Abstract
Homeobox transcript antisense RNA (HOTAIR), as a long intergenic non-coding RNA (lincRNA), is upregulated in various cancers and involved in diverse cellular functions. However, its role in liver fibrosis is unclear. In this study, HOTAIR expression was upregulated in hepatic stellate cells (HSCs) in vivo and in vitro during liver fibrosis. HOTAIR knockdown suppressed HSC activation including α-smooth muscle actin (α-SMA) and typeIcollagen in vitro and in vivo. Both HSC proliferation and cell cycle were inhibited by HOTAIR knockdown. Notably, inhibition of HOTAIR led to an increase in PTEN, associated with the loss of DNA methylation. miR-29b-mediated control of PTEN methylation was involved in the effects of HOTAIR knockdown. HOTAIR was confirmed a target of miR-29b and lack of the miR-29b binding site in HOTAIR prevented the suppression of miR-29b, suggesting HOTAIR contributes to PTEN expression downregulation via sponging miR-29b. Interestingly, increased HOTAIR was also observed in hepatocytes during liver fibrosis. Loss of HOTAIR additionally led to the increase in PTEN and the reduction in typeIcollagen in hepatocytes. Collectively, we demonstrate that HOTAIR downregulates miR-29b expression and attenuates its control on epigenetic regulation, leading to enhanced PTEN methylation, which contributes to the progression of liver fibrosis.
Keywords: homeobox transcript antisense RNA, HOTAIR, microRNA-29b, DNA methylation, phosphatase and tensin homolog deleted on chromosome 10, PTEN, DNA methyltransferase, DNMT
Jianjian Zheng and colleagues found that HOTAIR was upregulated in activated HSCs during liver fibrosis and loss of HOTAIR suppressed HSC activation. They demonstrated that HOTAIR downregulates miR-29b and attenuates its control on DNMT3b, leading to restoration of DNMT3b and enhancement of PTEN methylation, which contributes to liver fibrosis.
Introduction
Liver fibrosis, characterized by excessive accumulation of extracellular matrix (ECM) proteins in response to chronic liver injury of any cause, is an integral part in the progression of chronic inflammatory liver disease.1, 2, 3 With the development of liver damage, the risk of cirrhosis and hepatocellular carcinoma (HCC) is increased. Generally, liver fibrosis is considered as a reversible disease and could be prevented from becoming advanced fibrotic process by effective treatments. During liver fibrosis, hepatic stellate cells (HSCs), which are mainly responsible for the accumulation of ECM proteins, become activated and undergo myofibroblastic transdifferentiation.4 Therefore, suppression of activated HSCs is considered as a potential target for liver fibrosis.
Regulatory non-coding RNAs (ncRNAs), such as microRNAs (miRNAs), have been demonstrated to play a key role in a variety of cellular processes including cell proliferation and differentiation.5 Emerging evidence has revealed that miRNAs are implicated in HSC activation and thereby regulate liver fibrosis.6, 7, 8, 9 For example, Wang et al. reported that miR-29b prevents liver fibrosis by suppressing HSC activation and inducing cell apoptosis via targeting PI3K/AKT pathway.3 Our previous study found that curcumin upregulates miR-29b expression, leading to the silencing of DNA methyltransferase 3b (DNMT3b) and the loss of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) methylation, which contributes to suppression of activated HSCs.10
Other ncRNAs, such as long intergenic non-coding RNAs (lincRNAs) and the heterogeneous group of long non-coding RNAs (lncRNAs), have also been reported to be involved in human diseases.11, 12, 13 LncRNA is most commonly defined as a non-protein-coding RNA molecule longer than 200 nucleotides. Increasing evidence has demonstrated that lncRNAs are involved in a wide range of biological processes, including differentiation, proliferation, and apoptosis.14 We previously found that Alu-mediated p21 transcriptional regulator (APTR) contributes to HSC activation.15 Homeobox (HOX) transcript antisense RNA (HOTAIR), as a lincRNA, is often deregulated in human neoplasia.16, 17 Accumulating studies have indicated that HOTAIR is upregulated in cancers including HCC.18, 19, 20 It has been reported that HOTAIR regulates gene expression via epigenetic modifications.21 For example, Rinn et al. demonstrated that HOTAIR interacts with polycomb-repressive complex 2 (PRC2) to increase the trimethylation of histone H3 lysine-27 (H3K27), resulting in the reduction of HOXD gene expression.21 However, the role of HOTAIR in liver fibrosis has never been studied.
Results
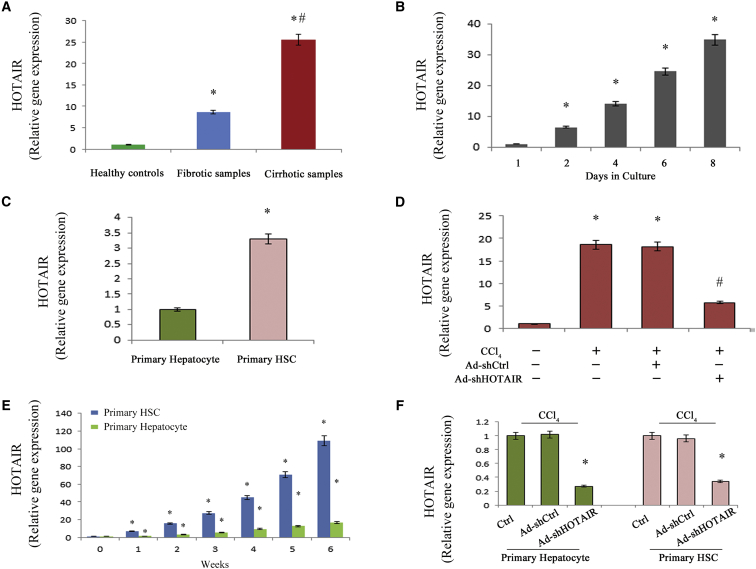
HOTAIR Upregulation Correlates with the Progression of Liver Fibrosis
To gain insights into the possible involvement of HOTAIR in liver fibrosis, HOTAIR expression was detected in liver tissues from healthy controls, fibrotic tissues, and cirrhotic tissues. Compared with the control, HOTAIR expression was enhanced in fibrotic samples and cirrhotic samples, with the highest level in cirrhotic samples (Figure 1A). Next, HSCs were isolated from the livers of healthy mice and cultured for up to 8 days. With time in culture, there was a significant increase in HOTAIR level (Figure 1B). Hepatocytes were additionally isolated from the livers of healthy mice. Interestingly, HOTAIR was higher in primary HSCs than that of primary hepatocytes (Figure 1C). In vivo, compared with the control mice, HOTAIR expression was increased in carbon tetrachloride (CCl4) mice (Figure 1D). During CCl4 treatment, there was also a significant increase in HOTAIR expression in isolated primary HSCs and primary hepatocytes, especially in HSCs, indicating that HOTAIR correlated with liver fibrosis (Figure 1E).
Figure 1.
