Abstract
Dizziness and hearing loss are among the most common disabilities. Many forms of hereditary balance and hearing disorders are caused by abnormal development of stereocilia, mechanosensory organelles on the apical surface of hair cells in the inner ear. The deaf whirler mouse, a model of human Usher syndrome (manifested by hearing loss, dizziness, and blindness), has a recessive mutation in the whirlin gene, which renders hair cell stereocilia short and dysfunctional. In this study, wild-type whirlin cDNA was delivered to the inner ears of neonatal whirler mice using adeno-associated virus serotype 2/8 (AAV8-whirlin) by injection into the posterior semicircular canal. Unilateral whirlin gene therapy injection was able to restore balance function as well as improve hearing in whirler mice for at least 4 months. Our data indicate that gene therapy is likely to become a treatment option for hereditary disorders of balance and hearing.
Keywords: whirlin, whirler, vestibular dysfunction
Introduction
The vestibular system is important for maintaining spatial orientation and balance of the human body.1 Dysfunction in the vestibular system is a common medical condition that can be severe and debilitating. According to the National Health and Nutrition Examination Survey, 35.4% of US adults aged 40 years and older have some degree of balance dysfunction.1 Loss of balance is a major etiology for falls in elderly persons, which can lead to additional morbidities and mortalities.1, 2, 3 The mechanosensory hair cells in the inner ear are critical for mediating vestibular and auditory functions. Hair cells have stereocilia bundles on their apical surfaces, which detect incoming auditory and vestibular stimuli that include gravity, rotational movement, and linear acceleration. Therefore, disease processes that affect hair cell stereocilia bundles can significantly impair balance and auditory functions.
Many of the genes associated with Usher syndrome have important roles in hair cell stereocilia development and maintenance. Usher syndrome is the most common cause of the combination of deafness and blindness worldwide, with an estimated prevalence of ∼5 in 100,000.4, 5, 6 Many patients with Usher syndrome also have vestibular dysfunction. To date, WHRN and 10 other genes have been associated with different types of Usher syndrome.4, 7 WHRN encodes a scaffolding protein called whirlin, which is required for normal hair cell stereocilia elongation.8 Mutations in the WHRN gene can cause Usher syndrome type 2D (OMIM 611383),9 as well as the non-syndromic autosomal recessive hearing loss DFNB31 (OMIM 607084).10 The whirler mouse (Whrnwi/wi) does not produce functional whirlin proteins in the inner ear,11 resulting in abnormally short stereocilia in cochlear and vestibular hair cells.8 Whirler mice are deaf and have significant balance dysfunction observable as nearly constant circling movements and head bobbing.12, 13 In a previous study, we showed that when adeno-associated virus serotype 2/8 (AAV8-whirlin) gene therapy was injected into the round window of the cochlea, the infected whirler inner hair cells had elongated stereocilia.14 However, the inner hair cell infection rate was low (11%–16%) and there was no hearing recovery in the treated whirler mice. In the present study, we demonstrate that whirlin gene therapy delivered through the posterior semicircular canal results in a significantly higher number of hair cells with whirlin expression both in the vestibular system and in the cochlea, which leads to the restoration of balance function as well as improvement in hearing in whirler mice (Figure 1A). Thus, the current study is, to our knowledge, the first to report rescue of balance and hearing functions using gene therapy in this model of Usher syndrome.
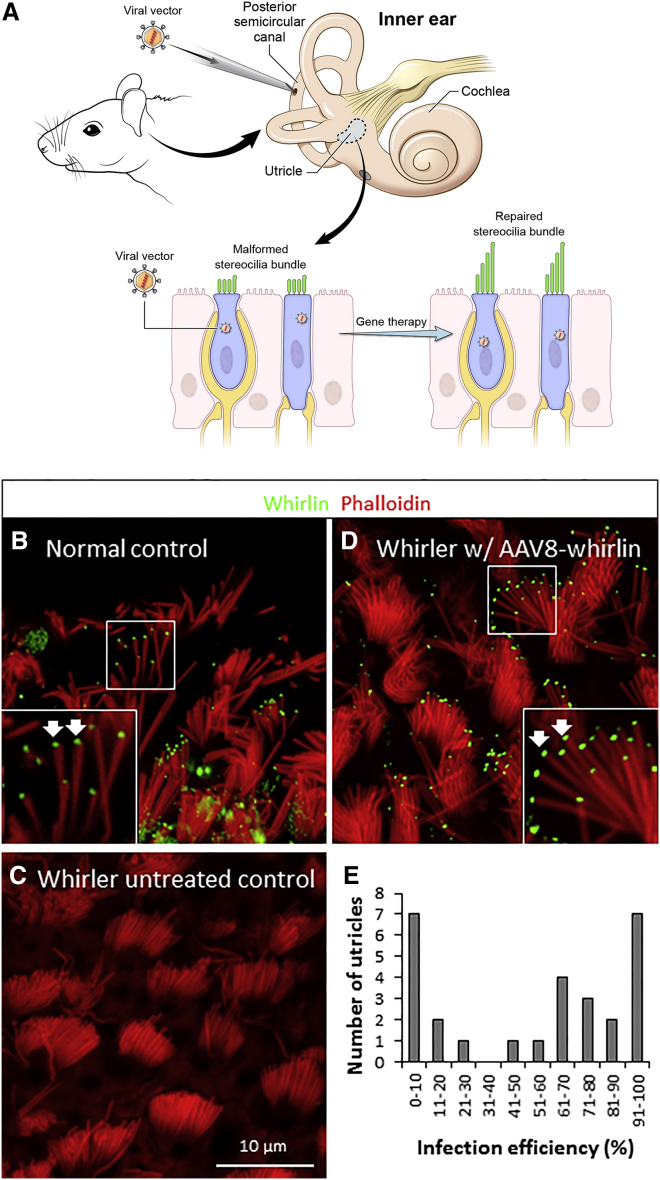
Figure 1.
AAV8-Whirlin Gene Therapy Restores Whirlin Expression in Infected Utricular Hair Cells
(A) Schematic of the study. The inner ear is important for balance function. Vestibular hair cells detect rotational inputs as well as linear and gravitational acceleration by the deflection of their stereocilia bundles. This information is transmitted by the superior and inferior vestibular nerves to the central vestibular system for processing. In this study, we examined whether AAV8-whirlin gene therapy delivered through the posterior semicircular canal can restore vestibular hair cell stereocilia morphology and balance function in whirler mice. The predominant long isoform of whirlin used in this study encodes a scaffolding protein with three PDZ domains and one proline-rich domain. The region recognized by the anti-whirlin antibody is indicated by the purple line. ab, antibody. (B) Utricular hair cells in a phenotypically normal control mouse (whrn+/wi, P30) show immunolocalization of whirlin (green) at the tips of the stereocilia (red). Examples of whirlin expression are shown with white arrows. (C) Utricular hair cells in a whirler untreated control mouse (whrnwi/wi, no gene therapy, P30) lack whirlin expression at the stereocilia tips. In addition, whirler utricular hair cells have aberrantly short stereocilia bundles. (D) AAV8-whirlin gene therapy delivered via the posterior semicircular canal at P4 restored whirlin expression at the stereocilia tips and initiated elongation of stereocilia in infected utricular hair cells in a P30 whirler mouse. Examples of whirlin expression are indicated with white arrows. (E) Distribution of utricular hair cell infection efficiency in all whirler ears that received AAV8-whirlin gene therapy.
Results
Gene Therapy Restores Whirlin Expression in Whirler Vestibular End-Organs
In phenotypically normal control mice (Whrn+/+ and Whrn+/wi), whirlin is localized at the tips of auditory and vestibular hair cell stereocilia (Figure 1B). In contrast, homozygous whirler mutant mice (Whrnwi/wi, referred to as the “whirler untreated control”) do not express whirlin (Figure 1C). The mouse Whrn gene expresses multiple transcript splice variants.11, 15, 16 The predominant longest isoform of wild-type whirlin encodes three PDZ domains and one proline-rich domain (Figure 1A). The cDNA of this isoform was used in the present study because it restores whirler stereocilia length in vitro.17 We used adeno-associated virus serotype 2/8 as the viral vector to deliver whirlin cDNA (AAV8-whirlin) to the whirler inner ear. AAV 2/8 can infect both cochlear and vestibular hair cells with high efficiency.18, 19 In this study, AAV8-whirlin was delivered to the mouse inner ear via the posterior semicircular canal approach, which has been shown to be a safe and effective route for inner ear gene delivery.20 When AAV8-whirlin was injected into whirler inner ears through the posterior semicircular canal, robust whirlin expression was observed at the stereocilia tips in many vestibular hair cells (Figure 1D). We did not observe whirlin protein expression in the microvilli. Whirlin expression was detected in all five vestibular end-organs in AAV8-whirlin-injected whirler mice (Figure S1).
