Abstract
Wiskott-Aldrich syndrome (WAS) is a life-threatening immunodeficiency caused by mutations within the WAS gene. Viral gene therapy to restore WAS protein (WASp) expression in hematopoietic cells of patients with WAS has the potential to improve outcomes relative to the current standard of care, allogeneic bone marrow transplantation. However, the development of viral vectors that are both safe and effective has been problematic. While use of viral transcriptional promoters may increase the risk of insertional mutagenesis, cellular promoters may not achieve WASp expression levels necessary for optimal therapeutic effect. Here we evaluate a self-inactivating (SIN) lentiviral vector combining a chromatin insulator upstream of a viral MND (MPSV LTR, NCR deleted, dl587 PBS) promoter driving WASp expression. Used as a gene therapeutic in Was−/− mice, this vector resulted in stable WASp+ cells in all hematopoietic lineages and rescue of T and B cell defects with a low number of viral integrations per cell, without evidence of insertional mutagenesis in serial bone marrow transplants. In a gene transfer experiment in non-human primates, the insulated MND promoter (driving GFP expression) demonstrated long-term polyclonal engraftment of GFP+ cells. These observations demonstrate that the insulated MND promoter safely and efficiently reconstitutes clinically effective WASp expression and should be considered for future WAS therapy.
Keywords: Wiskott-Aldrich Syndrome, gene therapy, insulated lentivirus
Introduction
Wiskott-Aldrich syndrome (WAS) is an X-linked primary immunodeficiency disease resulting from mutations within the WAS gene that result in the loss of WAS protein (WASp) or function. Subjects with WAS suffer from combined immunodeficiency, thrombocytopenia, and eczema and also frequently develop autoimmunity and lymphoid malignancies.1, 2, 3, 4 WASp is a scaffold protein involved in signal transduction pathways that activate the actin cytoskeleton downstream of multiple cell surface receptors, including the B and T cell antigen receptors.5, 6, 7 Although the disease phenotype can be alleviated with hematopoietic stem cell transplantation (HSCT), the success of this therapy is variable, depending on factors such as the patient’s age, donor compatibility, conditioning regimen, and the extent of reconstitution. In the absence of a histocompatibility leukocyte antigen (HLA)-matched donor, transplantation with a mismatched donor has a reduced survival rate.3, 8, 9 Since the phenotype of WAS deficiency impacts only hematopoietic cells, gene therapy is a possible alternative. In this approach, a WASp expression cassette is stably integrated into the chromatin of autologous hematopoietic stem cells (HSCs) using viral-based gene delivery.
Previous and ongoing clinical trials have demonstrated the efficacy of gene therapy for alleviating the pathologies of WAS.10, 11, 12 Importantly, following development of T cell leukemia due to insertional mutagenesis in γ-retroviral gene therapy trials for both severe combined immunodeficiency (SCID) and WAS,13, 14, 15 much research has focused on strategies for eliminating this risk. The use of self-inactivating (SIN) lentiviruses (LVs) for gene transfer is one critical improvement, combining a safer integration profile (less affinity for insertions near promoters than γ-retroviruses16, 17, 18) with the ability to select internal promoters that optimize transgene expression and safety.19 Because of the association between internal promoter strength and transformation potential,19 internal promoters are selected for their ability to recapitulate endogenous expression levels and regulation, as well as for the lack of transactivation potential both in vitro and in vivo. These considerations are particularly important for treating WAS based on the following findings: sub-endogenous levels of WASp expression may hinder the reconstitution of murine B cell, T cell, and myeloid subsets and platelets;20 insufficient WASp expression in B cells compared to T cells can drive acquisition of autoimmunity;21, 22, 23 and patients with WAS are predisposed to malignancies and clonal expansion.1, 3, 4 Current clinical trials for WAS utilize a SIN-LV with an internal promoter consisting of the proximal 1.6 kb of the endogenous WAS promoter (WS1.6) to drive human WASp (hWASp) expression.10, 12 Patients treated with this SIN-LV showed improvements in immunity to infections, resolved eczema, and protection from bleeding, without evidence of clonal expansion of cells10, 12 or loss of self-tolerance.24, 25 However, clinical improvement required relatively high levels of viral marking and alleviation of the WAS phenotype was incomplete with, most notably, limited or no improvement in platelet counts. In previous mouse gene therapy experiments, we found that the WS1.6 promoter did not effectively rescue WASp expression in all lineages including B cells and resulted in the acquisition of features of humoral autoimmunity.20 In contrast, an SIN-LV using a synthetic promoter derived from a γ-retrovirus called MND (MPSV LTR, NCR deleted, dl587 PBS)26 as an internal promoter rescued WASp expression in all affected lineages and reduced the risk of autoimmunity.20, 27, 28
In a clinical gene therapy trial for adrenoleukodystrophy, MND has been used as an internal promoter for LV gene therapy without adverse effects.29 Although strongly trans-activating promoters are associated with an enhanced risk of insertional transactivation, this risk can be diminished by including a chromatin insulator.28, 30, 31 Chromatin insulators act as boundary elements preventing interactions between adjacent chromatin domains.32, 33, 34 Candidate insulator constructs can function as “barrier only” elements (acting as barriers to epigenetic chromatin silencing) or “enhancer-blocker only” elements (that prevent promoter transactivation between the domains that they separate), or they can have both activities (as reviewed in Emery et al.35 and Raab et al.36). One of the best characterized insulators, chicken β globin hypersensitive site 4 (cHS4), exhibits both barrier and enhancer-blocker activity.37 The insulator properties of the canonical 1.2-kb cHS4 can be recapitulated by a 650-bp sequence consisting of the 250-bp cHS4 core and 400 bp of sequence at its 3′ end.37 Because the 3′ long terminal repeat (LTR) of the LV is copied and replaces the 5′ LTR upon integration into the host chromatin, the insulator sequence that is cloned within the 3′ LTR will result in the integrated LV being bordered with insulator sequences at both ends. In previous in vitro assays testing the safety of an insulated SIN-LV incorporating an MND internal promoter, we have shown that the 650-bp insulator significantly reduces transactivation of LMO2 when placed in close proximity to the LMO2 promoter.27 Additionally, the insulated MND LV did not promote a pre-leukemic block in differentiation of primary murine thymocytes following transduction and in vitro culture.38
Our group also previously tested a series of cHS4-insulated and non-insulated SIN-LV constructs containing various internal promoters (MND, EF1α, and WAS 1.6-kb and 0.5-kb promoters) driving GFP reporter gene expression in a mouse gene therapy model. HSCT with LV containing the 650-bp cHS4-insulated MND-GFP (650.MND.GFP) resulted in GFP expression in all hematopoietic lineages, including platelets, that was stable over time. The 650.MND.GFP LV also showed the highest GFP expression per viral integration.28 Therefore, based on our combined efficacy and expression data, a producer cell clone capable of generating high-titer LV with the 650-bp cHS4 insulator and MND-WASp expression cassette (650.MND.hWASp) was developed for clinical use.27 In the current study, we utilize this clinical vector to perform an expanded efficacy and safety study. Our analysis also includes a direct comparison of LV with and without the 650-bp cHS4 insulator. Efficacy and safety was assessed in a large cohort of Was−/− mice, including serial transplants. We also evaluated the safety profile of these SIN-LV vectors in non-human primates (NHPs), expressing GFP instead of hWASp to improve assay sensitivity. Our combined observations strongly support future use of the insulated MND promoter for clinical WAS gene delivery.
Results
650.MND.hWASp LV Efficiently Rescues WASp Expression in Affected Hematopoietic Lineages
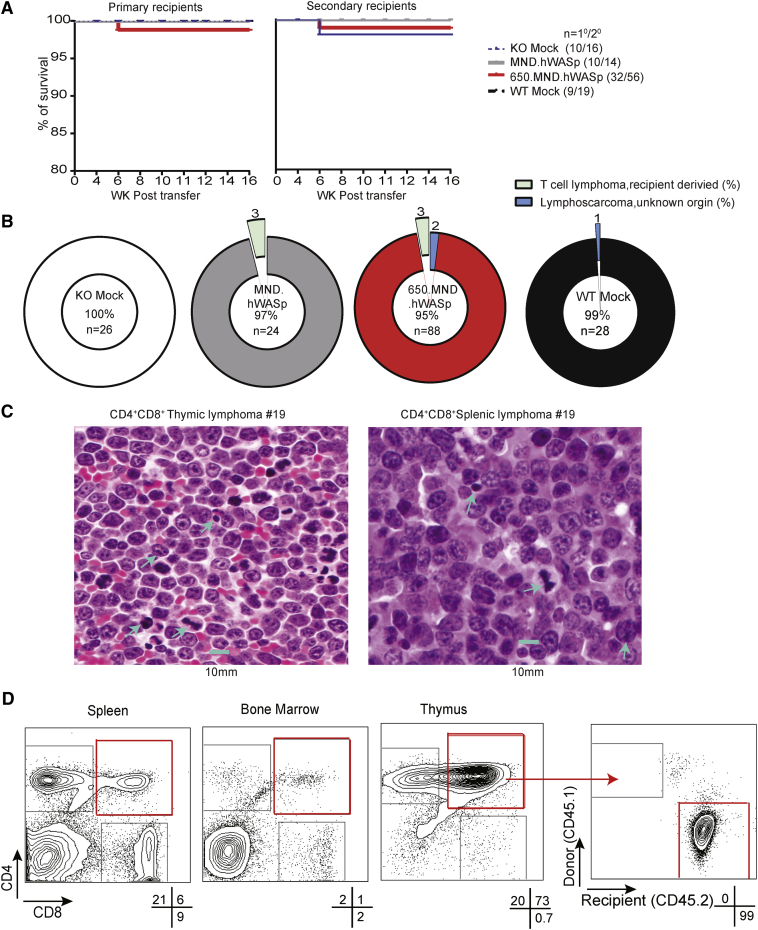
We evaluated the ability of the insulated SIN-LV 650.MND.hWASp (Figure 1A), generated from a clinical-grade producer cell line,27 to stably reconstitute WASp expression in the appropriate hematopoietic cell lineages using a Was−/− mouse gene therapy model. Expression from this LV was compared, in parallel, to the non-insulated version, MND.hWASp, that previously demonstrated reconstitution of Was−/− hematopoietic cell defects.20 Lineage-depleted (Lin−) bone marrow (BM) cells from Was−/− (KO) mice were transduced with SIN-LV at a MOI of 50 and transplanted into lethally irradiated Was−/− mice. In parallel, mock transduced Was−/− or C57BL/6 Lin− BM cells were transplanted as negative (KO mock) and positive (WT mock) controls, respectively. Cells from donor mice were distinguishable from recipients by surface staining for the congenic CD45.1/CD45.2 alleles. We also performed secondary transplants to more rigorously test vector safety and to verify the reconstitution of WASp in long-term repopulating HSCs. At 12–15 weeks post-transplant, peripheral blood (PB) samples were obtained for analysis of platelets; 16 weeks post-transplant, mice were euthanized and a complete necropsy, as well as extensive phenotyping and functional assessment of hematopoietic cells within the thymus, BM, and spleen, was performed (Figure 1B). Secondary recipients were similarly assessed; however, thymus tissue was assessed only in the event that expansion of T cells was identified in the spleen or BM.
