Abstract
Endoscopic lung volume reduction (ELVR) is being adopted as a treatment option for carefully selected patients suffering from severe emphysema. ELVR with the one-way endobronchial Zephyr valves (EBV) has been demonstrated to improve pulmonary function, exercise capacity, and quality of life in patients with both heterogeneous and homogenous emphysema without collateral ventilation. In this “expert best practices” review, we will highlight the practical aspects of this therapy. Key selection criteria for ELVR are hyperinflation with a residual volume >175% of predicted, forced expiratory volume <50% of predicted, and a 6-min walking distance >100 m. Patients with repeated infectious complications, severe bronchiectasis, and those with unstable cardiovascular comorbidities should be excluded from EBV treatment. The procedure may be performed with either conscious sedation or general anesthesia and positive pressure mechanical ventilation using a flexible endotracheal tube or a rigid bronchoscope. Chartis and EBV placement should be performed in 1 procedure when possible. The sequence of valve placement should be orchestrated to avoid obstruction and delivery of subsequent valves. If atelectasis has not occurred by 1 month after procedure, evaluate valve position on CT and consider replacing the valves that are not optimally positioned. Pneumothorax is a common complication and typically occurs in the first 2 days following treatment. A management algorithm for pneumothorax has been previously published. Long-term sequelae from EBV therapy do occur but are easily manageable.
Keywords: Bronchoscopic lung volume reduction, Chronic obstructive pulmonary disease, Collateral ventilation, Emphysema, Endobronchial valves, Hyperinflation, Bronchoscopy
Introduction
Endoscopic lung volume reduction (ELVR) is being adopted as a treatment option for carefully selected patients suffering from severe emphysema. The Zephyr endobronchial valve (EBV; Pulmonx Corp., Redwood City, CA, USA) is an implantable device designed to occlude bronchi in diseased regions of the lung and to allow for the expiration of air from the treated lobe of the lung. When placed correctly and in appropriate patients, EBV reduces hyperinflation which manifests in clinical improvement [1]. The effectiveness of EBV is dependent on the absence of collateral ventilation between the lobe with valves and the ipsilateral lobe. The Chartis Diagnostic System (Pulmonx) enables accurate and precise assessment of collateral ventilation status [2]. Used together, the Chartis Diagnostic System and Zephyr EBVs have been demonstrated to provide meaningful benefits of improved pulmonary function, exercise capacity and quality of life in patients with both heterogeneous [3] and homogenous emphysema [4]. Table 1 provides an overview of the most relevant EBVs for emphysema trials in the field.
Table 1.
Overview of the most relevant endobronchial valves for emphysema trials in the field
| Trial [Ref.] (months of follow-up) | ΔRV (L) (treatment vs. control) | p value | ΔFEV1% (treatment vs. control) | p value | Δ6MWT, m (treatment – control) | p value | ΔSGRQ, points (treatment – control) | p value | Pneumothorax rate, % of total treated |
|---|---|---|---|---|---|---|---|---|---|
| IMPACT [4] (3) | –0.48 (–0.84, −0.11) | 0.011 | 17.0 (8.1, 25.8) | <0.001 | 40 (15, 65) | 0.002 | –9.64 (–14.1, −5.2) | <0.001 | 25.6 |
| STELVIO [9] (6) | –0.83a (–1.10, −0.56) | <0.001 | 17.8 (7.6, 28) | 0.001 | 74 (47, 100) | <0.001 | –14.7a (–21.8, −7.6) | <0.001 | 18.0 |
| BELIEVER [3] (3) | –0.37 (–0.72, −0.03) | 0.079 | 20.9 (4.3, 37.5) | 0.033 | 33 (−3, 69) | 0.012 | –5.1 (–14.4, 4.3) | 0.345 | 8.0 |
| VENTb [11] (6) | –0.46 | 0.022 | 24.8 | <0.001 | 28 | 0.065 | –8.4 (–13.8, −3.0) | 0.003 | 11.4c |
Figures in parentheses indicate 95% CI. FEV 1, forced expiratory volume in 1 s; RV, residual volume; 6MWD, 6-min walk distance; SGRQ, Saint George's Respiratory Questionnaire total score.
