Abstract
Psoriasis is a complex inflammatory disease that occurs in genetically susceptible individuals and presents with the development of erythematous scaly plaques on the skin. Interleukins (ILs) in the Th17 pathway play a pivotal role in the pathogenesis of psoriasis and have thus become targets for recent biologic drug development. Secukinumab is a human monoclonal IgG1k antibody that has been developed to target and block the actions of IL-17A. Secukinumab recently approved for use as first-line systemic therapy in a patient with moderate to severe psoriasis has been studied first in psoriasis before other diseases. Both Phase II and III clinical trials have demonstrated the effectiveness of secukinumab in the treatment of moderate-to-severe plaque psoriasis, and it has demonstrated superiority to other comparable biologics on the market, including the tumor necrosis factor inhibitor etanercept. Secukinumab has also shown superiority to ustekinumab, a relatively recent biologic introduced for the treatment of psoriasis. Besides demonstrating better efficacy compared to etanercept and ustekinumab, secukinumab has also demonstrated a greater impact of the quality of life of patients with a comparable safety profile. Secukinumab shows great promise in having a tremendous impact on the treatment of plaque psoriasis based on its ability to produce similar, if not better, clinical outcomes than other biologic antipsoriasis medications.
Keywords: Biologics, Interleukin-17, monoclonal antibody, PASI, psoriasis, secukinumab
Introduction
What was known?
Introduction of biologics has brought a revolution in the management of psoriasis
TNF inhibitors like etanercept achieve PASI 90, the new treatment success threshold, only in 20% of patients.
Psoriasis is one of the most common dermatological disorders in India. It affects 1.4%–2.0% of the population and comprises 2.6% of skin-related visits to primary care physicians or between 0.3% and 0.6% of all visits to family physicians.[1] In India, the prevalence of psoriasis varies from 0.44% to 2.8%, it is twice more common in males compared to females, and most of the patients are in their third or fourth decade at the time of presentation.[2]
Psoriasis presents in various forms, most commonly as chronic plaque psoriasis (90% of patients), also called psoriasis vulgaris. Psoriasis has a significant impact on the quality of life (QoL) with patients with the severe disease having a reduced life expectancy of approximately 4 years.[3]
Patients with plaque psoriasis that cannot be controlled with topical agents/phototherapy progress to traditional systemic agents (cyclosporine, methotrexate, acitretin, and fumaric acid esters). The frequency of prescribing systemic therapies for patients with severe psoriasis is suboptimal; in other words, patients who should receive systemic therapy do not receive appropriate therapy, leading to a significant unmet need.[3]
Psoriasis has had its therapeutic armamentarium transformed through the development of biologic agents. Biologics were introduced up to 10 years ago for the treatment of psoriasis and have revolutionized the management of patients with moderate to severe psoriasis. Advances in the understanding of psoriasis pathogenesis have allowed the development of biologics targeting cytokines involved in the psoriatic inflammatory process.[4]
Rationale for Targeting Interleukin-17 in Psoriasis
The cytokine interleukin (IL)-17A is elevated in lesions of psoriasis. Th17 cells are the main producers of IL-17A. IL-17A has many functions that are relevant to psoriasis, including the enhancement of angiogenesis, the promotion of the release of other inflammatory cytokines (tumor necrosis factor [TNF]-α, IL-1, and IL-6), and the direct activation of keratinocytes leading to increased production of chemokines. ILs in the Th17 pathway play a pivotal role in the pathogenesis of psoriasis and have become targets for drug development.[5] Blocking IL-17A results in amelioration of psoriasis-like pathology in animal models.[4]
The neutralization of this function by the blockage of this union to the receptor, or by the administration of a specific antibody against IL, entails the possibility of a new biological treatment of autoimmune diseases such as psoriasis. The introduction of a new biological, with a new mechanism of action, means that the history of biological therapies for psoriasis and other autoimmune diseases will be re-written.[6]
Secukinumab - First in Class Interleukin-17A Inhibitor
Secukinumab is unique from other approved therapies for psoriasis as it is a first in class recombinant high-affinity, fully human monoclonal antibody of the IgG1/kappa isotype that selectively targets IL-17A. Secukinumab interferes in the psoriasis pathologic process by selectively binding to IL-17A and thereby preventing IL-17A interaction with the IL-17 receptor expressed on, for example, keratinocytes. By its mechanism of action, secukinumab prevents and reverses key pathologic processes in psoriasis leading to normalization of skin histology.
