between B-1 and B-2 cells (the reported CD19+CD27+CD43+ population includes B-1 cells, pre-plasmablasts, and plasmablasts), the data suggested reconstitution of both human B-1 and B-2 cells had occurred in HSC recipients. We collected peripheral blood samples from HSC recipients before and after transplantation (see Materials and Methods), and found that, irrespective of the type of transplantation, as early as 8 weeks post-transplantation B-1-phenotype cells (CD20+CD27+CD43+CD38lo/int) developed in the recipients as soon or shortly after the appearance of CD19+ B cells (~1% of total CD19+ B cells were B-1 cells, Fig. 5A–B). Our data presented here suggest that, similar to xenogeneic transplantation, mobilized HSC used to transplant patients with hematologic malignancies also reconstitute both B-1 and B-2 cell populations. The significance, if any, of the observed changes in the frequencies of B-1 cells during follow-up remains to be determined.
Figure 5. Reconstitution of B-1 and B-2 cell compartments in patients undergoing HSC transplantation for treatment of hematologic malignancy.
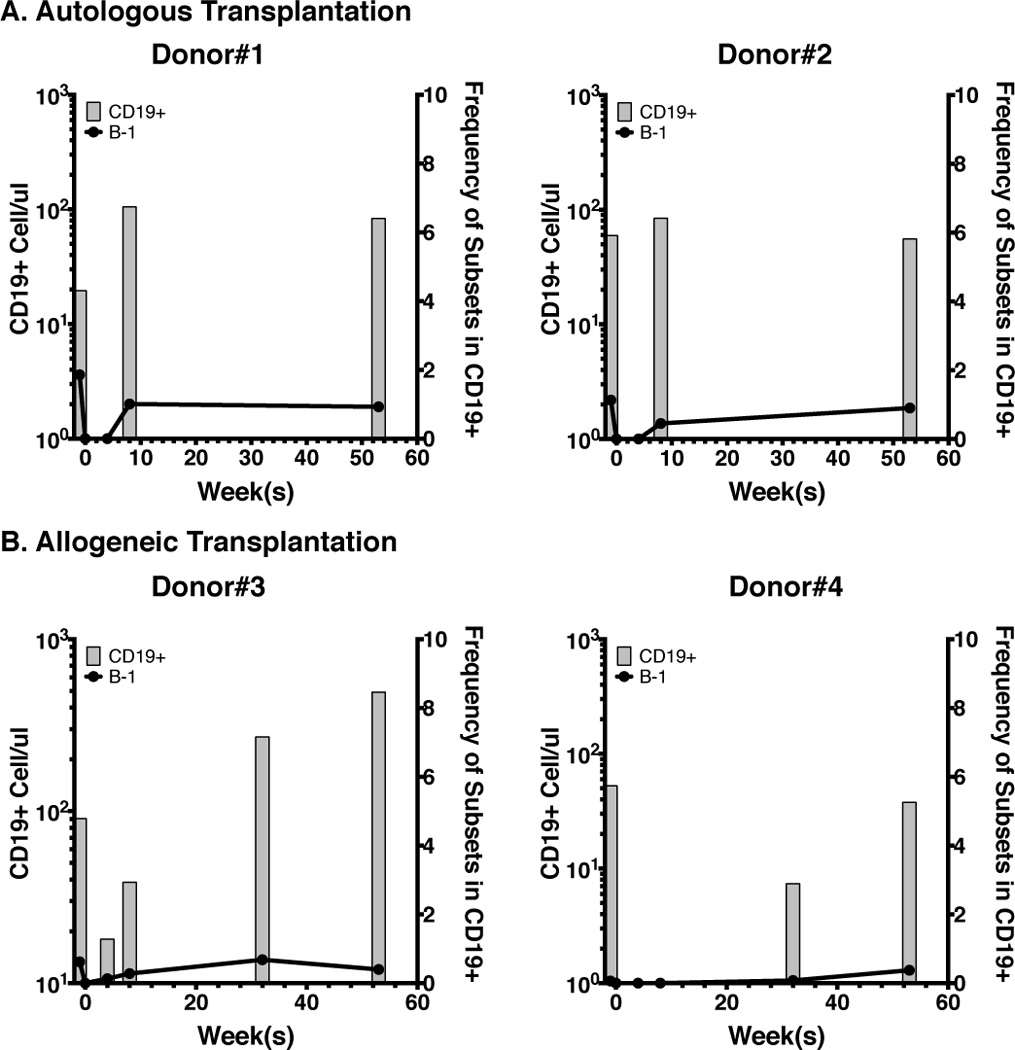
A–B. Numbers of CD19+ B cells (grey bars) from 4 HSC recipients before transplant (time 0) and 4, 8, 32, and 52 weeks after transplant. (in A, patients received autologous HSCs, and in B, patients received allogeneic HSCs). Black lines indicate the frequencies of B-1 cells in total CD19+ B cells at different time points.