Detailed analyses of the effects of AAV8-whirlin on the vestibular system focused primarily on the utricle. Hair cell infection was defined by the expression of whirlin at the stereocilia tips. Quantification of the efficiency of AAV8-whirlin infection in whirler utricular hair cells revealed a bimodal distribution among the specimens (Figure 1E): those with a high infection efficiency (>40%, n = 18) and those with a low infection efficiency (<30%, n = 10). The overall mean utricular hair cell infection efficiency was 53.1% (SD = 38.1%).
Gene Therapy Restores Stereocilia Morphology in Whirler Utricular Hair Cells
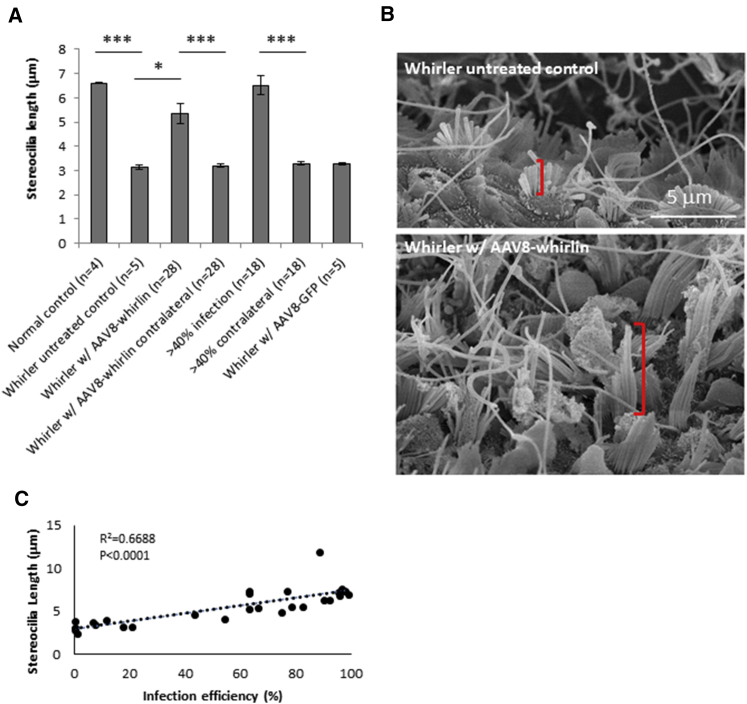
Utricular hair cell stereocilia bundles help to detect changes in head positioning. The average utricular stereocilia length in the phenotypically normal control littermates (Whrn+/+ and Whrn+/wi) was 6.61 ± 0.07 μm, as measured using confocal microscopy images with 3-dimensional (3D) rendering (Figure 2A). In contrast, untreated whirler (Whrnwi /wi) utricular hair cells have aberrantly short stereocilia measuring 3.14 ± 0.19 μm, due to the absence of whirlin expression (Figures 1C, 2A, and 2B). In whirler ears that received AAV8-whirlin gene therapy, the utricular hair cells had significantly increased mean stereocilia length (5.35 ± 2.11 μm, p = 0.027) compared to whirler untreated controls that did not receive gene therapy (Figures 2A and 2B). Mean stereocilia length in the AAV8-whirlin-infected whirler utricles was also longer than the contralateral non-infected ears of the same animals (3.20 ± 0.34 μm, p < 0.00001). Injection of AAV8-GFP to the whirler inner ears was performed as a sham surgery control, and there was no increase in mean stereocilia length in utricular hair cells in these control animals (3.27 ± 0.08 μm) (Figures 2A and S2). In the high-infection group (>40%), average stereocilia length was similar to that of the normal control littermates (6.52 ± 1.72 μm, p = 0.92) (Figure 2A). Linear regression analysis (Figure 2C) revealed a strong positive correlation (R2 = 0.67, p < 0.0001) between stereocilia length and utricular hair cell infection efficiency, indicating that AAV8-whirlin gene therapy restored stereocilia morphology in whirler vestibular end-organs when sufficient numbers of hair cells were infected.
Figure 2.
AAV8-Whirlin Gene Therapy Restores Stereocilia Length in Whirler Utricles
(A) Stereocilia length measurements of utricular hair cells. The “normal control” group includes data from wild-type and heterozygous littermates (whrn+/+ and whrn+/wi, n = 4). The “whirler untreated control” group includes data from whirler mice (whrnwi/wi) that did not receive AAV8-whirlin gene therapy (n = 5). The “whirler w/ AAV8-whirlin” group includes data from all whirler ears (whrnwi/wi) that received AAV8-whirlin gene therapy, including ones with low or no infection (n = 28). The “AAV8-whirlin contralateral” group includes data from the contralateral whirler ears (whrnwi/wi) that did not receive AAV8-whirlin gene therapy (n = 28). The “>40% infection” group includes data from whirlers with >40% AAV8-whirlin infection in utricular hair cells (n = 18). The “>40% contralateral” group includes data from the contralateral whirler ears that did not receive AAV8-whirlin gene therapy in the >40% infection group (n = 18). The “whirler w/ AAV8-GFP” group includes data from whirlers that received AAV8-GFP as a sham surgery control. All stereocilia length measurements were made using confocal images with 3D rendering. Error bars represent SEs. *p < 0.05, ***p < 0.001. (B) Scanning electron microscopy images showing whirler untreated control (whrnwi/wi, no gene therapy) vestibular hair cells that have aberrantly short stereocilia bundles (top panel), and whirler vestibular hair cells with elongated stereocilia bundles after AAV8-whirlin gene therapy (bottom panel). Examples of stereocilia length are shown with red brackets. (C) There was a strong positive correlation (R2 = 0.67) between utricular hair cell infection efficiency and stereocilia length.
Gene Therapy Improves Balance Function in Whirler Mice
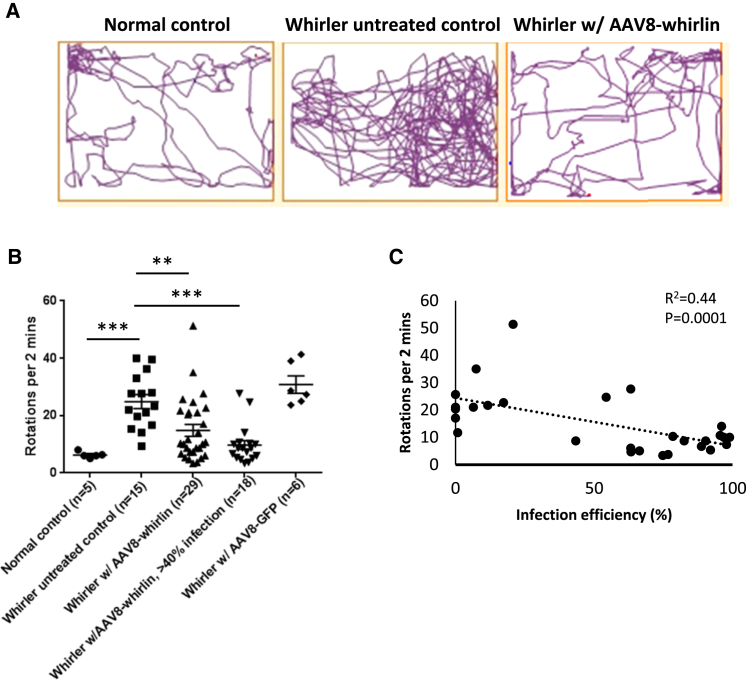
Whirler mice have significant balance dysfunction, as evidenced by their circling behavior. We quantified circling behavior by calculating the number of rotations the animals made in a given amount of time in the testing apparatus. On average, phenotypically normal control littermates (Whrn+/+ and Whrn+/wi) made 6.27 ± 1.09 rotations every 2 min, whereas whirler untreated control mice (Whrnwi/wi, no gene therapy) made 24.9 ± 9.41 rotations in the 2-min time frame (p = 0.004) (Figures 3A and 3B). In whirler mice that received AAV8-whirlin, there was a significant reduction in the number of rotations (14.8 ± 10.9 rotations/2 min, p = 0.004) compared to whirler untreated control mice (Figures 3A and 3B). When the circling behavior was quantified in the high-infection group (>40% hair cells infected) of whirler mice that received AAV8-whirlin, the average number of rotations was not statistically different from phenotypically normal control littermates (9.74 ± 6.61, p = 0.26) (Figure 3B). Injection of the negative control virus AAV8-GFP to whirler inner ears did not improve circling behavior (30.8 ± 7.47 rotations/2 min). Linear regression analysis revealed a strong negative correlation (R2 = 0.44, p = 0.0001) between circling behavior and utricular hair cell infection efficiency (Figure 3C), indicating that when hair cell infection was high enough, AAV8-whirlin gene therapy normalized the circling behavior in whirler mice.