At the therapeutic concentrations used in psoriasis, secukinumab fully neutralizes the activity of IL-17A, does not neutralize IL-17F, leaves other functions of Th17 cells intact, and does not directly influence the Th1 pathway. This specific mechanism of action is unique to IL-17 inhibitors and leads to the normalization of skin histology, including achievement of clear to almost clear skin for the majority of patients. Furthermore, this specificity offers the potential for fewer off-target effects when compared to other available current treatment options.[5]
Pharmacokinetics
Secukinumab's pharmacokinetics (PK) profile is typical of a fully human immunoglobulin IgG1 molecule: Slow subcutaneous (SC) absorption, low clearance, and long half-life. There is no evidence of a time-dependent change in clearance.[5]
Clearance of secukinumab in geriatric patients and patients <65 years of age was similar. Because secukinumab is a human IgG with a large molecular size (~150 kDa) and because intact immunoglobulin is filtered by the kidney only to a very small degree, only very small amounts of antibody are expected to be excreted in the urine. Thus, renal impairment is not likely to influence urinary excretion and the overall PK profile. Finally, hepatic impairment would not be expected to influence metabolism or excretion of human IgG.[5]
Secukinumab's main route of elimination is through intracellular catabolism, and thus the potential for drug interactions between secukinumab and small drug molecules is low. Other monoclonal antibodies used in treating psoriasis, including infliximab and adalimumab, have been noted to have decreased clearances when administered with methotrexate.[5]
Efficacy studies
Phase III studies
Phase III clinical trials have demonstrated the efficacy of secukinumab in the treatment of moderate-to-severe plaque psoriasis.
The Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis (ERASURE) study compared secukinumab with placebo, and the Full Year Investigative Examination of Secukinumab versus Etanercept using Two Dosing Regimens to Determine Efficacy in Psoriasis (FIXTURE) study compared secukinumab with placebo and etanercept, the first TNF inhibitor approved by the Food and Drug Administration for moderate-to-severe plaque psoriasis. These two randomized, Phase III trials assessed the efficacy and safety of secukinumab, at a dose of 300 mg or 150 mg, administered as induction therapy (with assessment at week 12) and maintenance therapy (with assessment at week 52) in patients with moderate-to-severe plaque psoriasis.[7]
Secukinumab was associated with a rapid reduction in psoriasis symptoms, the median time to 50% reduction in mean Psoriasis Area and Severity Index (PASI) score was 3.0 weeks and 3.9 weeks in the 300 mg and 150 mg secukinumab groups respectively, versus 7 weeks in the etanercept group (P < 0.001).[7]
The proportion of patients who met the criterion for PASI 75/90/100 at week 12 was higher with each secukinumab dose than with placebo or etanercept [Figures 1–3].[7]
Figure 1.
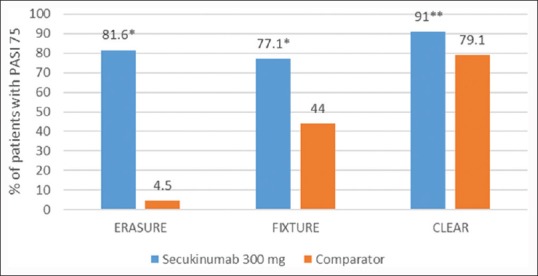
Psoriasis Area and Severity Index 75 at week 12 across three Phase III studiesa with secukinumab 300 mg in patients with moderate-to-severe psoriasis. *P < 0.001, **P < 0.0001, aComparator: Placebo in ERASURE, Etanercept in FIXTURE and ustekinumab in CLEAR. ERASURE: Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis; FIXTURE: Full Year Investigative Examination of Secukinumab versus Etanercept using Two Dosing Regimens to Determine Efficacy in Psoriasis, CLEAR: Comparison to assess Long-term Efficacy, sAfety and toleRability of secukinumab vs. ustekinumab
Figure 3.
