Abstract
Vitamin D3 (D3) can be metabolized by cytochrome P450scc (CYP11A1) into 20S-hydroxyvitamin D3 (20D3) as a major metabolite. This bioactive metabolite has shown strong antiproliferative, antifibrotic, pro-differentiation and anti-inflammatory effects while being non-toxic (non-calcemic) at high concentrations. Since D3 analogs with two symmetric side chains (Gemini analogs) result in potent activation of the vitamin D receptor (VDR), we hypothesized that the chain length and composition of these types of analogs also containing a 20-hydroxyl group would affect their biological activities. In this study, we designed and synthesized a series of Gemini 20D3 analogs. Biological tests showed that some of these analogs are partial VDR activators and can significantly stimulate the expression of mRNA for VDR and VDR-regulated genes including CYP24A1 and transient receptor potential cation channel V6 (TRPV6). These analogs inhibited the proliferation of melanoma cells with potency comparable to that of 1α,25-dihydroxyvitamin D3. Moreover, these analogs reduced the level of interferon γ and up-regulated the expression of leukocyte associated immunoglobulin-like receptor 1 in splenocytes, indicating that they have potent anti-inflammatory activities. There are no clear correlations between the Gemini chain length and their VDR activation or biological activities, consistent with the high flexibility of the ligand-binding pocket of the VDR.
Keywords: 20-Hydroxyvitamin D3, anti-inflammation, anti-proliferation, Gemini analogs, CYP24A1, vitamin D receptor
The production, activation and metabolism of vitamin D3 (D3) involves the participation of a variety of tissues and organs (1). In the epidermis of the skin, D3 is produced from ultraviolet (UV) irradiation causing photoconvesion of 7-dehydrocholesterol (7DHC) to pre-D3, which undergoes thermal isomerization to D3. On the systemic level, activation of D3 involves initial hydroxylation in the liver by a 25-hydroxylase [cytochrome P450 2R1 (CYP2R1) or cytochrome P450 (CYP27A1)] followed by 1α-hydroxylation by CYP27B1 in the kidney to form its biological active form, 1α,25-dihydroxyvitamin D3 (1,25D3, Figure 1). 1,25D3 acts through the vitamin D receptor (VDR) and regulates the expression of a variety of genes involved in immunomodulation, anti-inflammation, anti-proliferation, pro-differentiation, anti-angiogenesis, pro-apoptosis, mineral homeostasis and vitamin D catabolism (2, 3). One of these genes, CYP24A1, encodes D3 24-hydroxylase which sequentially oxidizes the side chain of 1,25D3 producing 1α,24R,25-trihydroxyvitamin D3 (1,24,25D3), 24-oxo-1,25D3, 24-oxo-1α,23,25-trihydroxyvitamin D3, 23-oxo-24,25,26,27-tetranor-1α,-hydroxyvitamin D3, and finally calcitroic acid for excretion (4, 5).
Figure 1.
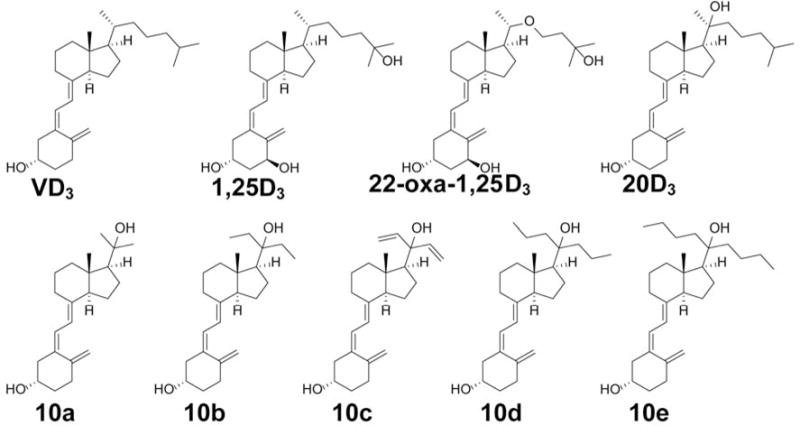
Chemical structures of vitamin D3 (VD3), 1α,25-dihydroxyvitamin D3 (1,25D3), 22-oxa-1α,25-dihydroxyvitamin D3 (22-oxa-1,25D3), 20S-hydroxyvitamin D3 (20D3) and its five Gemini analogs (10a–10e).
We previously reported the identification and characterization of a new metabolic pathway, in addition to the classical pathway described above, for activation of D3 (6–9). This pathway is initiated by mammalian CYP11A1 (also known as cytochrome P450scc) acting on D3 to produce 20S-hydroxyvitamin D3 (20D3) as the major product (7, 10), which can be further metabolized into di- and tri-hydroxy products by CYP11A1 and other vitamin D-metabolizing enzymes such as CYP24A1, CYP27A1 and CYP27B1 (4, 7, 11). Importantly, 20D3 and its di- and tri-hydroxymetabolites accumulate in vivo in the human epidermis and serum (12). 20D3 produces a number of biological effects similar to those of 1,25D3, acting as a biased agonist on the VDR (7, 13), but lacks the calcemic effect. High doses of 20D3 (up to 30 μg/kg) do not cause hypercalcemia in rats or mice, while 1,25D3 has substantial hypercalcemic effects (toxicity) at a dose as low as only 2 μg/kg (14, 15). Thus 20D3 has the potential to be used at therapeutic doses without toxicity. 20D3 displays antiproliferative, pro-differentiation and anti-inflammatory properties in many cell lines (7, 16). In addition, 20D3 is able to inhibit the growth of solid tumors (17) and leukemia (14), indicating its tumorostatic activities, and it has shown antifibrotic activity in vitro and in vivo (15, 18, 19).
Gemini analogs of D3 are characterized by having two symmetric side chains at C20. Many Gemini D3 analogs have been synthesized to investigate the contribution of the extra side chain to their drug-like properties (20, 21). To determine the effects of chain length and composition in the Gemini analogs also possessing a 20-hydroxyl group, we designed and synthesized a series of 20D3 Gemini analogs (Figure 2) based on our established synthetic route (16). Biological activities including VDR activation, expression of VDR-regulated genes, and inhibition of proliferation and inflammation were investigated for these Gemini analogs by comparison of their properties with those of positive controls.
Figure 2.
