Abstract
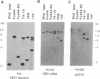
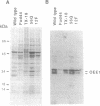
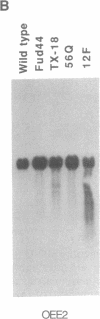
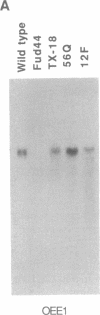
We have developed a stable nuclear transformation system for the unicellular green alga Chlamydomonas reinhardtii. Transformation was accomplished by introducing the cloned C. reinhardtii oxygen-evolving enhancer protein 1 (OEE1) gene into C. reinhardtii cells by bombardment with DNA-coated tungsten particles. The recipient strain was an OEE1-deficient, nonphotosynthetic, acetate-requiring mutant, which recovered photosynthetic competence after transformation, and was therefore able to grow in the absence of acetate. Analysis of several transformants indicates that transformation has proceeded via second-site integration of the cloned gene, leaving the endogenous mutant gene intact. In genetic crosses of transformants with wild type, both mutant and wild-type phenotypes were recovered, showing that the photosynthetic competence of transformants was due not to reversion of the original locus but rather to expression of the introduced gene. We suggest that the success of the present system is largely due to using a homologous C. reinhardtii gene, leading to stable maintenance and expression of the gene. Transformation with heterologous genes may be problematic because of poor expression due to an unusual codon bias in C. reinhardtii.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blowers A. D., Bogorad L., Shark K. B., Sanford J. C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989 Jan;1(1):123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Day A., Schirmer-Rahire M., Kuchka M. R., Mayfield S. P., Rochaix J. D. A transposon with an unusual arrangement of long terminal repeats in the green alga Chlamydomonas reinhardtii. EMBO J. 1988 Jul;7(7):1917–1927. doi: 10.1002/j.1460-2075.1988.tb03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain S. E., Manavathu E. K., Leung W. C. DNA-mediated transformation of Chlamydomonas reinhardi cells: use of aminoglycoside 3'-phosphotransferase as a selectable marker. Mol Cell Biol. 1985 Dec;5(12):3647–3650. doi: 10.1128/mcb.5.12.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Kindle K. L., Schnell R. A., Fernández E., Lefebvre P. A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol. 1989 Dec;109(6 Pt 1):2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A., Rosenbaum J. L. Regulation of the synthesis and assembly of ciliary and flagellar proteins during regeneration. Annu Rev Cell Biol. 1986;2:517–546. doi: 10.1146/annurev.cb.02.110186.002505. [DOI] [PubMed] [Google Scholar]
- Luck D. J. Genetic and biochemical dissection of the eucaryotic flagellum. J Cell Biol. 1984 Mar;98(3):789–794. doi: 10.1083/jcb.98.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Bennoun P., Rochaix J. D. Expression of the nuclear encoded OEE1 protein is required for oxygen evolution and stability of photosystem II particles in Chlamydomonas reinhardtii. EMBO J. 1987 Feb;6(2):313–318. doi: 10.1002/j.1460-2075.1987.tb04756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P., Rahire M., Frank G., Zuber H., Rochaix J. D. Expression of the nuclear gene encoding oxygen-evolving enhancer protein 2 is required for high levels of photosynthetic oxygen evolution in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1987 Feb;84(3):749–753. doi: 10.1073/pnas.84.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T., Harpster M. H., Mayfield S. P., Taylor W. C. Light-regulated gene expression during maize leaf development. J Cell Biol. 1984 Feb;98(2):558–564. doi: 10.1083/jcb.98.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., Erickson J. Function and assembly of photosystem II: genetic and molecular analysis. Trends Biochem Sci. 1988 Feb;13(2):56–59. doi: 10.1016/0968-0004(88)90029-1. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J., Rahire M. Construction and characterization of autonomously replicating plasmids in the green unicellular alga Chlamydomonas reinhardii. Cell. 1984 Apr;36(4):925–931. doi: 10.1016/0092-8674(84)90042-4. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J. Transformation of the green alga Chlamydomonas reinhardii with yeast DNA. Nature. 1982 Mar 4;296(5852):70–72. doi: 10.1038/296070a0. [DOI] [PubMed] [Google Scholar]
- Youngblom J., Schloss J. A., Silflow C. D. The two beta-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984 Dec;4(12):2686–2696. doi: 10.1128/mcb.4.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]