HOTAIR Is Upregulated during Liver Fibrosis
(A) HOTAIR was increased in liver cirrhotic samples (n = 15) and fibrotic samples (n = 15) when compared to healthy controls (n = 15). (B) Relative HOTAIR gene expression was detected in primary HSCs during culture days. (C) HOTAIR was detected in primary hepatocytes and primary HSCs from the livers of healthy mice. (D) HOTAIR was analyzed in the liver from CCl4 mice after Ad-shHOTAIR treatment. (E) HOTAIR was detected in isolated primary HSCs and primary hepatocytes from CCl4 mice at different weeks. (F) HOTAIR was detected in isolated primary hepatocytes and primary HSCs from CCl4 mice after Ad-shHOTAIR treatment. *p < 0.05 compared to the control and #p < 0.05 compared to fibrotic samples or CCl4 group. Each value is the mean ± SD of three experiments.
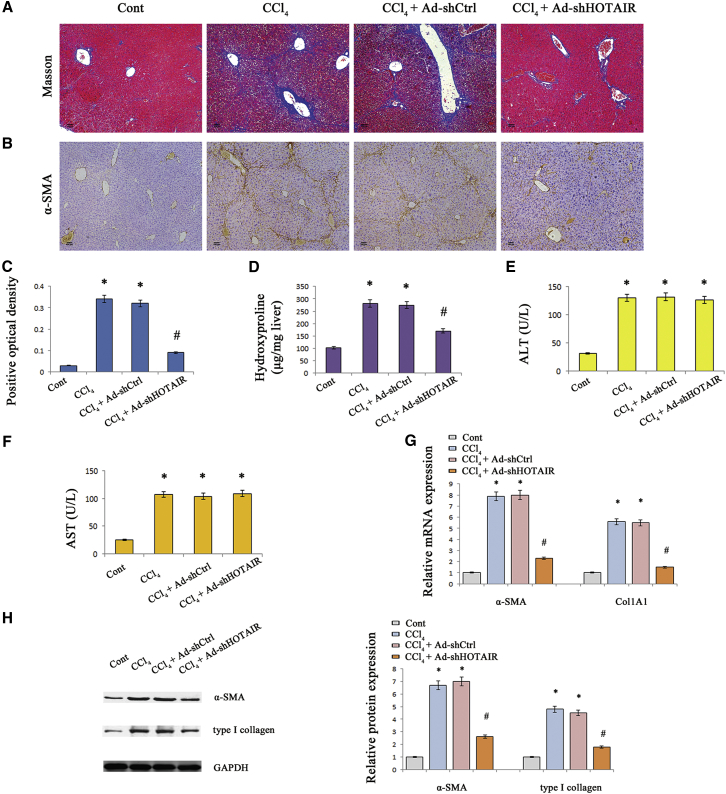
Loss of HOTAIR Ameliorates Liver Fibrosis In Vivo
To determine the functions of HOTAIR in liver fibrosis, the effects of HOTAIR knockdown on CCl4-induced liver fibrosis were explored. Delivery of adenoviral vectors expressing shRNA against HOTAIR (Ad-shHOTAIR) significantly inhibited HOTAIR level in vivo (Figure 1D). In isolated primary hepatocytes and primary HSCs from CCl4 mice after Ad-shHOTAIR treatment, there was also a significant reduction in HOTAIR expression (Figure 1F). As shown in Masson staining and immunohistochemical images, loss of HOTAIR significantly attenuated accumulated collagen and α-smooth muscle actin (α-SMA) levels caused by CCl4 (Figures 2A–2C). Moreover, CCl4-induced hydroxyproline was inhibited by Ad-shHOTAIR (Figure 2D). But Ad-shHOTAIR had no effect on ALT/AST values caused by CCl4 (Figures 2E and 2F). HOTAIR knockdown significantly resulted in the suppression of α-SMA and type I collagen (Figures 2G and 2H).
Figure 2.
HOTAIR Downregulation Suppressed CCl4-Induced Liver Fibrosis in Mice
(A) Accumulation of collagen was assessed by Masson staining. The scale bar represents 100 μm. (B) α-SMA level was analyzed by immunohistochemistry. The scale bar represents 100 μm. The levels of α-SMA positive optical density (C) and hydroxyproline (D) were analyzed in CCl4 mice after Ad-shHOTAIR treatment. The levels of ALT (E) and AST (F) were analyzed after Ad-shHOTAIR treatment. The mRNA (G) and protein (H) expressions of α-SMA and Col1A1 were analyzed after Ad-shHOTAIR treatment. *p < 0.05 compared to the control and #p < 0.05 compared to CCl4 group. Each value is the mean ± SD of three experiments.
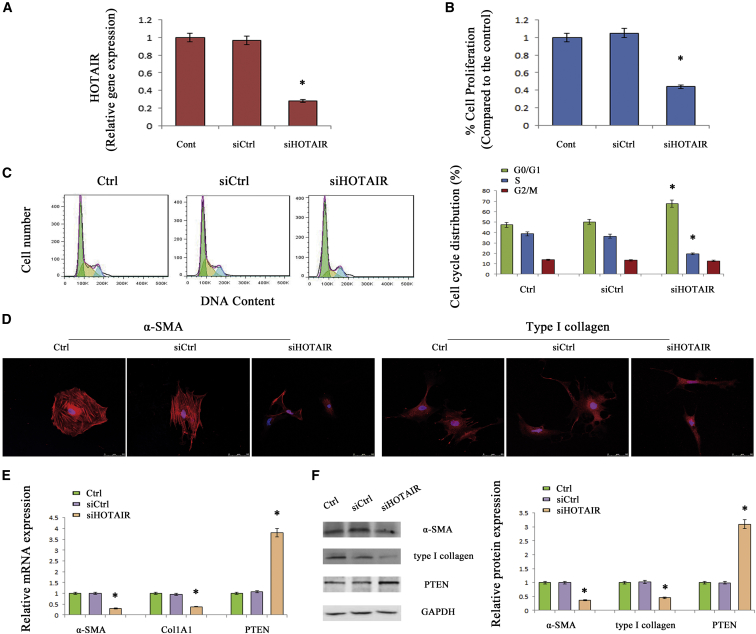
HOTAIR Downregulation Suppresses HSC Activation
Next, we examined the effects of HOTAIR knockdown on cell proliferation, cell cycle, collagen expression, and HSC transdifferentiation. In primary HSCs, it was found that HOTAIR was significantly inhibited by the HOTAIR-specific siRNA (siHOTAIR) (Figure 3A). Using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assays, HSC proliferation was suppressed by HOTAIR knockdown (Figure 3B), and the inhibitory effects of loss of HOTAIR on HSC proliferation were confirmed by 5-Ethyny-2′-deoxyuridine (EdU) assays (Figure S1). By cell cycle analysis, loss of HOTAIR induced a proportion of cells in the G0/G1 phase and suppressed the number of cells in the S phase (Figure 3C). Immunofluorescence analysis indicated that HOTAIR knockdown resulted in a reduction in type I collagen and α-SMA (Figure 3D). As indicated by the results of quantitative (q)real-time PCR and western blot, type I collagen and α-SMA were markedly reduced in the presence of siHOTAIR (Figures 3E and 3F). On the contrary, HOTAIR overexpression promoted HSC proliferation, α-SMA, and alpha-1(I) collagen (Col1A1) expressions (Figure S2).
Figure 3.