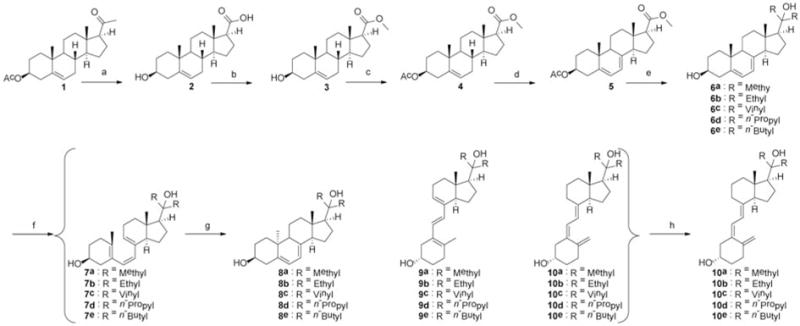
Synthetic route for producing Gemini analogs of 20-hdroxyvitamin D3. Reagents and conditions: (a) NaOH, Br2, 0°C then warmed to r.t., overnight. (b) H2SO4, methanol, reflux 2 h. (c) Acetic anhydride, pyridine, 4-dimethylaminopyridine (DMAP), 6 h. (d) Dibromantin, azobisisobutyronitrile (AIBN), benzene: hexane (1:1), reflux 20 min; tetra-n-butylammonium bromide (TBAB), tetrahydrofuran (THF), r.t., 75 min, then tetra-n-butylammonium fluoride (TBAF), r.t., 50 min. (e) Grignard reagent in THF, THF, 0°C then warmed up to r.t., 8 h (6c: vinyl magnesium bromide, CeCl3, −78°C then warmed up to r.t., 24 h). (f) Ultraviolet B (UVB), diethyl ether, 15 min. (g) Ethanol, reflux, 3 h. (h) High-performance liquid chromatography, acetonitrile:H2O.
Materials and Methods
Chemicals
The starting material pregnenolone acetate was purchased from Bosche Scientific LLC (New Brunswick, NJ, USA) with a purity above 98% as determined by high-performance liquid chromatography (HPLC). HPLC-grade acetonitrile was purchased from Fisher Scientific (Hampton, NH, USA). De-ionized water was prepared by a Milli-Q purification system for the HPLC mobile phases. Vitamin D3 (Sigma-Aldrich, St. Louis, MO, USA) was used as standard reference to generate HPLC standard curves to quantify small aliquots of vitamin D3 analogs.
General methods for chemistry
All reagents and solvents for the synthesis and separation were purchased from commercial sources and were used as received. Reactions of 5,7-diene compounds were carried out in the dark by wrapping the flasks with aluminum foils. Moisture- or air-sensitive reactions were performed under an argon atmosphere. All reactions were routinely monitored by thin layer chromatography (TLC) on silica gel using ethyl acetate and hexane as mobile phases, and visualized by 5% phosphomolybdic acid in ethanol or UV lights. Mass spectra of all compounds were obtained by a Bruker ESQUIRE-LC/MS system (Bruker Corporation, Billerica, MA, USA) equipped with an electrospray ionization (ESI) source. Nuclear magnetic resonance (NMR) spectra were recorded by either a Bruker Avance III 400 MHz or an Agilent Unity Inova 500 MHz spectrometer. High-resolution mass spectrometry (HRMS) was carried out based on our previous methods (22, 23) by a Waters Acquity™ ultra-performance liquid chromatography system (Milford, MA, USA) equipped with a Waters Xevo™ G2-S quadrupole time-of-flight mass spectrometer and an ESI source in positive mode. Ethyl acetate was used for extraction of reaction mixtures and then dried over anhydrous Na2SO4, filtered and removed using a rotary evaporator under reduced pressure.
In brief, starting material 1 underwent haloform reaction with high yield (96%) to give the 3-hydroxyl acid 2 following our previously published procedure (24). To generate the 20-hydroxyl group, the acid of 2 was firstly esterified, with quantitative yield, by methanol in the presence of sulfuric acid, to produce methyl ester 3. The ester was then ready for the introduction of the same two aliphatic side chains in one Grignard reaction. The 3-hydroxyl group on 3 was then protected to give ester 4, following our established acetylation procedure (3), for the introduction of C7 double bond. The 5,7-diene structure was then introduced into 4 by free radical reaction under dibromatin/azobisisobutyronitrile/tetra-n-butylammonium bromide/tetra-n-butylammonium fluoride conditions to provide the protected methyl ester 5 with acceptable yield (45%). Under dark conditions, the Grignard reaction was carefully carried out using different Grignard reagents in THF to introduce the two side chains to the C20 position where the 20-hydroxyl was formed as a result. Irradiation (15 min) of the 7DHC analogs in ethyl ether by UVB, followed by 3 hours reflux in ethanol gave D3 structures (10) together with their parent 7DHC, pre-vitamin D3 (7), lumisterol (8) and tachysterol (9) structures as related impurities. The separation of compounds in the reaction mixture after irradiation was achieved by preparative HPLC using a gradient of acetonitrile in water to afford the final D3 compounds (10) with high purity (>98%) for biological testing.
HPLC conditions
An Agilent HPLC 1100 series system consisting of a binary pump, a column oven, a degasser, a diode array detector and an autosampler was used for chromatographic analysis. The purity check of 20D3 analogs was carried out on a Phenomenex Luna-PFP C18 column (5 μm, 250 mm × 4.6 mm; Phenomenex) maintained at 25°C. The flow rate was 1.0 ml/min. The UV absorption at 263 nm was set for displaying the chromatograms. Isocratic elution using water (A) and acetonitrile (B) was as follows: 60% B for compound 10a, 70% B for compound 10b, 70% B for compound 10c, 80% B for compound 10d and 90% B for compound 10e.
Cell culture
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with glucose, L-glutamine, pyridoxine hydrochloride, 5% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin antibiotic solution (Sigma-Aldrich, St. Louis, MO, USA) was used to culture immortalized human keratinocytes (HaCaT). Jurkat cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% antibiotic solution. Eagle’s minimal essential medium (EMEM) containing 9% charcoal-stripped FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, non-essential amino acids, 2.5 mM 2-mercaptoethanol and 2.5 mM L-glutamine was used to culture splenocytes from DBA/1 mice. All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
VDRE-luciferase reporter assay
Jurkat cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were transduced with lentiviral VDRE luciferase using a Cignal Lenti VDRE Reporter (luc) Kit according to the manufacturer’s protocol (QIAGEN, Valencia, CA, USA). The cells then went through 1-week selection under puromycin (1.0 μg/ml) treatment. During the selection, the media were changed every other day. For the biological test, transduced Jurkat cells were washed with PBS (1×) and then seeded in a 96-well plate (10,000 cells/well, 100 μl/well) diluted by FBS-free media. All cells were then synchronized by a 24 h incubation. DMSO solutions (10%; 1.0 μl) of each secosteroid were added to each well of cells with final concentrations of 1000, 300, 100, 30, 10, 3 and 1 nM, which were then incubated for another 24 h. The luciferase signal of the cells was measured by the ONE-Glo™ Luciferase Assay System (Promega, Madison, WI, USA) according to the manufacturer’s suggested procedure. DMSO (10%) in water was used as the vehicle control and the final concentration of DMSO in culture media was 0.1% during the activity test. Each concentration of analog was tested in triplicate (n=3).