Completed cases data.
Subgroup analysis on subjects with complete fissures (n = 61 in both groups) and lobar occlusion (n = 61 in EBV treatment group).
Includes 3 subjects with “ex-vacuo” (stable) pneumothorax.
Based on the extensive experience of the panel members from many of the most experienced centers in Europe, this monograph discusses practical and effective approaches to optimizing patient outcomes, including patient selection, patient preparation, patient management, and postoperative care and follow-up.
Patient Selection
As with every medical procedure, patient selection remains an integral part of the successful outcomes. Important selection criteria include:
Spirometry and Hyperinflation
There are no absolute spirometry cutoffs when considering patients for EBV treatment. In clinical practice and clinical trials, however, most patients have a postbronchodilator forced expiratory volume in 1 s (FEV1) below 50% of predicted [1,5], including some patients with FEV1 values as low as 15% of predicted. More importantly, because ELVR is thought to work primarily by reducing lung hyperinflation, it is imperative to select patients for EBV therapy that have hyperinflated lungs (Fig. 1). Consistent with previous trials and expert recommendations, patients should display evidence of hyperinflation as measured by a TLC >100% and RV >175%, both measured by body plethysmography [1,6].
Fig. 1.

X-ray of a typical EBV candidate. Note hyperinflation and subsequent flattening of the diaphragm.
Collateral Ventilation
The absence of collateral ventilation between the treated and ipsilateral lobes is critical for procedural success. The Chartis System is the most studied diagnostic tool for identifying potential responders to EBV treatment based on the absence of collateral ventilation in the target lobe [2]. Because of the considerable interoperator variability of determining lobar fissure integrity by visual assessment on high-resolution computed tomography (HRCT) scans, this approach is not generally recommended as a means of ruling patients in or out for EBV treatment. Quantitative CT analysis has shown early promise, with predictive capabilities in the same range as Chartis in small data sets [7]. More recent data [8] support the use of quantitative CT measurements to screen in patients for further analysis and/or treatment with fissure completeness ≥80%. Patients with fissure integrity <80% are not considered for EBV treatment. If quantitative CT analysis shows fissure completeness between 80 and 95%, performing an additional Chartis measurement to confirm absence of collateral flow is very important to avoid nonresponders to the treatment. If the fissure completeness is >95%, EBV treatment can be directly performed, with Chartis being optional. A typical quantitative CT reconstruction of fissure completeness is displayed in Figure 2.
Fig. 2.
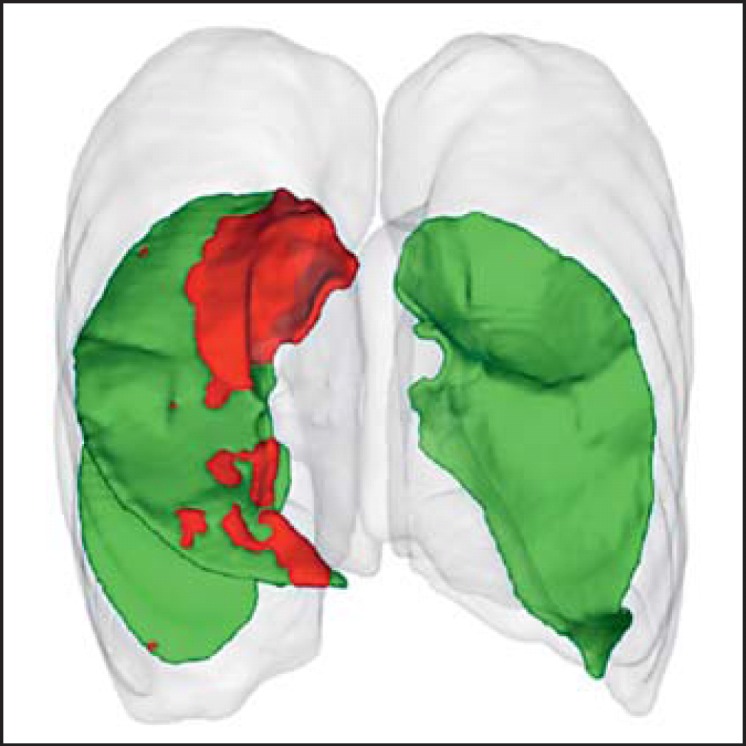
Quantitative CT reconstruction with fissure rendering illustrating a complete major fissure of left lung, a nearly complete major fissure of the right lung (>95%), and an incomplete minor fissure (<80%). Numbers indicate percent of fissure completeness for the lobe mentioned.