Figure 3.
AAV8-Whirlin Gene Therapy Reduces Circling Behavior in Whirler Mice
(A) Representative track plots from a phenotypically normal control (whrn+/wi), a whirler untreated control (whrnwi/wi, no gene therapy), and a whirler mouse that received AAV8-whirlin gene therapy, demonstrating that AAV8-whirlin gene therapy significantly reduced circling behavior in whirler mice. (B) Quantification of circling behavior testing was performed at ∼P30. Error bars represent SEs. **p < 0.01, ***p < 0.001. (C) Linear regression analysis revealed a strong negative correlation (R2 = 0.44) between utricular hair cell infection efficiency and circling behavior, indicating the greater the infection efficiency, the less the circling behavior.
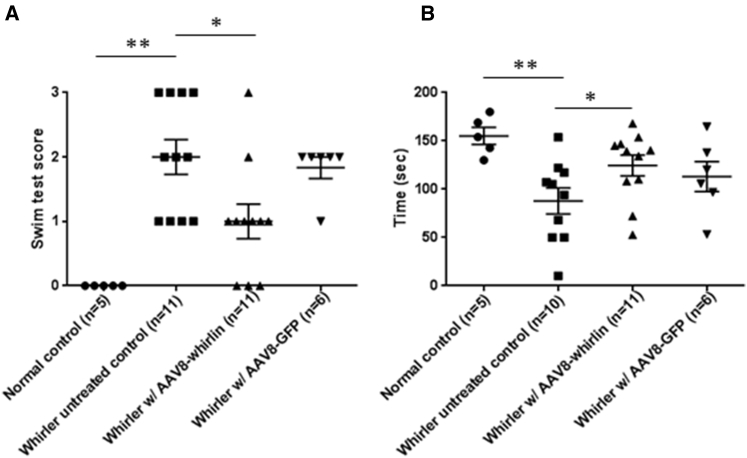
Mice with inner ear vestibular dysfunction also have difficulty with swimming and often demonstrate diminished balance on the rotarod test.21, 22 In the swim test, a well-established 0–3 scoring system was used to measure swim performance, with 0 indicating normal swimming ability, and an increasing score indicating increased swimming difficulty as described in the Materials and Methods.23 All phenotypically normal control mice (Whrn+/+ and Whrn+/wi) had swim scores of 0. Whirler untreated control mice (Whrnwi/wi, no gene therapy) had difficulty swimming, with the average swim score of 2.0 (SD = 0.89) (Figure 4A). When whirler mice received AAV8-whirlin, on average their swim scores improved to 1.0 (SD = 0.89) (Figure 4A). Of note, 3 of 11 whirler mice (27%) had normal swim scores (0) after AAV8-whirlin gene therapy. The only whirler mouse that had a swim score of 3 after AAV8-whirlin gene therapy had low AAV8-whirlin infection efficiency in the utricle (11.7%). The improvement in swim scores in whirler mice that received AAV8-whirlin gene therapy was statistically significant compared to the whirler untreated controls (p = 0.018, Wilcoxon rank-sum test). Injection of AAV8-GFP to the whirler inner ears did not improve swim scores (1.83 ± 0.41) (Figure 4A).
Figure 4.
AAV8-Whirlin Gene Therapy Improves Swimming and Rotarod Performance in Whirler Mice
(A) A well-established swim scoring system was used to measure swim performance, with 0 representing normal swimming and 3 representing underwater tumbling requiring immediate rescue.23 Testing was performed between P90 and P120. (B) The duration of time each animal remained balanced on the rotarod apparatus was recorded. Testing was performed between P90 and P120. Error bars in both figures represent SEs. *p < 0.05, **p < 0.01.
The rotarod test is a measure of balance function in which mice are placed on a rod that rotates with increasing velocity, and the length of time the mouse can remain balanced on the rotating rod is recorded.24 In phenotypically normal control littermates (Whrn+/+ and Whrn+/wi), the average duration on the rotarod was 155 ± 20.0 s, whereas whirler untreated control mice (Whrnwi/wi, no gene therapy) remained on the rotarod for only 87.8 ± 42.6 s (p = 0.006) (Figure 4B). Injection of AAV8-whirlin resulted in significantly improved rotarod performance in whirler mice, with the average rotarod time increased to 125 ± 35.4 s (p = 0.04) (Figure 4B). Although the whirler mice that received AAV8-whirlin had shorter rotarod times compared to phenotypically normal control littermates, this difference was not statistically significant (p = 0.10). Injection of AAV8-GFP to the whirler inner ears did not significantly improve rotarod performance (113 ± 38.0 s, p = 0.25) (Figure 4B).
Although the three balance tests reported above are informative for assessing balance function in mice, they do not specifically assess the function of the inner ear vestibular system. To directly examine the effects of whirlin gene therapy on the vestibular periphery, vestibular evoked potentials (VsEPs) were recorded (Figure S3). It has been reported that ∼50% of whirler mice do not have measurable VsEP thresholds.21 However, eight of nine whirler control mice that did not receive AAV8-whirlin had measurable but elevated VsEP thresholds (−3.56 ± 5.66 dB, Figure S3A). This was not statistically different from the VsEP thresholds of normal control littermates (−9.30 ± 1.64 dB, p = 0.05) or whirlers that received unilateral AAV8-whirlin gene therapy (−5.25 ± 6.65 dB, p = 0.64). The VsEP waveforms in the whirler mice that received unilateral AAV8-whirlin injection were abnormal in both P1-N1 amplitude and P1 latency compared to normal control littermates (Figures S3B and S3C). Since VsEPs measure bilateral vestibular function, we postulated that the abnormal VsEP waveforms seen in whirler mice that received unilateral AAV8-whirlin injection may be explained by the fact that the contralateral non-injected ear remains abnormal. Therefore, VsEP measurements were recorded from whirler mice that underwent bilateral AAV8-whirlin injections. All whirler mice that underwent bilateral AAV8-whirlin injections had normalized circling behavior (7.44 ± 1.46 rotations/2 min, n = 4). All of these mice also had measurable VsEP thresholds (−1.5 ± 0.0 dB). The VsEP P1-N1 amplitude showed a stimulus-level-dependent increase in whirlers that received bilateral AAV8-whirlin injections, whereas the amplitudes remained unchanged with increasing stimulus levels in the whirler controls that did not receive AAV8-whrilin (Figure S3B). In addition, there was a statistically significant improvement in P1 latency in bilaterally treated whirler mice compared to whirler controls that did not receive AAV8-whirlin gene therapy (p = 0.0009, p = 0.006, and p = 0.01 at the 6-dB, 3-dB, and 0-dB stimulus levels, respectively; Figure S3C). Our results indicate that AAV8-whirlin gene therapy was able to improve VsEP P1-N1 amplitude and shorten P1 latency in whirlers when injected into both ears. Taken together, these data indicate that AAV8-whirlin gene therapy significantly improved balance function in whirler mice.