Real-time polymerase chain reaction (RT-PCR)-based expression analysis
HaCaT cells were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and were cultured as described above. The RNA from HaCaT keratinocytes treated with 10a–e, 1,25D3, 22-oxa-1,25D3 or DMSO was isolated using the Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA, USA). Reverse transcription (100 ng RNA/reaction) was performed with the Transcriptor First Strand cDNA Synthesis Kit (Roche Inc., Mannheim, Germany). Real-time PCR was performed using cDNA diluted 10-fold in sterile water and a SYBR Green PCR Master Mix. The primers for both forward and reverse lines for VDR, CYP24A1 and TRPV6 genes were designed based on the mouse and rat sequences using Primer Quest software (Integrated Device Technology, San Jose, CA, USA). Reactions (in triplicate) were performed at 50°C for 2 min, 95°C for 10 min and then 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. Data were collected and analyzed on a Roche Light Cycler 480. The amount of the final amplified product for each gene was compared and normalized to the amount of β-actin as a housekeeping gene using a comparative Ct method (25).
Antiproliferative assay
Antiproliferative assay was performed using the (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)/phenazine methosulfate (MTS/PMS) solution (Promega, Madison, WI, USA) as per the manufacturer’s instructions (26). Briefly, SKMEL-188 cells were plated in on 96-well plates with Ham’s F10 media containing 5% charcoal treated FBS (Atlanta Biologicals, Inc. Flowery Branch, GA, USA). After overnight culture, the medium was changed to serum-free medium to synchronize the cells for 24 h. Subsequently, the cells were incubated with compounds 10a–e for 48 h. Finally, 20 μl of MTS/PMS solution was added to the cells and they were incubated for another 4 h at 37°C then the absorbance was recorded at 490 nm using Cytation 5 Cell Imaging Multi-Mode Reader (Winooski, VT, USA).
Interferon-γ (IFNγ)-inhibition assay
Compounds 10a–e and 1,25D3 were solubilized in absolute EtOH at 10−4 M and diluted to 10–6 M by adding EMEM as described above (27). Splenocytes from DBA/1 mice were isolated, erythrocytes lysed by hypotonic shock, then cells were washed twice with EMEM, and suspended at 2×106/ml in supplemented EMEM described above. To each well of a 48-well tissue culture plate, 450 μl of the splenocytes were added. Analogs (50 μl of the 10−6 M stock), or EtOH diluted 1:100 with the above culture medium as negative control, were added to triplicate wells and then incubated at 37°C in 5% CO2 in a humidified tissue culture incubator for 2 h, after which 1.0 μg/well of rat monoclonal antibody to mouse cluster of differentiation 3 (CD3) was added. After a 72 h incubation, supernatants from each well were harvested and analyzed by the enzyme-linked immunosorbent assay (ELISA) to determine the levels of D-murine IFNγ (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. The concentration of IFNγ in supernatants from cultures containing analogs were compared with that of EtOH-treated control cultures, by ANOVA. Results are expressed as the mean of triplicate determinations±SEM.
Assay of leukocyte-associated immunoglobulin-like receptor 1 (LAIR1) by flow cytometry
Splenocytes from DBA/1 mice were isolated (3) and the level of expression of LAIR1 was determined following overnight culture with each analog at a concentration of 10−7 M (or ethanol as vehicle control) by multi-parameter flow cytometry using an LSRII flow cytometer (BD Biosciences, San Jose, CA, USA). Cells were labeled with fluorochrome antibodies specific for CD4 (BD Biosciences) and for LAIR1 (eBioscience, San Diego, CA, USA). Gating was performed on CD4+ cells and the data were displayed as mean fluorescence±standard deviation. A minimum of 10,000 cells were analyzed from each treated sample and the final analysis was performed using Flow software (Tree Star, Ashland, OR, USA). Results are expressed as the mean of duplicate values±SEM.
Statistical analysis
The values are reported as the means±SD (or SE). The significance of the differences between different treatments was estimated by unpaired, two-tailed Student’s t-test with p<0.05 considered as being statistically significant. All statistical analyses were performed and some of the figures were produced using GraphPad Prism 6.0 (Graph-Pad Software, San Diego, CA, USA).
Results
The designed 20D3 analogs were synthesized starting from commercially available pregnenolone acetate (1). Due to the sensitivity of 5,7-diene structure to light, heat and acidic conditions, we chose to introduce the double bond at the C7 position in later steps (3).
Characterization of 20D3 analogs gave the following results
(3S,8S,10R,13S,14S,17S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-17-carboxylic acid (2)
1H NMR (400 MHz, Chloroform-d) δ 5.19 (dd, J=4.9, 2.2 Hz, 1H), 3.31 (tt, J=10.7, 4.8 Hz, 1H), 3.20 (p, J=1.6 Hz, 2H), 2.19 (t, J=9.3 Hz, 1H), 2.09 (qdd, J=13.1, 9.0, 4.0 Hz, 2H), 1.99−1.79 (m, 3H), 1.72 (t, J=3.6 Hz, 1H), 1.55 (tdd, J=12.2, 6.9, 3.6 Hz, 1H), 1.42 (ddt, J=16.5, 12.7, 3.0 Hz, 2H), 1.37−1.23 (m, 3H), 1.12 (tdd, J=12.0, 7.8, 5.1 Hz, 2H), 1.03−0.88 (m, 2H), 0.86 (s, 3H), 0.84−0.77 (m, 1H), 0.57 (s, 3H). MS (ESI) m/z 317.1 [M-H]–.
(3S,8S,10R,13S,14S,17S)-methyl 3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-17-carboxylate (3)
1H NMR (400 MHz, Chloroform-d) δ 5.36 (dt, J=5.4, 2.0 Hz, 1H), 3.68 (s, 3H), 3.53 (tt, J=11.2, 4.7 Hz, 1H), 2.36 (t, J=9.3 Hz, 1H), 2.33−2.17 (m, 2H), 2.17−2.08 (m, 1H), 2.00 (ddt, J=7.5, 4.9, 2.7 Hz, 1H), 1.84 (dddd, J=19.4, 13.8, 7.1, 3.3 Hz, 3H), 1.77−1.65 (m, 1H), 1.65−1.56 (m, 2H), 1.56−1.45 (m, 2H), 1.45−1.38 (m, 1H), 1.36−1.21 (m, 2H), 1.17−1.04 (m, 2H), 1.02 (s, 3H), 1.00−0.93 (m, 1H), 0.92−0.80 (m, 1H), 0.68 (s, 3H). MS (ESI) m/z 355.4 [M+Na]+.
(3S,8S,10R,13S,14S,17S)-methyl 3-acetoxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-17-carboxylate (4)
1H NMR (400 MHz, Chloroform-d) δ 5.37 (dt, J=5.1, 1.6 Hz, 1H), 4.71−4.50 (m, 1H), 3.67 (s, 3H), 2.40−2.32 (m, 2H), 2.32−2.25 (m, 1H), 2.22−2.08 (m, 1H), 2.05 (s, 1H), 2.04 (s, 1H), 2.02−1.95 (m, 1H), 1.92−1.83 (m, 2H), 1.83−1.75 (m, 1H), 1.75−1.67 (m, 1H), 1.67−1.63 (m, 1H), 1.63−1.53 (m, 2H), 1.53−1.37 (m, 2H), 1.35−1.21 (m, 3H), 1.20−1.06 (m, 2H), 1.02 (s, 3H), 1.01−0.94 (m, 1H), 0.92−0.77 (m, 1H), 0.67 (s, 3H). MS (ESI) m/z 397.4 [M+Na]+.
