Abstract
Mutations in cartilage oligomeric matrix protein cause pseudoachondroplasia, a severe disproportionate short stature disorder. Mutant cartilage oligomeric matrix protein produces massive intracellular retention of cartilage oligomeric matrix protein, stimulating ER and oxidative stresses and inflammation, culminating in post-natal loss of growth plate chondrocytes, which compromises linear bone growth. Treatments for pseudoachondroplasia are limited because cartilage is relatively avascular and considered inaccessible. Here we report successful delivery and treatment using antisense oligonucleotide technology in our transgenic pseudoachondroplasia mouse model. We demonstrate delivery of human cartilage oligomeric matrix protein-specific antisense oligonucleotides to cartilage and reduction of cartilage oligomeric matrix protein expression, which largely alleviates pseudoachondroplasia growth plate chondrocyte pathology. One antisense oligonucleotide reduced steady-state levels of cartilage oligomeric matrix protein mRNA and dampened intracellular retention of mutant cartilage oligomeric matrix protein, leading to a reduction of inflammatory markers and cell death and partial restoration of proliferation. This novel and exciting work demonstrates that antisense-based therapy is a viable approach for treating pseudoachondroplasia and other human cartilage disorders.
Keywords: antisense oligonucleotides, cartilage, pseudoachondroplasia, COMP
Mutations in COMP cause pseudoachondroplasia, a dwarfing condition. Mutant COMP accumulates in the ER of growth plate chondrocytes, which causes premature chondrocyte death and ultimately reduces long bone growth. Posey et al. show successful delivery of COMP ASOs to the avascular growth plate, which largely alleviates pseudoachondroplasia growth plate chondrocyte pathology.
Introduction
Treatments of skeletal dysplasia/dwarfing conditions, including pseudoachondroplasia (PSACH), are few and fraught with problems because of the inaccessibility of chondrocytes within the cartilage matrix and the relative avascular nature of the cartilage environment. PSACH is clinically characterized by disproportionate short stature, rhizomelic shortening of the long bones, brachydactyly, and extreme joint laxity.1, 2 Joint pain in childhood and osteoarthritis (OA) in early adulthood are the most debilitating features of PSACH; only symptomatic treatments are available and have limited efficacy.2, 3 The diagnosis is made between 2–3 years of age when linear growth slows and a waddling gait develops.1, 2 Because the diagnosis, in most cases, is made post-natally, treatments aimed at resolving the pathology will have to be started immediately after diagnosis.
PSACH is caused by mutations in cartilage oligomeric matrix protein (COMP), a pentameric extracellular matrix (ECM) glycoprotein abundant in musculoskeletal tissues.4, 5 Functionally, COMP facilitates type II collagen fibril assembly, enhances chondrocyte attachment in vitro, and interacts with other ECM proteins, including type II and IX collagens, matrilin 3, and SPARC.6, 7, 8, 9, 10, 11, 12 In contrast, mutations in COMP cause misfolding of the protein, preventing export from the chondrocyte and resulting in massive retention within the endoplasmic reticulum (ER).10, 13, 14, 15 Although this finding has long been appreciated, there was little understanding of the pathologic molecular mechanisms, which are critical to develop mechanism-driven therapeutics. To circumvent this problem, we generated the mutant (MT)-COMP mouse with the common D469del PSACH mutation that expresses human MT-COMP in chondrocytes in the presence of the inducing agent doxycycline (DOX).16, 17, 18, 19 MT-COMP mice recapitulate the PSACH clinical findings of reduced growth and cellular abnormalities, including massive intracellular retention of COMP and other ECM proteins.16, 17, 19 The intracellular retention of MT-COMP activates the protein kinase RNA-like endoplasmic reticulum kinase (PERK) arm of the unfolded protein response, which is the cellular response mechanism that is activated by misfolded proteins.20, 21 By 3 weeks of age, all murine MT-COMP growth plate chondrocytes have mutant COMP in the ER, causing chronic ER stress, inflammation, oxidative stress, and DNA damage that drives the chondrocytes to necroptosis.17 This becomes a self-perpetuating pathological process, with each pathologic component exacerbating the others.22, 23, 24, 25, 26 We have shown that aspirin, ibuprofen, and resveratrol dampen the PSACH chondrocyte pathology, reducing inflammation and/or oxidative stress.16 This important and novel therapeutic approach interrupts the pathological loop.
An alternative therapeutic approach would be to limit the amount of COMP expression, thereby treating the PSACH chondrocyte pathology at its origin. Support for this approach comes from COMP knockout mice that are normal in all growth parameters, with only mild growth plate disturbances and flattening of the articular cartilage surface after exercise.27 This suggests that COMP is not necessary for normal skeletal development, longevity, or fertility in mice,28 whereas the presence of mutant COMP is detrimental to skeletal growth. Therefore, decreasing COMP expression should limit the pathology. Using a short hairpin RNA (shRNA) that targets COMP, we showed that RNAi reduces COMP mRNA, intracellular retention, and ER stress in vitro.29 To develop a therapeutic platform based on these findings, we tested the delivery and efficacy of antisense oligonucleotides (ASOs) complementary to human COMP mRNA expressed in the MT-COMP mouse. In this work, we show that ASOs are delivered to the growth plate and effectively reduce COMP mRNA, COMP intracellular retention, and the inflammatory process elicited by MT-COMP expression.