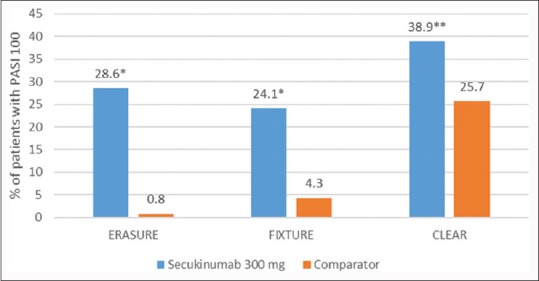
Psoriasis Area and Severity Index 100 at week 12 across three Phase III studiesa with secukinumab 300 mg in patients with moderate-to-severe psoriasis. *P < 0.001, **P = 0.0003, aComparator in Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis was placebo, in Full Year Investigative Examination of Secukinumab versus Etanercept using Two Dosing Regimens to Determine Efficacy in Psoriasis was etanercept and in Cancer Lifestyle and Evaluation of Risk was ustekinumab
Figure 2.
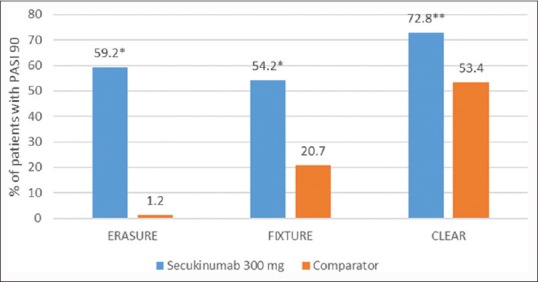
Psoriasis Area and Severity Index 90 at week 12 across three Phase III studiesa with secukinumab 300 mg in patients with moderate-to-severe psoriasis. *P < 0.001, **P < 0.0001, aComparator: Placebo in ERASURE, Etanercept in FIXTURE and ustekinumab in CLEAR. ERASURE: Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis; FIXTURE: Full Year Investigative Examination of Secukinumab versus Etanercept using Two Dosing Regimens to Determine Efficacy in Psoriasis, CLEAR: Comparison to assess Long-term Efficacy, sAfety and toleRability of secukinumab vs. ustekinumab
The proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1, indicating no impairment of health-related QoL (HRQoL), was significantly higher at week 12 in each secukinumab dose group than in the etanercept or placebo group (P < 0.001 for all comparisons).[7]
The results of these Phase III studies validate IL-17A as an important therapeutic target in moderate-to-severe plaque psoriasis, confirming earlier findings from basic research and Phase II trials of secukinumab that suggested that IL-17A plays a role in the pathogenesis of psoriasis.[7]
Comparison to assess Long-term Efficacy, sAfety and toleRability of secukinumab vs. ustekinumab (CLEAR), a Phase IIIb trial, the second head-to-head trial of secukinumab, directly compared the efficacy and safety of secukinumab with ustekinumab in subjects with moderate to severe plaque psoriasis.[8] In this 52-week, double-blind study, 676 subjects were randomized 1:1 to SC injection of secukinumab 300 mg or ustekinumab. PASI 75 at week 4 was superior for secukinumab (50.0%) versus ustekinumab (20.6%) (P < 0.0001). Secukinumab was superior to ustekinumab as assessed by PASI 75/90/100 response at week 12 [Figures 1–3]. The percentage of subjects with the DLQI score 0/1 (week 16) was significantly higher with secukinumab (71.9%) than ustekinumab (57.4%) (P < 0.0001).[8]
SCULPTURE was a Phase III study specifically designed to evaluate the noninferiority of maintenance therapy using a retreatment-as needed regimen versus a fixed-interval regimen at two doses (300 mg and 150 mg SC) of secukinumab in patients with moderate to severe plaque psoriasis.[9]
Secukinumab induced high responses by week 12 (84.4%–91.1% PASI 75 responders). From week 12 to week 52, more patients on fixed interval (78.2%, 300 mg; 62.1%, 150 mg) maintained PASI 75 versus retreatment as needed (67.7%; 52.4%); statistical noninferiority of retreatment as needed was not established.[9]
The DLQI indicated greater improvement in HRQoL in the fixed interval groups versus the retreatment-as-needed groups. DLQI scores in the retreatment-as-needed groups worsened at first start of relapse visit and improved when PASI 75 was regained.[9]
Secukinumab fixed interval showed clear benefit versus the study-specified retreatment-as-needed regimen for maintaining efficacy. Current evidence supports regular secukinumab administration every 4 weeks, without interruption, to maintain optimal disease control and QoL.[9]
Safety
The two Phase III clinical trials mentioned, ERASURE and FIXTURE, included a total of 2044 patients. During the 12-week induction period in the ERASURE study, 55.1% of patients had at least one adverse event (AE) in the 300 mg secukinumab group versus 60.4% in the 150 mg secukinumab group. On the other hand, 47.0% of patients in the placebo group had at least one AE. In the 300 mg secukinumab group and the 150 mg secukinumab group, 29.4% and 26.9% of the subjects, respectively, had infections compared with 16.2% of the placebo group during the 12-week induction period. The most common AEs in the 12-week induction period and the entire treatment period overall were upper respiratory tract infection, nasopharyngitis, and headache. The incidence of adverse effects in all other system organ classes was similar between the study groups.[7]
In the FIXTURE study, the incidences of AEs during the induction period and the entire treatment period were similar in the secukinumab and etanercept groups. In contrast to the ERASURE study, nasopharyngitis, headache, and diarrhea were the most common AEs in the secukinumab groups during the 12-week induction and the entire treatment period. Seven patients (0.7%) in the combined secukinumab groups versus 36 patients (11.1%) in the etanercept group experienced injection-site reactions during the entire study [Table 1].[7]
Table 1.