Gene Therapy Improves Auditory Function in Whirler Mice
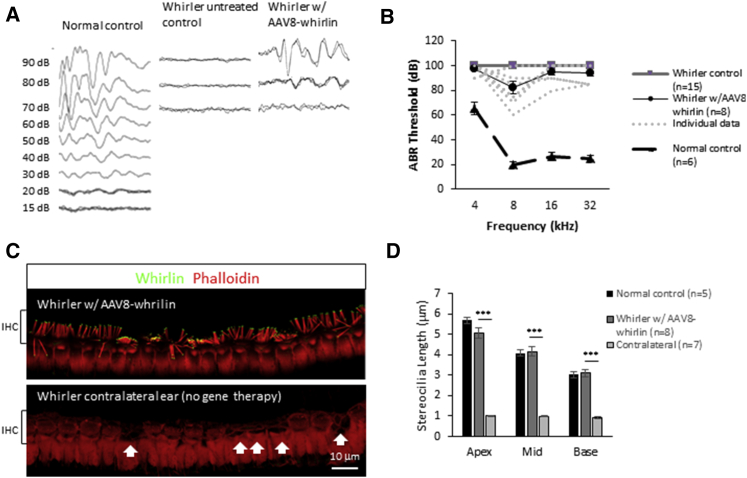
Gene delivery into the mouse posterior semicircular canal can also reach the cochlea.25 In whirler mice that underwent AAV8-GFP injections into the posterior semicircular canal, the average cochlear inner hair cell infection efficiency was 36.1% ± 23.6% (n = 4; Figure S4). Therefore, we examined hearing function in the whirler mice that received AAV8-whirlin gene therapy via this route. Eight of 29 whirler mice (28%) showed some hearing recovery as measured by auditory brainstem response (ABR) threshold testing after AAV8-whirlin delivery to the posterior semicircular canal, while none of the whirler untreated control mice (Whrnwi/wi, no gene therapy) had measurable ABR thresholds (0 of 15, p = 0.024, Fisher’s exact test) (Figures 5A and 5B). Improvement in hearing was seen at all four tested frequencies (4, 8, 16, and 32 kHz), with most of the hearing improvement at 8 kHz, where recorded ABR thresholds were as low as 60 dB sound pressure level (SPL). Examination of cochleas from these eight animals revealed robust AAV8-whirlin infection in both inner and outer hair cells (Figures 5C and S5). The average inner hair cell (IHC) infection efficiency for these eight mice was 71.7% ± 26.0% at the apex, 81.2% ± 15.3% at the middle turn, and 75.2% ± 17.6% at the base of the cochlea. The overall IHC infection efficiency was 77.1% ± 12.7% in the eight hearing mice. In contrast, the average IHC infection efficiency in whirler mice that received AAV8-whirlin but did not have hearing recovery was 25.9% ± 34.8% at the apex, 31.2% ± 37.0% at the middle turn, and 13.4% ± 25.9% at the base of the cochlea (n = 15). The overall IHC infection efficiency in these non-hearing mice was 23.9% ± 29.2% (p < 0.0001). In general, the number of IHCs with whirlin expression achieved with the posterior semicircular canal injection was significantly higher than the round window injection approach reported in our previous study (11%–16%).14 The average outer hair cell (OHC) infection efficiency for the eight whirler mice with hearing improvement was 10.4% ± 6.38% at the apex, 8.64% ± 13.2% at the middle turn, and 3.21% ± 5.95% at the base of the cochlea. The overall OHC infection efficiency was 13.3% ± 9.82%. This is significantly lower than the IHC infection efficiency and is likely the explanation for the partial hearing recovery seen in these animals. The ABR waveforms were also analyzed in the eight whirler mice with hearing improvement. The average ABR wave 1 amplitude in these eight whirler mice was 0.47 ± 0.32 μV (90 dB SPL, 8 kHz). This is lower compared to the measurement from six normal control littermates, although this difference did not reach statistical significance (1.46 ± 1.23 μV, p = 0.09). The ABR wave 1 latency was also longer in the eight whirler mice (3.52 ± 0.66 ms) compared to normal control littermates (1.96 ± 0.22 ms, p = 0.0003). Given the fact that only partial hearing recovery was observed, it is not surprising that the ABR waveforms were not completely normalized in these eight whirler mice. However, since whirler mice are deaf and have no measurable ABR waveforms, recovery of any measurable ABR wave 1 amplitude by AAV8-whirlin gene therapy is a major step forward for hearing restoration in these animals.
Figure 5.
AAV8-Whirlin Gene Therapy Improves Hearing in Whirler Mice
(A) Representative ABR recordings from a phenotypically normal control (whrn+/wi), a whirler untreated control (whrnwi/wi, no gene therapy), and a whirler that received AAV8-whirlin gene therapy. In 8 of 29 whirler mutant mice, measurable ABR thresholds were obtained after AAV8-whirlin gene therapy. (B) ABR thresholds at the four measured frequencies (4, 8, 16, and 32 kHz). The ABR thresholds from the eight whirler mice that had improved hearing are shown individually (dashed lines). An ABR threshold of 100 dB represents no hearing. ABR testing was done at ∼P30. (C) Representative confocal images of the inner hair cells (IHCs) from the cochlear apex in a whirler mouse that received AAV8-whirlin gene therapy in the left ear (top panel) and the contralateral ear that did not receive AAV8-whirlin gene therapy (bottom panel). Images were taken at P120. The treated ear has robust whirlin expression (green) at the stereocilia tips in all IHCs, whereas the contralateral non-treated ear shows no whirlin expression. In addition, several IHCs have degenerated in the non-treated ear (white arrows). The stereocilia (red) lengths in infected whirler IHCs are significantly longer than the non-infected ones. (D) Stereocilia length measurements at different regions of the cochlea in AAV8-whirlin-treated and contralateral (non-treated) ears. The whirler ears that received AAV8-whirlin had significantly longer stereocilia compared to the contralateral non-treated ears. In one animal, the contralateral cochlea was damaged during dissection, leaving seven cochleas for analyses. The stereocilia length measurements from normal controls from a previous study are also shown for comparison (these measurements were made in the exact same way by the same investigators).14 Error bars represent SEs. ***p < 0.001.
Similar to the vestibular system, we found that the infected whirler cochlear IHCs had significantly longer stereocilia bundles compared to non-infected IHCs in the contralateral (untreated) ears: 5.04 ± 0.72 μm versus 1.01 ± 0.08 μm in the apex (p < 0.0001), 4.16 ± 0.64 μm versus 0.99 ± 0.07 μm in the middle turn (p < 0.0001), and 3.10 ± 0.47 μm versus 0.93 ± 0.10 μm in the base (p < 0.0001), respectively (Figure 5D). Stereocilia lengths in AAV8-whirlin-treated whirler cochleas are similar to phenotypically normal controls in all regions of the cochlea (Figure 5D). These results indicate that compared to round window delivery, AAV8-whirlin gene therapy delivered via the posterior semicircular canal resulted in a much higher number of cochlear hair cells with whirlin expression and significantly improved hearing sensitivity and stereocilia morphology in whirler mice.14
Functional Improvements after Whirlin Gene Therapy Are Long Lasting
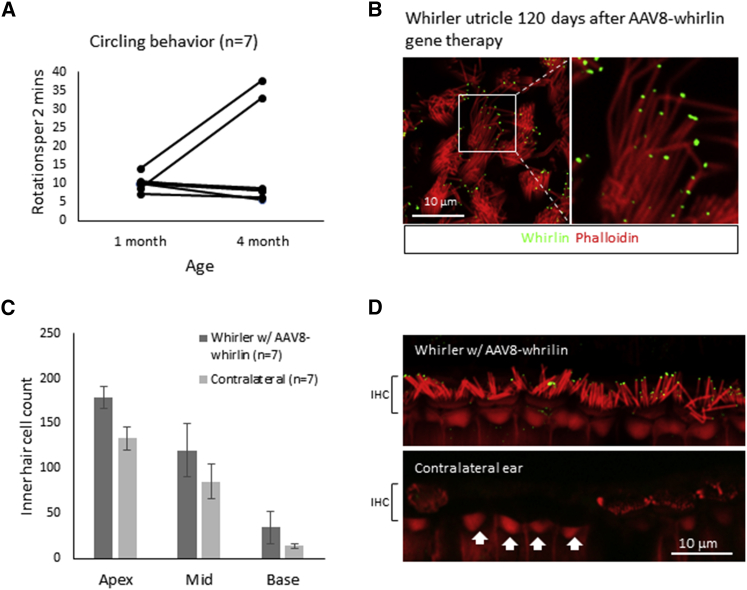
In order to assess the duration of improved function after AAV8-whirlin gene therapy, seven whirler mice that received AAV8-whirlin were examined at ∼P120 for circling behavior. Five of seven whirler mice (71.4%) that received AAV8-whirlin continued to show reduced circling behavior at 4 months of age (Figure 6A). Examination of the utricles from these mice revealed robust whirlin expression at the stereocilia tips with elongated stereocilia bundles (Figure 6B). These data indicate that improvements in both stereocilia morphology and balance function remained stable for at least 4 months after AAV8-whirlin gene therapy.
Figure 6.
The Effects of AAV8-Whirlin Gene Therapy Are Long Lasting
(A) Comparison of circling behavior at 1 month and 4 months of age in seven whirler mice that received AAV8-whirlin gene therapy. Five of seven whirler mutants (71.4%) had sustained reductions in circling behavior. (B) Utricular hair cells in a whirler mouse at P120 showing robust whirlin expression (green) at the stereocilia tips with elongated stereocilia (red). The inset (right) shows stereocilia at higher magnification. (C) IHC counts from AAV8-whirlin-treated whirler ears and the contralateral non-treated ears at P120. The AAV8-whirlin-treated whirler ears had more surviving inner hair cells (IHCs) compared to the contralateral non-treated ears across the entire cochlea. Error bars represent SEs. (D) Representative confocal images of the IHCs from cochlear middle turn in a whirler mouse that received AAV8-whirlin gene therapy in the left ear (top panel) and the contralateral ear that did not receive AAV8-whirlin gene therapy (bottom panel). Images were taken at P120. The treated ear has robust whirlin expression (green) at the stereocilia tips in all IHCs, whereas the contralateral non-treated ear shows no whirlin expression. In addition, several IHCs have degenerated in the non-treated ear (white arrows). Stereocilia (red) lengths in infected whirler IHCs were significantly longer than the non-infected ones.