Exercise Capacity
Patients should have some level of preserved exercise capacity in order to tolerate the procedure and potential complications. Patients with a 6-min walk distance (6MWD) between 100 and 500 m should be considered for EBV treatment). In patients with a 6MWD below 200 m, reassessment should be considered after pulmonary rehabilitation.
Emphysema Morphology
A low-dose thin-slice (0.5-1.5 mm) volumetric HRCT scan should be used to evaluate the degree and distribution of emphysema. Though most clinical research and experience to date has been focused on the benefit of EBV treatment in patients with heterogeneous emphysema, recent findings suggest that EBV benefits patients with homogeneous emphysema [9] and thus, emphysema heterogeneity should no longer be considered an exclusion criterion for this therapy [4,9]. Coronal, sagittal, and axial reconstructions of HRCT scans can help determine which lobes have greater destruction for purposes of choosing a primary – and if applicable – secondary target lobe. In patients with a more homogeneous disease distribution, a perfusion scan should be performed to provide additional information to select the appropriate target lobe that should exhibit low perfusion compared with the ipsilateral untreated lobe [10].
The physician should carefully review the CT scan for findings, such as bulla in or adjacent to the target lobe (which may suggest the need for additional vigilance after procedure for a pneumothorax), pathologies or nodules (requiring further assessment and/or follow-up), infiltrations or cavity (suggesting active infection, which should be treated before procedure), or disqualifying criteria (such as severe bronchiectasis, severe paraseptal emphysema, extensive fibrosis or other conditions that may significantly impair outcomes; see Fig. 3).
Fig. 3.
CT analysis revealing emphysema morphology not recommended for treatment.
Prior Surgery
For patients who have had previous pulmonary surgeries, a key consideration is whether the target lobe is on the same side of the lung as the previous surgery or the contralateral lung. Patients who have had bullectomies, lobar, segmental-, or wedge resections on the contralateral lung may still be considered candidates if they meet the other screening criteria. Patients with prior surgery on the same side as the target lobe (including bilateral lung transplant or previous bilateral LVRS), or patients with previous pleurodesis should be excluded for safety reasons due to possible lack of compliance in the remaining lobes.
Hypercapnia
Patients with severe hypercapnia (>60 mm Hg on room air) and/or severe hypoxemia (<45 mm Hg on room air) should be excluded from EBV treatment [11,12]. However, in patients that display evidence of hypercapnia, reassessment after a trial of at least 3 months of regular noninvasive ventilation may be warranted.
Medical History and Stability
Available clinical data have shown that EBV treatment benefits patients that are α1-antitrypsin deficient [13], have homogeneous distribution of emphysema [4,9], have upper or lower lobe predominant emphysema [14], and have pulmonary hypertension [1,15]. Patients with unstable cardiovascular disease such as severe heart failure (left ventricular ejection fraction <35% despite optimal medical management), unstable cardiac arrhythmia, myocardial infarction, or stroke within the past 6 months should be excluded until stabilized or improved. Although not an absolute contraindication, patients with pulmonary hypertension with RVSP >45 mm Hg (using echocardiography or right heart catheter measurement) should be treated with caution. Patients that are clinically unstable despite optimal medical management with more than 3 exacerbations resulting in hospitalizations in the previous 12 months are excluded until further stabilized. ELVR should not be performed in patients with significant symptomatic bronchiectasis and chronic sputum production with microbiological colonization, such as pseudomonas or MRSA.
Overall, any one single criterion should not automatically disqualify a candidate if other measures appear favorable, and physicians should exercise caution and sound clinical judgment in selecting appropriate candidates.