Comparative safety profile of secukinumab compared to etanercept and ustekinumab
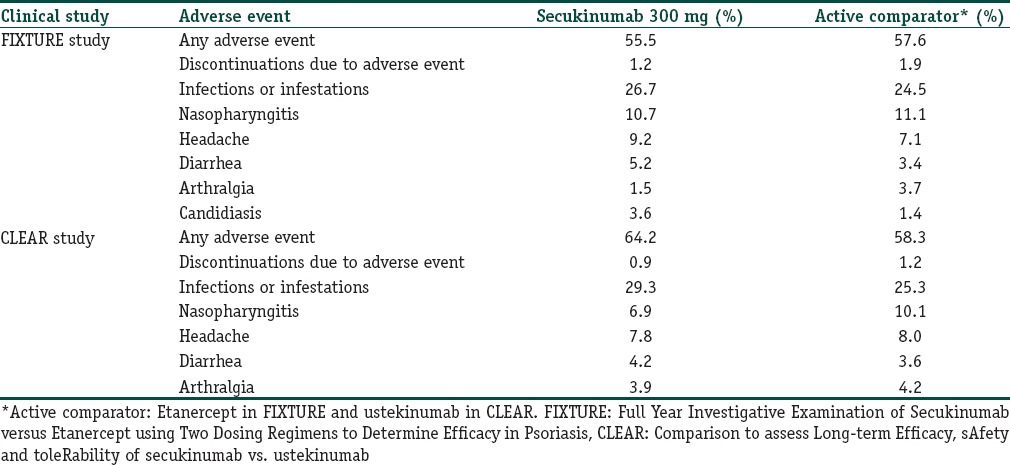
Candida infections were more common with secukinumab than with etanercept during the entire treatment period [Table 1]. All of the infections resolved on their own or with standard therapy and none resulted in chronic mucocutaneous candidiasis or discontinuation of secukinumab.[7]
Anti-secukinumab antibodies were detected during treatment in 2 of 702 patients (0.3%) receiving secukinumab in the ERASURE study and 4 of 980 (0.4%) in the FIXTURE study. The presence of anti-secukinumab antibodies was not associated with AEs or reduced efficacy.[7]
In the CLEAR study, the proportion of subjects experiencing at least 1 AE was 64.2% in these secukinumab group and 58.3% in the ustekinumab group. AEs in the system organ class of “Infections and Infestations” were reported most often [Table 1]; however, most infectious AEs were nonserious, of mild to moderate severity, easily manageable, and did not lead to study drug discontinuation. The most common AEs were a headache and mild to moderate nasopharyngitis in both groups [Table 1].[7,8]
In terms of interactions with concurrent vaccinations, secukinumab was found to have no adverse effects on vaccine administration.[5] Eighty percent of subjects exposed to secukinumab and 76% of subjects exposed to no treatment achieved a >4-fold protective immune response to both vaccinations at 4 weeks. Therefore, patients receiving secukinumab may receive concurrent inactivated or nonlive vaccinations. Live vaccines, however, should not be given concomitantly with secukinumab.[5]
There is limited information regarding secukinumab and the use of biologics during pregnancy. Specifically, information is missing concerning fetal malformation and incidence of spontaneous abortions. Routine pharmacovigilance may currently be the best option for pregnant women receiving secukinumab treatment.[5]
Secukinumab - Place in Therapy
Targeted biologic therapies such as TNF-α inhibitors infliximab, etanercept, and adalimumab and the IL-12/IL-23 antagonist ustekinumab have greatly improved the treatment of moderate to severe plaque psoriasis. In randomized controlled trials, approximately 50%–80% of subjects receiving these biologics achieved PASI 75 after 10–16 weeks of treatment.[8]
However, the threshold for treatment success in psoriasis has now changed. A 90% improvement from baseline PASI score is now defined as the threshold of treatment success as per the European Medicines Agency and a “measure of optimal response” by the American Academy of Dermatology. This goal, however, was considered a “very stringent requirement and a target not always possible to obtain in clinical practice.”[10] Of note, PASI 90 response was only achieved by approximately 20% of patients treated with etanercept, and approximately 40%–50% with infliximab, adalimumab, and ustekinumab.[8] With secukinumab, the data show that a PASI 90 response is now an achievable goal in a majority of subjects.