Effects of siHOTAIR on HSC Activation
HSCs were transfected with siHOTAIR for 48 hr. The effects of siHOTAIR on HOTAIR level (A), HSC proliferation (B), and cell cycle (C) are shown. HSC proliferation was detected by MTT assay. (D) Immunofluorescence staining for α-SMA (red) and type I collagen (red) were evaluated by confocal laser microscopy. DAPI stained the nuclei blue. The scale bar represents 50 μm. The mRNA (E) and protein (F) expressions of α-SMA, Col1A1, and PTEN were analyzed. *p < 0.05 compared to the control and each value is the mean ± SD of three experiments.
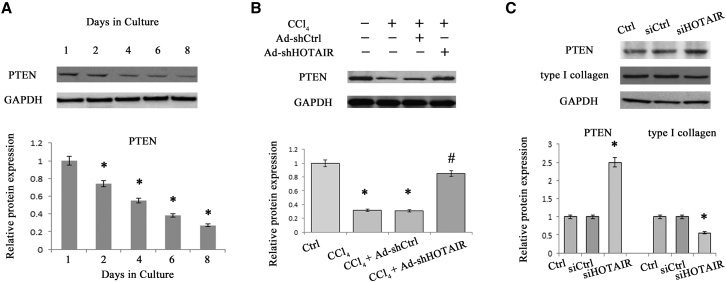
PTEN Upregulation Induced by HOTAIR Knockdown Is Associated with Promoter Methylation
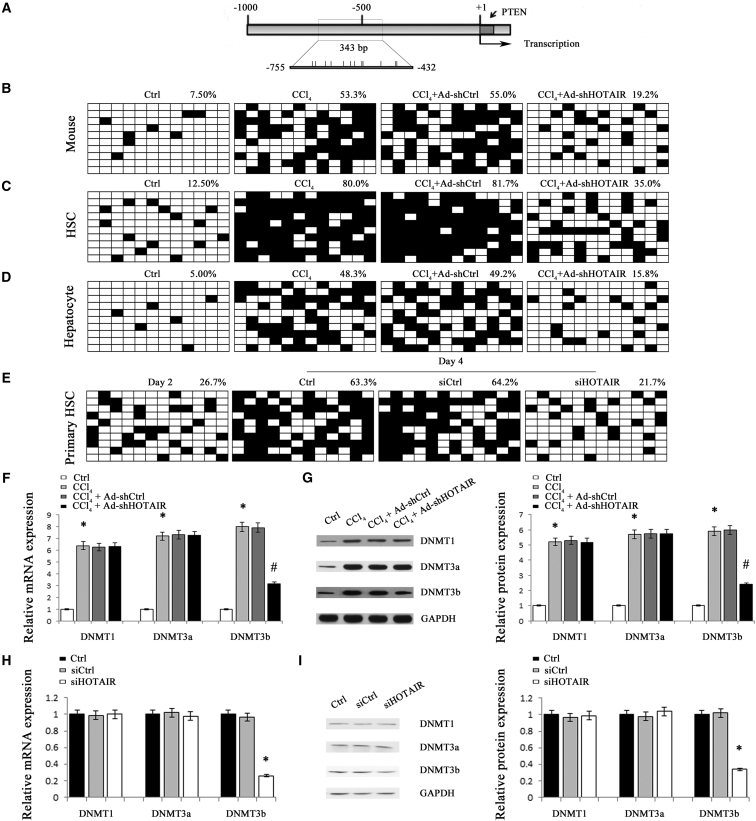
PTEN is often downregulated during liver fibrosis and has been reported to play a vital role in liver fibrosis.22 Upregulation of PTEN contributes to the suppression of HSC activation and proliferation.23 It was found that PTEN was gradually reduced in primary HSCs during culture days and inhibited by CCl4 in vivo (Figures 4A and 4B). Then, the effect of HOTAIR downregulation on PTEN expression in liver fibrosis was examined. In vivo, reduced PTEN caused by CCl4 was inhibited by HOTAIR knockdown (Figure 4B). Likewise, in HSCs, loss of HOTAIR promoted an increase in PTEN (Figures 3E and 3F). In hepatocytes, HOTAIR knockdown increased PTEN expression and decreased collagen expression (Figure 4C). Conversely, HOTAIR overexpression inhibited PTEN expression (Figures S2B and S3). Recently, Li et al. reported that HOTAIR regulates PTEN methylation in laryngeal squamous cell carcinoma.16 To confirm whether HOTAIR knockdown-induced PTEN is associated with its promoter methylation, we examined the methylation level at 12 CpG sites within the CpG island in the PTEN locus by bisulfite-sequencing analysis (Figure 5A). Compared with the control mice, there was a significant increase in PTEN methylation in CCl4 mice (Figure 5B). However, PTEN hypermethylation induced by CCl4 was almost reversed by Ad-shHOTAIR (Figure 5B). Similarly, HOTAIR knockdown inhibited CCl4-induced PTEN methylation in isolated primary HSCs and primary hepatocytes (Figures 5C and 5D). Furthermore, PTEN hypermethylation in day 4 was markedly inhibited by siHOTAIR (Figure 5E). Generally, DNMTs, including DNMT1, DNMT3a, and DNMT3b, are responsible for the regulation of the global patterns of DNA methylation. To determine whether DNMTs were involved in regulation of PTEN methylation by HOTAIR, DNMTs expressions were detected in vivo and in vitro. HOTAIR knockdown caused a reduction in DNMT3b expression in CCl4 mice with no effect on DNMT1 and DNMT3a expressions (Figures 5F and 5G). Similarly, only DNMT3b was decreased by siHOTAIR in vitro (Figures 5H and 5I). Interestingly, loss of DNMT3b contributed to the suppression of PTEN hypermethylation induced by HOTAIR overexpression, indicating that DNMT3b is involved in PTEN methylation induced by HOTAIR (Figure S4).
Figure 4.
Effects of siHOTAIR on PTEN Expression
(A) PTEN protein expression was detected in primary HSCs. (B) PTEN protein expression was detected in CCl4 mice after Ad-shHOTAIR. (C) PTEN protein and type I collagen were analyzed in primary hepatocytes transfected with siHOTAIR. *p < 0.05 compared to the control and #p < 0.05 compared to CCl4 group. Each value is the mean ± SD of three experiments.
Figure 5.
PTEN Expression Was Regulated by Promoter DNA Methylation
CCl4 mice were treated with Ad-shHOTAIR. (A) A schematic representation of the promoter region amplified by bisulfide sequencing. Each vertical bar represents the presence of a CpG dinucleotide. The average percentage of PTEN methylation was shown in the livers from mice (B), as well as isolated primary HSCs (C), and primary hepatocytes (D). (E) The average percentage of PTEN methylation was shown in HSCs with siHOTAIR. The mRNA (F) and protein (G) expression levels of DNMT1, DNMT3a, and DNMT3b were analyzed in CCl4 mice after Ad-shHOTAIR treatment. The mRNA (H) and protein (I) expression levels of DNMT1, DNMT3a, and DNMT3b were analyzed in primary HSCs transfected with siHOTAIR. *p < 0.05 compared to the control and #p < 0.05 compared to CCl4 group. Each value is the mean ± SD of three experiments.