DISCUSSION
The results presented here demonstrate that cord blood and adult bone marrow HSCs with a Lin−CD34+CD38lo phenotype have the ability to generate both B-1 and B-2 cells. The experiments were conducted using a xenogeneic transplantation system in which human HSC populations were transplanted into neonates of immune-deficient NSG mice. Engrafted B-1 cells expressed rearranged immunoglobulin genes with features recapitulating those of B-1 cells from human cord blood. Our results demonstrate that a population of human Lin−CD34+CD38lo stem cells reconstituted B-1 and B-2 cells, suggesting human B-1 and B-2 cells derive from the same progenitor cell population; however, it remains possible that adoptively transferred Lin−CD34+CD38lo stem cells consisted of a mixture of progenitors separately committed to B-1 and B-2 cell differentiation at this early stage. To rule out this latter possibility, it will be necessary either to identify markers that subdivide this population or to assess the differentiation potential of individual cells.
In mice, there are two major subsets of B-1 cells, which are distinguished on the basis of CD5 expression (B-1a cells express CD5, and B-1b cells lack CD5 expression) (72) and function (B-1a cells produce natural IgM antibodies and provide innate immune responses, and B-1b cells produce isotype switched antibodies and provide adaptive immune responses) (3, 73). Insofar as they have been characterized, human B-1 cells display characteristics of both murine B-1a and B-1b cells, including having both natural and adaptive antibody features (30, 36, 37, 40); therefore, we anticipate that the development of human B-1 cells could have characteristics mimicking both murine B-1a and B-1b development. In mice, it has been shown that in an adoptive transfer experiment with single HSC, adult BM failed to give rise to B-1a cells, whereas HSC deficient yolk sac gave rise to B-1a but not conventional B-2 cells (74–76). These data suggest that murine B-1a cells are HSC independent. However, other reports also show that adult BM cells give rise to B-1a cells, B-1b cells and B-2 cells (63–65, 77). Consistent with the later, our study here focuses on the development of human B-1 cells from human cord blood and adult bone marrow stem cells; however, it remains to be determined whether distinct progenitors of human B-1 and B-2 cells occur during further development as found in mice (5).