We next examined the long-term stability of hearing function after whirlin gene therapy. Of the eight whirler mice that received AAV8-whirlin gene therapy and had measureable ABR thresholds at P30, seven were kept alive until ∼P120 (the eighth was examined at P30; see Figure S5). The ABR thresholds measured at P120 in these seven mice are shown in Figure S6. Two of the seven (28.6%) retained measurable ABR thresholds at P120. Comparison of IHC numbers in AAV8-whirlin-treated ears and the contralateral non-treated ears revealed significantly higher numbers of surviving IHCs on the treated side (p = 0.009, two-way ANOVA) (Figure 6C), with the surviving IHCs expressing whirlin at the stereocilia tips (Figure 6D, also see Figure 5C). These data indicate that AAV8-whirlin gene therapy resulted in whirlin expression and restoration of stereocilia bundle morphology in both the utricle and the cochlea at P120. Improved balance function remained stable in 71.4% of animals and improved hearing function remained stable in 28.6% of animals for at least 4 months.
Discussion
Application of gene therapy as a treatment for inner ear pathologies is a nascent, burgeoning field. While attention has been given to cochlear gene therapy, few studies have assessed the efficacy of gene therapy in the inner ear vestibular system.26, 27, 28, 29 To our knowledge, the current study is the first to examine the effects of gene replacement therapy in a mouse model of hereditary balance and hearing dysfunction. Injection of AAV8-whirlin into the posterior semicircular canal successfully infected numerous hair cells throughout the vestibular system. This resulted in a wild-type whirlin localization at the stereocilia tips in infected hair cells, followed by initiation of stereocilia elongation and the restoration of a wild-type stereocilia length that is critical for inner ear hair cell function. There was a positive correlation between stereocilia length and AAV8-whirlin infection efficiency, and in whirler mice with >40% of hair cells infected, stereocilia length was completely restored to that of phenotypically normal control littermates (Whrn+/+ and Whrn+/wi).
Several behavioral tests were used to examine balance function in whirler mice treated with AAV8-whirlin gene therapy, and treated mice showed improved balance function relative to untreated mice on each of these measures. AAV8-whirlin gene therapy significantly reduced circling behavior in whirler mice. In fact, animals with high infection efficiency (>40% AAV8-whirlin infection of utricular hair cells) were indistinguishable from their wild-type littermates. AAV8-whirlin gene therapy also significantly improved swim scores and rotarod performance in whirler mice, with many animals exhibiting a normal phenotype. The behavioral test results were particularly compelling in animals that had high AAV8-whirlin infection efficiency compared to those with low infection efficiency. It is possible that the bimodal distribution of vestibular hair cell infection efficiency reflected differences in injection location: perilymph (low infection efficiency) versus endolymph (high infection efficiency). The small size of the neonatal mouse posterior canal makes it presently not possible to determine whether the gene therapy was injected into the perilymph or into the endolymph in each animal. Despite some variability seen in AAV8-whirlin infection efficiency, our data indicate that AAV8-whirlin gene therapy restored balance function in whirler mice.
The improvement in whirler balance function is remarkable considering that only one ear of each whirler mouse was injected with AAV8-whirlin gene therapy. It is well known that patients with unilateral vestibular loss (e.g., after labyrinthectomy surgery in which the vestibular end-organs in one ear are surgically removed) can recover their balance function by a process of vestibular compensation.30 The exact mechanism(s) underlying vestibular compensation are unknown, but it is thought to be mediated by adaptive changes in brainstem vestibular nuclei.30 Therefore, it is possible that the restoration of unilateral vestibular function by AAV8-whirlin gene therapy is sufficient to allow vestibular compensation to take place in whirler mice, leading to recovery of balance function. It is important to point out that in our experimental model, whirlin gene therapy improved vestibular function in whirler mice de novo (since whirler mice have congenital bilateral vestibular dysfunction). This is in contrast to the vestibular compensation that occurs in patients that initially have intact vestibular function but suffer from acute vestibular loss. Our data do not address the potential role of vestibular compensation in the improved vestibular function we observed in whirler mice after whirlin gene therapy.
Even though the behavioral tests performed in this study (circling behavior, swim and rotarod tests) are commonly used to assess the inner ear vestibular system,21, 31, 32 they are indirect measures of inner ear vestibular function. To test the vestibular system more directly, VsEP measurements were made in whirler mice that underwent AAV8-whirlin gene therapy. All whirler mice that received unilateral and bilateral AAV8-whirlin gene therapy had measurable VsEP thresholds. While whirler mice that received unilateral AAV8-whirlin continued to have abnormal VsEP waveforms, mice that received bilateral AAV8-whirlin injections had significant improvement in VsEP P1-N1 amplitude and P1 latency. In a previous study by Gaines,33 VsEPs were measured in normal C57BL/6 mice after unilateral labyrinthectomy. The author found that VsEP amplitudes were reduced while the latencies remained unaffected. This is in contrast to whirler mice that received unilateral AAV8-whirlin gene therapy, where the VsEP amplitude and latency remained abnormal. The lack of completely normalized VsEP waveforms in whirler mice could be explained by the fact that whirlin is expressed not only in the inner ear but also in the central nervous system.34, 35, 36 It is possible that restoration of whirlin expression in the vestibular hair cells is sufficient to restore balance function, but not enough to completely normalize VsEP waveforms. The reduced VsEP amplitudes may be explained by the lack of synchrony in the vestibular nerve fibers or the reduced number of vestibular nerve fibers activated after gene therapy delivery. Despite the lack of completely normalized VsEP waveforms, the fact that AAV8-whirlin gene therapy improved VsEP responses in whirler mice, along with the significant improvement in all balance function tests performed, indicates that AAV8-whirlin gene therapy was highly effective at improving balance function in these animals.
We also tested whether AAV8-whirlin injection into the posterior semicircular canal improved hearing in whirler mice. Eight of 29 whirler mice (28%) that received AAV8-whirlin gene therapy via the posterior semicircular canal showed improvement in hearing compared to whirler untreated controls, as measured by ABR. AAV8-whirlin infection in both inner and outer hair cells in the cochlea was seen in these animals, with average IHC infection efficiencies between 71.7% and 81.2%. This is in contrast with our previous study, in which none of the whirler mice had hearing recovery when AAV8-whirlin was injected through the round window, and the number of IHCs with whirlin expression was much lower (∼15%).14 These results indicate that improved auditory function is possible when high infection efficiency is achieved.
Since the round window is closer anatomically to the cochlea than the posterior semicircular canal, it may seem counterintuitive that hearing recovery and a higher number of IHCs with whirlin expression were achieved with a posterior semicircular canal gene delivery approach compared to round window injection. It has been shown that drug delivery in rodent cochlea is much more effective when injected through the semicircular canal than through the round window, because rodents have a large cochlear aqueduct, which is located at the base of the cochlea, next to the round window.37, 38 The AAV8-whirlin delivered through the round window may be diluted by cerebrospinal fluid from the cochlear aqueduct, or it may escape through the cochlear aqueduct, which decreases its concentration and limits its ability to infect the entire cochlea.38
When AAV8-whirlin gene therapy was delivered through the posterior semicircular canal, the average IHC stereocilia lengths in the eight whirler mice that showed hearing recovery were much longer than in our previous study in which AAV8-whirlin was injected through the round window.14 This is likely due to the significantly higher number of hair cells with whirlin expression achieved in the present study (the AAV8-whirlin used in this study is the same as in our previous study). These results indicate that AAV8-whirlin gene therapy does improve hearing in whirler mice when sufficiently high hair cell infection efficiency is achieved. Several factors may have prevented complete hearing restoration after AAV8-whirlin gene therapy, including (1) the observed lower infection efficiency in outer hair cells and (2) the fact that only one isoform (the predominant longest isoform) of whirlin was used in this study.11, 16 While the hearing improvement seen in this study was incomplete (60–90 dB SPL), it is important to remember that clinically, the recovery of some hearing in deaf patients can make a significant difference in patients’ quality of life. The hearing improvement seen in this study is also greater than that in a recent study in which TMC1 gene therapy was applied to a mouse model of hereditary hearing loss (90–110 dB SPL).28
Gene therapy in the form of antisense oligonucleotide (ASO) has been applied to another mouse model of Usher syndrome, where ASO was used to correct a recessive mutation in the Ush1c gene that results in a cryptic splice site in the mutant mouse.31 Intraperitoneal injection of the ASO to the Ush1c mutant mice corrected splicing of the mutant pre-mRNA, resulting in some functional recovery in hearing and balance.31 While the ASO gene therapy approach is promising, its application may be limited to mutations that create cryptic splice sites, whereas the gene replacement therapy approach utilized in our study may have broader clinical applications for all types of mutations.