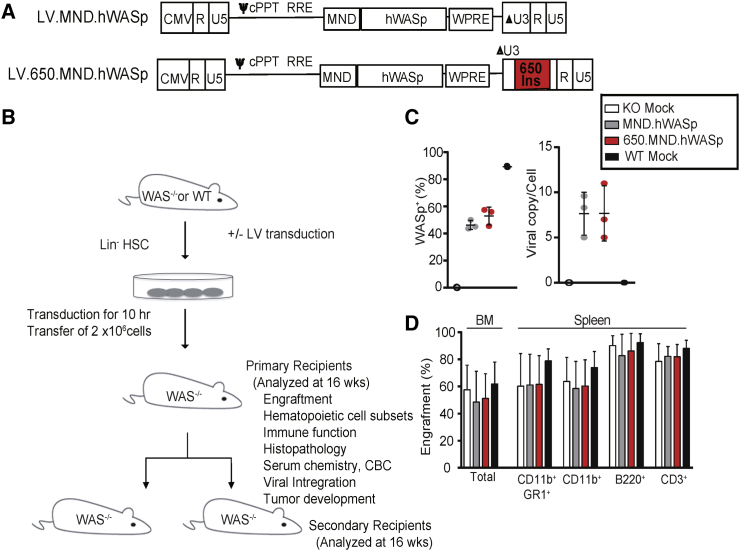
Figure 1.
Evaluation of a Clinical SIN-LV for the Treatment of WAS in a Mouse Gene Therapy Model
(A) Configuration of SIN-LVs used. Expression of hWASp (with a 3′ UTR consisting of the woodchuck hepatitis virus post-transcriptional regulatory element [WPRE]) is driven by an MND promoter. Both of the 5′ long terminal repeats (LTRs) are composed of the following: a cytomegalovirus (CMV) promoter and HIV-1 R and U5 regions; the 3′ LTR of both viruses have R and U5 regions and a modified U3 region (promoter deleted; ΔU3); the insulated version (below) has a 650-bp fragment derived from the cHS4 element inserted into the ΔU3 region of the LV LTR (650 INS; red box). cPPT, central polypurine track; ψ, Psi packaging sequence; RRE, Rev response element. (B) Experimental design for in vivo studies; further details are described in the Materials and Methods. (C) Percentage of gene therapy-treated Lin− cells expressing hWASp (left) and the lentiviral copy number (VCN) per cell (right) of an aliquot of input cells cultured for 7 days. (D) Percentage of donor cells (expressing donor CD45 allele) in total BM and splenic subsets 16 weeks after transplantation with gene therapy-treated Lin− BM. Means ± SD are shown.
Analysis of LV-transduced donor Lin− BM cells demonstrated an equal viral copy number (VCN) and WASp expression for both SIN-LV vectors at transplantation. WASp-expressing Lin− BM cells were 44% and 51% (compared to 89% for WT mock) after LV MND.hWASp and 650.MND.hWASp transduction, respectively (Figure 1C). At 16 weeks post-transplant, we identified high donor engraftment levels (based on the percentage of cells congenic for the donor CD45 allele) in the BM (49%–61%) and in splenic neutrophil (60%–78%), monocyte (59%–73%), B cell (82%–92%), and T cell (78%–88%) compartments in all treatment groups (Figure 1D).
Using a WASp-specific antibody for intracellular staining, we found that MND.hWASp and 650.MND.hWASp experimental groups exhibited WT levels of WASp expression in BM and splenic populations, including neutrophils, monocytes, B cells, CD4 and CD8 T cells, and natural killer (NK) cells (Figures 2A–2D, S1A, and S1B), with expression levels significantly increased relative to KO mock recipients. There was no statistically significant difference in the mean fluorescence intensity (MFI) of WASp intracellular staining among the two LV-treated groups (Figure S1C). Myeloid cells lack a selective advantage for WASp expression; thus, the fraction of WASp+ cells at this time point likely represents the fraction of early progenitors transduced with LV.39, 40 An increase in the fraction of WASp+ cells in a peripheral subset over that observed in myeloid-derived neutrophils (or monocytes) therefore implies a selective advantage for WASp+ cells. In each cohort, we found that splenic B cells, CD4 and CD8 T cells, and NK cells had a significantly higher percentage of WASp+ cells compared to splenic neutrophils (17% and 27% in the insulated and non-insulated LVs, respectively; solid lines in Figures 2A–2D). Interestingly, we also observed a significant increase in splenic WASp+ NK1.1+ CD3+ cells in the 650.MND.hWASp (48%) versus the MND.hWASp (35%) experimental groups (Figure 2A), suggesting an increased selection for NK cells using the insulated LV.
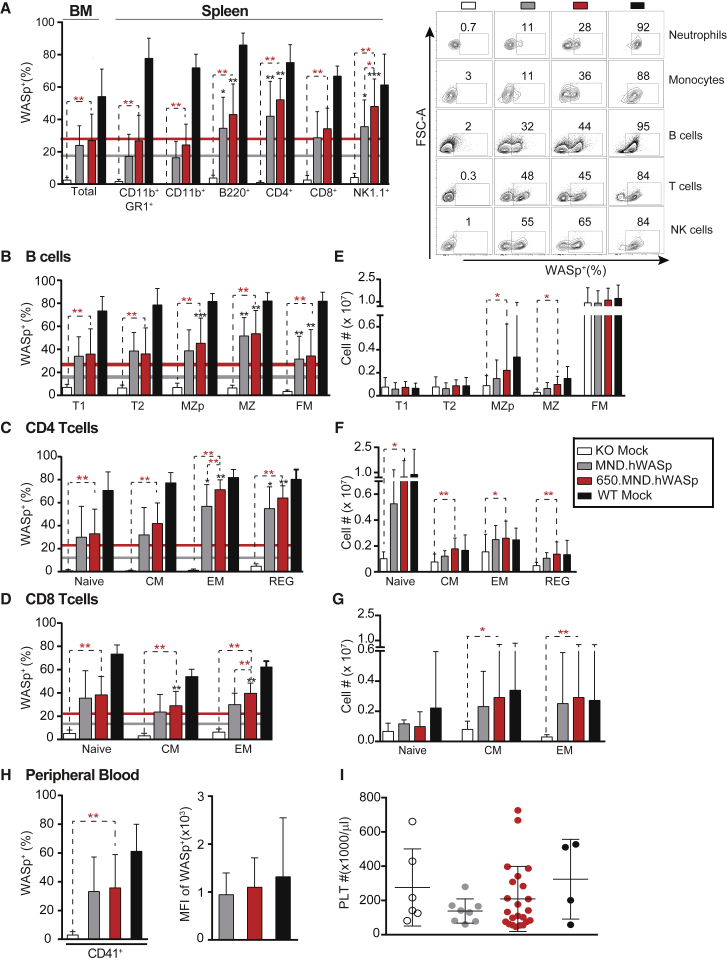
Figure 2.
Restoration of WASp Expression in Affected Hematopoietic Lineages following LV Gene Therapy in WAS−/− Primary Recipient Mice
Shown are the proportion of WAS+ cells and numerical reconstitution of lymphocytes and platelets 16 weeks post-transplant. (A) WASp-expressing cells in total BM and in hematopoietic lineages of the spleen. Colored horizontal lines in this and subsequent panels show the average percentage of WASp+ splenic neutrophils (CD11b+ GR-1+) in the MND.hWASp-treated (gray) and 650.MND.hWASp-treated (red) cohorts for comparison. The right panel shows representative flow cytometry analysis of splenocytes. (B–D) WASp+ cells within developmental subsets of splenic B (B) and T cells (C and D). B cells are plotted in order from least to more mature subsets: transitional 1 (T1), transitional 2 (T2), marginal zone precursor (MZP), marginal zone (MZ), and follicular mature (FM). T cell subsets are designated as naive, central memory (CM), effector memory (EM), or regulatory (REG). (E–G) Absolute number of specific developmental subsets of splenic B cells (E) and CD4+ (F) or CD8+ (G) T cells. (H) Percentage (left) and MFI (right) of WASp+ platelets in PB. (I) Number of platelets per microliter of PB. Bars and error bars represent the mean ± SD. In (A)–(D) and (H), n = 10 (KO mock, MND.hWASp), 28 (650.MND.hWASp), and 9 (WT mock) from three independent experiments. In (E) to (G), n = 3 (KO and WT mock), 5 (MND.hWASp), and 12 (650.MND.hWASp) from one experiment. In (I) n = 4 (WT mock), 7 (KO mock), 8 (MND.hWASp), and 22 (650.MND.hWASp) from one experiment. *p < 0.04, **p < 0.002, ***p < 0.0001. Asterisks in red indicate p values of comparisons indicated by the dashed lines; asterisks in black show p values comparing the mean value indicated with that of the monocytes (gray or red horizontal line) in the same cohort, to test the significance of selective advantage.
We also analyzed splenic lymphocytes by surface markers that allowed us to define B and T cell sub-populations along with intracellular WASp levels (Figures 2B–2D). Our findings mostly replicated those reported previously for a non-insulated MND.hWASp LV.20 In both gene therapy cohorts, we observed higher percentages of WASp+ cells in each of the B and T cell subsets analyzed, compared to that in the myeloid compartment, indicating selection for WAS expression. Those that were statistically significant for both gene therapy cohorts were post-transitional B cells, including MZ precursors (MZps), marginal zone (MZ) and follicular mature (FM) B cells, and CD4+ effector memory (TEM) and regulatory (TReg) subsets. A statistically significant selective advantage for CD8+ T cells was observed only in central memory (TCM) and TEM subsets for the 650.MND.hWASp cohort. The numerical reconstitution of splenic lymphocyte subsets was consistent with dependence on WASp expression (Figures 2E–2G): lymphocyte sub-populations where WASp expression provided selective advantage in general had increased numbers in the LV-treated cohorts. There were no significant differences in cell numbers when comparing the insulated versus non-insulated LV. Based on the above observations, we conclude that gene therapy using the 650.MND.hWASp LV rescues development of affected hematopoietic cell lineages comparably to the non-insulated vector.