(3S,10R,13S,14R,17S)-methyl 3-acetoxy-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-17-carboxylate (5)
1H NMR (400 MHz, Methanol-d4) δ 5.59 (dd, J=5.8, 2.6 Hz, 1H), 5.48−5.41 (m, 1H), 4.66 (tdd, J=11.4, 4.9, 3.9 Hz, 1H), 3.69 (s, 3H), 2.55−2.50 (m, 1H), 2.49 (q, J=2.9 Hz, 1H), 2.38 (ddd, J=14.3, 11.9, 2.3 Hz, 1H), 2.24−2.15 (m, 1H), 2.12 (ddd, J=12.8, 3.9, 2.5 Hz, 1H), 2.08−2.05 (m, 3H), 2.04 (s, 3H), 1.99−1.80 (m, 3H), 1.71 (ddt, J=11.4, 9.8, 5.9 Hz, 2H), 1.65−1.52 (m, 2H), 1.39 (tt, J=14.6, 5.4 Hz, 2H), 0.97 (s, 3H), 0.63 (s, 3H). MS (ESI) m/z 395.4 [M+Na]+.
(3S,10R,13S,14R,17S)-17-(2-hydroxypropan-2-yl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol (6a)
1H NMR (400 MHz, Chloroform-d) δ 5.58 (dd, J=5.7, 2.6 Hz, 1H), 5.40 (dt, J=5.6, 2.8 Hz, 1H), 3.64 (tt, J=11.2, 4.2 Hz, 1H), 2.47 (ddd, J=14.4, 4.8, 2.3 Hz, 1H), 2.28 (dddd, J=18.9, 11.8, 5.9, 3.7 Hz, 1H), 2.18 (ddt, J=10.6, 5.7, 2.9 Hz, 1H), 1.97 (ddt, J=9.4, 7.1, 2.3 Hz, 1H), 1.93−1.84 (m, 2H), 1.84−1.67 (m, 3H), 1.67−1.53 (m, 2H), 1.53−1.39 (m, 2H), 1.39−1.34 (m, 1H), 1.33 (s, 3H), 1.32−1.22 (m, 2H), 1.22 (s, 3H), 1.2 (m, 1H), 0.95 (s, 3H), 0.78 (s, 3H). MS (ESI) m/z 353.4 [M+Na]+. Yield 91%. HPLC purity > 98%. HRMS (ESI) m/z 313.2521 [M+H-H2O]+ (error: −3.2 ppm).
(3S,10R,13S,14R,17S)-17-(3-hydroxypentan-3-yl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol (6b)
1H NMR (400 MHz, Chloroform-d) δ 5.58 (dd, J=5.8, 2.5 Hz, 1H), 5.40 (dt, J=5.6, 2.8 Hz, 1H), 3.64 (tt, J=11.2, 4.1 Hz, 1H), 2.47 (ddd, J=14.4, 4.8, 2.3 Hz, 1H), 2.38−2.22 (m, 1H), 2.22−2.11 (m, 1H), 2.01−1.79 (m, 3H), 1.79−1.60 (m, 4H), 1.56−1.41 (m, 3H), 1.41−1.30 (m, 1H), 1.18−0.99 (m, 1H), 0.97 (d, J=6.6 Hz, 2H), 0.95 (s, 3H), 0.90 (m, 2H), 0.88 (s, 2H), 0.88−0.85 (m, 3H), 0.81 (s, 3H), 0.79 (t, J=7.6 Hz, 3H). MS (ESI) m/z 381.6 [M+Na]+. Yield 85%. HPLC purity >98%. HRMS (ESI) m/z 321.2844 [M+H-H2O]+ (error: 0.0 ppm).
(3S,10R,13S,14R,17S)-17-(3-hydroxypenta-1,4-dien-3-yl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol (6c)
1H NMR (400 MHz, Chloroform-d) δ 6.06 (dd, J=17.3, 10.7 Hz, 1H), 5.96 (dd, J=17.3, 10.7 Hz, 1H), 5.57 (dd, J=5.7, 2.5 Hz, 1H), 5.39 (dt, J=5.6, 2.7 Hz, 1H), 5.23 (ddd, J=37.5, 17.3, 1.3 Hz, 2H), 5.06 (ddd, J=27.4, 10.7, 1.3 Hz, 2H), 3.71−3.55 (m, 0H), 2.47 (ddd, J=14.3, 4.9, 2.4 Hz, 1H), 2.28 (t, J=12.9 Hz, 1H), 2.18 (ddd, J=12.6, 4.8, 2.5 Hz, 1H), 1.95 (t, J=9.7 Hz, 1H), 1.91−1.80 (m, 2H), 1.79−1.62 (m, 3H), 1.57 (m, 1H), 1.49−1.38 (m, 2H), 1.34−1.23 (m, 3H), 1.18 (td, J=13.0, 4.9 Hz, 1H), 0.93 (s, 3H), 0.88−0.82 (m, 1H), 0.73 (s, 3H). MS (ESI) m/z 377.4 [M+Na]+. HPLC purity >98%. HRMS (ESI) m/z 337.2528 [M+H-H2O]+ (error: −0.9 ppm).
(3S,10R,13S,14R,17S)-17-(4-hydroxyheptan-4-yl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol (6d)
1H NMR (400 MHz, Chloroform-d) δ 5.58 (dd, J=5.7, 2.5 Hz, 1H), 5.40 (dt, J=5.6, 2.7 Hz, 1H), 3.64 (tt, J=11.3, 4.1 Hz, 1H), 2.47 (ddd, J=14.3, 4.8, 2.3 Hz, 1H), 2.29 (ddq, J=13.7, 11.2, 2.1 Hz, 1H), 2.15 (ddd, J=12.6, 4.8, 2.6 Hz, 1H), 2.02−1.92 (m, 1H), 1.92−1.79 (m, 3H), 1.79−1.68 (m, 2H), 1.67−1.53 (m, 3H), 1.53−1.47 (m, 1H), 1.47−1.39 (m, 2H), 1.39−1.27 (m, 3H), 1.27−1.17 (m, 2H), 0.95 (s, 3H), 0.94−0.85 (m, 14H), 0.81 (s, 3H). MS (ESI) m/z 409.5 [M+Na]+. Yield 63%. HPLC purity >98%. HRMS (ESI) m/z 369.3157 [M+H-H2O]+ (error: 0.0 ppm).