It is important to remember there are differences between the mouse and human inner ears. For example, the human inner ear is fully developed at birth, whereas mouse auditory function is measurable only after approximately P11.39 Since most inner ear gene therapy studies that have successfully improved hearing in mice required neonatal gene delivery (∼P2),27, 28, 29 it is possible that inner ear gene therapy would need to be delivered in utero for humans. In addition, while many patients with Usher syndrome have significant vestibular dysfunction,40 it is unclear whether patients with whirlin mutations (DFNB31 or Usher 2D) have vestibular deficits.9, 10, 21, 41, 42, 43 None of the studies that reported on the phenotypes of these patients included detailed vestibular function tests. Therefore, additional studies are required to investigate the vestibular function in patients with whirlin mutations in order to gauge the potential therapeutic benefits of whirlin gene therapy on balance function in these patients.
In our previous study, when AAV8-whirlin was injected through the round window in neonatal whirler mice, we found that few IHCs expressed whirlin at the stereocilia tips (∼15%).14 We did not see any OHCs with whirlin expression. Even though the stereocilia length was elongated in infected whirler IHCs, the overall stereocilia length was still shorter than the normal control littermates. Due to the low hair cell infection efficiency with the round window injection, no hearing recovery was observed in those mice. The current study demonstrates that AAV8-whirlin introduced into the inner ear via the posterior semicircular canal resulted in a significantly larger number of hair cells with whirlin expression (>70% of IHCs) to restore balance function and improve hearing in the whirler mouse model of human deafness and vestibular dysfunction. The stereocilia lengths in both vestibular and cochlear hair cells were restored to normal after AAV8-whirlin gene therapy. In addition, after gene therapy, IHC survival in the treated whirler cochleas was prolonged compared to the contralateral non-treated cochleas.14 Improved hearing and balance function in whirler mice remained stable for at least 4 months. These data indicate that inner ear gene therapy delivered via the posterior canal approach holds promise for treating a variety of human inherited vestibular and hearing disorders, including Usher syndrome.
Materials and Methods
AAV Vector Construction
The AAV (serotype 2/8) vector was constructed and obtained purified from Vector Biolabs as previously described.14 The AAV8 vector contained the predominant longest isoform of whirlin cDNA (NCBI accession no. AY739114) and was driven by a cytomegalovirus (CMV) promoter. The carrying capacity of this vector is estimated to be about 4,500 bp.44 The predominant longest isoform of whirlin cDNA is 2,724 bp and fits within the AAV8 vector. The concentration of the stock viral solution was 1 × 1013 genome copies per milliliter. The AAV8-GFP vector (Vector Biolabs) also has a CMV promoter and the concentration was 1 × 1013 genome copies per milliliter.
Animal Surgery
All animal procedures were approved by the Animal Care and Use Committee at the National Institute on Deafness and Other Communication Disorders (NIDCD ASP1378-15). Hypothermia was used to induce and maintain anesthesia in neonatal mice.27 Surgery was performed only on the left ear of each animal. The right ear served as a negative control. On the left ear, a post-auricular incision was made, and tissue was dissected to expose the posterior semicircular canal. Care was taken to avoid the facial nerve during the dissection. A nanoliter microinjection system (Nanoliter2000; World Precision Instruments) was used to load AAV8-whirlin into the glass micropipette (∼10 μm in diameter). A total of 0.98 μL AAV8-whirlin was injected over approximately 40 s. Sham surgeries were performed as above with AAV8-GFP as a negative control virus.
Behavioral Measures of Balance Function
Circling Behavior
The circling behavior of mice was quantified using optical tracking and the ANY-maze tracking software (version 4.96; Stoelting). A 38-cm × 58-cm box was attached to a video camera (Fujinon YV5X2.7R4B-2 1/3-inch 2.7–13.5 mm F1.3 Day/Night Aspherical Vari-Focal Lens; Fujifilm). The ANY-maze video tracking software was set to track the head of mice placed within the box. Each mouse was placed into the box and allowed to acclimate to the new environment for 2 min. Complete rotations were recorded and quantified for the next 2 min, followed by a 1-min “cool-down” period in which rotations were not tracked. Each mouse was assessed three times on the same day, and the average was taken. Testing was performed at ∼P30 in all animals (5 normal control mice, 15 whirler untreated control mice, 29 whirlers that received AAV8-whirlin gene therapy, and 6 whirlers that received AAV8-GFP). In a group of seven whirlers that received AAV8-whirlin gene therapy, repeat testing was done at ∼P120 to assess the longevity of the gene therapy effects.
Swim Testing
Mice were placed in a large container filled with room temperature water. Their swimming behavior was recorded using a video camera over 2 min. The videos were de-identified and scored by an observer who was blinded to the genotype and treatment and who was also not involved with the initial video recording. A well-established 0–3 scoring system was used to assess the swim performance.23 Briefly, a score of 0 indicates normal swimming behavior. A score of 1 indicates mild swimming abnormality (circling, vertical swimming). A score of 2 indicates moderate swimming abnormality (immobile floating). A score of 3 indicates significant swimming abnormality in which the mouse needs to be rescued immediately (underwater tumbling). Testing was done between 3 and 4 months of age (5 normal control mice, 11 whirler untreated control mice, 11 whirlers that received AAV8-whirlin gene therapy, and 6 whirlers that received AAV8-GFP).
Rotarod Testing
The rotarod test was performed using a Rotamex 4/8 system (Columbus Instruments). Mice were placed in the testing room for at least 30 min for acclimation. During each trial, a mouse was placed on the rotating rod for a maximum of 180 s. The rotating rod began at a speed of 5 rpm and the speed was gradually increased by 20 rpm/min, with the maximum speed of 20 rpm. The amount of time each animal remained on the rotating rod was recorded. Three trials were performed on each animal on the same day. The average from the three trials was taken as the final score. Testing was done between 3 and 4 months of age (5 normal control mice, 10 whirler untreated control mice, 11 whirlers that received AAV8-whirlin gene therapy, and 6 whirlers that received AAV8-GFP).
Vestibular Evoked Potentials
The VsEP measurements were previously reported in detail.45 All mice were shipped to Dr. Jones’ laboratory at the University of Nebraska for testing. Briefly, mice were anesthetized with an intraperitoneal injection of ketamine/xylazine (18:2 mg/mL, 5–7 μL/g body weight) and electrodes were placed subcutaneously at the nuchal crest (non-inverting), behind the right pinna (inverting) and at the hip (ground). Linear acceleration pulses (2-ms duration, 17 pulses per second) were applied to the cranium in the naso-occipital axis using a commercial electromechanical shaker (Labworks). A calibrated accelerometer was used to monitor the jerk component (i.e., the first derivative of acceleration) of the stimulus and quantify it in decibels relative to 1.0 g/ms (1.0 g = 9.8 μm/ms2). Stimulus levels ranged from +6 dB to −18 dB. Electrophysiological activity was amplified (200,000×), filtered (300–3,000 Hz, −6 dB points), and digitized (125 kHz) beginning at stimulus onset. Two-hundred fifty-six responses were averaged to produce one response trace and all responses were replicated. Waveforms were collected in the presence of an intense forward masker (50–50,000 Hz, 96 dB SPL) to verify the absence of auditory responses. The first positive and negative response peaks were analyzed. Peak latency was measured in milliseconds from the onset of the stimulus to the first positive response peak (P1). Peak-to-peak amplitude was measured in microvolts from P1 to its respective negative peak (N1). Threshold measured in decibels re: 1.0 g/ms was defined as the stimulus level midway between the stimulus level just producing a discernible response and the level which did not.