A prominent finding in patients with WAS is bleeding due to a deficit in platelet numbers and function. An important consideration for WAS gene therapy then is the restoration of WASp levels in platelets.3, 4 It should be noted that the platelet defect in Was−/− mice is mild relative to that seen in human subjects, so a change in blood platelet counts was not expected.41, 42 We obtained platelets from PB 12–15 weeks post-transplantation for analysis by flow cytometry for intracellular WASp expression (Figures 2H and 2I). We found that both LVs increased the percentage of WASp+ platelets relative to KO mock-treated animals. The MFI of WASp+ platelets in both gene therapy cohorts was similar to that of wild-type platelets, confirming the ability of the MND promoter to drive efficient transgene expression in this lineage.
650.MND.hWASp LV Promotes Reconstitution of Immune Cell Functions
Improvement in T Cell Receptor Signaling
WASp has a role in actin polymerization downstream of the T cell receptor (TCR)6 and its loss impacts TCR responses including proliferation of murine CD4 T cells.41, 42 Note that CD8 T cell TCR responses are only partially impacted by the loss of WASp. We tested the proliferation of murine splenic T cells in vitro in response to anti-CD3 and anti-CD28 stimulation (αCD3/28), read as dilution of CellTrace Violet (a fluorescent succinimidyl ester dye) from stained cells by flow cytometry. As expected, KO mock CD4+ T cells proliferated minimally in response to αCD3/28, while a substantial fraction of those from LV-treated or WT cohorts had proliferated by 72 hr. The percentage of cells that had proliferated at this time point was not significantly different between the two SIN-LV treatments. We consistently found that Was−/− CD8+ T cells proliferated in response to TCR stimulation even without gene therapy treatment, but LV treatment increased the overall proportion of cells proliferating in response to CD3 (Figures 3A and 3B). Control CD4+ and CD8+ T cells from all treatment groups proliferated equally well in response to phorbol 12-myristate 13-acetate (PMA)-ionomycin (P/I), which activates the TCR signaling pathway downstream of WASp.
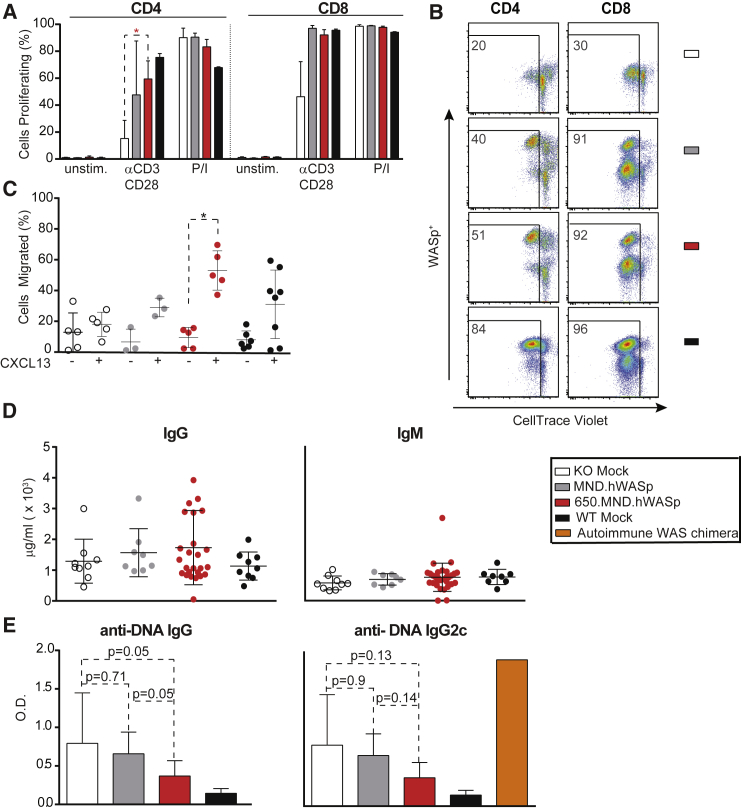
Figure 3.
Reconstitution of Immune Cell Functions following LV Gene Therapy in Primary Recipients
Assays of immune cell function 16 weeks after BM transplantation. Bars show the mean ± SD. (A) The percentage of cells that underwent ≥1 cell division 72 hr after incubation with CD3/CD28 antibodies or P/I (as measured by CellTrace Violet dilution). Shown are cells within CD4 (left) or CD8 (right) staining gates by flow cytometry. Data are from one of the three independent experiments: n = 3 (KO and WT mock, MND.hWASp) and 5 (650.MND.hWASp). (B) Flow cytometry analysis showing CellTrace Violet labeled splenocytes 72 hr post-CD3/CD28 stimulation, gated on live and either CD4+ or CD8+ populations. Numbers indicate the percentages that have proliferated after CellTrace Violet labeling. (C) B cell (CD43− splenocyte) migration in response to media only (−) or media supplemented with CXCL13 chemokine (+). Each dot indicates the percentage of B cells from a single mouse that migrated through the 5-μm-pore transwell. Data are from two independent experiments: n = 5 (KO mock, 650.MND.hWASp), 3 (MND.hWASp), and 8 (WT mock). (D) IgG and IgM and (E) anti-double-stranded DNA antibody levels in the serum of primary recipients as determined by ELISA 16 weeks post-transplant. Data are from three independent experiments: n = 5 (KO and WT mock), 4 (MND.hWASp), 2 (WT) (D only), and 12 (650.MND.hWASp). Serum from an autoimmune WAS chimera21 (E only) with high serum anti-DNA antibodies was used as a positive control. The p value was determined by unpaired two-tailed t test. *p < 0.024.
Restoration of B Cell Motility
Contrary to T cells, WASp deficiency renders B cells hyper-responsive to antigen receptor stimulation.21, 43 However, WASp deficiency impacts B cell motility and homing in response to chemotactic agents, ultimately affecting B cell development and selection.44 To determine whether SIN-LVs corrected this motility defect, we measured the number of total splenic B cells (CD43-depleted splenocytes) migrating across a transwell membrane into media with or without the chemokine CXCL13. For both gene therapy cohorts, the addition of CXCL13 increased the percentage of B cells migrating across the transwell membrane (Figure 3C). A significantly higher fraction of B cells from the 650.MND.hWASp LV cohort migrated relative to KO mock B cells, and this was comparable to the fraction of WT B cells that had migrated. Thus, 650.MND.hWASp LV rescues the homing defect in Was−/− B cells.
Reduction in Autoantibody Production
In the presence of WASp-expressing T cells, WASp-deficient B cells trigger spontaneous germinal center responses that lead to autoantibody production and autoimmune disease in mice.21, 22, 23 These findings are likely to explain the increased rates of autoimmune disease in human patients with WAS with low-level donor chimerism following HSCT.9 As quantified by ELISA in this study, we found that total serum immunoglobulin G (IgG) and IgM levels were similar across treatment groups (Figure 3D). In contrast, there was a trend for reduced anti-DNA autoantibodies present in KO mock recipients (assessed as total IgG or by the pathogenic IgG2c subclass) in MND-LV-treated cohorts, approaching levels present in WT mock in 650.MND.hWASp LV-treated mice (Figure 3E). These findings suggest that 650.MND.hWASp LV drives WASp expression at levels that may limit the subsequent risk for autoimmune disease.
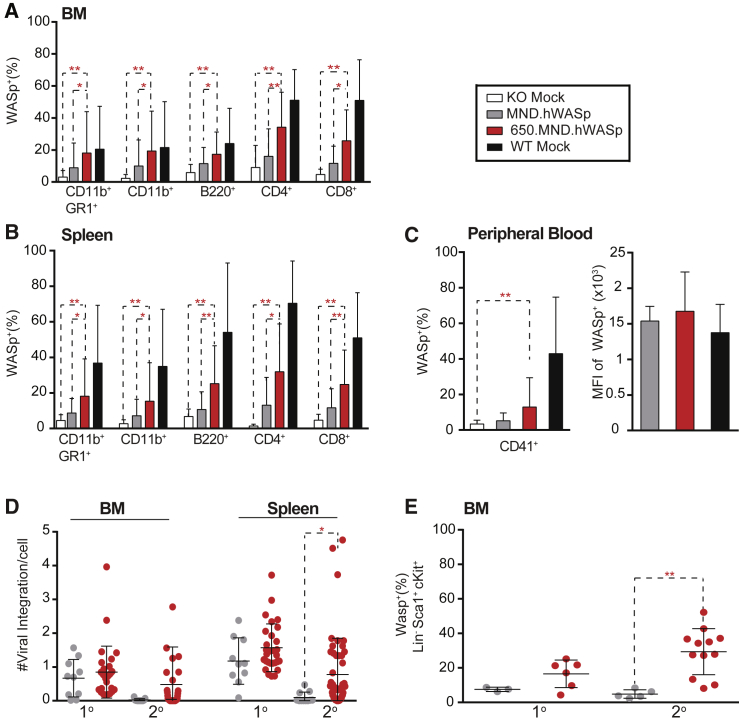
Sustained 650.MND.hWASp LV Marking and Expression following Serial Transplantation
Secondary transplants were performed to determine whether 650.MND.hWASp targeted long-term repopulating HSCs and to test relative safety.45, 46 BM cells from each primary recipient (16 weeks post-primary transplant) were transplanted into two irradiated secondary recipients (10 × 106 cells/recipient). We performed extensive analysis of the secondary recipients at week 16 by flow cytometry, histology, hematology, and clinical chemistry. Compared to primary donors, secondary recipients from all donors (including WT mock controls) had a smaller proportion of WASp+ cells within the myeloid, B cell, and T cell compartments in the BM and spleen (Figures 4A and 4B). Interestingly, secondary recipients from the 650.MND.hWASp group had higher proportions of WASp+ cells compared to the MND.hWASp group in hematopoietic lineages of the BM (Figure 4A) and spleen (Figure 4B). Additionally, only the insulated LV improved WAS expression in platelets (Figure 4C), although for both vectors, platelets that did express WASp did so at equivalent levels (MFI) to those from the WT mock cohorts. We next determined the average VCN by qPCR in total splenocytes and BM from primary and secondary recipients (16 weeks after their respective BM transplants; Table 1). In primary recipients, the average VCN per cell was increased in the splenic compartment relative to the BM, reflecting positive selection of WASp+ lymphocytes (Figure 4D), and the differences between the two LV groups were not statistically significant. The VCN within the BM of secondary recipients was lower than that of the primary recipients, but again increased in the spleen. Notably, 650.MND.hWASp LV-treated secondary recipients exhibited a significantly higher VCN within the spleen and a larger proportion of WASp+, Lin− HSCs within the BM compared to animals treated with MND-hWASp LV (Figures 4D and 4E, respectively). These combined findings imply that inclusion of the insulator exerts a significant impact by allowing stable WASp transgene expression, thus providing a selective advantage to cells with the insulated WASp transgene.
Figure 4.