(3S,10R,13S,14R,17S)-17-(5-hydroxynonan-5-yl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol (6e)
1H NMR (400 MHz, Chloroform-d) δ 5.51 (dd, J=5.8, 2.5 Hz, 1H), 5.33 (dt, J=5.6, 2.8 Hz, 1H), 3.57 (tt, J=11.2, 4.0 Hz, 1H), 2.45−2.34 (m, 1H), 2.22 (t, J=12.7 Hz, 1H), 2.12−2.05 (m, 1H), 1.89 (t, J=9.5 Hz, 1H), 1.85−1.72 (m, 3H), 1.72−1.61 (m, 2H), 1.61−1.47 (m, 6H), 1.46−1.31 (m, 3H), 1.31−1.16 (m, 6H), 1.16−1.09 (m, 1H), 1.08 (s, 2H), 0.88 (s, 3H), 0.87−0.76 (m, 14H), 0.74 (s, 3H). MS (ESI) m/z 437.5 [M+Na]+. Yield 55%. HPLC purity > 98%. HRMS (ESI) m/z 397.3468 [M+H-H2O]+ (error: −0.5 ppm).
(S,Z)-3-((E)-2-((1S,3aS,7aS)-1-(2-hydroxypropan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexanol (10a)
1H NMR (400 MHz, Chloroform-d) δ 6.15 (d, J=11.3 Hz, 1H), 5.96 (d, J=11.2 Hz, 1H), 4.97 (dt, J=2.6, 1.4 Hz, 1H), 4.74 (d, J=2.5 Hz, 1H), 3.88 (s, 1H), 2.75 (dd, J=12.1, 4.3 Hz, 1H), 2.56−2.44 (m, 1H), 2.32 (ddd, J=13.1, 7.9, 4.8 Hz, 1H), 2.21 (dd, J=13.1, 7.5 Hz, 1H), 2.10 (ddd, J=13.6, 8.5, 4.7 Hz, 1H), 2.05−1.97 (m, 1H), 1.96−1.88 (m, 1H), 1.88−1.79 (m, 1H), 1.76−1.52 (m, 4H), 1.45 (m, 2H), 1.25 (s, 1H), 1.23 (s, 3H), 1.17 (s, 2H), 1.13 (m, 1H), 1.12 (s, 3H), 0.63 (s, 3H). MS (ESI) m/z 353.4 [M+Na]+. HPLC purity >98%. HRMS (ESI) m/z 313.2533 [M+H-H2O]+ (error: 0.6 ppm).
(S,Z)-3-((E)-2-((1S,3aS,7aS)-1-(3-hydroxypentan-3-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexanol (10b)
1H NMR (400 MHz, Methanol-d4) δ 6.24 (d, J=11.2 Hz, 1H), 6.04 (d, J=11.2 Hz, 1H), 5.09−5.02 (m, 1H), 4.77 (dd, J=2.8, 1.2 Hz, 1H), 3.78 (tt, J=8.8, 4.0 Hz, 1H), 2.87 (dd, J=11.8, 3.9 Hz, 1H), 2.55 (dd, J=12.9, 4.1 Hz, 1H), 2.43 (dt, J=13.6, 5.0 Hz, 1H), 2.26−2.16 (m, 1H), 2.16−2.07 (m, 2H), 2.07−1.92 (m, 2H), 1.92−1.79 (m, 1H), 1.79−1.61 (m, 6H), 1.61−1.45 (m, 6H), 1.45−1.35 (m, 2H), 0.93−0.79 (m, 1H), 0.89 (t, J=7.5 Hz, 1H), 0.83 (t, J=7.5 Hz, 3H), 0.74 (s, 3H). MS (ESI) m/z 381.6 [M+Na]+. HPLC purity >98%. HRMS (ESI) m/z 321.2842 [M+H-H2O]+ (error: −0.6 ppm).
(S,Z)-3-((E)-2-((1S,3aS,7aS)-1-(3-hydroxypenta-1,4-dien-3-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene) ethylidene)-4-methylenecyclohexanol (10c)
1H NMR (400 MHz, Methanol-d4) δ 6.22 (d, J=11.2 Hz, 1H), 6.12−5.96 (m, 3H), 5.22 (ddd, J=32.5, 17.3, 1.7 Hz, 2H), 5.05 (dd, J=2.8, 1.2 Hz, 1H), 5.00 (ddd, J=32.5, 17.3, 1.7 Hz, 2H), 4.76 (dd, J=2.8, 1.2 Hz, 1H), 3.78 (tt, J=9.1, 3.9 Hz, 1H), 2.90−2.79 (m, 1H), 2.60−2.49 (m, 1H), 2.42 (dt, J=13.6, 5.0 Hz, 1H), 2.27−2.19 (m, 1H), 2.14 (dddd, J=13.7, 10.6, 4.6, 1.6 Hz, 2H), 1.99 (q, J=9.2 Hz, 2H), 1.88−1.74 (m, 2H), 1.74−1.60 (m, 2H), 1.60−1.38 (m, 3H), 1.28 (td, J=12.8, 3.9 Hz, 2H), 1.03−0.67 (m, 1H), 0.65 (s, 3H). MS (ESI) m/z 377.4 [M+Na]+. HPLC purity > 98%. HRMS (ESI) m/z 337.2528 [M+H-H2O]+ (error: −0.9 ppm).
(S,Z)-3-((E)-2-((1S,3aS,7aS)-1-(4-hydroxyheptan-4-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexanol (10d)
1H NMR (400 MHz, Chloroform-d) δ 6.23 (d, J=11.2 Hz, 1H), 6.03 (d, J=11.3 Hz, 1H), 5.05 (dt, J=2.6, 1.4 Hz, 1H), 4.82 (d, J=2.5 Hz, 1H), 3.95 (s, 1H), 2.82 (dd, J=11.7, 4.1 Hz, 1H), 2.57 (dd, J=13.2, 3.8 Hz, 1H), 2.40 (ddd, J=13.1, 7.9, 4.8 Hz, 1H), 2.29 (dd, J=13.2, 7.5 Hz, 1H), 2.18 (ddd, J=13.6, 8.5, 4.7 Hz, 1H), 2.12−2.02 (m, 1H), 2.02−1.86 (m, 2H), 1.87−1.75 (m, 1H), 1.69 (ddd, J=11.6, 9.0, 5.6 Hz, 5H), 1.56 (d, J=9.9 Hz, 3H), 1.45−1.27 (m, 5H), 1.27−1.15 (m, 2H), 1.09 (s, 1H), 0.90 (dt, J=10.2, 7.1 Hz, 7H), 0.74 (s, 3H). MS (ESI) m/z 409.5 [M+Na]+. HPLC purity >98%. HRMS (ESI) m/z 369.3152 [M+H-H2O]+ (error: −1.4 ppm).
(S,Z)-3-((E)-2-((1S,3aS,7aS)-1-(5-hydroxynonan-5-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexanol (10e)
1H NMR (500 MHz, Chloroform-d) δ 6.23 (d, J=11.2 Hz, 1H), 6.03 (d, J=11.3 Hz, 1H), 5.05 (s, 1H), 4.82 (s, 1H), 3.95 (s, 1H), 2.82 (d, J=13.1 Hz, 1H), 2.57 (d, J=13.1 Hz, 1H), 2.39 (dd, J=13.5, 6.6 Hz, 1H), 2.29 (dd, J=13.2, 7.5 Hz, 1H), 2.23−2.11 (m, 1H), 2.06 (d, J=15.8 Hz, 1H), 1.99 (t, J=9.5 Hz, 1H), 1.92 (s, 1H), 1.84−1.75 (m, 1H), 1.74−1.63 (m, 4H), 1.56 (m, 2H), 1.44−1.33 (m, 1H), 1.33−1.22 (m, 8H), 1.19 (q, J=7.2 Hz, 1H), 1.08 (s, 1H), 0.91 (q, J=6.9 Hz, 10H), 0.74 (s, 3H). MS (ESI) m/z 437.5 [M+Na]+. HPLC purity > 98%. HRMS (ESI) m/z 397.3469 [M+H-H2O]+ (error: −0.3 ppm).