Results
Loss of Endogenous Mouse COMP Decreases MT-COMP Chondrocyte Pathology
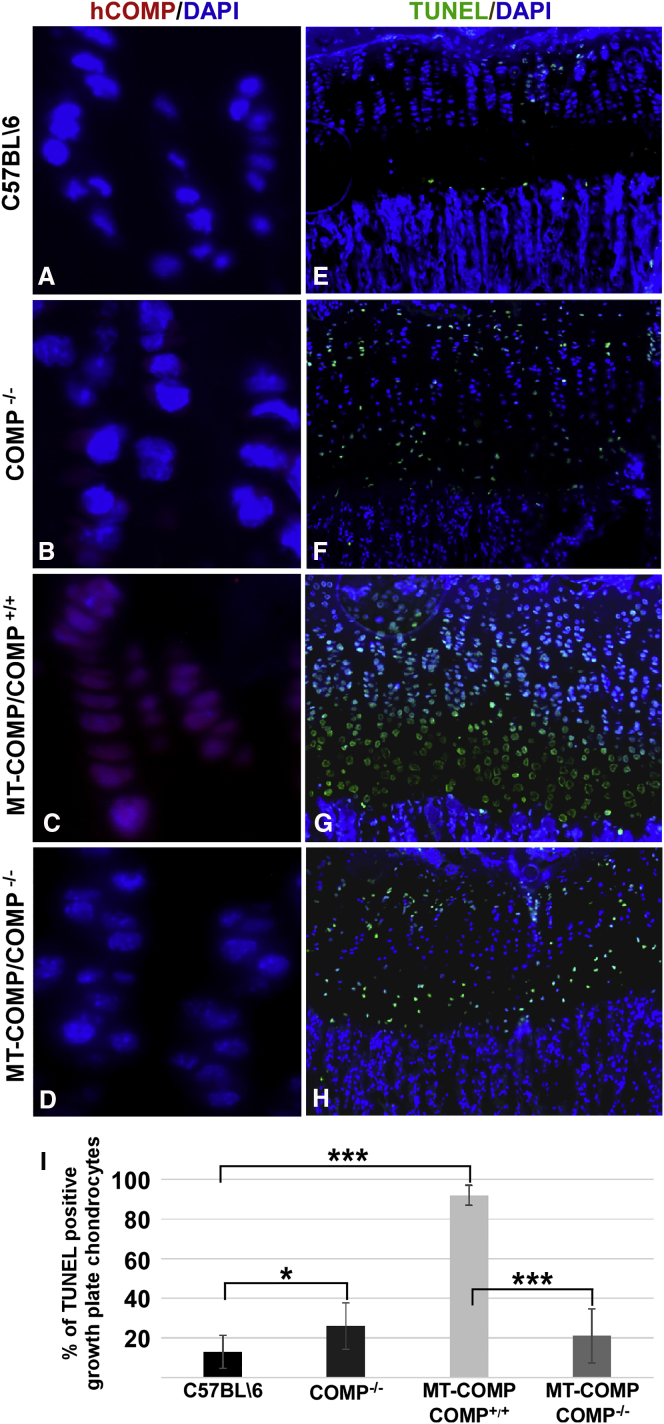
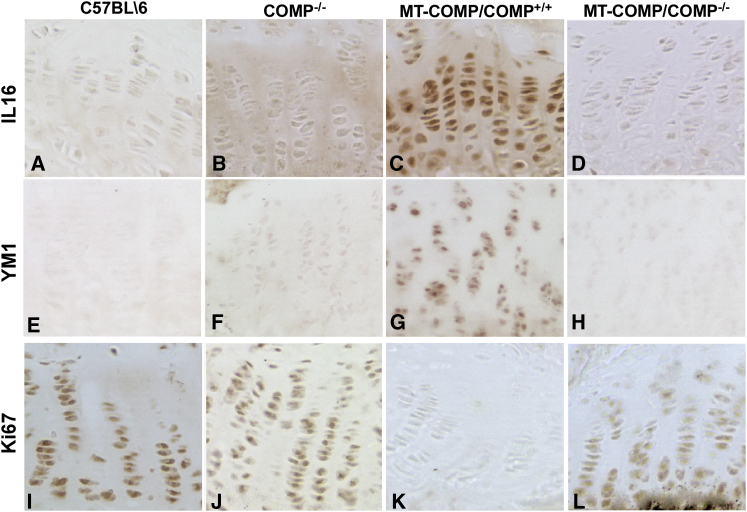
We have shown that MT-COMP accumulates in growth plate chondrocytes, generating ER and oxidative stress and an inflammatory process, which leads to premature chondrocyte death and decreased long bone growth in mice.16, 17, 18, 19 COMP pentamers often include both wild-type and misfolded mutant subunits, with an estimated 97% of the protein stalling and getting trapped in the ER.21, 30 This suggests that, in the presence of MT-COMP, wild-type (WT) COMP subunits may contribute to the cytotoxic intracellular retention. To determine the role of WT-COMP in this process, we evaluated whether reducing the total amount of murine COMP would dampen the MT-COMP chondrocyte phenotype. To accomplish this goal, we generated an MT-COMP/COMP−/− mouse that expresses MT-COMP in a COMP-null background using standard breeding. We found that human MT-COMP intracellular retention was reduced in the absence of endogenous wild-type mouse COMP (mCOMP; compare Figure 1C with Figure1D). As expected, no human COMP was observed in the C57BL\6 or COMP−/− growth plates because human COMP (hCOMP) is not present in these mice (Figures 1A and 1B; hCOMP is shown in red). The decrease in intracellular human MT-COMP in the MT-COMP/COMP−/− mice was accompanied by decreased terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL)-positive cells (Figure 1I; from 87% to 20%), indicating a reduction in growth plate chondrocyte death (compare Figure 1G with Figure 1H). Consistent with dampening of the MT-COMP chondrocyte phenotype, the inflammation-related proteins IL-16 and YM1 were reduced in the MT-COMP/COMP−/− growth plates (compare Figures 2D and 2H with Figures 2C and 2G). Although YM1 mRNA only trended downward, interleukin-16 (IL-16) mRNA was significantly reduced by 58% (p > 0.05). Moreover, proliferation, as monitored by Ki67 signal, was partially restored in MT-COMP/COMP−/− compared with MT-COMP growth plate chondrocytes (compare Figure 2L with Figure 2K). MT-COMP growth plates have much less Ki67 signal than the COMP−/− and C57BL\6 controls (Figures 2I and 2J). These results indicate that WT-COMP plays a significant role in the pathologic process; therefore, decreasing total COMP expression could be a therapeutic approach to mitigate the negative effects of MT-COMP on growth plate chondrocytes.
Figure 1.
The Absence of Wild-Type Endogenous Mouse COMP Decreases MT-COMP Intracellular Retention and Chondrocyte Death
(A–I) Human COMP-specific (A–D) and TUNEL (E–H) immunostaining of P28 growth plates from control (C57BL\6), COMP−/−, MT-COMP (MT-COMP/COMP+/+), and MT-COMP/COMP−/− mice is shown. DAPI (blue) was used to visualize nuclei. Growth plates from at least eight mice from each genotype were examined in each group. No human COMP is observed in either the control (C57BL\6) or COMP−/− growth plate (A and B). MT-COMP intracellular retention is decreased in the absence of endogenous mouse COMP (compare D with C). TUNEL staining is present in only a few chondrocytes of control mice (E), whereas, in the MT-COMP growth plate, most chondrocytes are TUNEL-positive (G). TUNEL staining is markedly decreased in the MT-COMP/COMP−/− mouse growth plate (compare H with G and I).
Figure 2.
Inflammatory Proteins Are Decreased and Proliferation Is Partially Restored by the Absence of Endogenous Wild-Type Murine COMP in MT-COMP/COMP−/− Mice
(A–L) Immunostaining of P28 growth plates from control (C57BL\6), COMP−/−, MT-COMP (MT-COMP/COMP+/+), and MT-COMP/COMP−/− mice is shown. Growth plates from at least eight mice from each genotype were examined in each group. Expression of the inflammatory proteins IL-16 and YM1 was reduced in MT-COMP/COMP−/− growth plate chondrocytes (D and H) compared with MT-COMP (C and G) but were similar to control (A and E) and COMP−/− (B and F) growth plate chondrocytes. Expression of Ki67, a marker of DNA proliferation, was present in control and COMP−/− (I and J), absent in MT-COMP (K), and partially restored in MT-COMP/COMP−/− (L) growth plate chondrocytes.