Hearing Testing via Auditory Brainstem Response Measurements
Auditory brainstem response testing was used to evaluate hearing sensitivity. Testing was done in all animals at ∼P30 (18 normal control mice, 15 whirler untreated control mice, and 29 whirlers that received AAV8-whirlin gene therapy). In seven whirlers that received AAV8-whirlin therapy and had hearing recovery at P30, repeat testing was done at P120. Animals were anesthetized with ketamine (100 mg/kg) and dexmedetomidine (0.5 mg/mL) via intraperitoneal injections and placed on a warming pad inside a sound booth (ETS-Lindgren Acoustic Systems). The animal’s temperature was maintained using a closed feedback loop and monitored using a rectal probe (ATC-1000; World Precision Instruments). Sub-dermal needle electrodes were inserted at the vertex (+) and test-ear mastoid (−) with a ground electrode under the contralateral ear. Stimulus generation and ABR recordings were completed using Tucker Davis Technologies hardware (RZ6 Multi I/O Processor) and software (BioSigRx, version 5.1). ABR thresholds were measured at 4, 8, 16, and 32 kHz using 3-ms, Blackman-gated tone pips presented at 29.9/s with alternating stimulus polarity. At each stimulus level, 512–1,024 responses were averaged. Thresholds were determined by visual inspection of the waveforms and were defined as the lowest stimulus level at which any wave could be reliably detected. The maximal stimulus level tested was at 90 dB SPL. A minimum of two waveforms were obtained at the threshold level to ensure repeatability of the response. Physiological results were analyzed for individual frequencies, and then averaged for each of these frequencies from 4 to 32 kHz.
Immunohistochemistry
After completion of behavioral and auditory testing, mice were euthanized by CO2 asphyxiation followed by decapitation. Temporal bones were harvested and fixed overnight with 4% paraformaldehyde (diluted in 1× PBS) followed by decalcification in 120 mM EDTA (diluted in 1× PBS) for 4 days. The vestibular end-organs and cochlear sensory epithelia were micro-dissected, blocked, and labeled with mouse anti-myosin 7a antibody to label hair cells (1:250, product no. 138-1; Developmental Studies Hybridoma Bank) and with rabbit anti-whirlin antibody at 1:300, as previously reported.17 Primary and secondary antibodies were diluted in blocking solution. Rhodamine-conjugated phalloidin was used to label filamentous actin in stereocilia (1:50; Life Technologies). Images were obtained using a Zeiss LSM780 confocal microscope at ×10 and ×63 using z stacks (∼3- and 0.3-mm thickness, respectively).
Utricular and Cochlear Stereocilia Length and Infection Efficiency Measurements
For each experimental animal, two ×63 images were obtained from the utricle in the extra-striolar region. The imaging location was consistent across all specimens. To measure stereocilia lengths, three 20-μm × 20-μm boxes were placed within each ×63 image (the location of these boxes was consistent across all specimens). All hair cells that fell within a box had their five longest stereocilia measured (excluding the kinocilium) in 3-dimensional space using Volocity 3D image analysis software (PerkinElmer). Approximately five hair cells were measured in each box, making the total hair cells measured per specimen to be ∼30 hair cells (three boxes per image, and two images per utricle). For stereocilia measurements in the cochlea, one ×63 image was obtained from the center of the apex, the middle turn, and the base of the cochlea. Five IHCs near the center of the image had their longest five stereocilia measured in the same way as described above (15 IHCs per cochlea). In some specimens where hair cell degeneration was evident (mostly seen at the base), all available hair cells in the image were measured.
To measure infection efficiency in the utricle, all hair cells in each ×63 image were counted. To measure the infection efficiency in the cochlea, ×63 images were taken throughout the entire cochlea and all IHCs were counted in each cochlea. A hair cell was considered infected by AAV8-whirlin if its stereocilia bundle contained at least one stereocilia that showed whirlin immunostaining at its tip. Even though a total of 29 whirler mice were injected with AAV8-whirlin, 1 specimen was damaged during dissection, so only 28 specimens were available for stereocilia and infection efficiency analyses.
Scanning Electron Microscopy
The micro-dissected vestibular sensory epithelia were fixed in 4% paraformaldehyde in 1× PBS supplemented with calcium and magnesium and processed for immunocytochemistry. Subsequently, for scanning electron microscopy imaging, the coverslip was removed from the slides and sensory epithelia specimens were washed in 0.1 M sodium cacodylate buffer followed by immersion in fixative solution containing 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.3, with 2 mM CaCl2 and 5% sucrose for 2 hr at room temperature. Then samples were washed in cacodylate buffer three times (5 min each wash), rinsed in distilled water, and dehydrated in graded series of ethanol (EtOH). Specimens were then transferred from 100% EtOH to 100% acetone, placed into metal mesh baskets (Ted Pella), critical point dried from liquid CO2 (CPD030 Critical Point Dryer; Bal-Tec), and finally sputter-coated with a 4-nm-thick platinum layer using turbo-pumped sputter coater Q150T (Quorum Technologies). Samples were mounted on aluminum studs (Electron Microscopy Sciences) and imaged using a field emission scanning electron microscope (S-4800; Hitachi).
Statistics
The Student’s t test was used to analyze utricular stereocilia length, circling behavior, and rotarod test results. Ranked data (swim score) were analyzed using Wilcoxon rank-sum test. The statistical significance of hearing recovery in whirler mice was tested using the Fisher exact test. Cochlear stereocilia length data were analyzed using two-way ANOVA. A p value of < 0.05 indicates statistical significance.
Author Contributions
The project was conceived by W.W.C. and L.L.C. Experiments were designed by W.W.C., L.L.C., I.A.B., M.C.D., T.B.F., and A.J.G. and performed by W.W.C., K.I., J.W.S., I.A.B., and M.C.D. Data analyses were done by W.W.C., K.I., and J.W.S. Animal work was done by W.W.C., K.I., and J.W.S. Vestibular and auditory testing was done by K.I. and J.W.S. S.M.J. provided support for vestibular testing. T.S.F. provided support for ABR testing. The paper was written by W.W.C., J.W.S., K.I., and L.L.C. with input from all co-authors.
Conflicts of Interest
The authors have no competing financial interests to report.
Acknowledgments
We are grateful to the NIDCD animal facility staff for caring for our animals. This work was supported by funds from the NIH NIDCD Division of Intramural Research (DC000082-02 to W.W.C., DC000079-03 to L.L.C., DC000048-19 to T.B.F., DC000080 to T.S.F., and DC000060-13 to A.J.G.) and from the Nebraska Tobacco Settlement Biomedical Research Development Fund (to S.M.J.).
Footnotes
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.01.007.
Supplemental Information
References
- 1.Agrawal Y., Carey J.P., Della Santina C.C., Schubert M.C., Minor L.B. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch. Intern. Med. 2009;169:938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 2.Alexander B.H., Rivara F.P., Wolf M.E. The cost and frequency of hospitalization for fall-related injuries in older adults. Am. J. Public Health. 1992;82:1020–1023. doi: 10.2105/ajph.82.7.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterling D.A., O’Connor J.A., Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J. Trauma. 2001;50:116–119. doi: 10.1097/00005373-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Friedman T.B., Schultz J.M., Ahmed Z.M., Tsilou E.T., Brewer C.C. Usher syndrome: hearing loss with vision loss. Adv. Otorhinolaryngol. 2011;70:56–65. doi: 10.1159/000322473. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg T., Haim M., Hauch A.M., Parving A. The prevalence of Usher syndrome and other retinal dystrophy-hearing impairment associations. Clin. Genet. 1997;51:314–321. doi: 10.1111/j.1399-0004.1997.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 6.Spandau U.H., Rohrschneider K. Prevalence and geographical distribution of Usher syndrome in Germany. Graefes Arch. Clin. Exp. Ophthalmol. 2002;240:495–498. doi: 10.1007/s00417-002-0485-8. [DOI] [PubMed] [Google Scholar]
- 7.Eppsteiner R.W., Smith R.J. Genetic disorders of the vestibular system. Curr. Opin. Otolaryngol. Head Neck Surg. 2011;19:397–402. doi: 10.1097/MOO.0b013e32834a9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mburu P., Mustapha M., Varela A., Weil D., El-Amraoui A., Holme R.H., Rump A., Hardisty R.E., Blanchard S., Coimbra R.S. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- 9.Ebermann I., Scholl H.P., Charbel Issa P., Becirovic E., Lamprecht J., Jurklies B., Millán J.M., Aller E., Mitter D., Bolz H. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum. Genet. 2007;121:203–211. doi: 10.1007/s00439-006-0304-0. [DOI] [PubMed] [Google Scholar]