Maintenance of VCN and WASp+ Expression in HSC and Their Progeny in 650.MND.hWASp LV-Treated Animals following Secondary Transplantation
Proportion of WASp+ cells in the BM (A) and spleen (B) and in PB platelets (C) of secondary recipients (16 weeks post-transplantation with BM from primary recipients). Data represent three individual experiments: n = 12 (KO mock), 16 (MND.hWASp), 51 (650.MND.hWASp) (n = 50 in C), and 15 (WT mock). In (C), the percentage (left) and MFI (right) of WAS+ platelets is shown. (D) Comparison of viral copy number (VCN) determined by qPCR of gDNA extracted from total BM and splenic cells of primary (1°) and secondary (2°) recipient mice; each dot represents a single animal. Data are from three independent experiments for both primary (n = 10 for MND.hWASp and n = 28 for 650.MND.hWASp) and secondary recipients (n = 16 for MND.hWASp, 51 for 650.MND.hWASp). (E) Percentage of WASp+ cells in HSCs in BM of primary (1°) and secondary (2°) gene therapy recipients. Data represents two unique experiments for both primary (n = 3 for MND.hWASp and n = 6 for 650.MND.hWASp) and secondary recipients (n = 6 for MND.hWASp and 12 for 650.MND.hWASp). In all panels, the mean ± SD value is shown. p values were calculated using the unpaired two-tailed t test. *p < 0.04, **p < 0.005.
Table 1.
Safety Parameters of Pre-clinical LV Gene Therapy
| LV | Total No. of Mice | VCN (Mean ± SD) |
No. with Malignancy Derived from |
No. of Deaths (<4 Weeks Post-transfer) | |||
|---|---|---|---|---|---|---|---|
| Spleen | BM | Donor | Recipient | Unknown | |||
| Primary | |||||||
| KO mock | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| WT mock | 9 | 0 | 0 | 0 | 0 | 1 | 0 |
| MND.hWASp | 10 | 1.17 ± 0.21 | 0.66 ± 0.17 | 0 | 0 | 0 | 0 |
| 650.MND.hWASp | 32 | 1.15 ± 0.13 | 0.84 ± 0.14 | 0 | 1 | 0 | 3 |
| Secondary | |||||||
| KO mock | 16 | 0 | 0 | 0 | 0 | 0 | 2 |
| WT mock | 19 | 0 | 0 | 0 | 0 | 0 | 0 |
| MND.hWASp | 14 | 0.09 ± 0.04 | 0.02 ± 0.01 | 0 | 3 | 0 | 0 |
| 650.MND.hWASp | 56 | 0.77 ± 0.15 | 0.43 ± 0.16 | 0 | 2 | 2 | 3 |
Assessing the Safety of 650.MND.hWASp LV Gene Therapy
Mice transplanted with 650.MND.hWASp SIN-LV-treated cells (either as primary or secondary transplants) had no statistically significant change in survival rates compared with the other gene therapy cohorts (Figures 5A and 5B). Table 1 summarizes the mortality and morbidity events occurring in the course of this study. Of 61 primary transplanted mice, three died from unknown causes within 4 weeks of their transplant, all from the larger 650.MND.hWASp LV cohort. Note that 32 of the primary recipient mice received 650.MND.hWASp LV gene therapy; the other 29 were divided into the remaining three cohorts. Mortality events occurring within 4 weeks post-transplant are likely to be due to the conditioning regimen, and the rates they occurred in this study as a whole are lower than those historically seen in previous LV gene therapy models we have performed (7%), although the rate for the insulated LV group alone (9.4%) is higher. At the 16-week study endpoint, we identified two mice with thymic lymphomas: one received 650.MND.hWASp gene therapy-treated cells (mouse 19), and the other received WT mock cells (mouse 36). The CD4+CD8+ lymphoma of mouse 19 was identified both by flow cytometry and by histopathology of thymus and spleen (Figure 5C). Using flow cytometry and antibodies specific to donor and recipient congenic markers, we determined that this lymphoma was recipient derived (Figure 5D); consistent with this conclusion, there were no viral integrations (by qPCR) within genomic DNA (gDNA) obtained from thymic tissue of this mouse (Table 2). Mouse 36 from the WT mock cohort developed a thymic lymphoma identified during histopathology screening but not detected by flow cytometry analysis. We were unable to determine whether this tumor was donor or recipient derived using congenic antibodies. Flow cytometry of harvested hematopoietic tissues and extensive histological analysis post-transplantation did not reveal evidence of clonal expansion within any of the other primary transplant recipients. Complete blood counts (CBCs) and serum chemistries of PB were identical between the treatment groups, except for one of the mice with lymphoma (mouse 19; Figures S2 and S3).
Figure 5.
Overall Survival and Absence of Donor-Derived Malignancies in LV-Treated Mice
(A) Kaplan-Meier survival curves for primary (left) and secondary (right) recipients during the 16-week post-BM transplant periods. (B) Pie chart showing tumor incidence as a percentage of all (primary and secondary) recipients from each treatment cohort. The percentage of mice with no tumors is shown in center of each chart. (C) Hematoxylin and eosin-stained sections of thymus and spleen of a secondary recipient from the 650.MND.hWASp cohort with a host-derived CD4+CD8+ lymphoma at ×60 magnification. The scale bar represents 10 μm. Blue arrowheads point to mitotic figures. (D) Flow cytometry plots of cells from the affected mouse shown in (C). Shown are CD4 and CD8 surface expression in indicated tissues. The recipient’s tumor origin was identified by congenic marker stain after gating on CD4+CD8+ thymic cells (plots at right). Percentages of live cells within each gate are indicated beneath the plots.
Table 2.
Features of Malignancies Found in Experimental Mice
| LV (Mouse Number) | Flow Cytometry |
Histopathology | VCN | ||
|---|---|---|---|---|---|
| Phenotype | Donor (%) | Recipient (%) | |||
| Primary Recipient | |||||
| WT Mock (36) | ND | ND | ND | + (thymus, lungs) | ND |
| 650.MND.hWASp (19) | CD4+CD8+ | 0 | 93.8 | + (multiple organs) | 0 |
| Secondary Recipient | |||||
| 650.MND.hWASp (116) | CD4+CD8+ | 0 | 99.7 | + (multiple organs) | 0 |
| 650.MND.hWASp (155) | CD8+ | 0 | 99.5 | + (multiple organs) | 0 |
| 650.MND.hWASp (111) | ND | ND | ND | + (thymus, lungs) | ND |
| 650.MND.hWASp (153) | ND | ND | ND | + (thymus) | ND |
| MND.hWASp (68) | CD4+CD8+ | 0 | 99.8 | ND | 0 |
| MND.hWASp (69) | CD4+CD8+ | 0 | 99.8 | ND | 0 |
| MND.hWASp (128) | CD4+CD8+ | 0 | 99.4 | ND | 0 |
ND, not determined.
The lifespans of secondary recipients were not significantly different than those of the primary recipients. Five of the 105 secondary recipients died within 4 weeks of the transplant procedure from unknown causes: three of these mice were from the 650.MND.hWASp gene therapy cohort, and two were from the KO mock cohort (Table 1). At the 16-week endpoint, we identified five secondary transplanted mice with clonal expansion of T cells by flow-based phenotyping (Table 2): two were from the 650.MND.hWASp cohort and consisted of recipient-derived (based on congenic markers and lack of VCN) CD4+CD8+ (mouse 116) and CD8+ (mouse 155) T cells; three animals in the MND.hWASp cohort (mice 68, 69, and 128) developed CD4+CD8+ recipient-derived lymphomas (and also had abnormal hematological and clinical chemistries; Figures S4 and S5). We identified two additional probable lymphomas by histological analyses (not detected by flow cytometry) within the 650.MND.hWASp gene therapy group (mice 111 and 153). Because these tumors were identified only by histopathology, after tissue was fixed and embedded, VCN and congenic marker expression could not be assessed.
A variety of radiation-associated lesions were identified during histological examination (Table S1); these were uniformly distributed across all groups. The prevalence of recipient-derived lymphomas in this WAS gene therapy model is consistent with the oncogenicity of the conditioning regimen in a genetically susceptible immunodeficient (Was−/−) mouse in the C57BL/6 background.46 Overall, we found no evidence of clonal toxicity associated with integration of the 650.MND.hWASp LV in this extensive pre-clinical study.
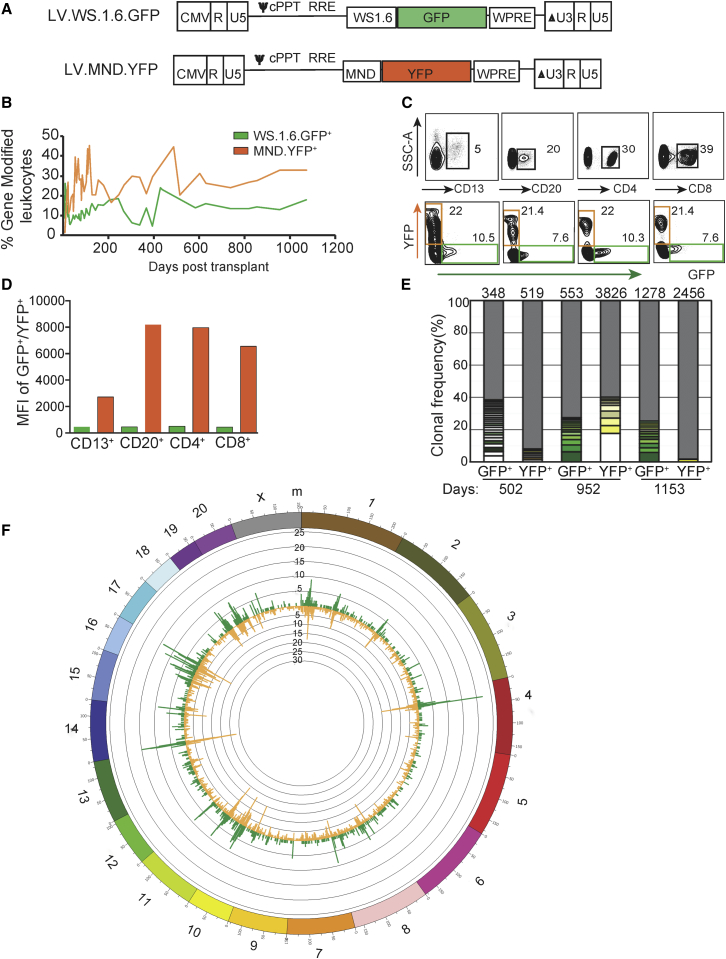
MND Promoter-Based LVs Exhibit Robust Expression and Polyclonal Hematopoiesis in a NHP Gene Therapy Model
To assess the safety and the integration profile of SIN-LV with various internal promoters in a large animal model, we performed gene therapy in healthy juvenile pigtailed macaques (Macaca nemestrina) using a CD34+ autologous HSC myeloablative transplant protocol.47 Similar to our mouse gene therapy studies,39 we first compared the efficacy and safety of LV containing either the human 1.6-kb WAS promoter (WS1.6; currently in use in clinical trials)10, 12 or the MND promoter (Figure 6A). In a competitive repopulation experiment, CD34+ BM cells were separately transduced with WS1.6. GFP or MND.YFP SIN-LV. Equivalent numbers of marked cells (7.2 × 106 CD34+ cells from each transduction/kg; total of 14.4 × 106/kg body weight) were infused into animal F09002 following 1,020-cGy total body irradiation. PB samples demonstrated hematopoietic recovery and engraftment of gene-modified cells within 3 weeks following transplant and stable CBCs and serum chemistries over a >30-month follow-up period (Figures S6 and S7). Initial flow cytometry showed gene-modified cell levels of ∼30% and ∼15% for the MND (yellow fluorescent protein [YFP+]) and WAS1.6 (GFP+) promoter-encoding LV-transduced cells among PB leukocytes, and these values were stably maintained for more than 1,000 days (Figure 6B). We observed stable granulocyte and lymphocyte marking up to the latest time point of follow-up (1,153 days), with 38.7% YFP+ and 15.6% GFP+ granulocytes and 27.0% YFP+ and 15% GFP+ lymphocytes (data not shown). We also observed a marked increase in the MFI for YFP+ cells compared to GFP+ cells (Figures 6C and 6D), consistent with the robust expression expected from the MND promoter compared to the WAS1.6 promoter.20
Figure 6.