COMP ASO1 Is Delivered to Growth Plate Chondrocytes
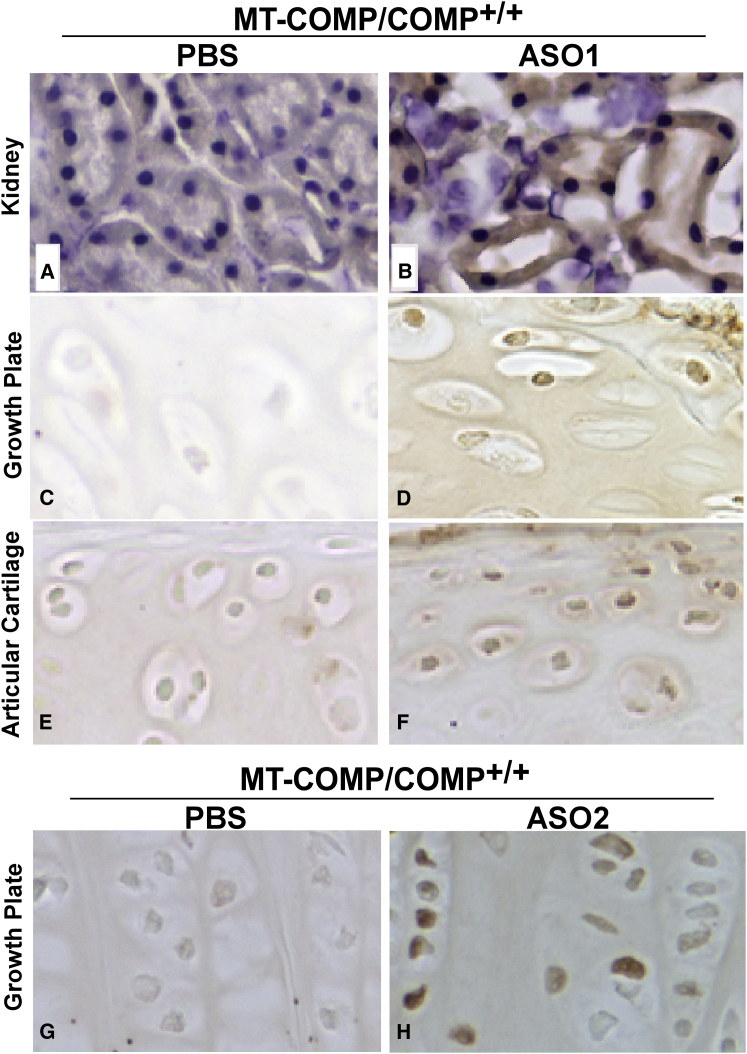
We have shown previously that COMP shRNAs reduced intracellular retention of MT-COMP, ER stress, and co-retention of extracellular proteins in rat chondrosarcoma (RCS) cells in vitro.29 In contrast to tissue culture experiments, the growth plate cartilage has long been considered difficult to target with drugs or antisense molecules because of its relative avascularity compared with the highly vascular liver and kidney.31 However, our success with drug therapy in the growth plate16, 18 led us to ask whether ASOs with high-affinity nucleoside modifications32 could be delivered to growth plate chondrocytes and reduce MT-COMP intracellular retention. Recent studies have shown that chemically modified ASOs can reduce mRNA targets efficiently in extra-hepatic tissues and show pharmacological benefits in disease models.33, 34 COMP-targeted ASOs were generated for testing in the MT-COMP mouse as described in Materials and Methods. Two ASOs were selected for therapeutic efficacy after in vitro testing and were administered starting at post-natal day 7 (P7) by subcutaneous injection of 60 mg/kg three times weekly for 3 weeks. Nine experiments were conducted with ASO1 (Figure 3), and two experiments were performed with ASO2 (data not shown). Diluent PBS-injected MT-COMP and non-treated C57BL\6 mice were controls for the experiments. Hindlimb joints and kidneys were collected on P29, 1 day after the last injection, and processed as described in Materials and Methods. Immunostaining with ASO DNA Ect3 backbone-specific antibody35 showed the presence of the ASO backbone in the positive control kidney tubules (Figure 4B) and chondrocytes of the growth plate (Figures 4D, ASO1, and 4H, ASO2) and articular cartilage (Figure 4F). No signal was detected in any of these tissues from phosphate buffer (diluent) (Figures 4A, 4C, 4E, and 4G) or untreated C57BL\6 controls (data not shown).
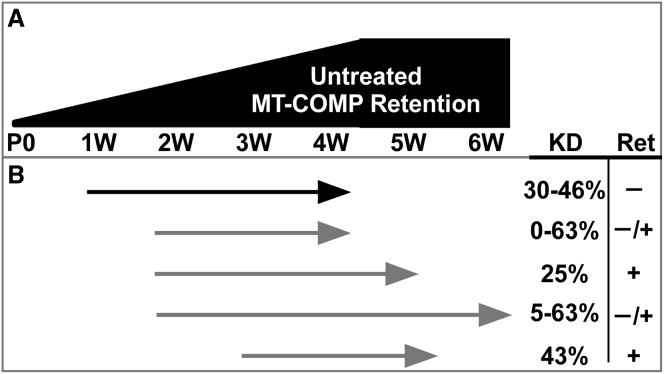
Figure 3.
Age at, Duration, and Outcomes of ASO1 Treatment
(A) The relative level of MT-COMP intracellular retention increases with age in untreated MT-COMP mice. (B) COMP ASO1 treatment start and stop points (arrows) with percent knockdown (KD) and intracellular retention (Ret = ± intracellular retention) are shown. Early treatment, beginning at 1 week, gave the best outcomes.
Figure 4.
Delivery of COMP-Targeted ASO1 to the Growth Plate and Articular Cartilage Chondrocytes
(A–H) Immunostaining with Ect3 antibody that recognizes the ASO DNA backbone in P29 kidney, growth plate, and articular cartilage from MT-COMP with and without COMP ASO1 or ASO2 treatment is shown. Sections from PBS-injected mice show little to no signal (A, C, E, and G). COMP ASO1 was administered by subcutaneous injection and, as expected, was present in the kidney (positive control) (D). COMP ASO1 and ASO2 were detected in both the growth plate (D and H) and articular chondrocytes (F; data not shown for ASO2).