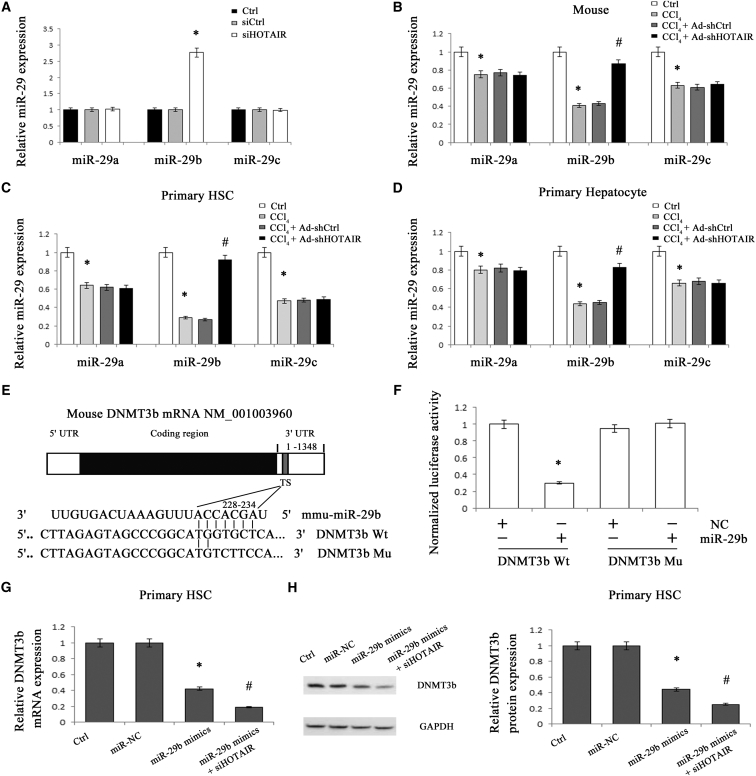
miR-29b Regulates PTEN Expression by Targeting DNMT3b and Is Involved in the Effects of HOTAIR on PTEN
Previously, we demonstrated that miR-29b epigenetically regulates PTEN expression via DNMT3b.10 The miR-29 family consists of miR-29a, miR-29b, and miR-29c, which differ only in two or three bases.24, 25 Recent studies showed that DNMTs may be a target of miR-29 family.26, 27 Based on these, the effects of loss of HOTAIR on the miR-29 family were examined. In vivo and in vitro, HOTAIR knockdown increased miR-29b level with no effect on miR-29a and miR-29c levels (Figures 6A and 6B). Enhanced miR-29b expression by Ad-shHOTAIR was confirmed in isolated primary HSCs and primary hepatocytes (Figures 6C and 6D). By contrast, HOTAIR overexpression caused a reduction in miR-29b level (Figure S3). Then, miR-29b was selected for the next experiment, and we further determined whether miR-29b could regulate DNMT3b. Using bioinformatic analysis (miRDB), DNMT3b is predicted as a potential target of miR-29b, and we generated a DNMT3b 3′-UTR luciferase reporter containing the miR-29b-binding sites (DNMT3b Wt) or mutated sites (DNMT3b Mu) (Figure 6E). miR-29b reduced DNMT3b Wt luciferase activity, whereas miR-29b did not cause obvious changes in DNMT3b Mu luciferase activity (Figure 6F). Also, DNMT3b expression was significantly decreased by miR-29b mimics in HSCs (Figures 6G and 6H). These data suggest that DNMT3b is a target of miR-29b. Notably, miR-29b led to a significant reduction in Col1A1 and a significant increase in PTEN in primary HSCs and primary hepatocytes (Figures S5A and S5B). Interestingly, PTEN is also predicted as a direct target of miR-29b (Figure S5C). However, our results showed that compared with the control, miR-29b caused no significant changes in PTEN-Wt-1 luciferase activity as well as PTEN-Wt-2 luciferase activity (Figure S5D). Combined with these, our results suggest that miR-29b can’t directly regulate PTEN and may regulate PTEN via DNMT3b. Next, miR-29b expression was also examined in primary HSCs and primary hepatocytes. It was found that miR-29b was gradually reduced in primary HSCs during culture days (Figure S6A). Moreover, miR-29b was lower in primary HSCs than that of primary hepatocytes (Figure S6B). Combined with the data of HOTAIR in primary HSCs and primary hepatocytes, there may be a negative relation between HOTAIR and miR-29b in liver fibrosis. Further studies showed that the effects of miR-29b on DNMT3b, Col1A1, and PTEN were enhanced by the loss of HOTAIR (Figures 6G, 6H, S5A, and S5B). Taken together, our data indicate that miR-29b may be involved in the effects of HOTAIR on PTEN.
Figure 6.
Silencing HOTAIR Enhanced miR-29b Expression
(A) The levels of miR-29a, miR-29b, and miR-29c were analyzed in HSCs transfected with siHOTAIR. CCl4 mice were treated with Ad-shHOTAIR. The levels of miR-29a, miR-29b, and miR-29c were detected in the livers from mice (B), as well as isolated primary HSCs (C), and primary hepatocytes (D). (E) Putative miR-29b binding sites (TS) within the mouse DNMT3b 3′-UTR are shown. The position of the binding sites was numbered relative to the first nucleotide of the 3′-UTR. Mutations were introduced into DNMT3b 3′-UTR that matched the seed region of miR-29b as shown in DNMT3b Mu. (F) Dual-luciferase assay was performed in HEK293T co-transfected with luciferase constructs containing the DNMT3b wild-type or Mu 3′-UTR and miR-29b mimics or scrambled oligonucleotides as the negative control. The mRNA (G) and protein (H) levels of DNMT3b in primary HSCs were reduced by miR-29b mimics, which were further decreased by siHOTAIR. *p < 0.05 compared to the control and #p < 0.05 compared to CCl4 or miR-29b mimics group. Each value is the mean ± SD of three experiments.