Achieving PASI 90 response in patients with psoriasis is highly clinically relevant, given the direct relationship between PASI score improvement and HRQoL.[11] In a study, 4–6 out of 10 patients achieving PASI 90 and 100 respectively reported no impact of their skin problems on HRQoL, versus 2 out of 10 of those with PASI 75 to less than PASI 90 response. These results support the importance of achieving PASI 90 to PASI 100 responses in patients with psoriasis for the maximal improvement in HRQoL.[11]
Treatment of plaque psoriasis with secukinumab yields relatively rapid improvements in disease course and sustained improved results. Data from ERASURE, FIXTURE, and other studies demonstrate that the 300 mg SC dose achieves peak effects at week 16 and sustains efficacy to week 52 of treatment. Moreover, speed of response data from the FIXTURE trial, in particular, demonstrated that patients in the 300 mg secukinumab group achieved a 50% reduction from mean baseline PASI score at 3 weeks of treatment, compared to 7 weeks for the etanercept group.[7]
Results from the CLEAR study add to the evidence from the pivotal Phase III program supporting that secukinumab can better deliver clear or almost clear skin and improved HRQoL when compared with other existing therapies, making it the new reference standard treatment for patients with moderate to severe plaque psoriasis.[8]
Conclusion
Secukinumab has been evaluated in an extensive clinical program for the treatment of patients with moderate to severe plaque psoriasis. As the first in class, a new mode of action product, specifically neutralizing IL-17A, secukinumab provides an important addition to existing treatment options for this serious disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
What is new?
Secukinumab is the first biological drug approved for the first-line treatment of patients with psoriasis eligible for systemic therapy
Secukinumab sets a new benchmark for clinical efficacy with a PASI 90 response rate of around 80% by week 16.
References
- 1.Sarkar S, Sarkar A, Saha R, Sarkar T. Psoriasis and psychiatric morbidity: A profile from a tertiary care centre of Eastern India. J Family Med Prim Care. 2014;3:29–32. doi: 10.4103/2249-4863.130267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dogra S, Yadav S. Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76:595–601. doi: 10.4103/0378-6323.72443. [DOI] [PubMed] [Google Scholar]
- 3.Lynch M, Kirby B, Warren RB. Treating moderate to severe psoriasis – Best use of biologics. Expert Rev Clin Immunol. 2014;10:269–79. doi: 10.1586/1744666X.2014.873701. [DOI] [PubMed] [Google Scholar]
- 4.Gisondi P, Dalle Vedove C, Girolomoni G. Efficacy and safety of secukinumab in chronic plaque psoriasis and psoriatic arthritis therapy. Dermatol Ther (Heidelb) 2014;4:1–9. doi: 10.1007/s13555-014-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roman M, Madkan VK, Chiu MW. Profile of secukinumab in the treatment of psoriasis: Current perspectives. Ther Clin Risk Manag. 2015;11:1767–77. doi: 10.2147/TCRM.S79053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheinberg M. A new era in psoriasis and psoriatic arthritis therapy: New mechanisms of action and the introduction of biogeneric drugs. Rev Bras Reumatol. 2015;55:469–70. doi: 10.1016/j.rbr.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis – Results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 8.Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–9. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE) J Am Acad Dermatol. 2015;73:27–36.e1. doi: 10.1016/j.jaad.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP). Guideline on Clinical Investigation of Medicinal Products Indicated for the Treatment of Psoriasis. [Last accessed on 2016 Mar 07]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf .
- 11.Revicki DA, Willian MK, Menter A, Saurat JH, Harnam N, Kaul M. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology. 2008;216:260–70. doi: 10.1159/000113150. [DOI] [PubMed] [Google Scholar]