COMP ASO1 Dampens the MT-COMP Pathology in Growth Plate Chondrocytes
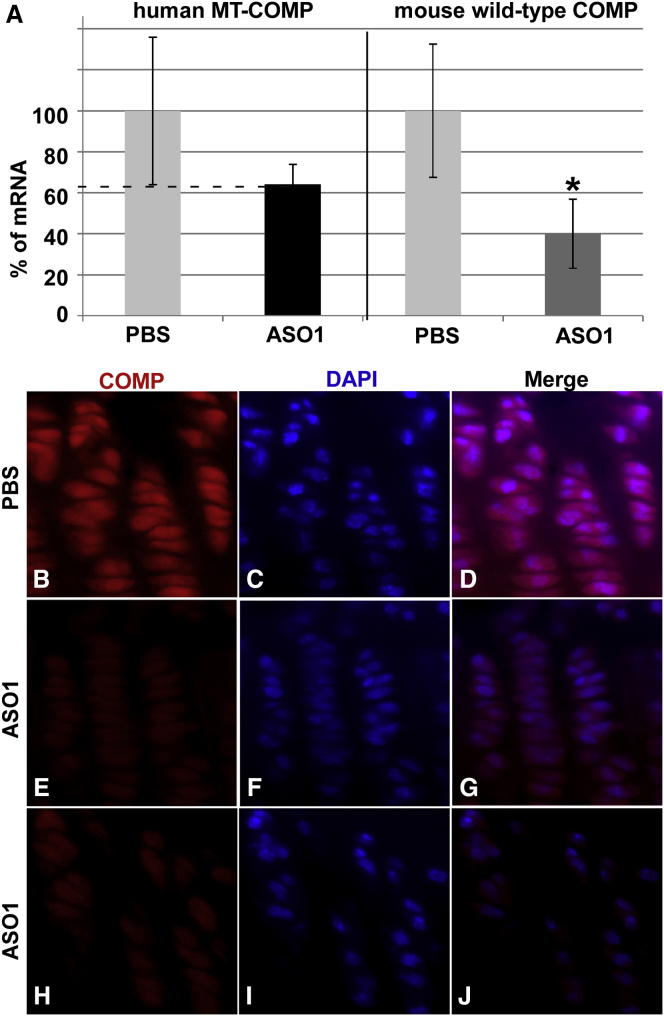
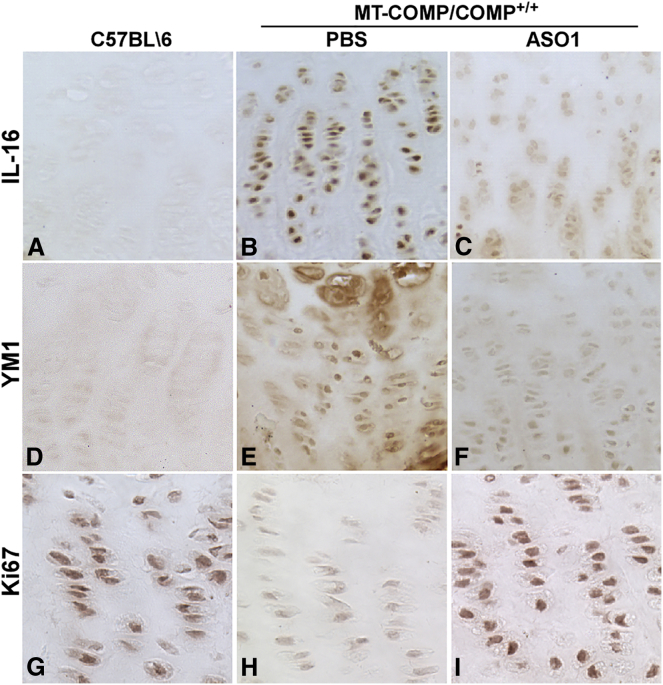
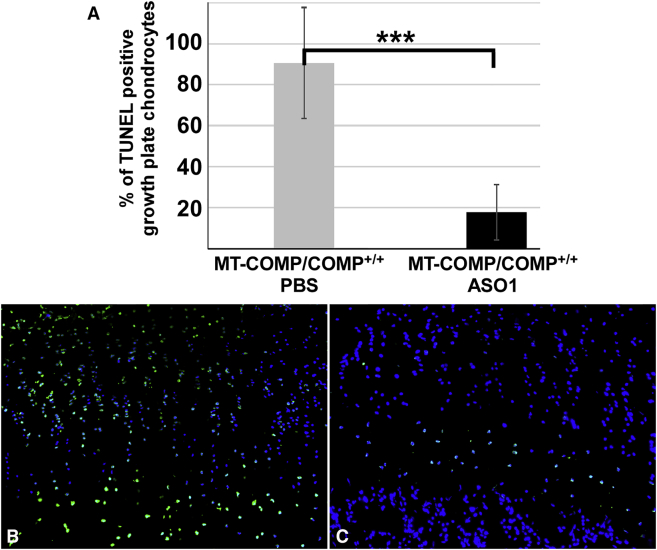
We next assessed the effect of COMP/ASO1 on steady-state COMP mRNA levels, MT-COMP intracellular retention, inflammatory markers, viability, and proliferation in growth plate chondrocytes. As shown in Figure 5A, ASO1 decreased human MT-COMP by 38% (range, 30%–46%). The reduction in steady-state MT-COMP mRNA markedly reduced the amount of intracellular (ER) MT-COMP retention (Figures 5G and 5J) compared with PBS-treated (Figure 5D) and untreated (data not shown) MT-COMP mice. Additionally, a 60% reduction in endogenous mouse COMP was observed in the MT-COMP mouse treated with ASO1. Expression of IL-16 and YM1, inflammatory markers, were decreased by ASO1 treatment (Figures 6C and 6F) compared with PBS-treated MT-COMP (Figures 6B and 6E), untreated MT-COMP (data not shown), and untreated C57BL\6 mice (Figures 6A and 6D). Additionally, IL-16 and YM1 mRNA levels were reduced 40% (p > 0.05) and 26% (p > 0.05), respectively. Consistent with a decrease in inflammation-related proteins, proliferation was restored by ASO1 treatment (compare Figure 6G with Figures 6H and 6I), suggesting that ASO1 treatment restored chondrocyte homeostasis in the growth plate. Additionally, the percentage of TUNEL-positive chondrocytes was reduced 4-fold by ASO1 treatment (Figure 7A). In the MT-COMP ASO1 growth plate, TUNEL-positive chondrocytes were primarily restricted to the hypertrophic zone in contrast to MT-COMP PBS, in which TUNEL-positive chondrocytes were observed throughout all zones of the growth plate (Figures 7B and 7C). ASO2 decreased human MT-COMP mRNA by 23% but did not reduce endogenous mouse COMP mRNA (data not shown). ASO2 did not reduce intracellular retention of MT-COMP (data not shown), and ASO2 was not evaluated further.
Figure 5.
COMP-Targeted ASO1 Decreases MT-COMP mRNA, Intracellular Retention of MT-COMP, and Growth Plate Chondrocyte Death
Quantitative real-time RT-PCR was used to assess steady-state levels of human and endogenous mouse COMP. Human COMP-specific antibody and TUNEL staining were used to assess intracellular retention and cell viability. DAPI (blue) was used to visualize nuclei. At least eight growth plates from PBS treated and untreated controls (C57BL\6), ASO1-treated, and untreated P29 mice were assessed in these experiments. (A) ASO1 reduced transgenic MT-COMP mRNA by 38% and endogenous wild-type mouse COMP by 60%. (B–D) Intracellular retention of MT-COMP is present in untreated MT-COMP (MT-COMP/COMP+/+) mice, as shown previously.16, 17, 19 (E–J) There is marked decreased MT-COMP intracellular retention with ASO1 treatment. ASO2 did not substantially reduce MT-COMP retention (data not shown). As expected, no human COMP is observed in the control (C57BL\6) (data not shown). *p > 0.05.
Figure 6.
COMP-Targeted ASO1 Treatment Reduces MT-COMP Inflammation in Growth Plate Chondrocytes
(A–I) Expression of IL-16 and YM1 was assessed in at least eight P29 growth plates from control (C57BL\6, no injection), MT-COMP (MT-COMP/COMP+/+) PBS-injected, and MT-COMP (MT-COMP/COMP+/+) COMP-ASO1-injected mice (A). IL-16 and YM1 expression was reduced by COMP-ASO1 treatment (compare C with B and F with E). In growth plate chondrocytes, Ki67, a DNA proliferation marker, was present in control C57BL\6 (G), reduced in MT-COMP PBS (H), and partially restored in MT-COMP by ASO1 treatment (I).
Figure 7.
Chondrocyte Death in MT-COMP Growth Plates Is Markedly Reduced by COMP-Targeted ASO1
(A–C) TUNEL immunostaining of P29 growth plates from MT-COMP (MT-COMP/COMP+/+) with and without ASO1 administration mice (B and C). The percentage of TUNEL-positive growth plate chondrocytes is compared (n ≥ 8 mice/group) (A). The percentage of TUNEL-positive MT-COMP growth plate chondrocytes is dramatically reduced by ASO1 treatment from 90% to 19%. Representative images of MT-COMP PBS (B) and ASO1 (C) treated growth plates are also shown. DAPI (blue) was used to visualize nuclei, and the TUNEL signal is shown in green. ***p > 0.0005.