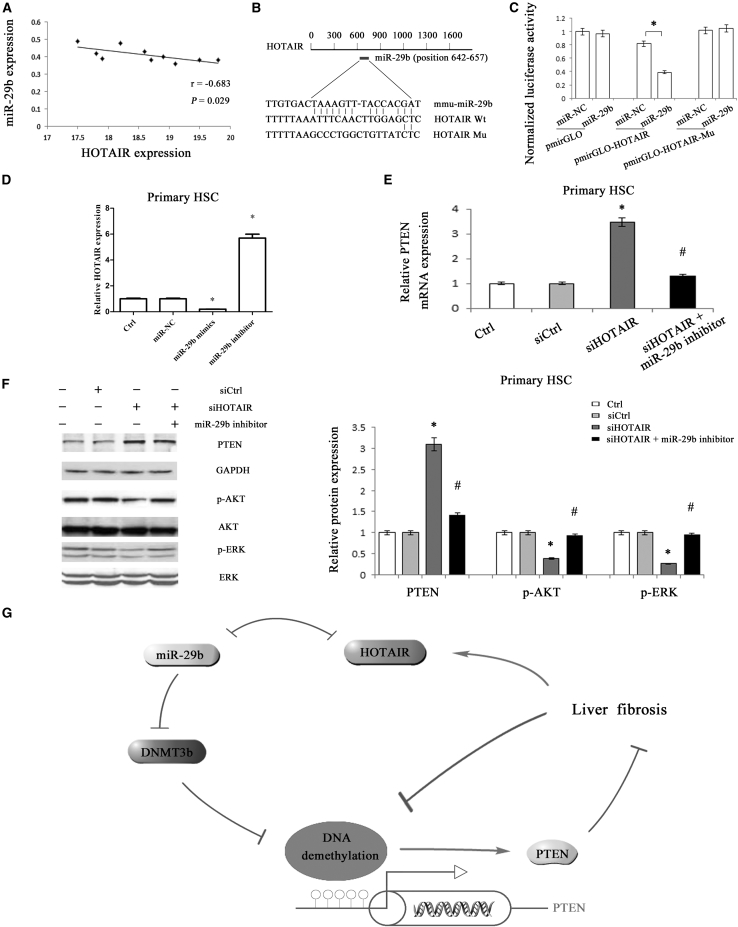
HOTAIR Is a Target of miR-29b and Contributes to the Activation of the ERK and AKT Pathways
There was a strong negative correlation between HOTAIR level and miR-29b expression in liver tissues samples from CCl4 mice (r = −0.683, p = 0.029) (Figure 7A). There might be a relation between HOTAIR and miR-29b, and this hypothesis was confirmed by luciferase reporter assays. HOTAIR contains one target site for miR-29b using bioinformatic analysis (RNA22) (Figure 7B). Using pmirGLO construct, we generated a HOTAIR luciferase reporter containing the miR-29-binding sites (pmirGLO-HOTAIR-Wt) or mutated sites (pmirGLO-HOTAIR-Mu) (Figure 7B). miR-29b mimics induced a reduction in luciferase activity of pmirGLO-HOTAIR (Figure 7C). By contrast, miR-29b inhibitor caused an increase in pmirGLO-HOTAIR luciferase activity (Figure S7A). But both miR-29b mimics and inhibitor had no effect on pmirGLO-HOTAIR-Mu luciferase activity (Figures 7C and S7A). The results indicate that HOTAIR is a target of miR-29b. Consistent with this result, HOTAIR was reduced by miR-29b mimics and enhanced by miR-29b inhibitor (Figure 7D). Notably, HOTAIR-Wt overexpression reduced miR-29b expression, whereas HOTAIR-Mu overexpression with the mutation of the miR-29b binding site did not suppress miR-29b expression, indicating that lack of the miR-29b binding site in HOTAIR prevents the suppression of miR-29b expression (Figure S7B). Next, the role of miR-29b in the effect of HOTAIR on PTEN expression was further examined. It was found that PTEN induced by siHOTAIR was almost inhibited by miR-29b inhibitor (Figures 7E and 7F). Moreover, the reduced expressions of p-AKT and p-ERK caused by HOTAIR knockdown were restored by miR-29b inhibitor (Figure 7F). These data suggest that HOTAIR modulates PTEN level and contributes to the activation of the ERK and AKT pathways via miR-29b.
Figure 7.
The Effect of HOTAIR on PTEN Expression Is through Competitively Binding miR-29b
(A) Correlation between HOTAIR level and miR-29b expression in liver fibrosis tissue samples from CCl4 mice (n = 10) was subjected to Pearson correlation analysis. (B) Schematic diagram of the miR-29b binding site in the HOTAIR based on RNA22 software. (C) Relative luciferase activities of luciferase reporters bearing wild-type or mutant HOTAIR were analyzed 48 hr following transfection with the indicated miR-29b mimics or miR-NC in HEK293T. Relative gene expressions of HOTAIR (D) and PTEN (E) were analyzed by quantitative real-time PCR. (F) PTEN, phosphorylation of ERK (T202/Y204) and Akt (S473) were analyzed by western blotting. (G) The signal pathway was discovered in liver fibrosis. *p < 0.05 compared to the control and #p < 0.05 compared to siHOTAIR group. Each value is the mean ± SD of three experiments.
Discussion
Recently, lncRNAs have been reported to be involved in liver diseases including liver fibrosis and HCC.11, 12, 28 For example, lncRNA maternally expressed gene 3 (MEG3) has been found to inhibit liver fibrosis via p53.11 In this study, HOTAIR was found to be upregulated in HSCs in vivo and in vitro during liver fibrosis. HOTAIR knockdown resulted in the suppression of HSC activation including α-SMA and type I collagen in vivo and in vitro. In addition, HOTAIR knockdown led to the inhibition of HSC proliferation and cell cycle. Notably, loss of HOTAIR contributed to suppress liver fibrosis via restoration of miR-29b and inhibition of DNMT3b, with a reduction in PTEN methylation and an increase in PTEN level. Owing to the restoration of PTEN, HSC activation including cell proliferation, collagen, and α-SMA expression was inhibited. Meanwhile, HOTAIR overexpression exhibited the opposite effects. Further studies demonstrated that miR-29b could directly target DNMT3b and HOTAIR. These data revealed that HOTAIR contributed to HSC activation, at least in part, through suppressing miR-29b-mediated PTEN.
Activation of HSCs to myofibroblast-like cells plays a vital role in the initiation and progression of hepatic fibrosis.29 However, the numbers of HSCs in liver are far less than that of hepatocytes, which are the main cells in the liver. Herein, HOTAIR could be detected in hepatocytes and was upregulated during CCl4 treatment. Although Ad-shHOTAIR was designed to target HSC, HOTAIR was reduced in isolated primary hepatocytes from CCl4 mice after Ad-shHOTAIR treatment. Therefore, delivery of Ad-shHOTAIR into mice is targeted toward not only HSCs, but also hepatocytes. Both hepatocytes and HSCs were affected by Ad-shHOTAIR. It may be the reason why Ad-shHOTAIR resulted in a significant increase in PTEN in CCl4 mice. In HSCs, inhibition of HOTAIR increased PTEN level and reduced collagen expression. Moreover, Ad-shHOTAIR contributed to the increase in miR-29b and the reduction in PTEN methylation in isolated HSCs from CCl4 mice. The similar effects were observed in primary hepatocytes. All these data suggest that HOTAIR also plays a vital role in hepatocytes during liver fibrosis. However, the effects of siHOTAIR on other cells such as Kupffer were not studied and further studies are warranted to prove it.
The roles of lncRNAs in human disease are correlated with their effects on impacting different cellular processes via diverse molecular mechanisms.14, 30 For example, biological processes such as proliferation and apoptosis can be regulated by lncRNAs through chromatin modification, transcriptional regulation, and post-transcriptional regulation.14 Interestingly, lncRNAs can act as competing endogenous RNAs (ceRNAs) to sponge miRNAs, consequently modulating the derepression of miRNA targets.31 For instance, Xia et al.32 found that lncRNA fer-1-like family member 4 (FER1L4) acts as a ceRNA to regulate the expression of PTEN by taking up miR-106a-5p in gastric cancer. As confirmed by situ hybridization assay, HOTAIR was distributed in both nucleus and cytoplasm33, and has been reported to be able to bind miRNAs in cancers.31, 34 In this study, it was found that loss of DNMT3b inhibited HOTAIR-induced PTEN methylation. In addition, lack of the miR-29b binding site in HOTAIR prevented the suppression of miR-29b expression. Our data demonstrated that HOTAIR-mediated PTEN promoter methylation was through sponging miR-29b and indirectly enhanced DNMT3b. Loss of HOTAIR caused an increase in miR-29b, whereas HOTAIR overexpression reduced miR-29b expression. Moreover, overexpression of miR-29b increased PTEN level, which was further enhanced by siHOTAIR. Conversely, miR-29b inhibitor suppressed HOTAIR knockdown-induced PTEN. These data confirm the existence of the HOTAIR/miR-29b/PTEN signaling network. Meanwhile, collagen expression was inhibited by miR-29b, and this inhibition was further enhanced by siHOTAIR, indicating that collagen level was regulated by the HOTAIR/miR-29b/PTEN axis. It is known that loss of PTEN gene expression causes aberrant activation of the PI3K/AKT and ERK pathways and consequently leads to cancer cell proliferation and ultimately stimulates tumorigenesis.35 HOTAIR knockdown induced a reduction in p-AKT and p-ERK levels, which was reversed by miR-29b inhibitor. All these data suggest that HOTAIR can bind miR-29b and subsequently upregulate DNMT3b, leading to PTEN methylation and activation of ERK and AKT pathways.