Stable Promoter Activity and Polyclonal Hematopoiesis Using the Non-insulated MND LV in a Long-Term NHP Gene Therapy Model
(A) Schematic diagram of SIN-LV used in competitive repopulation study performed with NHP autologous BM, GFP, yellow fluorescent protein (YFP), and the 1.6-kb proximal human WAS promoter (WS1.6).57 (B) Percentage of cells expressing the indicated fluorescent protein in peripheral blood mononuclear cells (PBMCs) over time after transplant. (C) Representative flow plots showing marking (bottom row) within specific PB subsets (top row) obtained 326 days post-transplant. (D) The MFI for each sub-population in this sample is shown. (E) Frequency of MSG-PCR clones identified in sorted GFP+ or YFP+ PB cells at 502, 952, or 1,153 days after transplant. The total number of clones sequenced is indicated at the top of each bar in the chart. Thickness of bar segments indicates the frequency of a particular clone; the color given to each clone is consistent for comparison between time points. (F) Circos plot mapping RIS positions identified in GFP+ (green histogram) and YFP+ (yellow histogram) PB cells (obtained between 502 and 1,153 days after gene therapy) to the Rhesus genome (outer circle showing chromosome numbers). The Rhesus genome was divided into 1-Mbp bins; the heights of the histograms indicate the number of integrations found in a 1-Mbp bin.
To assess the diversity of clonal contributions to this pool, we performed high-throughput retroviral integration site (RIS) analysis on flow-sorted YFP+ and GFP+ PB leukocytes 502 and 1,153 days post-transplant. Modified genome sequencing (MGS)-PCR of gDNA from sorted cells was used to determine genomic sites of provirus integration.48 We observed highly diverse integration sites in all samples assayed, with distinct clonal contributions in each transduced cell population (Figure 6E). In total, we identified 4,699 and 1,688 unique integration sites of the YFP+ and GFP+ LVs, respectively. The most abundant clone constituted 1.5% (YFP+) and 3.7% (GFP+) of the integration site sequences identified, implying a highly polyclonal expansion of progenitors. We identified a higher number of distinct clones in the YFP+ cell population, suggesting that increased gene marking in this population is not due to expansion of a single or small number of clones. RISs were mapped on all chromosomes, and comparison of the global genomic distribution of integration sites in these samples (Figure 6F) showed no promoter-specific changes in distribution pattern. This animal displayed no evidence for adverse events at nearly 4 years post-transplantation, and gene marking remained stable.
Owing to the correlation between retroviral promoter activity and insertional mutagenesis observed in γ-retrovirus-mediated gene therapy trials for both X-SCID and WAS,13, 14, 15 we next tested whether inclusion of a chromatin insulator in the MND promoter SIN-LV could improve safety while maintaining efficient gene transfer, engraftment, and clonal diversity in this animal model. We performed a three-arm competitive repopulation assay (Figure S8A) using CD34+ cells transduced with either cHS4.MND.GFP (using the 1.2-kb full-length chicken β-globin insulator element), 650.MND.mCherry, or 400.MND.BFP (an ∼400-bp insulator fragment).37 We observed only small differences in in vitro transduction efficiency of CD34+ cells between the three LV vectors (38% GFP+, 23% mCherry+, and 35% blue fluorescent protein [BFP]+). Transduced cells were transplanted into the autologous myeloablated animal A11207 (3.5, 4.3, and 2.9 × 106 transduced cells/kg for cHS4.MND.GFP, 650.MND.mCherry, and 400.MND.BFP SIN-LVs, respectively; total of 10.7 × 106 CD34+ cells/kg of body weight). The transplanted animal demonstrated hematopoietic recovery and engraftment of gene-modified cells within 1 week after transplant. All three arms engrafted equivalently, as evidenced by the levels of each fluorescent protein in PB. This sustained expression and gene marking supported stable hematopoiesis in this animal for nearly 2 years with no adverse events (Figures S6–S8B). Stable granulocyte and lymphocyte marking was observed up to the latest time point of follow-up (782 days), with 1.5% GFP+, 1.4% mCherry+, and 0.9% BFP+ granulocytes and 0.9% GFP+, 0.4% mCherry+, and 1.7% BFP+ lymphocytes (data not shown).
To assess the diversity of clonal contributions to this pool, we collected PB and BM at approximately the same time point after transplant (days 221 and 223 post-transplant for PB for BM, respectively) and we used fluorescence-activated cell sorting (FACS) to sort PB leukocytes by fluorescent protein expression to distinguish between the three insulator elements. CD34+ leukocytes from the BM were also purified to correlate clonal contributions observed in the PB fluorescent protein-sorted populations. RIS analysis of gDNA isolated from sorted and purified cells was then performed by MGS-PCR. Highly diverse integration sites were observed in all samples assayed, with distinct clonal contributions in each transduced cell population (Figure S8C). We identified 265–392 unique RISs for sorted GFP, mCherry, and BFP + PB leukocytes and 396 unique RISs in total BM CD34+ cells. There were no significant differences in the number of clones observed for each sample, which is consistent with the similar levels of gene marking in each cell population. We identified a total of six clones in the BM CD34+ cell population that were also identified in one of the three PB fluorescent protein-sorted populations, demonstrating the contribution from a CD34+ cell residing in the BM to a marked PB leukocyte. Of these, three were GFP+ (full-length insulator), two were mCherry+ (650-bp insulator), and one was BFP+ (400-bp insulator). Of the CD34+ BM clones identified, the two most prevalent contributors were both from the GFP+ lineage (full-length insulator).
After establishing the genomic positions of all LV integration sites for both animals (F09002 and A11207), we compared their positions to the 2010 rheMac3 RefSeq gene list available from the UCSC Genome Browser using a custom python script.49 For each insertion site, we determined the distance to the nearest transcription start site (TSS) relative to the direction of transcription (Figures S9A and S9B) and gene name. If the insertion fell within a gene, the gene name and genomic feature (i.e., within an intron, etc.) were recorded. In both animals F09002 and A11207, we observed an equivalent number of integration events within 100 kb of TSS regardless of the promoter (F09002) or insulator element (A11207) tested. For animal A11207, genes proximal to the integration sites identified at 96, 221, and 392 days after transplant were compared to the established list of oncogenes from the Cosmic Cancer Gene Database (http://cancer.sanger.ac.uk/cosmic) (Table S2).50 Of 21 integrations identified, all were within unique proto-oncogenes; only one identical integration was found at two time points (96 and 392 days post-transplant; FBXW7, F-Box and WD repeat domain containing 7, E3 ubiquitin protein ligase). The integration was 69.4 kb downstream of the transcription start site of this tumor suppressor gene, within intron 3. The contribution of this clone was <0.1% of overall hematopoiesis at both time points, implying a lack of a biological impact from this integration during this time period. Together, these data support the transduction efficiency, engraftment, and clonal diversity of insulated LV vector-transduced autologous CD34+ hematopoietic cells in the clinically relevant non-human primate animal model, without evidence of insertional mutagenesis.
Discussion
Here we report evidence of long-term efficacy and safety of an SIN-LV with an insulated MND internal promoter for WAS gene therapy in Was−/− mice and for a non-human primate model using a reporter gene. In primary recipient Was−/− mice, we found similar features of restored immune cell function in both gene therapy cohorts (650.MND.hWASp and MND.hWASp), including stable engraftment of WASp-expressing cells in all hematopoietic lineages examined; progressive selection for WASp+ cells among T, NK, and B cell lineages; correction of T and B cell development and function; and restoration of wild-type levels of WASp expression in platelets. These findings expand upon those of a previous murine gene therapy study using a non-insulated MND.hWASp SIN-LV that used a different LV backbone than used here.20 In the current study, mice receiving the insulated 650.MND.hWASp gene therapy also exhibited a trend for lower levels of autoimmune-associated anti-DNA autoantibodies of the IgG and IgG2c subtypes compared to animals treated with the non-insulated LV construct.
In our murine gene therapy experiments, serial BM transfers into secondary Was−/− recipients were performed to verify long-term hematopoietic stem cell (LT-HSC) transduction and long-term efficacy of the viral vectors, and to increase the sensitivity of detecting vector-induced transformation events in vivo.45 We found that the 650.MND.hWASp gene therapy vector resulted in superior immune cell reconstitution in the secondary recipients compared with the non-insulated MND.hWASp LV, including significantly higher percentages of WASp+ immune cells in BM, spleen, and PB. Additionally, although the number of viral integrations per cell in BM and splenic cells of primary recipients were similar for both LV gene therapy cohorts, both the number of viral integrations and WASp expression in HSCs and progeny was significantly higher in 650.MND.hWASp-treated secondary recipients by the 16-week experimental endpoint. These striking observations are consistent with the interpretation that the insulated vector more effectively prevents epigenetic silencing in transduced long-term repopulating HSCs. In this scenario, higher-level WASp expression in donor HSCs (derived from primary recipients) is predicted to facilitate increased competitive repopulation in secondary recipients, an outcome that would also promote the observed selective expansion of WASp+ differentiated lymphoid subsets in 650.MND.hWASp secondary recipients. This idea is supported by previous work showing that migration defects in WASp-deficient HSCs hamper long-term hematopoietic reconstitution in a competitive repopulation setting.51 Together, these findings strongly imply that inclusion of the 650-insulator element is likely to improve sustained expression in LT-HSCs in a clinical application.