COMP ASO1 Does Not Cause Liver Toxicity
Gross examination of liver size and color did not reveal any changes in ASO1- and PBS-treated and uninjected control mice (data not shown). The serum levels of albumin (ABL), aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T.Bil), creatinine (CREAT), and blood urea nitrogen (BUN) were within normal limits and did not indicate any compromise of murine liver function (Table S1).36, 37, 38, 39, 40
Discussion
In this study, we show that ASOs can be delivered to the growth plate and decrease MT-COMP mRNA steady-state levels and the associated chondrocyte pathology in the MT-COMP mouse (Figure 4).17, 18, 19, 41, 42, 43 This is a new and exciting approach not previously considered for cartilage-specific disorders because the growth plate has been considered to be an inaccessible avascular tissue. Here and in our previous antioxidant/anti-inflammatory therapeutic approaches, we show that the growth plate and articular cartilage are accessible and amenable to different therapies. Because absence of COMP has no effect on fertility and longevity in mice, COMP ASOs are a potential innovative and important treatment approach for PSACH.27, 28
ASO technology is a promising therapeutic approach for suppressing disease-associated genes, but delivery to tissues that do not have a rich blood supply can be challenging. Newer generations of chemically modified ASOs have enhanced RNA-binding affinity, nuclease stability, and pharmacokinetic properties that enable broad distribution to peripheral tissues in animals.44 Indeed, both ASO1 and ASO2 were detected in the growth plates of MT-COMP mice, expanding this therapeutic approach beyond the heart, liver, and kidney (Figure 4).45 Delivery to the growth plate was optimized by testing the method and frequency of injection, dosage, age at treatment, and duration of administration. Importantly, early administration was critical to therapeutic success. Most likely, for cartilage-targeted ASOs, these parameters will need to be optimized for each condition. In addition to optimizing delivery of the ASO, determining which particular ASO achieves reduction of the target mRNA in vivo without side effects requires screening a number of candidates (>300 COMP ASOs in this study).
Our treatment approaches are based on understanding the molecular pathology underlying PSACH. PSACH is an autosomal dominant condition caused by heterozygous mutations in COMP.41, 42, 43, 46 Functional COMP protein consists of five subunits bound together at the N-terminal domain.4, 46 The pentamerization domain in MT-COMP is normal, and, therefore, mutant and wild-type monomers are assembled in the same pentameric COMP molecule.21, 30 It is estimated that 97% of the COMP pentamers will have one to five mutant subunits, suggesting that, when MT-COMP is present, wild-type COMP may participate in the cytotoxic retention of COMP, and, therefore, the total load of COMP protein may play a role in PSACH chondrocyte pathology. Most mutations are in the calcium-binding domain that is critical for correct protein folding and structure.21, 30, 47, 48 Misfolded COMP causes massive retention of COMP and other extracellular proteins in the ER, which forms an inappropriate intracellular matrix eliciting oxidative and inflammatory stress, which is cytotoxic to the chondrocyte.3, 5, 14, 16, 17, 18, 43, 49 We have shown that treatments that target inflammation (aspirin), oxidative stress (resveratrol), and ER stress (absence of C/EBP homologous protein [CHOP] in MT-COMP mice, valproate) reduce the MT-COMP chondrocyte pathology.16, 17 Moreover, we have shown that shRNAs in vitro reduce COMP expression, ER stress, and intracellular retention.29 Therefore, treatment(s) aimed at the origin of the pathology should have a therapeutic benefit. Indeed, using ASOs, we show partial resolution of molecular pathology without side effects. Further improvements in ASO technology should enhance therapeutic outcomes. Additionally, combining ASOs with anti-inflammatory and/or antioxidant treatments may improve the efficacy over a single treatment agent.
To determine whether reducing the COMP protein load would diminish the chondrocyte pathology, we crossed the MT-COMP mouse into a COMP−/− background. As shown in Figures 1 and 2, the absence of endogenous mouse COMP in the presence of human MT-COMP (MT-COMP/COMP−/−) decreased intracellular retention of MT-COMP, growth plate chondrocyte death, and inflammatory markers and increased proliferation. This is consistent with the concept that COMP endogenous expression levels in the presence of mutant COMP are necessary to observe the PSACH chondrocyte phenotype in mice, especially because there is a shorter period of skeletal growth (8–10 weeks) in mice. This is further supported by the minimal chondrocyte pathology observed in two knockin COMP mutation mouse models that do not completely recapitulate PSACH chondrocyte pathology and clinical manifestations.50, 51, 52, 53 Altogether, this suggests that the total amount of COMP is an important factor in MT-COMP pathology and that reduction of total COMP with ASOs ameliorates the disease process.
We show that mitigation of the chondrocyte pathology was achieved by administering ASO1 for 3 weeks at 180 mg/kg/week. This level of COMP knockdown (38% hCOMP and 60% mCOMP) reduced intracellular retention of MT-COMP, chondrocyte death, and inflammatory markers and partially restored normal levels of chondrocyte proliferation (Figures 5B–5J and 6). This treatment protocol was selected because the level of knockdown was consistent, and treatment beginning on P7 in mice is roughly equivalent to 2 years in humans, the average age of PSACH diagnosis. For conditions in which there is a dominant-negative effect causing cellular stress, the reduction of both mutant and wild-type proteins needs to be considered in the therapeutic approach.
ASOs are designed to bind their cognate RNA by Watson-Crick base-pairing and modulate its function to produce a pharmacological reduction in the target RNA.54 The ASOs used in this study were fully modified with the phosphorothioate backbone modification and had a central gap region of ten DNA nucleotides flanked on either end with three constrained ethyl (cEt) nucleotides.32, 34 The phosphorothioate modification enhances ASO metabolic stability and promotes binding to plasma and cell surface proteins,55 which helps the ASO distribute from the site of injection to peripheral tissues. The DNA gap region supports RNase H-mediated cleavage of cRNA, whereas the cEt nucleotides enhance ASO metabolic stability and boost binding affinity for cRNA. One ASO, Kynamro,56 was recently approved by the Food and Drug Administration (FDA) for the treatment of homozygous familial hypercholesterolemia, and more than 35 RNase H-active ASOs are at various stages of clinical development. Interestingly, we found that ASO1 targeted to human COMP also reduces mouse COMP. One explanation is that the partial complementarity between ASO1 and mouse COMP mRNA at critical positions allows knockdown. In this scenario, the therapeutic outcome was enhanced. However, this will not apply in human clinical trials.
Taken together these findings suggest that ASOs should be explored further as a clinical therapeutic approach in PSACH, osteoarthritis, and joint injury. Increased human longevity has also increased the burden of cartilage-related disease (http://www.cdc.gov/arthritis/basics/osteoarthritis.htm). Cartilage recovery from trauma or severe wear and tear is limited compared with the healing capacity of other tissues.57 ASOs could be used to repress counter-productive chronic inflammation and to slow the cartilage degeneration processes by targeting pro-inflammatory mRNAs and mRNAs that code for degradative enzymes and, thereby, perhaps promote tissue recovery and regeneration. An effective treatment for cartilage injuries could potentially prevent the post-traumatic osteoarthritis that frequently develops many years after the initial joint damage. Improvements in osteoarthritis therapeutics have the potential to keep our aging population healthy and active in society for longer, not only improving individual health but also decreasing the cost of healthcare.