Recent studies have shown that ceRNAs should have a certain number of miRNA target-sites to act as a miRNA sponge.36 In our study, HOTAIR had only one target-site for miR-29b. Of note, increased HOTAIR level was found in liver fibrosis. Due to the high level of HOTAIR, we considered that the total number of miRNA target-sites provided by HOTAIR may be enough for sponging miR-29b. It may be the reason why HOTAIR could act as a miRNA sponge for miR-29b. Therefore, ceRNAs, containing one site for targeted miRNA, can act as a miRNA sponge, which is consistent with the previous studies.37, 38
In conclusion, we demonstrate that HOTAIR downregulates miR-29b and attenuates its control on DNMT3b, leading to restoration of DNMT3b and enhancement of PTEN methylation, which contributes to liver fibrosis. Our results provide a new insight of the HOTAIR-mediated PTEN epigenetic mechanisms in the progression of liver fibrosis and confirm HOTAIR/miR-29b/PTEN as a new signaling network in liver fibrosis (Figure 7G).
Materials and Methods
Materials
CCl4 was obtained from Sigma. Antibodies against DNMT3a, DNMT3b, type I collagen, and α-SMA were obtained from Abcam. Antibodies against PTEN, DNMT1, and GAPDH were purchased from Santa Cruz Biotechnology. Antibodies targeting AKT, phosphorylated AKT (S473), ERK, and phosphorylated ERK (T202/Y204) were purchased from Cell Signaling. Chemically synthesized RNAs, including negative control (miR-NC), miR-29b mimics, and miR-29b inhibitor, were obtained from GenePharma Biotechnology. For transfection, the cells were transfected with 1 μg of the chemically synthesized RNA. Moreover, siDNMT3b, siHOTAIR, the scrambled siRNA (siCtrl), Ad-shHOTAIR, adenoviral vectors expressing the scrambled shRNA (Ad-shCtrl), adenoviral vectors expressing HOTAIR (Ad-HOTAIR), and adenoviral vectors expressing a control scrambled sequence (Ad-Ctrl) were purchased from GenePharma Biotechnology.
Human Specimens
Written informed consent was received from all patients prior to liver tissues. In this study, 15 healthy controls, 15 chronic hepatitis B (CHB) patients, and 15 liver cirrhosis patients undergoing partial liver resection or liver biopsy were selected from the First Affiliated Hospital of Wenzhou Medical University. For CHB patients, liver fibrosis was diagnosed by liver biopsy. Liver cirrhosis was diagnosed by liver biopsy and/or a typical appearance of the liver on abdominal ultrasound and/or computed tomography scan. This study was performed in compliance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Isolation and Culture of Primary HSCs and Hepatocytes
Primary HSCs were isolated as described previously.39 The isolated cells were seeded in tissue culture plates and cultured in DMEM (Gibco) with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The purity of cultures was confirmed by immunocytochemical staining for α-SMA and the purity reached >98%. Hepatocytes were isolated using a two-step collagenase perfusion technique.40 Then, gene expression levels including F4/80, CD32b, and CYP3A11 were measured by quantitative real-time PCR. Hepatocyte purity was found to be greater than 95%.
CCl4 Liver Injury Model
CCl4 (diluted 1:9 in olive oil) or vehicle (olive oil) was administered by intraperitoneal injection at a dose of 7 mL/kg of body weight two times weekly for 6 weeks to induce liver fibrosis. During CCl4 treatment, Ad-shHOTAIR (1 × 109 pfu/100 μL) or Ad-HOTAIR (1 × 109 pfu/100 μL) was injected into mice every 2 weeks by way of the tail vein for 6 weeks. There were 60 mice that were randomly divided into six groups including olive oil, CCl4, CCl4 plus Ad-shCtrl, CCl4 plus Ad-shHOTAIR, CCl4 plus Ad-Ctrl, and CCl4 plus Ad-HOTAIR. For targeting HSCs, the α-SMA promoter was amplified from the pSMP8 plasmid and the PCR product cloned into the pTOPO plasmid (Invitrogen) as described previously.41 Then, the α-SMA promoter was excised from the pTOPO plasmid using EcoRI and cloned into the EcoRI site in pDNR plasmid (BD Bioscience) upstream of the shHOTAIR insert. Sequencing confirmed orientation and integrity of the insert. The recombinant adenovirus was generated using the BD Adeno-x Expression System Promoterless Vector (BD Bioscience) according to the manufacturers’ protocol. All animals were provided by the Experimental Animal Center of Wenzhou Medical University. The animal experimental protocol was approved by the University Animal Care and Use Committee. Mice were sacrificed under anesthesia at the end of 6 weeks and the livers were removed for further analysis. The liver tissues were used for Masson staining by fixation with 10% formalin.
Immunohistochemistry
Immunohistochemical staining was performed on the sections (3 μm thick) from the liver tissues, as described previously.7, 42 Briefly, after deparaffinization, hydration, and antigen retrieval, samples were incubated overnight at 4°C with a primary antibody against α-SMA (1:100) and then with a biotinylated secondary antibody. α-SMA expression was visualized by 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining. Slides were counterstained with hematoxylin before dehydration and mounting α-SMA-positive areas within the fibrotic region were then observed. Quantitative analysis was calculated from five fields for each liver slice.
Immunofluorescence Microscopy
Primary HSCs were plated on 18-mm cover glasses in DMEM and incubated for 24 hr. Then, cells were transfected with siHOTAIR for 48 hr, washed with PBS, and fixed in an acetic acid: ethanol (1:3) solution for 5 min at −20°C. Non-specific binding was blocked with 5% goat serum in PBS for 1 hr at room temperature, and the cells were then incubated with primary antibodies against α-SMA or type I collagen (Abcam) in a humidified chamber. After washing twice in PBS, the cells were incubated with fluorescein-labeled secondary antibody (1:50 dilution; Dianova) in antibody dilution solution for 1 hr at room temperature in the dark. The nuclei were stained with DAPI in the dark for 30 min at room temperature. The slides were washed twice with PBS, covered with DABCO (Sigma-Aldrich), and examined by confocal laser scanning microscopy (Olympus) at 568 nm.
Hepatic Hydroxyproline Content
Liver tissues (50 mg) were homogenized in HCl and hydrolyzed at 120°C overnight. After lysate centrifugation at 12,000 g for 10 min at 4°C, the supernatant was evaporated to dryness under vacuum. The hepatic hydroxyproline content was assessed using the Hydroxyproline Colorimetric Assay Kit (BioVision). Data were normalized to liver weight.