To evaluate the capability of these analogs to interact with the VDR, a previously established Jurkat cell line transduced with a VDRE luciferase vector construct was used to carry out the reporter assays. We compared the activity of five analogs with that of two positive controls (1,25D3 and 22-oxa-1,25D3) to activate the VDR via binding to the synthetic VDRE in this construct. In preliminary studies, these analogs lacked the ability to activate the VDR at a concentration of 0.1 μM, however, as shown in Figure 3A, all five analogs significantly activated VDR at a concentration of 1.0 μM. The detected luciferase signal increased 31%, 20%, 27%, 20% and 33% as compared with blank controls for compounds 10a, 10b, 10c, 10d and 10e, respectively. Compared to 1,25D3 and 22-oxa-1,25D3, these analogs caused weak activation of the VDR causing 33- and 30-times less activation respectively, at 0.1 μM.
Figure 3.
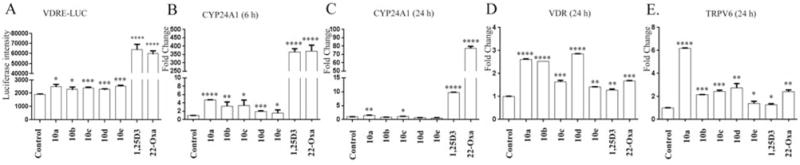
Gemini analogs of 20S-hydroxyvitamin D3 (20D3) activate the VDR in a vitamin D response element-luciferase (VDRE-LUC) reporter assay and regulate cytochrome P450 24A1 (CYP24A1), vitamin D receptor (VDR) and transient receptor potential cation channel V6 (TRPV6) genes. A: Jurkat cells transduced with a VDRE-LUC reporter construct were treated for 24 h with Gemini analogs (10a–10e, 1.0 μM), 1α,25-dihydroxyvitamin D3 (1,25D3) (0.1 μM), 22-oxa-1,25D3 (22-Oxa) (0.1 μM) or 10% dimethyl sulfoxide (DMSO) (final concentration 0.1% DMSO) as a negative control. Gemini 20D3 analogs increased mRNA levels for CYP24A1 (B and C), VDR (D) and TRPV6 (E). HaCaT cells were treated with 100 nM of Gemini analogs, 1,25D3, 22-Oxa or DMSO only (solvent) as a control. The mRNA was isolated and the real-time polymerase chain reaction was performed using specific primers for CYP24A1, VDR and TRPV6 genes. Data are presented as mean±SE (n=3). *p<0.05, **p<0.01, ***p<0.005 ****p<0.001 compared to the control.
We compared the activity of the five synthetic analogs on the expression of CYP24A1 gene in HaCaT cells to that of 1,25D3 and 22-oxa-1,25D3. As shown in Figure 3B, after 6 h treatment with 0.1 μM of each analog, relative mRNA levels for CYP24A1 were 4.7-, 3.2-, 3.4-, 1.9- and 1.6-fold higher than that of the negative control for analogs 10a, 10b, 10c, 10d and 10e, respectively. In comparison, cells treated with 0.1 μM 1,25D3 or 22-oxa-1,25D3 showed a 366- or 370-fold increase in mRNA for CYP24A1, respectively. Following 24 h of treatment (Figure 3C), the positive controls still caused greater stimulation of CYP24A1 gene expression than did the Gemini analogs, with the degree of stimulation for all compounds being less than at 6 h. Only 10a and 10c significantly stimulated expression over that of the control while 1,25D3 and 22-oxa-1,25D3 caused 10- and 78-fold stimulation, respectively.
The ability of the new Gemini analogs to regulate the expression of the VDR gene was studied using HaCaT cells. As shown in Figure 3D, the mRNA expression level was stimulated by 1.4- to 3-fold by the Gemini analogs following 24 h of treatment with 0.1 μM. In addition, the two positive controls, 1,25D3 and 22-oxa-1,25D3, also stimulated VDR expression by 1.3- and 1.7-fold, respectively, in comparison with the negative control. Interestingly, compound 10c and 10e caused significantly higher stimulation of the expression of the VDR gene than did 1,25D3 (p<0.05), and analogs 10a, 10b and 10d displayed much higher stimulation of VDR expression than both 1,25D3 and 22-oxa-1,25D3 (p<0.01). These results suggest that the new analogs may increase D3 catabolism, not only by mild stimulation of the expression of the hydroxy-D3-catabolizing enzyme (CYP24A1), but also through increased expression of its own receptor, the VDR.
A well-known target of 1,25D3 is the stimulation of the expression of the TRPV6 gene (encoding an intestinal calcium channel) (28). Because calcium plays an important role in keratinocytes differentiation, we evaluated the effects of the new Gemini 20D3 analogs on the expression of this gene by immortalized human epidermal keratinocytes (HaCaT cells). The mRNA levels of TRPV6 after a 24-h treatment were increased by 1.4- and 2.6-fold on 1,25D3 and 22-oxa-1,25D3 treatment, respectively (Figure 3E). In contrast, the mRNA level for TRPV6 was increased by 5.9-fold for analog 10a relative to the negative control, but only by 1.5- to 2.7-fold for the other Gemini analogs, similarly to that for 1,25D3 and 22-oxa-1,25D3. Interestingly the analog with the shortest side chain (10a) caused 4-fold higher expression of the TRPV6 gene expression than analog 10e with the longest side chain, indicating that the short aliphatic side chain is most favorable for regulation of the TRPV6 gene.
One of the 20D3 analogs, 10e, inhibited SKMEL-188 melanoma cell proliferation with comparable potency (1.24×10−9 M) to that seen for 1,25D3 (1.05×10−9 M), while analog 10d had 15-fold lower potency than 1,25D3. Analogs 10a, 10b and 10c displayed no significant antiproliferative activity against the growth of SKMEL-188 melanoma cells. These results suggest that a long side chain is required to inhibit proliferation with a potency similarly to that of 1,25D3.
1,25D3 acts as an immunomodulatory agent and displays anti-inflammatory activity (3, 5). Thus many analogs of 1,25D3 have been developed with the hope that they can be used as anti-inflammatory agents (5). To test whether the new Gemini-20D3 analogs can exert anti-inflammatory effects, IFNγ concentrations in the medium used to culture mouse splenocytes were measured using our established assay (3). The Gemini analogs were tested at a concentration of 0.1 μM (Figure 4B). 1,25D3 reduced the IFNγ concentration by 60% compared to the ethanol control. All the analogs significantly reduced IFNγ levels, with the most active compound being 10b, which caused a 62% reduction in the IFNγ concentration (62%), comparable to that seen for 1,25D3.