Chondrocytes produce COMP during skeletal growth, maturation, and cartilage maintenance, and, therefore, rescue of human PSACH chondrocytes will require long-term therapy.11, 58, 59, 60, 61, 62, 63 Tolerability and toxicity must be carefully considered with long-term administration, whereas these factors are less important with short-duration therapies. Collectively, the observations reported here demonstrate that early ASO1 systemic administration dampens the MT-COMP growth plate chondrocyte phenotype. This is the first demonstration of ASO molecules reaching cartilage in vivo in therapeutically relevant concentrations and effecting a benefit to the growth plate. This is a major milestone for cartilage disorders, where treatments aimed at the growth plate and articular cartilage chondrocytes have been hampered by inadequate delivery systems and the sequestered and avascular environment of the cartilage. These important results provide opportunities for a novel treatment not only for PSACH but other skeletal disorders caused by mutations in genes affecting cartilage and bone and common conditions such as osteoarthritis.
Materials and Methods
MT-COMP and MT-COMP/COMP−/− Mice
This study complied with the Guide for the Care and Use of Laboratory Animals, eighth edition (ISBN-10, 0-309-15396-4) and was approved by the Animal Welfare Committee at the University of Texas Medical School at Houston. Mutant MT-COMP (MT-COMP/COMP+/+) mice were generated by introducing DNA containing expression cassettes derived from two plasmids, pTRE-MT-COMP (D469del COMP mutation with a FLAG tag) and pTET-On-Col II, as described previously.19 Mice were administered DOX (500 ng/mL) through the drinking water (with 5% w/v sucrose) pre- and post-natally. The COMP−/− mice originally generated by Dr. A. Oldberg (Lund University) do not express endogenous mouse COMP or specifically display features of skeletal dysplasia or PSACH.28 C57BL/6 mice were used as controls because the WT-COMP mice showed no phenotypic differences in our previous studies.19
Histology and Immunohistochemistry
Hindlimbs from P28 or P29 MT-COMP, C57BL/6, COMP−/−, and MT-COMP/COMP−/− mice were collected, and tibial growth plates were analyzed as described previously.19 Briefly, the limbs were fixed in 95% v/v ethanol for immunostaining for human-specific COMP (Thermo Fisher Scientific, ab11056-rat, 1:100), YM1/eosinophil chemotactic factor-L protein (ECF-L; STEMCELL Technologies, 01404, 1:100), IL-16 (Santa Cruz Biotechnology, SC-7902, 1:100), Ki67 M19 (Santa Cruz, SC-7846 1:200), or Ionis ASO DNA backbone Ect3 antibody (Ionis Pharmaceuticals, proprietary, 1:20,000). Sections immunostained with Ionis ASO DNA backbone antibody were quenched (endogenous peroxide) with 3% hydrogen peroxide incubation for 10–15 min, followed by two rinses in PBS with tween (PBST) for 5 min each. Antigens were retrieved with proteinase K (Dako, 3020) treatment for 2–10 min at room temperature, followed by two rinses in PBST for 5 min each. Tissue was blocked with Cyto-Q background buster (Innovex Biosciences, NB 306) for 20 min, followed by two rinses in PBST for 5 min each. Sections were incubated overnight at 4°C with primary antibody (1:20,000) followed by two rinses in PBST for 5 min each. Donkey anti-rabbit secondary antibody (1:1,000) was incubated with tissues for 1 hr at room temperature, followed by three rinses in PBST for 5 min each. 3,3'-diaminobenzidine (DAB) chromagen was used to visualize the primary antibody signal. Limbs were fixed in 10% w/v formalin for TUNEL staining. At least eight mice were examined from each group in these experiments.
Quantitative Real-Time RT-PCR
Hindlimb knee joints were collected from mice at 4 weeks of age. Soft tissue was removed, and the remaining joint was homogenized in Trizol and purified using the manufacturer’s instructions (Life Technologies). RNA was further purified using QIAGEN RNAeasy columns. Quantitative real-time RT-PCR was performed utilizing the ABI-7900 RT-PCR system (Applied Biosystems). DNA contamination was removed using amplification-grade DNase (Life Technologies) following the manufacturer’s instructions. Each assay was replicated three times, and each sample was measured in triplicate, including a control without reverse transcriptase. The final data were normalized to HPRT1a (percent of the normalizer transcript). The following primers were used in this study: human COMP, 5′-GCAATGACACCATCCCAGAG-3′; FLAG tag primer, 5′-CTTGTCATCGTCGTCCTTGTAGTC-3′; mouse COMP, 5′-TGCTGCGAGAACTTCAGGA-3 and 5′-CCT CGT GTC GCA ACA GCT-3′; and HPRT1a, 5′-CCTCATGGACTGATTATGGACAG-3′ and 5′-TCAGCAAAGAACTTATAGCCCC-3′. The following primers were used to confirm qRT-PCR results: human COMP, 5′-CAGGGAGATCACGTTCCTGA-3′and 5′-GGCCGGTGCGTACTGAC-3′; mouse COMP, 5′-AGTCCCTAACGAGCAAG-3′ and 5′-GCTACATTTCGTATTCGGTCGCCATCT-3′ (data not shown).
Design and Screening of COMP ASOs
Three hundred ASOs were designed to target human COMP (accession number NM_000095) and tested in HEPG2 cells and human tenocytes cultured under standard culturing conditions with DMEM supplemented with fetal bovine serum (FBS), antibiotics, and antimycotics. Fifty COMP-ASOs were screened for dosage response in human tenocytes (concentration of an inhibitor where the response [or binding] is reduced by half [IC50], 0.3–0.8 μM). Twelve leads from the dose-response screen were screened in C57Bl\6 adult mice at 100 mg/kg for 3 weeks. The two best-tolerated ASOs, designated ASO1 and ASO2, were tested in MT-COMP mice at 180 mg/kg for 3 weeks. ASO1 5′TCCGGCGGGTCCTCAC3′ binds to the 5′ UTR and ASO2 5′CGGTAACGCAGGTTGG3′ binds in the C-terminal globular coding region and would be predicted to cause human COMP mRNA degradation.
ASO Administration
To identify the best injection method and to establish delivery to the cartilage, an ASO that targets metastasis associated lung adenocarcinoma transcript 1 (MALAT1), a non-coding RNA, was tested by subcutaneous, intramuscular, and intraperitoneal injection. Relative MALAT1 RNA levels from growth plates were compared, and liver knockdown was monitored to confirm ASO delivery. Subcutaneous injection resulted in 2- to 3-fold less mRNA, whereas intramuscular and intraperitoneal injections did not reduce MALAT1 mRNA levels. We next tested five concentrations of ASO1 (50, 100, 150, 180, 200, and 300 mg/kg/week) ASOs reconstituted in PBS buffer at 5 mg/mL and found that 180 mg/kg/week gave the maximum knockdown. Optimal timing was determined by varying when a treatment began and its duration. Figure 3 shows that administration of ASO1 for 3 weeks beginning at 1 week of age produced the best and most consistent level of knockdown. This is consistent with our previous findings, which indicate that 2 weeks of MT-COMP expression elicits intracellular retention and growth plate chondrocyte death18 and, therefore, that earlier treatment results in a better outcome. To establish the most effective frequency of administration, one, two, or three weekly injections were tested, and three weekly injections gave a more consistent level of knockdown. Based on these results, ASOs 1 and 2 were administered by subcutaneous injections of 60 mg/kg three times weekly beginning on P7 until P28, with a minimum of three replicates for each experiment. Murine blood, liver, kidney, and hindlimb joints were collected 1–2 days after the last injection and prepared as described above for analyses.
Statistical Method
Student’s t test was used to compare outcomes. F tests were first performed to determine whether the variance was equal, and then the appropriate t test was performed based on the outcome of the f test.