There were no statistically significant differences in survival rates between treatment groups in this study. Despite large cohorts receiving gene therapy-treated HSCs (42 primary and 70 secondary transplant recipients), six incidences of clonally expanded cells were identified by flow cytometry at autopsy (one primary and five secondary recipients); all originated from recipient progenitor cells, with no viral integrations detected by PCR. In addition, three cases of thymic lymphoma, including two in the secondary 650.MND.hWASp LV and one in the primary WT mock treatment groups, were identified based only on detailed examination of histological sections. While we were unable to definitively determine whether these latter tumors were donor or recipient derived, this rate of lymphoma development is consistent with our previous baseline observations in WAS-deficient recipient mice.
Compared with mice, large animal gene therapy trials more closely model the number of transduced LT-HSCs that must be transplanted into a human subject, as well as their higher proliferative demands over a longer time span.52 Because of the close genetic relationship between humans and NHPs, NHP HSCs respond to many of the recombinant human cytokines and other reagents used for clinical mobilization, selection, culture, transduction, and myeloablative total body irradiation (TBI).52, 53 In order to directly compare expression from two different internal promoters in the same animal, we used fluorescent reporter genes (GFP and YFP) transferred using SIN-LV gene therapy of BM from a pigtailed macaque, followed by autologous transplantation. Here we found that the proportion of cells in the periphery expressing either fluorescent protein was stable over 3 years. However, expression from the MND promoter was evident in a higher proportion and at a higher MFI in PB cells of all cell lineages compared with that of the WS1.6 promoter. Using a similar strategy in a second pigtailed macaque gene therapy experiment, we were able to simultaneously test three different fragments of the canonical 1.2-kb cHS4 insulator (upstream of the MND-fluorescent protein cassette) for their ability to prevent clonal dominance. As suggested by in vitro assays, insertional mutagenesis was not detected even after 2 years, and engraftment remained polyclonal with no evidence of clonal dominance (i.e., no individual clonal contribution of >20% to the total pool identified). These data imply that increased expression elicited by the viral MND promoter over the WS1.6 promoter in the context of a SIN lentivirus vector does not compromise transduction efficiency or clonal diversity of autologous CD34+ hematopoietic cells in the clinically relevant non-human primate animal model.28 Although there was no change in the level of engrafted gene-modified cells as a function of the different insulator elements used, inclusion of an insulator may reduce overall engraftment levels when the number of gene-modified PB leukocytes is compared between animals F09002 and A11207. Finally, although the non-insulated MND promoter showed insertional transactivation when inserted proximal to the LMO2 locus in Jurkat T cells by Cre-mediated cassette exchange and in a previous murine study,20, 28 we found no evidence for toxicity in our murine or NHP studies, suggesting that these animal models, as has been previously noted,46, 54 may lack the sensitivity to distinguish between LV vectors with low rates of genotoxic events.
Together, these studies suggest that the use of an insulated SIN-LV vector incorporating an MND internal promoter may strike a necessary balance for WAS gene therapy: driving sufficient expression of WASp to stably restore the immune cell deficits of WAS and WT levels of WASp expression in platelets while minimizing the potential of integrated proviral cassettes to create transactivational mutagenesis. Furthermore, our observations regarding the capacity of this vector to reconstitute both lymphocyte function and WASp expression in platelets strongly support consideration of the 650.MND.hWASp SIN-LV for clinical gene therapy trials for the treatment of WAS. Finally, our data suggest that use of insulated SIN-LV vectors harboring MND internal promoter expression cassettes may be considered for other hematopoietic disorders in which sustained high-level transgene expression in multiple lineages is required to achieve developmental and/or functional reconstitution.
Materials and Methods
Viral Vectors
The SIN-LV backbone pCL20cw has been described previously.55, 56 Plasmid pCL.HS4 has the full 1.2-kb HS4 insulator element from the chicken β-globin locus37 inserted into the 3′ U3 region of the viral LTR of pCL20cw. pCL400 contains 400 bp of the insulator sequence from the 3′ end of the 1.2-kb insulator fragment, while pCL650 has 650 bp of the insulator (comprising the 250-bp core and 400 bp from the 3′ end of the 1.2-kb insulator fragment) integrated into the 3′ LTR. We used the MND promoter26 as the internal promoter driving transgene expression in all vectors except pCL.WS1.6.GFP, which instead uses the 1.6-kb human WAS promoter to drive GFP expression.57 cDNA encoding human WASp was inserted downstream of the MND promoter in a pCL650 backbone to generate pCL650.MND.WASp, and WASp was replaced by GFP to make pCL650.MND.GFP. pCL.400,MND.mCherry had the coding region for mCherry in a pCL400 backbone and pCL.MND.YFP was created by insertion of an MND.YFP cassette into the pCL backbone without any insulator element. Third-generation LV stocks were generated by transient transfection of HEK293T cells with the vector plasmid, pCAGkGP1.1R (packaging), pCAG4-RTR2 (rev), and pCAG4 VSVG (envelope) plasmids.58, 59 Forty-eight hours post-transfection, the viral supernatant was filtered through a 0.22-μm filter and centrifuged at 8,500 × g overnight at 4°C. The virus was suspended in Hank’s balanced salt solution to achieve a 100-fold concentration, then stored at −80°C. LV stocks for pCL.650.MND.WASp were generated from a Tet-regulated version of the same (TL- CL.650.MND.WASp) by transfection of ligated vector concatamers into GPRT-G helper cells as described previously.27 LV titers were determined by infection of NALM-6 cells with serial dilutions of viral stocks and detection of proviral sequences by qPCR. Viral titers were calculated as proviral integrations using a standard curve generated from gDNA of a NALM-6 clone with a single copy of integrated provirus.60
Murine Lin− BM Transduction and Transplantation
Strains of mice used were C57BL/6 (WT), Was−/− (B6:129-Wastm1Sbs crossed into the C57Bl/6 background for nine generations41), and WT and Was−/− strains that were cross-bred with B6.SJL-Ptprca Pepcb/BoyJ (Jackson Laboratories) for congenic marking with the CD45.1+ (Ptprca) allele (C57BL/6 mice have the CD45.2+/Ptprcb allele). Mice were maintained in a specific pathogen-free facility at the Seattle Children’s Research Institute (SCRI). All studies were performed according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) standards and were approved by the SCRI Institutional Animal Care and Use Committee (IACUC).
Methods for collection and LV transduction of murine Lin− BM, and transplantation of LV-treated cells into irradiated recipients, were similar to those previously described.20, 60 BM cells isolated from euthanized 6- to 8-week-old donor mice (WT or Was−/−; congenically marked either CD45.1+ or CD45.2+) were enriched for lineage-negative cells using the EasySep Mouse Hematopoietic Progenitor Cell Enrichment Kit (StemCell Technologies). Lin− cells (4 × 106 cells/mL) in HSC media (StemSpan SFEM; StemCell Technologies) with murine stem cell factor (SCF) and thrombopoietin (50 and 20 ng/mL, respectively) were transduced without (mock) or with LV at a MOI of 50 for 10 hr (viral dose was administered twice, 5 hr apart). The LV used was either clinical-grade 650.MND.hWASp (titer: 2 × 109 viral particles [Vps]/mL) or non-insulated MND.hWASp (titer: 4.8 × 108 Vp/mL). After 10-hr viral transduction, donor cells were washed with PBS and 2 × 106 cells were transferred by intravenous (i.v.) injection into irradiated 6- to 8-week-old Was−/− recipients (the irradiation regimen was two 450-cGy doses administered 4 hr apart, the last one immediately prior to transplant). All Was−/− recipients had congenically distinct CD45 alleles from that of the donor cells. In parallel, 0.5 × 106 mock or LV-transduced Lin− BM cells were cultured in vitro in HSC media for 7 days to compare WASp expression and viral copy number of input cells using the two lentiviral constructs.
Assessing Gene Therapy-Treated Mice
The health status of all (primary and secondary) recipient animals post-transplant was evaluated daily by assessment of body condition. Mice that reached predefined humane endpoints were euthanized and analyzed for evidence of clonal expansion of donor or recipient immune cells, histopathology, and serum chemistry. All other recipients were euthanized 16 weeks post-transplant, and tissues were analyzed as described. BM collected from primary recipients at the 16-week time point was also serially transplanted into secondary recipients as described below.
Murine Serum Chemistry and Histological Analyses
PB samples were collected in lithium-coated tubes for CBC and serum chemistry analyses (mouse profile no. 892; Phoenix Central Laboratories) or in heparin for flow cytometry-based immunophenotyping (below). A full necropsy was performed, which included gross visual inspection of all organs and documentation of organomegaly. Major organs were weighed and then fixed in formalin; for the spleen and thymus, sections were removed for formalin fixation, and the remaining spleen and thymus were then re-weighed and processed for immunophenotyping and functional assays described below. Paraffin embedding, sectioning, and hematoxylin and eosin staining were performed by the Histology and Imaging Core at the University of Washington’s Department of Comparative Medicine. Organs examined included the heart, skeletal muscle, tongue, thyroid glands, lungs, esophagus, liver, kidneys, adrenal glands, thymus, spleen, salivary glands, stomach, small intestine, cecum, colon, pancreas, reproductive organs, skin, brain (cerebellum and cerebrum), pituitary glands, eyes, harderian glands, ears, and BM.
Murine Immunophenotyping
Thymus and spleen were processed for immunophenotyping and functional assays (below) as follows. Single cell suspensions were obtained by dissociating tissues with frosted glass slides, followed by erythrocytes lysis with ammonium-chloride-potassium buffer (ACK; 0.15 M ammonium chloride, 10.0 mM potassium bicarbonate, and 0.1 mM EDTA) and filtration through a 40-μm mesh. Serum from heparinized PB and BM collected in PBS were also subjected to erythrocyte lysis in ACK and filtration using a 40-μm mesh. Cells were stained with fluorochrome-labeled monoclonal antibodies against cell surface markers, as well as a polyclonal WASp antibody for intracellular WASp expression, using the Cytofix/Cytoperm kit (BD Biosciences) as described.20 Antibodies used are listed in Table S3. Stained cells were analyzed on an LSR II Flow Cytometer (BD Biosciences) and FACS data were analyzed with FlowJo software (Tree Star). The absolute numbers of splenic and thymic cells were calculated using AccuCount Rainbow Fluorescent beads (SpheroTech), extrapolating from the percentage of each tissue processed. Platelets were isolated from whole peripheral blood and stained for intracellular WASp and platelet surface marker CD41 expression, as described previously.20
Murine Secondary Transplantation Experiments
Total BM from each experimental and control gene therapy primary recipient mouse was transplanted into two secondary recipient mice (10 × 106 cells per mouse). Secondary recipients received the same conditioning regimen as described above and had the same congenic CD45 allele as the primary recipient. Primary recipients with less than 5% WASp expression in myeloid cells, or with less than 20% engraftment of donor myeloid cells, were excluded from secondary BM transfer experiments. Mice were monitored and analyzed at 16 weeks post-transplantation as described above for the primary recipient mice.