In a Borrellia hermsii infection study, Vuyyuru et al. showed that transplantation of the HSC CD34+ (positive selection by magnetic beads) population into NSG mice led to engraftment of human B cell subsets, including CD20+CD27+CD43+ B cells, proposed B-1 cells by Griffin et al. (27). These HIS mice were able to clear Borrellia hermsii infection whereas the non-transplanted control NSG mice failed to do so. Since clearing Borrelliosis infection is a T-cell independent process (78) and correlates with B-1b cell expansion (79), Vuyyuru et al. suggest that CD20+CD27+CD43+ cells (27, 37), are the key source of clearing the infection (40). These data suggested that proposed B-1 cell, CD20+CD27+CD43+, progenitors present in human the CD34+ stem cell compartment. Consistently, in our study when we separate the HSC Lin−CD34+ cell compartment into 2 HSC populations on the basis of CD38 expression, we find that only Lin−CD34+CD38lo cells give rise to B cells and Lin−CD34+CD38hi cells do not, suggesting that Lin−CD34+CD38lo cells are the source of B cells. In particular, they are also the source of CD20+CD27+CD43+ B cells reported in Vuyyuru et. al (40). Of note, on average, the total engrafted B cells in the spleen, bone marrow and peritoneal cavity, of our HIS mice transplanted with Lin−CD34+CD38lo cells comprised relatively low combined frequencies of B-1 cells and pre-plasmablast (CD20+CD38hi) cells (Figure 2C–E) whereas NSG mice transplanted with total CD34+ cells by Vuyyuru et al. developed a higher frequency of CD20+CD27+CD43+ cells (~35% of total CD19+ cells) (40). We speculate that the discrepancy in the frequency of B-1 cells in human HSC-reconstituted NSG mice between this study and Vuyyuru et al could be due to 1/ irradiation of NSG recipients used by Vuyyuru et al. that might contribute to a host environment that is more favorable to CD20+CD27+CD43+ B-1 cell development, or less favorable to B-2 cell development, or 2/ loss of support for the development of CD20+CD27+CD43+ B-1 cells provided by Lin−CD34+CD38hi, stem cells which were included in adoptive transfers carried out by Vuyyuru et al., but treated separately in our study.
In summary, our analysis of the development potential of human Lin−CD34+CD38lo and Lin−CD34+CD38hi stem cell populations from cord blood and adult bone marrow in an NSG xenotransplant model demonstrated that Lin−CD34+CD38lo cells reconstituted both B-1 and B-2 B cells. We also found that the Ig repertoire of B-1 cells reconstituted from Lin−CD34+CD38lo stem cells was consistent with distinctive features of the B-1 cell Ig repertoire found in native human B-1 cells. Thus it appears that NSG mice constitute a favorable host environment for xenogeneic development of human B-1 as well as B-2 cells, and that early human HSC give rise to both B-1 and B-2 cells in this model as well as during clinical transplantation. It remains to be determined whether B-1 cells specific progenitors appear later in development or in other tissues.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI029690 to TLR
We thank members of the Rothstein laboratory for helpful discussions and technical support. We thank Dr. Ruthee-Lu Bayer’s team for recruiting and collecting patient samples. We thank the Tissue Donation Program at the Feinstein and their staff for helping with Bone Marrow and Cord Blood collections. We give special thanks to our healthy donors and patient volunteers for their donations of peripheral blood, cord blood, and bone marrow.
REFERENCES
- 1.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TT, Elsner RA, Baumgarth N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol. 2015;194:1489–1502. doi: 10.4049/jimmunol.1401880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 8.Gagro A, McCloskey N, Challa A, Holder M, Grafton G, Pound JD, Gordon J. CD5-positive and CD5-negative human B cells converge to an indistinguishable population on signalling through B-cell receptors and CD40. Immunology. 2000;101:201–209. doi: 10.1046/j.1365-2567.2000.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5− B lymphocyte subset producing natural antibodies. J Immunol. 1992;148:2690–2702. [PMC free article] [PubMed] [Google Scholar]