Author Contributions
K.L.P. managed the project at UTH McGovern Medical School, planned experiments, analyzed data, and prepared the manuscript. P.P.S. managed the project at Ionis Pharmaceuticals, analyzed data, and provided ASO expertise. D.G. screened ASOs in cells to identify active leads, and S.B. screened ASOs in animals to identify well tolerated leads. F.C. performed the RT-PCR analyses, A.C.V. performed the immunostaining, M.H. managed the animal experiments, and J.L.A. contributed to manuscript preparation. J.T.H. oversaw the entire project, analyzed data, and prepared the manuscript.
Conflicts of Interest
P.P.S., D.G., and S.B. are employees of Ionis Pharmaceuticals, Inc. K.P., F.C., A.C.V., M.H., J.L.A., and J.T.H. have declared that no conflicts of interest exist.
Acknowledgments
We thank Seenya Vincent and Frankie Chiu for technical assistance. The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH under Award RO1-AR057117-05. Additional funding was provided by the Leah Lewis Family Foundation.
Footnotes
Supplemental Information includes one table and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.12.024.
Supplemental Information
References
- 1.McKeand J., Rotta J., Hecht J.T. Natural history study of pseudoachondroplasia. Am. J. Med. Genet. 1996;63:406–410. doi: 10.1002/(SICI)1096-8628(19960517)63:2<406::AID-AJMG16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 2.Unger S., Hecht J.T. Pseudoachondroplasia and multiple epiphyseal dysplasia: New etiologic developments. Am. J. Med. Genet. 2001;106:244–250. [PubMed] [Google Scholar]
- 3.Posey K.L., Hecht J.T. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets. 2008;9:869–877. doi: 10.2174/138945008785909293. [DOI] [PubMed] [Google Scholar]
- 4.Adams J.C. Thrombospondins: multifunctional regulators of cell interactions. Annu. Rev. Cell Dev. Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Hecht J.T., Deere M., Putnam E., Cole W., Vertel B., Chen H., Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17:269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 6.Bleasel J.F., Poole A.R., Heinegård D., Saxne T., Holderbaum D., Ionescu M., Jones P., Moskowitz R.W. Changes in serum cartilage marker levels indicate altered cartilage metabolism in families with the osteoarthritis-related type II collagen gene COL2A1 mutation. Arthritis Rheum. 1999;42:39–45. doi: 10.1002/1529-0131(199901)42:1<39::AID-ANR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Chen T.L., Posey K.L., Hecht J.T., Vertel B.M. COMP mutations: domain-dependent relationship between abnormal chondrocyte trafficking and clinical PSACH and MED phenotypes. J. Cell. Biochem. 2008;103:778–787. doi: 10.1002/jcb.21445. [DOI] [PubMed] [Google Scholar]
- 8.Lohmander L.S., Saxne T., Heinegård D.K. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann. Rheum. Dis. 1994;53:8–13. doi: 10.1136/ard.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann H.H., Ozbek S., Engel J., Paulsson M., Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J. Biol. Chem. 2004;279:25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- 10.Merritt T.M., Bick R., Poindexter B.J., Alcorn J.L., Hecht J.T. Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am. J. Pathol. 2007;170:293–300. doi: 10.2353/ajpath.2007.060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thur J., Rosenberg K., Nitsche D.P., Pihlajamaa T., Ala-Kokko L., Heinegård D., Paulsson M., Maurer P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- 12.Xu K., Zhang Y., Ilalov K., Carlson C.S., Feng J.Q., Di Cesare P.E., Liu C.J. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J. Biol. Chem. 2007;282:11347–11355. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 13.Hecht J.T., Makitie O., Hayes E., Haynes R., Susic M., Montufar-Solis D., Duke P.J., Cole W.G. Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate. J. Orthop. Res. 2004;22:759–767. doi: 10.1016/j.orthres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Hecht J.T., Montufar-Solis D., Decker G., Lawler J., Daniels K., Duke P.J. Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol. 1998;17:625–633. doi: 10.1016/s0945-053x(98)90113-5. [DOI] [PubMed] [Google Scholar]
- 15.Merritt T.M., Alcorn J.L., Haynes R., Hecht J.T. Expression of mutant cartilage oligomeric matrix protein in human chondrocytes induces the pseudoachondroplasia phenotype. J. Orthop. Res. 2006;24:700–707. doi: 10.1002/jor.20100. [DOI] [PubMed] [Google Scholar]
- 16.Posey K.L., Coustry F., Veerisetty A.C., Hossain M., Alcorn J.L., Hecht J.T. Antioxidant and anti-inflammatory agents mitigate pathology in a mouse model of pseudoachondroplasia. Hum. Mol. Genet. 2015;24:3918–3928. doi: 10.1093/hmg/ddv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posey K.L., Coustry F., Veerisetty A.C., Liu P., Alcorn J.L., Hecht J.T. Chop (Ddit3) is essential for D469del-COMP retention and cell death in chondrocytes in an inducible transgenic mouse model of pseudoachondroplasia. Am. J. Pathol. 2012;180:727–737. doi: 10.1016/j.ajpath.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posey K.L., Coustry F., Veerisetty A.C., Liu P., Alcorn J.L., Hecht J.T. Chondrocyte-specific pathology during skeletal growth and therapeutics in a murine model of pseudoachondroplasia. J. Bone Miner. Res. 2014;29:1258–1268. doi: 10.1002/jbmr.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posey K.L., Veerisetty A.C., Liu P., Wang H.R., Poindexter B.J., Bick R., Alcorn J.L., Hecht J.T. An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am. J. Pathol. 2009;175:1555–1563. doi: 10.2353/ajpath.2009.090184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs M.D., Hoffman S.M., King L.M., Olsen A.S., Mohrenweiser H., Leroy J.G., Mortier G.R., Rimoin D.L., Lachman R.S., Gaines E.S. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat. Genet. 1995;10:330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 21.Hecht J.T., Nelson L.D., Crowder E., Wang Y., Elder F.F., Harrison W.R., Francomano C.A., Prange C.K., Lennon G.G., Deere M. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat. Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 22.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 23.Gotoh T., Endo M., Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int. J. Inflamm. 2011;2011:259462. doi: 10.4061/2011/259462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cullinan S.B., Diehl J.A. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posey K.L., Hankenson K., Veerisetty A.C., Bornstein P., Lawler J., Hecht J.T. Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am. J. Pathol. 2008;172:1664–1674. doi: 10.2353/ajpath.2008.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svensson L., Aszódi A., Heinegård D., Hunziker E.B., Reinholt F.P., Fässler R., Oldberg A. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol. Cell. Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posey K.L., Liu P., Wang H.R., Veerisetty A.C., Alcorn J.L., Hecht J.T. RNAi reduces expression and intracellular retention of mutant cartilage oligomeric matrix protein. PLoS ONE. 2010;5:e10302. doi: 10.1371/journal.pone.0010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H., Deere M., Hecht J.T., Lawler J. Cartilage oligomeric matrix protein is a calcium-binding protein, and a mutation in its type 3 repeats causes conformational changes. J. Biol. Chem. 2000;275:26538–26544. doi: 10.1074/jbc.M909780199. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y., Tuan R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015;11:206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth P.P., Siwkowski A., Allerson C.R., Vasquez G., Lee S., Prakash T.P., Wancewicz E.V., Witchell D., Swayze E.E. Short antisense oligonucleotides with novel 2′-4′ conformationally restricted nucleoside analogues show improved potency without increased toxicity in animals. J. Med. Chem. 2009;52:10–13. doi: 10.1021/jm801294h. [DOI] [PubMed] [Google Scholar]