Viral Copy Number Determination
gDNA was extracted from total spleen or BM cells using the QIAmp DNA Blood Mini Kit (QIAGEN). Proviral integrations were detected using the following Gag-specific primers and probe: forward primer (5′-GGAGCTAGAACGATTCGCAGTTA-3′), reverse primer (5′-GGTTGTAGCTGTCCCAGTATTTGTC-3′), and probe (5′-[6FAM]ACAGCCTTCTGATGTTTCTAACAGGCCAGG[BHQ1]-3′). DNA content was normalized using the TaqMan gene expression array for murine β-actin (Life Technologies). Maxima Probe/ROX qPCR Master Mix (Fermentas) was used for amplification with 100 ng input DNA and qPCRs were run on ABI 7500 Real-Time PCR System (Applied Biosystems). Proviral integrations were quantified using a standard curve established using gDNA extracted from a murine B cell line harboring six LV integrations per diploid genome.61
Functional Assays of Murine Lymphocytes
Splenic cells were cultured in lymphocyte media (RPMI with 55 μM 2-mercaptoethanol and 10% fetal calf serum), and incubations were at 37°C with 5% CO2. For testing T cell proliferation, total splenic cells were labeled with CellTrace Violet (Thermo Fisher) and stimulated with 1 μg/mL plate-bound anti-CD3 and 0.25 μg/mL soluble CD28 (clones OKT-3 and 9.3, respectively; University of California, San Francisco Monoclonal Antibody Core), followed by a 72-hr incubation and then flow cytometry for WASp expression and CellTrace Violet dilution. As a positive control, some cells were stimulated with 2 ng/mL PMA and 500 ng/mL ionomycin. The B cell migration assays were performed using CD43-depleted splenocytes (CD43 microbeads; Miltenyi Biotec). One-hundred microliters of cells in lymphocyte media (1 × 106/mL) was seeded in duplicate upper wells of a 5-μm transwell plate (Corning Costar). The lower wells had 600 μL lymphocyte media and 300 nM recombinant murine CXCL13 (R&D Systems). Following a 1-hr incubation, the numbers of cells migrating to the lower well were counted, and the percentage of cells migrating was calculated based on the input cell number.
ELISA
Total serum IgG and IgM levels were quantified by ELISA as described previously.20 Serum anti-DNA IgG and IgG2c antibodies levels were quantified by ELISA as described.21
NHP BM Transplantation
Healthy juvenile pigtailed macaques (M. nemestrina) weighing between 3 and 8 kg were housed at the Washington Regional Primate Research Center under conditions approved by AAALAC. All studies and procedures were reviewed and approved by the University of Washington IACUC. Animals were given a 4-day (days −7 to −4) regimen of recombinant human (rh) cytokines including granulocyte colony-stimulating factor (rhG-CSF; 100 μg/kg; Amgen) and stem cell factor (rhSCF; 50 μg/kg; Amgen) as subcutaneous (s.c.) injections. One day later (day −3), marrow volumes totaling ≤10% body weight in kilograms were aspirated from the femora and/or humeri and collected in preservative-free heparin. CD34+ cells were enriched from marrow leukocytes by immunomagnetic column separation. Briefly, cells were incubated with immunoglobulin M monoclonal antibody (clone 12.8) at 4°C for 30 min, washed, and then incubated with rat monoclonal anti-mouse immunoglobulin M microbeads for an additional 30 min at 4°C, followed by immunomagnetic column separation (Miltenyi Biotec) per the manufacturer’s instructions. Resulting CD34+ cells were incubated at 37°C and 5% CO2 in a humidified incubator in StemSpan SFEM media containing 100 ng/mL each of rhSCF, Flt3-L, and thrombopoietin (TPO) for a period of 16–18 hr. Following this pre-stimulation, cells were then divided into equivalent aliquots and were transferred into non-tissue culture-treated vessels pre-coated with 2 μg/cm2 CH-296 (Retronectin). Cells were exposed to virus at an MOI of 5 infectious units (IU) per cell for 8 hr. An additional volume of virus equivalent to 5 IU/cell was added to each culture and cells were cultured overnight for a final MOI of 10 IU/cell. In preparation for transplantation, animals received myeloablative TBI (1,020 cGy) administered from a linear accelerator at 7 cGy/min in four equally divided fractions over 2 days (days −2 and −1). The following day, cells were harvested and washed and a sample was collected to determine colony-forming capacity and transduction efficiency prior to infusion (day 0) through a central vein catheter. Following transplantation, supportive treatments of rhG-CSF (100 μg/kg) were administered daily i.v. until a neutrophil count of >500/μL was attained for three consecutive counts. Blood product transfusions, antibiotics, and electrolytes were administered as needed.
Analysis of Gene-Modified Cell Engraftment in NHPs
Leukocytes were collected from heparinized PB and BM after ammonium chloride lysis at multiple time points after transplant. Expression of fluorescent proteins (GFP, YFP, mCherry, or BFP) was analyzed by flow cytometry on either a FACS Canto or LSR II cell analyzer (both from BD Biosciences). Fluorochrome expression in lineage-specific populations was determined by gating based on forward and right-angle light scatter characteristics or by expression of lineage-specific CD markers using FlowJo software (version 9 or 10; Tree Star).
Lentivirus Integration Site Analysis in NHP Gene Therapy
gDNA was extracted from leukocytes collected at various time points from either PB or BM or fluorochrome or CD marker-sorted populations by a QIAGEN Blood DNA Mini Kit per the manufacturer’s instructions. Lentivirus LTR-genome junctions were amplified by MGS-PCR as described.48 Resulting sequence libraries were subjected to Ion Torrent semiconductor sequencing and resulting sequence reads were analyzed using the Vector Integration Site Analysis (VISA) server (Grant Trobridge, Washington State University).62 Genomic sequences were mapped to the rhesus macaque genome (rheMac3) using a stand-alone version of BLAT available from the UCSC Genome Browser (http://genome.ucsc.edu/).49 Sequences corresponding to the same integration locus were grouped together to determine the total number of unique integration sites (clones) identified in the sample. Relative contributions of each clone were determined by the number of integration site (IS)-associated sequence reads corresponding to that clone. A quality control check was performed to reveal clones over-represented by PCR bias by comparing the number of IS-associated sequence reads with the number of different fragment lengths observed for each genomic locus.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism version 6 (GraphPad Software). Tests of statistical significance were performed using the unpaired two-tailed Student’s t test; p values less than 0.05 were considered significant.
Author Contributions
D.J.R. conceived, designed, and directed the overall project; H.-P.K., J.A., and A.N. designed selected experiments; S.S., I.K., S.K., B.S., M.W., D.L., and Z.N. performed experiments and interpreted results; S.S., K.S., J.A., and D.J.R. wrote the manuscript.
Conflicts of Interest
The authors declare that they have no competing financial interests.
Acknowledgments
Financial support for this work was from the NIH (award numbers R01-AI071163, P01-HL053749, and P01-AI097100), the Fred Hutchinson Cancer Research Center, and the Seattle Children’s Hospital Program for Cell and Gene Therapy.
Footnotes
Supplemental Information includes nine figures and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2016.11.001.
Supplemental Information
References
- 1.Bosticardo M., Marangoni F., Aiuti A., Villa A., Grazia Roncarolo M. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113:6288–6295. doi: 10.1182/blood-2008-12-115253. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M., Lagresle C., Hacein-Bey-Abina S., Fischer A. Gene therapy for severe combined immunodeficiency. Annu. Rev. Med. 2005;56:585–602. doi: 10.1146/annurev.med.56.090203.104142. [DOI] [PubMed] [Google Scholar]
- 3.Notarangelo L.D., Miao C.H., Ochs H.D. Wiskott-Aldrich syndrome. Curr. Opin. Hematol. 2008;15:30–36. doi: 10.1097/MOH.0b013e3282f30448. [DOI] [PubMed] [Google Scholar]
- 4.Ochs H.D., Thrasher A.J. The Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. 2006;117:725–738, quiz 739. doi: 10.1016/j.jaci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Galy A., Thrasher A.J. Gene therapy for the Wiskott-Aldrich syndrome. Curr. Opin. Allergy Clin. Immunol. 2011;11:545–550. doi: 10.1097/ACI.0b013e32834c230c. [DOI] [PubMed] [Google Scholar]
- 6.Ochs H.D., Notarangelo L.D. Structure and function of the Wiskott-Aldrich syndrome protein. Curr. Opin. Hematol. 2005;12:284–291. doi: 10.1097/01.moh.0000168520.98990.19. [DOI] [PubMed] [Google Scholar]
- 7.Thrasher A.J., Burns S.O. WASP: a key immunological multitasker. Nat. Rev. Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 8.Marangoni F., Bosticardo M., Charrier S., Draghici E., Locci M., Scaramuzza S., Panaroni C., Ponzoni M., Sanvito F., Doglioni C. Evidence for long-term efficacy and safety of gene therapy for Wiskott-Aldrich syndrome in preclinical models. Mol. Ther. 2009;17:1073–1082. doi: 10.1038/mt.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozsahin H., Cavazzana-Calvo M., Notarangelo L.D., Schulz A., Thrasher A.J., Mazzolari E., Slatter M.A., Le Deist F., Blanche S., Veys P. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111:439–445. doi: 10.1182/blood-2007-03-076679. [DOI] [PubMed] [Google Scholar]