Figure 4.
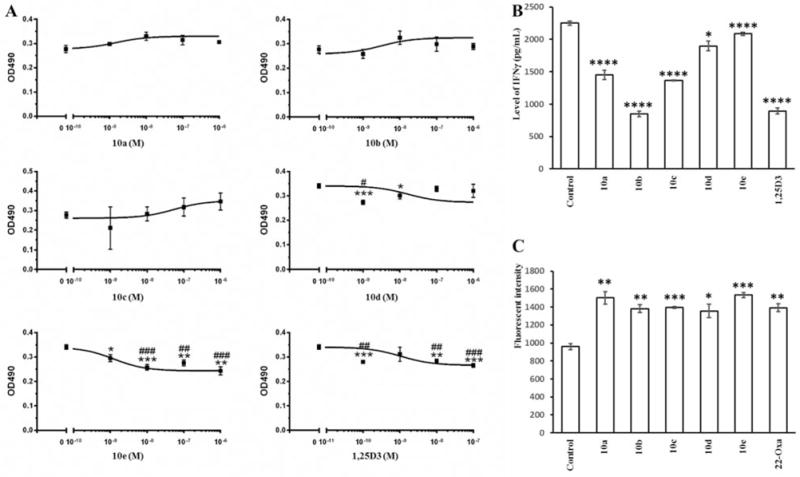
Antiproliferative effects of Gemini 20S-hydroxyvitamin D3 (20D3) analogs on human SKEML-188 melanoma cells and anti-inflammatory effects on splenocytes. A: SKEML-188 melanoma cells were treated with analogs, 1α,25-dihydroxyvitamin D3 (1,25D3) or dimethyl sulfoxide (DMSO) (solvent) as a control to assess their inhibitory effects on cell growth. Gemini 20D3 analogs reduced interferon-γ (IFNγ) concentration (B) and up-regulated leukocyte-associated immunoglobulin-like receptor 1 (LAIR1) levels (C) in mouse splenocytes. Splenic cells were treated by 100 nM of Gemini analogs, 1,25D3, 22-Oxa or EtOH only (solvent) as a control. Data are presented as mean±SE (n=3). *p<0.05, **p<0.01, ***p<0.005, ****p<0.001 compared to the control. Half-maximal inhibitory concentration (IC50): 1.65×10−8 M (10d), 1.24×10−9 M (10e) and 1.05×10−9 M (1,25D3).
Part of the anti-inflammatory activity of 1,25D3 is mediated by the up-regulation of LAIR1. LAIR1 is a receptor expressed on T-cells and other immune cells believed to down-regulate the immune response (29). The ability of the new Gemini analogs (100 nM) to up-regulate LAIR1 protein in splenocytes was compared with the EtOH negative control and a 22-oxa-1,25D3 (22-Oxa) positive control (Figure 4C). All the new Gemini analogs caused a significant increase in LAIR1 levels, generally comparable to that seen for the 22-Oxa positive control, with the highest stimulation being seen for analog 10e (59%). The data suggest that all the Gemini-20D3 analogs are strong anti-inflammatory agents.
Discussion
To completely remove the 3-acetyl group on intermediate 5, at least four equivalences of Grignard reagents were required to form the desired product 6. Among these 7DHC analogs, 6c was not obtained through a normal Grignard reaction in our initial trials due to the formation of an α,β-unsaturated ketone after the first attack of the vinyl side chain. Fortunately, the addition of anhydrous CeCl3 following a reported procedure (24) solved this problem and we ended up with an 86% yield. Grignard reagents with longer side chains (C≥5) were used to produce more 7DHC analogs (6), however after UVB irradiation, we were unable to separate the corresponding D3 structures from the mixture.
The Gemini-20D3 analogs showed similar ability to activate the VDR and bind to a synthetic VDRE reporter construct, to their parent compound (20D3) reported in our previous study (11). Receptor activation was less than for than classical VDR activators (1,25D3 and 22-oxa-1,25D3). These results are consistent with these analogs acting as biased agonists on the VDR, similar to 20D3 (7), where the ligand can influence the relative binding to different VDREs.
The CYP24A1 gene has two VDREs and is highly responsive to 1,25D3, but poorly responsive to 20D3 (7, 11). CYP24A1 is responsible for the catabolism of 25D3 and 1,25D3 and can act on numerous vitamin D analogs (4, 5). CYP24A1 initially hydroxylates the vitamin D side chain at C24 converting 1,25D3 into 1,24,25(OH)3D3 (3, 4). The ability of Gemini-20D3 analogs to stimulate the expression of the CYP24A1 gene, although only weakly, supports that they act through the VDR. Their inability to cause the massive induction seen with 1,25D3 and 22-oxa-1,25D3 likely results in lower CYP24A1 protein levels and thus lower rates of catabolism, therefore promoting prolonged action. However, the ability of CYP24A1 to metabolize these analogs remains to be determined. Our data show that the Gemini 20D3 analogs stimulate the expression of the VDR gene in HaCaT cells, suggesting they can up-regulate the basal expression level of their own receptor, the VDR. This might be an alternate way for them to exert their biological activities other than by directly modulating target genes, such as CYP24A1. Moreover, Gemini 20D3 analogs were able to up-regulate the mRNA level of TRPV6 which is a membrane calcium channel involved in the first step of calcium absorption in the intestine. The expression of TRPV6 is reported to be vitamin D-dependent in mice and humans, and is greatly decreased in animals that do not express VDR (30). In addition, TRPV6 is a direct target of the VDR and positively controls cell proliferation and apoptosis resistance in prostate cancer (31). Investigating the modulating effects of D3 analogs on TRPV6 gene is, thus, very important in order to understand the correlation between vitamin D compounds and their antiproliferative activity.
We have previously reported that 20D3 has antiproliferative activity using a colony-forming model. 20D3 showed comparable inhibition of colony formation to that of 1,25D3 (16, 32), suggesting there is a great potential to use 20D3 as a antitumor therapeutic agent, especially given its low calcemic activity. To evaluate the antiproliferative activity of our Gemini analogs, we tested them on the growth of SKMEL-188 cells. Compound 10e, and to a lesser extent 10d, had half-maximal inhibitory concentrations (IC50s) (Figure 4) similar to 1,25D3. However, analogs with shorter side chains did not show significant inhibitory effects, indicating that a longer aliphatic side chains is necessary for antiproliferative activity.