- 33.Murray S., Ittig D., Koller E., Berdeja A., Chappell A., Prakash T.P., Norrbom M., Swayze E.E., Leumann C.J., Seth P.P. TricycloDNA-modified oligo-2′-deoxyribonucleotides reduce scavenger receptor B1 mRNA in hepatic and extra-hepatic tissues--a comparative study of oligonucleotide length, design and chemistry. Nucleic Acids Res. 2012;40:6135–6143. doi: 10.1093/nar/gks273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler T.M., Leger A.J., Pandey S.K., MacLeod A.R., Nakamori M., Cheng S.H., Wentworth B.M., Bennett C.F., Thornton C.A. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature. 2012;488:111–115. doi: 10.1038/nature11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler M., Stecker K., Bennett C.F. Cellular distribution of phosphorothioate oligodeoxynucleotides in normal rodent tissues. Lab. Invest. 1997;77:379–388. [PubMed] [Google Scholar]
- 36.Hayes A.W. Raven Press; New York: 1982. Principles and Methods of Toxicology. [Google Scholar]
- 37.Hayes A.W. Raven Press; New York: 1989. Principles and Methods of Toxicology. [Google Scholar]
- 38.Hayes A.W. Taylor & Francis; Philadelphia, PA: 2001. Principles and Methods of Toxicology. [Google Scholar]
- 39.Hayes A.W. CRC Press/Taylor & Francis Group; Boca Raton, FL: 2008. Principles and Methods of Toxicology. [Google Scholar]
- 40.Hayes A.W., Kruger C.L. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2014. Hayes’ Principles and Methods of Toxicology. [Google Scholar]
- 41.Alcorn J.L., Merritt T.M., Farach-Carson M.C., Wang H.H., Hecht J.T. Ribozyme-mediated reduction of wild-type and mutant cartilage oligomeric matrix protein (COMP) mRNA and protein. RNA. 2009;15:686–695. doi: 10.1261/rna.1335909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T.L., Stevens J.W., Cole W.G., Hecht J.T., Vertel B.M. Cell-type specific trafficking of expressed mutant COMP in a cell culture model for PSACH. Matrix Biol. 2004;23:433–444. doi: 10.1016/j.matbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Posey K.L., Hayes E., Haynes R., Hecht J.T. Role of TSP-5/COMP in pseudoachondroplasia. Int. J. Biochem. Cell Biol. 2004;36:1005–1012. doi: 10.1016/j.biocel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Yu R.Z., Grundy J.S., Geary R.S. Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert Opin. Drug Metab. Toxicol. 2013;9:169–182. doi: 10.1517/17425255.2013.737320. [DOI] [PubMed] [Google Scholar]
- 45.Geary R.S., Norris D., Yu R., Bennett C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Adams J.C., Tucker R.P., Lawler J. R.G. Landes Co.; 1995. The thrombospondin gene family. [Google Scholar]
- 47.Délot E., Brodie S.G., King L.M., Wilcox W.R., Cohn D.H. Physiological and pathological secretion of cartilage oligomeric matrix protein by cells in culture. J. Biol. Chem. 1998;273:26692–26697. doi: 10.1074/jbc.273.41.26692. [DOI] [PubMed] [Google Scholar]
- 48.Délot E., King L.M., Briggs M.D., Wilcox W.R., Cohn D.H. Trinucleotide expansion mutations in the cartilage oligomeric matrix protein (COMP) gene. Hum. Mol. Genet. 1999;8:123–128. doi: 10.1093/hmg/8.1.123. [DOI] [PubMed] [Google Scholar]
- 49.Posey K.L., Alcorn J.L., Hecht J.T. Pseudoachondroplasia/COMP - translating from the bench to the bedside. Matrix Biol. 2014;37:167–173. doi: 10.1016/j.matbio.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suleman F., Gualeni B., Gregson H.J., Leighton M.P., Piróg K.A., Edwards S., Holden P., Boot-Handford R.P., Briggs M.D. A novel form of chondrocyte stress is triggered by a COMP mutation causing pseudoachondroplasia. Hum. Mutat. 2012;33:218–231. doi: 10.1002/humu.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piróg K.A., Irman A., Young S., Halai P., Bell P.A., Boot-Handford R.P., Briggs M.D. Abnormal chondrocyte apoptosis in the cartilage growth plate is influenced by genetic background and deletion of CHOP in a targeted mouse model of pseudoachondroplasia. PLoS ONE. 2014;9:e85145. doi: 10.1371/journal.pone.0085145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piróg K.A., Jaka O., Katakura Y., Meadows R.S., Kadler K.E., Boot-Handford R.P., Briggs M.D. A mouse model offers novel insights into the myopathy and tendinopathy often associated with pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Mol. Genet. 2010;19:52–64. doi: 10.1093/hmg/ddp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piróg-Garcia K.A., Meadows R.S., Knowles L., Heinegård D., Thornton D.J., Kadler K.E., Boot-Handford R.P., Briggs M.D. Reduced cell proliferation and increased apoptosis are significant pathological mechanisms in a murine model of mild pseudoachondroplasia resulting from a mutation in the C-terminal domain of COMP. Hum. Mol. Genet. 2007;16:2072–2088. doi: 10.1093/hmg/ddm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett C.F., Swayze E.E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 55.Miller C.M., Donner A.J., Blank E.E., Egger A.W., Kellar B.M., Østergaard M.E., Seth P.P., Harris E.N. Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res. 2016;44:2782–2794. doi: 10.1093/nar/gkw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crooke S.T., Geary R.S. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br. J. Clin. Pharmacol. 2013;76:269–276. doi: 10.1111/j.1365-2125.2012.04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Driscoll S.W. The healing and regeneration of articular cartilage. J. Bone Joint Surg. Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 58.Clark A.G., Jordan J.M., Vilim V., Renner J.B., Dragomir A.D., Luta G., Kraus V.B. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 59.DiCesare P.E., Mörgelin M., Carlson C.S., Pasumarti S., Paulsson M. Cartilage oligomeric matrix protein: isolation and characterization from human articular cartilage. J. Orthop. Res. 1995;13:422–428. doi: 10.1002/jor.1100130316. [DOI] [PubMed] [Google Scholar]
- 60.DiCesare P.E., Mörgelin M., Mann K., Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur. J. Biochem. 1994;223:927–937. doi: 10.1111/j.1432-1033.1994.tb19070.x. [DOI] [PubMed] [Google Scholar]
- 61.Fang C., Carlson C.S., Leslie M.P., Tulli H., Stolerman E., Perris R., Ni L., Di Cesare P.E. Molecular cloning, sequencing, and tissue and developmental expression of mouse cartilage oligomeric matrix protein (COMP) J. Orthop. Res. 2000;18:593–603. doi: 10.1002/jor.1100180412. [DOI] [PubMed] [Google Scholar]
- 62.Fang C., Johnson D., Leslie M.P., Carlson C.S., Robbins M., Di Cesare P.E. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J. Orthop. Res. 2001;19:634–641. doi: 10.1016/S0736-0266(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 63.Posey K.L., Davies S., Bales E.S., Haynes R., Sandell L.J., Hecht J.T. In vivo human Cartilage oligomeric matrix protein (COMP) promoter activity. Matrix Biol. 2005;24:539–549. doi: 10.1016/j.matbio.2005.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.