- 11.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- 12.Sutherland HJ, Eaves CJ, Eaves AC, Dragowska W, Lansdorp PM. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989;74:1563–1570. [PubMed] [Google Scholar]
- 13.Civin CI, Trischmann T, Kadan NS, Davis J, Noga S, Cohen K, Duffy B, Groenewegen I, Wiley J, Law P, Hardwick A, Oldham F, Gee A. Highly purified CD34-positive cells reconstitute hematopoiesis. J Clin Oncol. 1996;14:2224–2233. doi: 10.1200/JCO.1996.14.8.2224. [DOI] [PubMed] [Google Scholar]
- 14.Link H, Arseniev L, Bahre O, Kadar JG, Diedrich H, Poliwoda H. Transplantation of allogeneic CD34+ blood cells. Blood. 1996;87:4903–4909. [PubMed] [Google Scholar]
- 15.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34 + CD38− cells in cord blood and bone marrow. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 16.Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38− progenitor cells. Blood. 1991;77:1218–1227. [PubMed] [Google Scholar]
- 17.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa F, Livingston AG, Minamiguchi H, Wingard JR, Ogawa M. Human cord blood long-term engrafting cells are CD34+ CD38. Leukemia. 2003;17:960–964. doi: 10.1038/sj.leu.2402878. [DOI] [PubMed] [Google Scholar]
- 19.Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J Infect Dis. 1995;172:974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 20.Christianson SW, Greiner DL, Hesselton RA, Leif JH, Wagar EJ, Schweitzer IB, Rajan TV, Gott B, Roopenian DC, Shultz LD. Enhanced human CD4+ T cell engraftment in beta2-microglobulin-deficient NOD-scid mice. J Immunol. 1997;158:3578–3586. [PubMed] [Google Scholar]
- 21.Ishikawa F, Livingston AG, Wingard JR, Nishikawa S, Ogawa M. An assay for long-term engrafting human hematopoietic cells based on newborn NOD/SCID/beta2-microglobulin(null) mice. Exp Hematol. 2002;30:488–494. doi: 10.1016/s0301-472x(02)00784-1. [DOI] [PubMed] [Google Scholar]
- 22.Prochazka M, Gaskins HR, Shultz LD, Leiter EH. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci U S A. 1992;89:3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 24.Inui M, Hirota S, Hirano K, Fujii H, Sugahara-Tobinai A, Ishii T, Harigae H, Takai T. Human CD43+ B cells are closely related not only to memory B cells phenotypically but also to plasmablasts developmentally in healthy individuals. Int Immunol. 2015;27:345–355. doi: 10.1093/intimm/dxv009. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 27.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Descatoire M, Weill JC, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells are CD3−: A reply to “A human equivalent of mouse B-1 cells?” and” The nature of circulating CD27+CD43+ B cells”. The Journal of Experimental Medicine. 2011;208:2566–2569. doi: 10.1084/jem.20111761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leggat DJ, Khaskhely NM, Iyer AS, Mosakowski J, Thompson RS, Weinandy JD, Westerink MA. Pneumococcal polysaccharide vaccination induces polysaccharide-specific B cells in adult peripheral blood expressing CD19(+)CD20(+)CD3(−)CD70(−)CD27(+)IgM(+)CD43(+)CD5(+)/(−) Vaccine. 2013;31:4632–4640. doi: 10.1016/j.vaccine.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covens K, Verbinnen B, Geukens N, Meyts I, Schuit F, Van Lommel L, Jacquemin M, Bossuyt X. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121:5176–5183. doi: 10.1182/blood-2012-12-471953. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Batliwalla F, Rothstein TL. Human B-1 cells are not preplasmablasts: analysis of microarray data and other issues. Blood. 2013;122:3691–3693. doi: 10.1182/blood-2013-08-520031. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJ, Orfao A, van Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208:2565–2566. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbinnen B, Covens K, Moens L, Meyts I, Bossuyt X. Human CD20+CD43+CD27+CD5− B cells generate antibodies to capsular polysaccharides of Streptococcus pneumoniae. J Allergy Clin Immunol. 