Quantitative Real-Time PCR
Total RNA was extracted from primary mouse cells using the miRNeasy Mini Kit (QIAGEN). Also, 50 nanograms of total RNA was reverse-transcribed to cDNA using the ReverTra Ace qPCR RT Kit (Toyobo) in accordance with the manufacturer’s instructions. Gene expression (Table S1) was measured by quantitative real-time PCR using SYBR Green Real-Time PCR Master Mix (Toyobo). The primers of Col1A1, α-SMA, and GAPDH were designed as described previously.25, 43 To detect miR-29a, miR-29b, and miR-29c expressions, reverse transcription reactions were performed using the TaqMan MicroRNA Assay (Applied Biosystems) according to the manufacturer’s instructions. The GAPDH (Applied Biosystems) level was used to normalize the relative abundance of HOTAIR and mRNAs. U6 snRNA (Applied Biosystems) was used to normalize the relative abundance of miRNAs. The expression levels (2−ΔΔCt) of HOTAIR, mRNAs, and miR-29 were calculated as described previously.44
Western Blot Analysis
Tissues and cells were lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM 2-Mercaptoethanol, 2% w/v SDS, and 10% glycerol). Total proteins were quantified and separated by SDS-PAGE. Then western blot assay was performed as described previously.45 The levels of protein were normalized to total GAPDH.
Methylation Analysis
PTEN CpG island was searched in UCSC Genome Browser. About 0.5 μg genomic DNA was treated with sodium bisulfite and subjected to PCR. The PTEN primers for PCR were 5′-AC CCACTTTGTCCAACCAGG-3′ and 5′-GAACGGCTTTCATTCCC TGC-3′. The bisulfite-sequencing analysis was carried out as described previously.46
Proliferation Assay
Cell proliferation was determined by the MTT assay according to the instructions of a MTT Cell Proliferation Assay Kit (Beyotime Biotechnology). Briefly, the cells were seeded at a density of 1 × 103 cells per well in 96-well culture plates and transfected with siHOTAIR, Ad-HOTAIR, or negative control. Cell lysates were prepared after their respective treatment. The cells were incubated with 0.5% MTT for 4 hr. Upon removal of the supernatant, 150 μL DMSO was added and shaken for 5 min until the crystals were dissolved. The optical density (OD) was determined with a microplate reader (Bio-Rad 550) at 570 nm wavelength. Moreover, cell proliferation was assessed by EdU assays. Using Lipofectamine RNAiMAX, cells were transfected with siHOTAIR or siCtrl and then labeled with EdU for 12 hr. The proliferative rate was detected using a Cell-Light EdU In Vitro Imaging Detection Kit (Guangzhou RiboBio, cat# C10310-1) according to the manufacturer’s instructions.
Cell Cycle Analysis
For cell cycle analysis, we used a Cell Cycle Analysis Kit (Beyotime). Cells were fixed in 70% ethanol in PBS at −20°C for 24 hr and then labeled with 0.5 mL propidium iodide (PI) staining buffer containing 200 mg/mL RNase A and 50 μg/mL PI at 37°C for 40 min in the dark. Analyses were performed on a BD LSR flow cytometer (BD Biosciences) and experiments repeated at least three times.
Luciferase Activity Assay
Oligonucleotides containing target sequences of the HOTAIR and DNMT3b 3′-UTR were amplified and cloned into pmirGLO plasmids (Promega). HOTAIR for miR-29b forward, 5′-AGGTCCCCAACATCGGTAGA-3′ and reverse, 5′-GTTCCTTCCATCTGGACCCG-3′. DNMT3b 3′-UTR for miR-29b forward, 5′-GC TCAGACCTGGCTGCTTAG-3′ and reverse, 5′-CTCCAGCAAATGTGGAGCAC-3′. These plasmids were named as pmirGLO-HOTAIR and pmirGLO-DNMT3b, respectively. Empty plasmid pmirGLO was regarded as a negative control. Luciferase reporter plasmids plus miR-29b mimics or miR-NC were co-transfected into HEK293T using Lipofectamine 2000 (Invitrogen). At 48 hr after transfection, relative luciferase activity was examined in a luminometer using a Dual-Luciferase Reporter Assay System (Promega).
Statistical Analysis
Data from at least three independent experiments were expressed as the mean ± SD. Differences between multiple groups were evaluated using one-way ANOVA. Differences between two groups were compared using a Student’s t test. p < 0.05 was considered significant. Correlation between miR-29b expression and HOTAIR level in liver tissues was examined by Pearson’s correlation coefficient. All statistical analyses were performed with SPSS software (version 13; SPSS).
Author Contributions
F.Y. and J.Z. carried out most of the experiments; B.C. provided the statistical support; P.D. and J.Z. designed the study and analyzed the data; and B.C. and P.D. contributed to some of the experiments. All the authors contributed to the manuscript preparation and gave final approval of the submitted manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The project was supported by the National Natural Science Foundation of China (No. 81500458/H0317), Wenzhou Municipal Science and technology Bureau (No. Y20150091), and Zhejiang Provincial Natural Science Foundation of China (No. LY16H030012).
Footnotes
Supplemental Information includes seven figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.10.015.
Contributor Information
Peihong Dong, Email: dongpeihong111@163.com.
Jianjian Zheng, Email: 120378196@qq.com.