IFNγ, or type II interferon, is the only member in the type II class of IFN. It is well known for its immunostimulatory and immunomodulatory effects and is critical for both innate and adaptive immunity (33). For this reason, IFNγ is treated as a common inflammatory marker. Our previous studies have shown that D3 metabolites down-regulated IFNγ produced by mouse splenocytes (3, 7), inhibited interleukin 17 production by mouse T-lymphocytes (27) and down-regulated nuclear factor kappa-light-chain-enhancer of activated B-cells, which is a master regulator of pro-inflammatory actions (34, 35). The Gemini 20D3 analogs caused similar decreases in IFNγ concentrations in cultured splenocytes to that of other D3 metabolites. To further validate their anti-inflammatory effects, flow cytometric measurements of LAIR1 protein levels in splenocytes were made. LAIR1, also designated as CD305 (cluster of differentiation 305), is encoded by the LAIR1 gene and is an inhibitory receptor expressed in many peripheral cells in both innate and adaptive immune systems, such as natural killer cells, T-cells and B-cells (36, 37). It is an important anti-inflammatory marker due to its ability to prevent lysis of cells recognized as self during an immune response. Together with the inhibitory effects on IFNγ, the up-regulation of LAIR1 levels confirms a role for these analogs in the regulation of the immune responses and inflammation.
Conclusion
To conclude (Figure 5), the new Gemini 20D3 analogs were able to activate the VDR. Analysis of gene expression at the mRNA level showed that the analogs regulated CYP24A1, VDR and TRPV6 genes, consistent with their effects being mediated through activation of the VDR (7). In addition, these analogs displayed antiproliferative and anti-inflammatory activity, which might also correlate with their VDR activation process. This study suggests that Gemini 20D3 analogs have great potential as therapeutic agents on the immune system.
Figure 5.
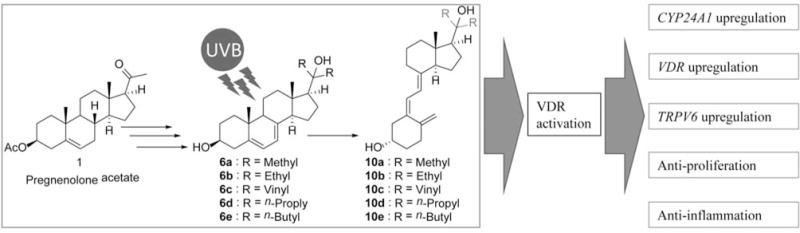
Summary of synthesis and biological activities of Gemini 20S-hydroxyvitamin D3 (20D3) analogs used in this study. The synthesis starts from pregnenolone acetate to obtain 7-dehydrocholesterol intermediates which are then irradiated by UVB to produce D3 structures. These analogs likely exert their activities, including gene up-regulation, antiproliferative and anti-inflammatory effects, through activation of the vitamin D receptor (VDR).
Acknowledgments
This work was supported by NIH grants 1R21AR063242-01A1 (W.L. and D.D.M.), 1S10OD010678-01 (W.L.), 1S10RR026377-01 (W.L.), and 2R01AR052190 (A.T.S.), R21 AR066505-01A1 (A.T.S.), 1R01AR056666-01A2 (A.T.S.) and VA Program Project Grant 1IPBX001607-01 (A.E.P. and A.T.S.). The content is solely the responsibility of the Authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
References
- 1.Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, Jurutka PW. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66:S98–112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- 2.Wierzbicka J, Piotrowska A, Zmijewski MA. The renaissance of vitamin D. Acta Biochim Pol. 2014;61:679–686. [PubMed] [Google Scholar]
- 3.Lin Z, Marepally SR, Ma D, Myers LK, Postlethwaite AE, Tuckey RC, Cheng CY, Kim TK, Yue J, Slominski AT, Miller DD, Li W. Chemical synthesis and biological activities of 20S,24S/R-dihydroxyvitamin D3 epimers and their 1alpha-hydroxyl derivatives. J Med Chem. 2015;58:7881–7887. doi: 10.1021/acs.jmedchem.5b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, Slominski A, Tuckey RC. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem Pharmacol. 2012;84:1696–1704. doi: 10.1016/j.bcp.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z, Li W. The roles of vitamin D and its analogs in inflammatory diseases. Curr Top Med Chem. 2016;16:1–20. doi: 10.2174/1568026615666150915111557. [DOI] [PubMed] [Google Scholar]
- 6.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Lin Z, Kim TK, Slominski AT, Miller DD, Li W. Total synthesis of biologically active 20S-hydroxyvitamin D3. Steroids. 2015;104:153–162. doi: 10.1016/j.steroids.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Scientific reports. 2015;5:14875. doi: 10.1038/srep14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013;5:7–19. doi: 10.4161/derm.23938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014;34:2153–2163. [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011;131:1167–1169. doi: 10.1038/jid.2010.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98:E298–303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pazos G, Rivadulla ML, Perez-Garcia X, Gandara Z, Perez M. Gemini analogs of vitamin D. Curr Top Med Chem. 2014;14:2388–2397. doi: 10.2174/1568026615666141208100411. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto R, Gery S, Kuwayama Y, Borregaard N, Ho Q, Alvarez R, Akagi T, Liu GY, Uskokovic MR, Koeffler HP. Novel Gemini vitamin D3 analogs: large structure/function analysis and ability to induce antimicrobial peptide. Int J Cancer. 2014;134:207–217. doi: 10.1002/ijc.28328. [DOI] [PubMed] [Google Scholar]
- 22.Pingili AK, Kara M, Khan NS, Estes AM, Lin Z, Li W, Gonzalez FJ, Malik KU. 6beta-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension. 2015;65:1279–1287. doi: 10.1161/HYPERTENSIONAHA.115.05396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Z, Yang R, Guan Z, Chen A, Li W. Ultra-performance LC separation and quadrupole time-of-flight MS identification of major alkaloids in Plumula nelumbinis. Phytochem Anal. 2014;25:485–494. doi: 10.1002/pca.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, Tuckey RC, Slominski AT, Li W, Miller DD. Design, synthesis, and biological action of 20R-hydroxyvitamin D3. J Med Chem. 2012;55:3573–3577. doi: 10.1021/jm201478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sittampalam GS, Gal-Edd N, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Lemmon V, Li Z. Cell Viability Assays. In: Sittampalam GS, Coussens NP, Nelson H, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Iversen PW, Li Z, McGee J, McManus O, Minor L, Napper A, Peltier JM, Riss T, Trask J Jr, Weidner J, editors. Assay Guidance Manual [Internet] Bethesda: Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004. [PubMed] [Google Scholar]
- 27.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 29.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 30.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehen’kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene. 2007;26:7380–7385. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 34.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23 Dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang X, Lu Z, Cui C, Deng M, Fan Y, Dong B, Han X, Xie F, Tyner JW, Coligan JE, Collins RH, Xiao X, You MJ, Zhang CC. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat Cell Biol. 2015;17:665–677. doi: 10.1038/ncb3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perbellini O, Falisi E, Giaretta I, Boscaro E, Novella E, Facco M, Fortuna S, Finotto S, Amati E, Maniscalco F, Montaldi A, Alghisi A, Aprili F, Bonaldi L, Paolini R, Scupoli MT, Trentin L, Ambrosetti A, Semenzato G, Pizzolo G, Rodeghiero F, Visco C. Clinical significance of LAIR1 (CD305) as assessed by flow cytometry in a prospective series of patients with chronic lymphocytic leukemia. Haematologica. 2014;99:881–887. doi: 10.3324/haematol.2013.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]