2012;130:272–275. doi: 10.1016/j.jaci.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 35.Reynaud CA, Weill JC. Gene profiling of CD11b(+) and CD11b(−) B1 cell subsets reveals potential cell sorting artifacts. J Exp Med. 2012;209:433–434. doi: 10.1084/jem.20120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelbertsen D, Vallejo J, Quach TD, Fredrikson GN, Alm R, Hedblad B, Bjorkbacka H, Rothstein TL, Nilsson J, Bengtsson E. Low Levels of IgM Antibodies against an Advanced Glycation Endproduct-Modified Apolipoprotein B100 Peptide Predict Cardiovascular Events in Nondiabetic Subjects. J Immunol. 2015;195:3020–3025. doi: 10.4049/jimmunol.1402869. [DOI] [PubMed] [Google Scholar]
- 37.Quach TD, Rodriguez-Zhurbenko N, Hopkins TJ, Guo X, Hernandez AM, Li W, Rothstein TL. Distinctions among Circulating Antibody-Secreting Cell Populations, Including B-1 Cells, in Human Adult Peripheral Blood. J Immunol. 2016;196:1060–1069. doi: 10.4049/jimmunol.1501843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson FK, Longhurst C, Chapman CJ, Ehrenstein M, Spellerberg MB, Hamblin TJ, Ravirajan CT, Latchman D, Isenberg D. Utilization of the VH4-21 gene segment by anti-DNA antibodies from patients with systemic lupus erythematosus. J Autoimmun. 1993;6:809–825. doi: 10.1006/jaut.1993.1066. [DOI] [PubMed] [Google Scholar]
- 39.Pascual V, Victor K, Lelsz D, Spellerberg MB, Hamblin TJ, Thompson KM, Randen I, Natvig J, Capra JD, Stevenson FK. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J Immunol. 1991;146:4385–4391. [PubMed] [Google Scholar]
- 40.Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichii M, Oritani K, Yokota T, Zhang Q, Garrett KP, Kanakura Y, Kincade PW. The density of CD10 corresponds to commitment and progression in the human B lymphoid lineage. PLoS One. 2010;5:e12954. doi: 10.1371/journal.pone.0012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berardi AC, Meffre E, Pflumio F, Katz A, Vainchenker W, Schiff C, Coulombel L. Individual CD34+CD38lowCD19−CD10− progenitor cells from human cord blood generate B lymphocytes and granulocytes. Blood. 1997;89:3554–3564. [PubMed] [Google Scholar]
- 43.Moore T, Huang S, Terstappen LW, Bennett M, Kumar V. Expression of CD43 on murine and human pluripotent hematopoietic stem cells. J Immunol. 1994;153:4978–4987. [PubMed] [Google Scholar]
- 44.Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerre TC, De Smet G, De Smedt M, Offner F, De Bosscher J, Plum J, Vandekerckhove B. Both CD34+38+ and CD34+38− cells home specifically to the bone marrow of NOD/LtSZ scid/scid mice but show different kinetics in expansion. J Immunol. 2001;167:3692–3698. doi: 10.4049/jimmunol.167.7.3692. [DOI] [PubMed] [Google Scholar]
- 46.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 47.Laurenti E, Dick JE. Molecular and functional characterization of early human hematopoiesis. Ann N Y Acad Sci. 2012;1266:68–71. doi: 10.1111/j.1749-6632.2012.06577.x. [DOI] [PubMed] [Google Scholar]
- 48.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH. Novel Human Transitional B Cell Populations Revealed by B Cell Depletion Therapy. J Immunol. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Current Biology. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 51.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant Autoantibody Production by Early Human B Cell Precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 53.Pinchuk GV, Alexander CM, Glas AM, Armitage RJ, Milner EC. VH repertoire in human B lymphocytes stimulated by CD40 ligand and IL-4: evidence for positive and negative selection mechanisms coupled to CD40 activation. Mol Immunol. 1996;33:1369–1376. doi: 10.1016/s0161-5890(96)00089-2. [DOI] [PubMed] [Google Scholar]
- 54.Kraj P, Friedman DF, Stevenson F, Silberstein LE. Evidence for the overexpression of the VH4-34 (VH4.21) Ig gene segment in the normal adult human peripheral blood B cell repertoire. J Immunol. 1995;154:6406–6420. [PubMed] [Google Scholar]