Supplemental Information
References
- 1.Kong X., Horiguchi N., Mori M., Gao B. Cytokines and STATs in Liver Fibrosis. Front. Physiol. 2012;3:69. doi: 10.3389/fphys.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwiecinski M., Noetel A., Elfimova N., Trebicka J., Schievenbusch S., Strack I., Molnar L., von Brandenstein M., Töx U., Nischt R. Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS ONE. 2011;6:e24568. doi: 10.1371/journal.pone.0024568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Chu E.S., Chen H.Y., Man K., Go M.Y., Huang X.R., Lan H.Y., Sung J.J., Yu J. microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget. 2015;6:7325–7338. doi: 10.18632/oncotarget.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schickel R., Boyerinas B., Park S.M., Peter M.E. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 6.Yu F., Guo Y., Chen B., Dong P., Zheng J. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab. Invest. 2015;95:781–789. doi: 10.1038/labinvest.2015.58. [DOI] [PubMed] [Google Scholar]
- 7.Zheng J., Wu C., Xu Z., Xia P., Dong P., Chen B., Yu F. Hepatic stellate cell is activated by microRNA-181b via PTEN/Akt pathway. Mol. Cell. Biochem. 2015;398:1–9. doi: 10.1007/s11010-014-2199-8. [DOI] [PubMed] [Google Scholar]
- 8.Zheng J., Lin Z., Dong P., Lu Z., Gao S., Chen X., Wu C., Yu F. Activation of hepatic stellate cells is suppressed by microRNA-150. Int. J. Mol. Med. 2013;32:17–24. doi: 10.3892/ijmm.2013.1356. [DOI] [PubMed] [Google Scholar]
- 9.Ji J., Zhang J., Huang G., Qian J., Wang X., Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Zheng J., Wu C., Lin Z., Guo Y., Shi L., Dong P., Lu Z., Gao S., Liao Y., Chen B., Yu F. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation--a novel mechanism suppressing liver fibrosis. FEBS J. 2014;281:88–103. doi: 10.1111/febs.12574. [DOI] [PubMed] [Google Scholar]
- 11.He Y., Wu Y.T., Huang C., Meng X.M., Ma T.T., Wu B.M., Xu F.Y., Zhang L., Lv X.W., Li J. Inhibitory effects of long noncoding RNA MEG3 on hepatic stellate cells activation and liver fibrogenesis. Biochim. Biophys. Acta. 2014;1842:2204–2215. doi: 10.1016/j.bbadis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Yuan J.H., Yang F., Wang F., Ma J.Z., Guo Y.J., Tao Q.F., Liu F., Pan W., Wang T.T., Zhou C.C. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Peng H., Ishida M., Li L., Saito A., Kamiya A., Hamilton J.P., Fu R., Olaru A.V., An F., Popescu I. Pseudogene INTS6P1 regulates its cognate gene INTS6 through competitive binding of miR-17-5p in hepatocellular carcinoma. Oncotarget. 2015;6:5666–5677. doi: 10.18632/oncotarget.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi X., Sun M., Liu H., Yao Y., Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Yu F., Zheng J., Mao Y., Dong P., Li G., Lu Z., Guo C., Liu Z., Fan X. Long non-coding RNA APTR promotes the activation of hepatic stellate cells and the progression of liver fibrosis. Biochem. Biophys. Res. Commun. 2015;463:679–685. doi: 10.1016/j.bbrc.2015.05.124. [DOI] [PubMed] [Google Scholar]
- 16.Li D., Feng J., Wu T., Wang Y., Sun Y., Ren J., Liu M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am. J. Pathol. 2013;182:64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Woo C.J., Kingston R.E. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–1259. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Lu L., Zhu G., Zhang C., Deng Q., Katsaros D., Mayne S.T., Risch H.A., Mu L., Canuto E.M., Gregori G. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res. Treat. 2012;136:875–883. doi: 10.1007/s10549-012-2314-z. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z., Zhou L., Wu L.M., Lai M.C., Xie H.Y., Zhang F., Zheng S.S. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann. Surg. Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 20.Kim K., Jutooru I., Chadalapaka G., Johnson G., Frank J., Burghardt R., Kim S., Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., Chang H.Y. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian E.B., Huang C., Ma T.T., Tao H., Zhang H., Cheng C., Lv X.W., Li J. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicol. Appl. Pharmacol. 2012;264:13–22. doi: 10.1016/j.taap.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Takashima M., Parsons C.J., Ikejima K., Watanabe S., White E.S., Rippe R.A. The tumor suppressor protein PTEN inhibits rat hepatic stellate cell activation. J. Gastroenterol. 2009;44:847–855. doi: 10.1007/s00535-009-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa T., Iizuka M., Sekiya Y., Yoshizato K., Ikeda K., Kawada N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem. Biophys. Res. Commun. 2010;391:316–321. doi: 10.1016/j.bbrc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya Y., Ogawa T., Yoshizato K., Ikeda K., Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem. Biophys. Res. Commun. 2011;412:74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Fabbri M., Garzon R., Cimmino A., Liu Z., Zanesi N., Callegari E., Liu S., Alder H., Costinean S., Fernandez-Cymering C. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garzon R., Liu S., Fabbri M., Liu Z., Heaphy C.E., Callegari E., Schwind S., Pang J., Yu J., Muthusamy N. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panzitt K., Tschernatsch M.M., Guelly C., Moustafa T., Stradner M., Strohmaier H.M., Buck C.R., Denk H., Schroeder R., Trauner M., Zatloukal K. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–342. doi: 10.1053/j.gastro.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 29.He Y., Huang C., Zhang S.P., Sun X., Long X.R., Li J. The potential of microRNAs in liver fibrosis. Cell. Signal. 2012;24:2268–2272. doi: 10.1016/j.cellsig.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing C.Y., Hu X.Q., Xie F.Y., Yu Z.J., Li H.Y., Bin-Zhou, Wu J.B., Tang L.Y., Gao S.M. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589:1981–1987. doi: 10.1016/j.febslet.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 32.Xia T., Chen S., Jiang Z., Shao Y., Jiang X., Li P., Xiao B., Guo J. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci. Rep. 2015;5:13445. doi: 10.1038/srep13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalil A.M., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D., Thomas K., Presser A., Bernstein B.E., van Oudenaarden A. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X.H., Sun M., Nie F.Q., Ge Y.B., Zhang E.B., Yin D.D., Kong R., Xia R., Lu K.H., Li J.H. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol. Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phuong N.T., Kim S.K., Lim S.C., Kim H.S., Kim T.H., Lee K.Y., Ahn S.G., Yoon J.H., Kang K.W. Role of PTEN promoter methylation in tamoxifen-resistant breast cancer cells. Breast Cancer Res. Treat. 2011;130:73–83. doi: 10.1007/s10549-010-1304-2. [DOI] [PubMed] [Google Scholar]
- 36.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S.H., Zhang W.J., Wu X.C., Zhang M.D., Weng M.Z., Zhou D., Wang J.D., Quan Z.W. Long non-coding RNA Malat1 promotes gallbladder cancer development by acting as a molecular sponge to regulate miR-206. Oncotarget. 2016;7:37857–37867. doi: 10.18632/oncotarget.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu F., Zheng J., Mao Y., Dong P., Lu Z., Li G., Guo C., Liu Z., Fan X. Long non-coding RNA growth arrest-specific Transcript 5 (GAS5) inhibits liver fibrogenesis through a mechanism of competing endogenous RNA. J. Biol. Chem. 2015;290:28286–28298. doi: 10.1074/jbc.M115.683813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang W., Yang M., Song L., Shen K., Wang H., Gao X., Li M., Niu W., Qin X. Isolation and culture of hepatic stellate cells from mouse liver. Acta Biochim. Biophys. Sin. (Shanghai) 2014;46:291–298. doi: 10.1093/abbs/gmt143. [DOI] [PubMed] [Google Scholar]
- 40.Bertolino P., Trescol-Biémont M.C., Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur. J. Immunol. 1998;28:221–236. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 41.Son G., Hines I.N., Lindquist J., Schrum L.W., Rippe R.A. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009;50:1512–1523. doi: 10.1002/hep.23186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawahara T., Kashiwagi E., Ide H., Li Y., Zheng Y., Miyamoto Y., Netto G.J., Ishiguro H., Miyamoto H. Cyclosporine A and tacrolimus inhibit bladder cancer growth through down-regulation of NFATc1. Oncotarget. 2015;6:1582–1593. doi: 10.18632/oncotarget.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi S.S., Syn W.K., Karaca G.F., Omenetti A., Moylan C.A., Witek R.P., Agboola K.M., Jung Y., Michelotti G.A., Diehl A.M. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J. Biol. Chem. 2010;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 45.Hua H.W., Jiang F., Huang Q., Liao Z., Ding G. MicroRNA-153 promotes Wnt/β-catenin activation in hepatocellular carcinoma through suppression of WWOX. Oncotarget. 2015;6:3840–3847. doi: 10.18632/oncotarget.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji W., Yang L., Yu L., Yuan J., Hu D., Zhang W., Yang J., Pang Y., Li W., Lu J. Epigenetic silencing of O6-methylguanine DNA methyltransferase gene in NiS-transformed cells. Carcinogenesis. 2008;29:1267–1275. doi: 10.1093/carcin/bgn012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.