- 10.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boztug K., Schmidt M., Schwarzer A., Banerjee P.P., Díez I.A., Dewey R.A., Böhm M., Nowrouzi A., Ball C.R., Glimm H. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 14.Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D. Gene therapy for Wiskott-Aldrich syndrome--long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell R.S., Beitzel B.F., Schroder A.R., Shinn P., Chen H., Berry C.C., Ecker J.R., Bushman F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schröder A.R., Shinn P., Chen H., Berry C., Ecker J.R., Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 18.Wu X., Li Y., Crise B., Burgess S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 19.Modlich U., Navarro S., Zychlinski D., Maetzig T., Knoess S., Brugman M.H., Schambach A., Charrier S., Galy A., Thrasher A.J. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Astrakhan A., Sather B.D., Ryu B.Y., Khim S., Singh S., Humblet-Baron S., Ochs H.D., Miao C.H., Rawlings D.J. Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich syndrome. Blood. 2012;119:4395–4407. doi: 10.1182/blood-2011-03-340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker-Herman S., Meyer-Bahlburg A., Schwartz M.A., Jackson S.W., Hudkins K.L., Liu C., Sather B.D., Khim S., Liggitt D., Song W. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 2011;208:2033–2042. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson S.W., Scharping N.E., Kolhatkar N.S., Khim S., Schwartz M.A., Li Q.Z., Hudkins K.L., Alpers C.E., Liggitt D., Rawlings D.J. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 2014;192:4525–4532. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Recher M., Burns S.O., de la Fuente M.A., Volpi S., Dahlberg C., Walter J.E., Moffitt K., Mathew D., Honke N., Lang P.A. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819–2828. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castiello M.C., Scaramuzza S., Pala F., Ferrua F., Uva P., Brigida I., Sereni L., van der Burg M., Ottaviano G., Albert M.H. B-cell reconstitution after lentiviral vector-mediated gene therapy in patients with Wiskott-Aldrich syndrome. J Allergy Clin. Immunol. 2015;136:692–702. doi: 10.1016/j.jaci.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pala F., Morbach H., Castiello M.C., Schickel J.N., Scaramuzza S., Chamberlain N., Cassani B., Glauzy S., Romberg N., Candotti F. Lentiviral-mediated gene therapy restores B cell tolerance in Wiskott-Aldrich syndrome patients. J. Clin. Invest. 2015;125:3941–3951. doi: 10.1172/JCI82249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Challita P.M., Skelton D., el-Khoueiry A., Yu X.J., Weinberg K., Kohn D.B. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J. Virol. 1995;69:748–755. doi: 10.1128/jvi.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wielgosz M.M., Kim Y.S., Carney G.G., Zhan J., Reddivari M., Coop T., Heath R.J., Brown S.A., Nienhuis A.W. Generation of a lentiviral vector producer cell clone for human Wiskott-Aldrich syndrome gene therapy. Mol. Ther. Methods Clin. Dev. 2015;2:14063. doi: 10.1038/mtm.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koldej R.M., Carney G., Wielgosz M.M., Zhou S., Zhan J., Sorrentino B.P., Nienhuis A.W. Comparison of insulators and promoters for expression of the Wiskott-Aldrich syndrome protein using lentiviral vectors. Hum. Gene Ther. Clin. Dev. 2013;24:77–85. doi: 10.1089/humc.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cartier N., Hacein-Bey-Abina S., Von Kalle C., Bougnères P., Fischer A., Cavazzana-Calvo M., Aubourg P. [Gene therapy of x-linked adrenoleukodystrophy using hematopoietic stem cells and a lentiviral vector] Bull. Acad Natl. Med. 2010;194:255–264, discussion 264–268. [PubMed] [Google Scholar]
- 30.Cesana D., Ranzani M., Volpin M., Bartholomae C., Duros C., Artus A., Merella S., Benedicenti F., Sergi Sergi L., Sanvito F. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014;22:774–785. doi: 10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryu B.Y., Evans-Galea M.V., Gray J.T., Bodine D.M., Persons D.A., Nienhuis A.W. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felsenfeld G., Burgess-Beusse B., Farrell C., Gaszner M., Ghirlando R., Huang S., Jin C., Litt M., Magdinier F., Mutskov V. Chromatin boundaries and chromatin domains. Cold Spring Harb. Symp. Quant. Biol. 2004;69:245–250. doi: 10.1101/sqb.2004.69.245. [DOI] [PubMed] [Google Scholar]
- 33.Gaszner M., Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 34.Neff T., Shotkoski F., Stamatoyannopoulos G. Stem cell gene therapy, position effects and chromatin insulators. Stem Cells. 1997;15(Suppl 1):265–271. doi: 10.1002/stem.5530150834. [DOI] [PubMed] [Google Scholar]
- 35.Emery D.W. The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum. Gene Ther. 2011;22:761–774. doi: 10.1089/hum.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raab J.R., Kamakaka R.T. Insulators and promoters: closer than we think. Nat. Rev. Genet. 2010;11:439–446. doi: 10.1038/nrg2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arumugam P.I., Urbinati F., Velu C.S., Higashimoto T., Grimes H.L., Malik P. The 3′ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PLoS ONE. 2009;4:e6995. doi: 10.1371/journal.pone.0006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S., Fatima S., Ma Z., Wang Y.D., Lu T., Janke L.J., Du Y., Sorrentino B.P. Evaluating the safety of retroviral vectors based on insertional oncogene activation and blocked differentiation in cultured thymocytes. Mol. Ther. 2016;24:1090–1099. doi: 10.1038/mt.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astrakhan A., Ochs H.D., Rawlings D.J. Wiskott-Aldrich syndrome protein is required for homeostasis and function of invariant NKT cells. J. Immunol. 2009;182:7370–7380. doi: 10.4049/jimmunol.0804256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humblet-Baron S., Sather B., Anover S., Becker-Herman S., Kasprowicz D.J., Khim S., Nguyen T., Hudkins-Loya K., Alpers C.E., Ziegler S.F. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J. Clin. Invest. 2007;117:407–418. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snapper S.B., Rosen F.S., Mizoguchi E., Cohen P., Khan W., Liu C.H., Hagemann T.L., Kwan S.P., Ferrini R., Davidson L. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Shehabeldin A., da Cruz L.A., Butler J., Somani A.K., McGavin M., Kozieradzki I., dos Santos A.O., Nagy A., Grinstein S. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J. Exp. Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolhatkar N.S., Scharping N.E., Sullivan J.M., Jacobs H.M., Schwartz M.A., Khim S., Notarangelo L.D., Thrasher A.J., Rawlings D.J., Jackson S.W. B-cell intrinsic TLR7 signals promote depletion of the marginal zone in a murine model of Wiskott-Aldrich syndrome. Eur. J. Immunol. 2015;45:2773–2779. doi: 10.1002/eji.201545644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westerberg L., Larsson M., Hardy S.J., Fernández C., Thrasher A.J., Severinson E. Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood. 2005;105:1144–1152. doi: 10.1182/blood-2004-03-1003. [DOI] [PubMed] [Google Scholar]
- 45.Modlich U., Schambach A., Brugman M.H., Wicke D.C., Knoess S., Li Z., Maetzig T., Rudolph C., Schlegelberger B., Baum C. Leukemia induction after a single retroviral vector insertion in Evi1 or Prdm16. Leukemia. 2008;22:1519–1528. doi: 10.1038/leu.2008.118. [DOI] [PubMed] [Google Scholar]
- 46.Zhou S., Ma Z., Lu T., Janke L., Gray J.T., Sorrentino B.P. Mouse transplant models for evaluating the oncogenic risk of a self-inactivating XSCID lentiviral vector. PLoS ONE. 2013;8:e62333. doi: 10.1371/journal.pone.0062333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiem H.P., Arumugam P.I., Burtner C.R., Fox C.F., Beard B.C., Dexheimer P., Adair J.E., Malik P. Pigtailed macaques as a model to study long-term safety of lentivirus vector-mediated gene therapy for hemoglobinopathies. Mol. Ther. Methods Clin. Dev. 2014;1:14055. doi: 10.1038/mtm.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beard B.C., Adair J.E., Trobridge G.D., Kiem H.P. High-throughput genomic mapping of vector integration sites in gene therapy studies. Methods Mol. Biol. 2014;1185:321–344. doi: 10.1007/978-1-4939-1133-2_22. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbloom K.R., Armstrong J., Barber G.P., Casper J., Clawson H., Diekhans M., Dreszer T.R., Fujita P.A., Guruvadoo L., Haeussler M. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 2015;43:D670–D681. doi: 10.1093/nar/gku1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forbes S.A., Beare D., Gunasekaran P., Leung K., Bindal N., Boutselakis H., Ding M., Bamford S., Cole C., Ward S. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacout C., Haddad E., Sabri S., Svinarchouk F., Garçon L., Capron C., Foudi A., Mzali R., Snapper S.B., Louache F. A defect in hematopoietic stem cell migration explains the nonrandom X-chromosome inactivation in carriers of Wiskott-Aldrich syndrome. Blood. 2003;102:1282–1289. doi: 10.1182/blood-2002-07-2099. [DOI] [PubMed] [Google Scholar]
- 52.Shepherd B.E., Kiem H.P., Lansdorp P.M., Dunbar C.E., Aubert G., LaRochelle A., Seggewiss R., Guttorp P., Abkowitz J.L. Hematopoietic stem-cell behavior in nonhuman primates. Blood. 2007;110:1806–1813. doi: 10.1182/blood-2007-02-075382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trobridge G.D., Kiem H.P. Large animal models of hematopoietic stem cell gene therapy. Gene Ther. 2010;17:939–948. doi: 10.1038/gt.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiem H.P., Baum C., Bushman F.D., Byrne B.J., Carter B.J., Cavagnaro J., Malech H.L., Mendell J.R., Naldini L.M., Sorrentino B.P. Charting a clear path: the ASGCT Standardized Pathways Conference. Mol. Ther. 2014;22:1235–1238. doi: 10.1038/mt.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanawa H., Hematti P., Keyvanfar K., Metzger M.E., Krouse A., Donahue R.E., Kepes S., Gray J., Dunbar C.E., Persons D.A., Nienhuis A.W. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- 56.Hanawa H., Yamamoto M., Zhao H., Shimada T., Persons D.A. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol. Ther. 2009;17:667–674. doi: 10.1038/mt.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrella A., Doti I., Agosti V., Giarrusso P.C., Vitale D., Bond H.M., Cuomo C., Tassone P., Franco B., Ballabio A. A 5′ regulatory sequence containing two Ets motifs controls the expression of the Wiskott-Aldrich syndrome protein (WASP) gene in human hematopoietic cells. Blood. 1998;91:4554–4560. [PubMed] [Google Scholar]
- 58.Hanawa H., Kelly P.F., Nathwani A.C., Persons D.A., Vandergriff J.A., Hargrove P., Vanin E.F., Nienhuis A.W. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol. Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 59.Hanawa H., Persons D.A., Nienhuis A.W. Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J. Virol. 2005;79:8410–8421. doi: 10.1128/JVI.79.13.8410-8421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerns H.M., Ryu B.Y., Stirling B.V., Sather B.D., Astrakhan A., Humblet-Baron S., Liggitt D., Rawlings D.J. B cell-specific lentiviral gene therapy leads to sustained B-cell functional recovery in a murine model of X-linked agammaglobulinemia. Blood. 2010;115:2146–2155. doi: 10.1182/blood-2009-09-241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sather B.D., Ryu B.Y., Stirling B.V., Garibov M., Kerns H.M., Humblet-Baron S., Astrakhan A., Rawlings D.J. Development of B-lineage predominant lentiviral vectors for use in genetic therapies for B cell disorders. Mol. Ther. 2011;19:515–525. doi: 10.1038/mt.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hocum J.D., Battrell L.R., Maynard R., Adair J.E., Beard B.C., Rawlings D.J., Kiem H.P., Miller D.G., Trobridge G.D. VISA--Vector Integration Site Analysis server: a web-based server to rapidly identify retroviral integration sites from next-generation sequencing. BMC Bioinformatics. 2015;16:212. doi: 10.1186/s12859-015-0653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.