- 55.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 56.Alt FW, Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982;79:4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 58.Feeney AJ. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990;172:1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desiderio SV, Yancopoulos GD, Paskind M, Thomas E, Boss MA, Landau N, Alt FW, Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984;311:752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- 60.Gregoire KE, Goldschneider I, Barton RW, Bollum FJ. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979;123:1347–1352. [PubMed] [Google Scholar]
- 61.Kantor AB. V-gene usage and N-region insertions in B-1a, B-1b and conventional B cells. Semin Immunol. 1996;8:29–35. doi: 10.1006/smim.1996.0005. [DOI] [PubMed] [Google Scholar]
- 62.Holodick NE, Vizconde T, Rothstein TL. B-1a cell diversity: nontemplated addition in B-1a cell Ig is determined by progenitor population and developmental location. J Immunol. 2014;192:2432–2441. doi: 10.4049/jimmunol.1300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E, Reth M, Buch T, Waisman A, Kretschmer K, Weiss S. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 64.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci U S A. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39:2383–2394. doi: 10.1002/eji.200838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Payne KJ, Crooks GM. Immune-cell lineage commitment: translation from mice to humans. Immunity. 2007;26:674–677. doi: 10.1016/j.immuni.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Thai TH, Purugganan MM, Roth DB, Kearney JF. Distinct and opposite diversifying activities of terminal transferase splice variants. Nat Immunol. 2002;3:457–462. doi: 10.1038/ni788. [DOI] [PubMed] [Google Scholar]
- 68.Schroeder HW., Jr Similarity and divergence in the development and expression of the mouse and human antibody repertoires. Dev Comp Immunol. 2006;30:119–135. doi: 10.1016/j.dci.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 69.Zemlin M, Bauer K, Hummel M, Pfeiffer S, Devers S, Zemlin C, Stein H, Versmold HT. The diversity of rearranged immunoglobulin heavy chain variable region genes in peripheral blood B cells of preterm infants is restricted by short third complementarity-determining regions but not by limited gene segment usage. Blood. 2001;97:1511–1513. doi: 10.1182/blood.v97.5.1511. [DOI] [PubMed] [Google Scholar]
- 70.Kim DK, Fujiki Y, Fukushima T, Ema H, Shibuya A, Nakauchi H. Comparison of hematopoietic activities of human bone marrow and umbilical cord blood CD34 positive and negative cells. Stem Cells. 1999;17:286–294. doi: 10.1002/stem.170286. [DOI] [PubMed] [Google Scholar]
- 71.Moins-Teisserenc H, Busson M, Herda A, Apete S, de Latour RP, Robin M, Xhaard A, Toubert A, Socie G. One-year CD19+CD5+ B-cell and B1 cell reconstitution following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Herzenberg LA, Stall AM, Lalor PA, Sidman C, Moore WA, Parks DR, Herzenberg LA. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 73.Binder CJ, Shaw PX, Chang MK, Boullier A, Hartvigsen K, Horkko S, Miller YI, Woelkers DA, Corr M, Witztum JL. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46:1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Ghosn EE, Yamamoto R, Hamanaka S, Yang Y, Herzenberg LA, Nakauchi H, Herzenberg LA. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci U S A. 2012;109:5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi M, Shelley WC, Seo W, Vemula S, Lin Y, Liu Y, Kapur R, Taniuchi I, Yoshimoto M. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proc Natl Acad Sci U S A. 2014;111:12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang CA, Henry C, Iacomini J, Imanishi-Kari T, Wortis HH. Adult bone marrow contains precursors for CD5+ B cells. Eur J Immunol. 1996;26:2537–2540. doi: 10.1002/eji.1830261039. [DOI] [PubMed] [Google Scholar]
- 78.Newman K, Jr, Johnson RC. T-cell-independent elimination of Borrelia turicatae. Infect Immun. 1984;45:572–576. doi: 10.1128/iai.45.3.572-576.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