- 10.Mustapha M., Chouery E., Chardenoux S., Naboulsi M., Paronnaud J., Lemainque A., Mégarbané A., Loiselet J., Weil D., Lathrop M., Petit C. DFNB31, a recessive form of sensorineural hearing loss, maps to chromosome 9q32-34. Eur. J. Hum. Genet. 2002;10:210–212. doi: 10.1038/sj.ejhg.5200780. [DOI] [PubMed] [Google Scholar]
- 11.Mathur P.D., Zou J., Zheng T., Almishaal A., Wang Y., Chen Q., Wang L., Vashist D., Brown S., Park A., Yang J. Distinct expression and function of whirlin isoforms in the inner ear and retina: an insight into pathogenesis of USH2D and DFNB31. Hum. Mol. Genet. 2015;24:6213–6228. doi: 10.1093/hmg/ddv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mogensen M.M., Rzadzinska A., Steel K.P. The deaf mouse mutant whirler suggests a role for whirlin in actin filament dynamics and stereocilia development. Cell Motil. Cytoskeleton. 2007;64:496–508. doi: 10.1002/cm.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane P.W. Whirler mice: a recessive behavior mutation in linkage group VIII. J. Hered. 1963;54:263–266. doi: 10.1093/oxfordjournals.jhered.a107262. [DOI] [PubMed] [Google Scholar]
- 14.Chien W.W., Isgrig K., Roy S., Belyantseva I.A., Drummond M.C., May L.A., Fitzgerald T.S., Friedman T.B., Cunningham L.L. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol. Ther. 2016;24:17–25. doi: 10.1038/mt.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright R.N., Hong D.H., Perkins B. RpgrORF15 connects to the usher protein network through direct interactions with multiple whirlin isoforms. Invest. Ophthalmol. Vis. Sci. 2012;53:1519–1529. doi: 10.1167/iovs.11-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahim S., Ingham N.J., Lewis M.A., Rogers M.J., Cui R., Kachar B., Pass J.C., Steel K.P. Alternative splice forms influence functions of whirlin in mechanosensory hair cell stereocilia. Cell Rep. 2016;15:935–943. doi: 10.1016/j.celrep.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belyantseva I.A., Boger E.T., Naz S., Frolenkov G.I., Sellers J.R., Ahmed Z.M., Griffith A.J., Friedman T.B. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat. Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick L.A., Li Q., Yang J., Goddard J.C., Fekete D.M., Lang H. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther. 2011;18:569–578. doi: 10.1038/gt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G.P., Guo J.Y., Peng Z., Liu Y.Y., Xie J., Gong S.S. Adeno-associated virus-mediated gene transfer targeting normal and traumatized mouse utricle. Gene Ther. 2014;21:958–966. doi: 10.1038/gt.2014.73. [DOI] [PubMed] [Google Scholar]
- 20.Kawamoto K., Oh S.H., Kanzaki S., Brown N., Raphael Y. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol. Ther. 2001;4:575–585. doi: 10.1006/mthe.2001.0490. [DOI] [PubMed] [Google Scholar]
- 21.Mathur P.D., Vijayakumar S., Vashist D., Jones S.M., Jones T.A., Yang J. A study of whirlin isoforms in the mouse vestibular system suggests potential vestibular dysfunction in DFNB31-deficient patients. Hum. Mol. Genet. 2015;24:7017–7030. doi: 10.1093/hmg/ddv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staecker H., Praetorius M., Baker K., Brough D.E. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol. Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 23.Hardisty-Hughes R.E., Parker A., Brown S.D. A hearing and vestibular phenotyping pipeline to identify mouse mutants with hearing impairment. Nat. Protoc. 2010;5:177–190. doi: 10.1038/nprot.2009.204. [DOI] [PubMed] [Google Scholar]
- 24.Deacon R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013;75:e2609. doi: 10.3791/2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada H., Iizuka T., Mochizuki H., Nihira T., Kamiya K., Inoshita A., Kasagi H., Kasai M., Ikeda K. Gene transfer targeting mouse vestibule using adenovirus and adeno-associated virus vectors. Otol. Neurotol. 2012;33:655–659. doi: 10.1097/MAO.0b013e31825368d1. [DOI] [PubMed] [Google Scholar]
- 26.Chien W.W., Monzack E.L., McDougald D.S., Cunningham L.L. Gene therapy for sensorineural hearing loss. Ear Hear. 2015;36:1–7. doi: 10.1097/AUD.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 27.Akil O., Seal R.P., Burke K., Wang C., Alemi A., During M., Edwards R.H., Lustig L.R. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askew C., Rochat C., Pan B., Asai Y., Ahmed H., Child E., Schneider B.L., Aebischer P., Holt J.R. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 2015;7:295ra108. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Q., Wang J., Li Q., Kim Y., Zhou B., Wang Y., Li H., Lin X. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol. Med. 2015;7:1077–1086. doi: 10.15252/emmm.201404929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutia M.B. Mechanisms of vestibular compensation: recent advances. Curr. Opin. Otolaryngol. Head Neck Surg. 2010;18:420–424. doi: 10.1097/MOO.0b013e32833de71f. [DOI] [PubMed] [Google Scholar]
- 31.Lentz J.J., Jodelka F.M., Hinrich A.J., McCaffrey K.E., Farris H.E., Spalitta M.J., Bazan N.G., Duelli D.M., Rigo F., Hastings M.L. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat. Med. 2013;19:345–350. doi: 10.1038/nm.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlecker C., Praetorius M., Brough D.E., Presler R.G., Jr., Hsu C., Plinkert P.K., Staecker H. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther. 2011;18:884–890. doi: 10.1038/gt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaines, G.C. (2012). Generators of mammalian vestibular surface responses to head motion. PhD thesis (East Carolina University).
- 34.de Nooij J.C., Simon C.M., Simon A., Doobar S., Steel K.P., Banks R.W., Mentis G.Z., Bewick G.S., Jessell T.M. The PDZ-domain protein Whirlin facilitates mechanosensory signaling in mammalian proprioceptors. J. Neurosci. 2015;35:3073–3084. doi: 10.1523/JNEUROSCI.3699-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap C.C., Liang F., Yamazaki Y., Muto Y., Kishida H., Hayashida T., Hashikawa T., Yano R. CIP98, a novel PDZ domain protein, is expressed in the central nervous system and interacts with calmodulin-dependent serine kinase. J. Neurochem. 2003;85:123–134. doi: 10.1046/j.1471-4159.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 36.Green J.A., Yang J., Grati M., Kachar B., Bhat M.A. Whirlin, a cytoskeletal scaffolding protein, stabilizes the paranodal region and axonal cytoskeleton in myelinated axons. BMC Neurosci. 2013;14:96. doi: 10.1186/1471-2202-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirose K., Hartsock J.J., Johnson S., Santi P., Salt A.N. Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J. Assoc. Res. Otolaryngol. 2014;15:707–719. doi: 10.1007/s10162-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salt A.N., Gill R.M., Hartsock J.J. Perilymph kinetics of FITC-dextran reveals homeostasis dominated by the cochlear aqueduct and cerebrospinal fluid. J. Assoc. Res. Otolaryngol. 2015;16:357–371. doi: 10.1007/s10162-015-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraus H.J., Aulbach-Kraus K. Morphological changes in the cochlea of the mouse after the onset of hearing. Hear. Res. 1981;4:89–102. doi: 10.1016/0378-5955(81)90038-1. [DOI] [PubMed] [Google Scholar]
- 40.Magliulo G., Iannella G., Gagliardi S., Iozzo N., Plateroti R., Plateroti P., Re M., Vingolo E.M. Usher’s syndrome: evaluation of the vestibular system with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. Otol. Neurotol. 2015;36:1421–1427. doi: 10.1097/MAO.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 41.Audo I., Bujakowska K., Mohand-Saïd S., Tronche S., Lancelot M.E., Antonio A., Germain A., Lonjou C., Carpentier W., Sahel J.A. A novel DFNB31 mutation associated with Usher type 2 syndrome showing variable degrees of auditory loss in a consanguineous Portuguese family. Mol. Vis. 2011;17:1598–1606. [PMC free article] [PubMed] [Google Scholar]
- 42.Besnard T., Vaché C., Baux D., Larrieu L., Abadie C., Blanchet C., Odent S., Blanchet P., Calvas P., Hamel C. Non-USH2A mutations in USH2 patients. Hum. Mutat. 2012;33:504–510. doi: 10.1002/humu.22004. [DOI] [PubMed] [Google Scholar]
- 43.Tlili A., Charfedine I., Lahmar I., Benzina Z., Mohamed B.A., Weil D., Idriss N., Drira M., Masmoudi S., Ayadi H. Identification of a novel frameshift mutation in the DFNB31/WHRN gene in a Tunisian consanguineous family with hereditary non-syndromic recessive hearing loss. Hum. Mutat. 2005;25:503. doi: 10.1002/humu.9333. [DOI] [PubMed] [Google Scholar]
- 44.Daya S., Berns K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones S.M., Subramanian G., Avniel W., Guo Y., Burkard R.F., Jones T.A. Stimulus and recording variables and their effects on mammalian vestibular evoked potentials. J. Neurosci. Methods. 2002;118:23–31. doi: 10.1016/s0165-0270(02)00125-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.