Thus, patients for EBV treatment should be selected based on the following minimal criteria:
Residual volume >175% predicted
FEV1 between 15 and 50% of predicted
No evidence of significant coexistent pulmonary pathology on HRCT
Little or no collateral ventilation in the targeted lobe for treatment
Clinically stable prior to the procedure
Able to safely undergo sedation or general anesthesia and bronchoscopy
Cessation of smoking
Key Messages
Residual volume and collateral ventilation are the most important inclusion criteria; patients with emphysema and RV >175% predicted should be evaluated for collateral ventilation
Patients with either homogeneous or heterogeneous emphysema who are able to tolerate the procedure and any potential complications should be considered candidates for valve treatment
Bronchoscopy and the Chartis Procedure
Both the Chartis procedure and subsequent placement of the EBVs in patients without collateral ventilation negative (CV-) are performed during a bronchoscopy. Once the patient is ready to undergo the bronchoscopy, there are a number of important nuances that can make the procedure easier. It is important that the patient is adequately medicated to provide anxiolysis, analgesia, and topical anesthesia to ensure patient comfort and minimize movement. The approach to patient preparation and management is outlined below.
Patient Management
General Anesthesia
This is the preferred and recommended method both for the Chartis measurement and the subsequent valve placement due to the ease of airway and patient management. Positive pressure ventilation minimizes distortion to the Chartis measurements [8] particularly with low ventilation frequency (8-10×/min) and long expiratory settings (I/E ratio 1:3-1:4).
Where applicable, depending on the sedation approach used, it is also often useful to apply topical lidocaine (1-2%) in the airways, particularly in the lobar bronchus of the target lobe prior to performing the Chartis assessment.
Sedation
It is critically important to achieve or maintain an optimal level of sedation. Individual Institutional sedation guidelines should be followed. Optimal sedation during the Chartis procedure is critical, as the patient must exhibit enough tidal breathing in order to validate the detection of a CV-negative target, but must be adequately sedated to avoid coughing and/or secretion production. An important consideration is that with increasing sedation, a majority of these patients can develop respiratory failure and increased carbon dioxide retention. Thus, moderate sedation is ideal, and deep sedation should be considered at facilities with appropriate support and recovery resources. The choice of medications for good patient management is typically determined by physician preference as well as country or institution-specific guidelines and practices. The most common approach for sedation is using a combination of a short-acting benzodiazepine and a narcotic, which can be easily titrated and readily reversed. Typical combinations for sedation include:
Propofol + (remi-)fentanyl sedation, and topical lidocaine
Midazolam + fentanyl + topical lidocaine
Secretion Management
Secretions can be adequately managed with minimal amounts of saline and suction to enhance visibility and device placement. However, excessive suctioning or imprecise scope control can lead to airway edema, mucosal bleeding and inflammation, which can adversely affect valve sizing decisions. The use of antisialogogue agents such as glycopyrrolate or atropine to blunt the production of airway secretions can be considered. Use of either of these drying agents should follow institutional guidelines, and physician's caution should be exercised due to their chronotropic risks.
The Chartis Procedure
The introduction of the Chartis balloon catheter into the bronchoscope is helped by application of a lubricating gel to the front end of the catheter. When the balloon is inserted into the bronchoscope, the obturator should be retained within the balloon catheter to prevent the catheter from kinking and prevent secretions from entering the airflow channel. It is recommended that the balloon and bronchoscope are positioned outside of the ostium of the target lobe with the black marker of the balloon catheter visible prior to balloon inflation. Inflate the balloon just in front of the ostium, then use the bronchoscope to maneuver the balloon into place to initiate contact with the ostium. Carefully inspect the positioning of the catheter to ensure that there is adequate contact between the balloon and the airway wall. An adequate seal is indicated by circumferential blanching of the mucosa. Once verified, the bronchoscope should be gently advanced over the Chartis catheter and rested against the balloon to allow visualization thru the balloon and ensure that the distal end of the catheter is not being obstructed by secretions, the airway wall, or septum (Fig. 4).
Fig. 4.
Correct Chartis balloon catheter placement to occlude the right lower lobe (RLL) airway by inflating the balloon and placing it onto the ostium. Note the circumferential blanching of the bronchial wall, indicating the balloon seal and correct position of the catheter tip. ML, middle lobe.
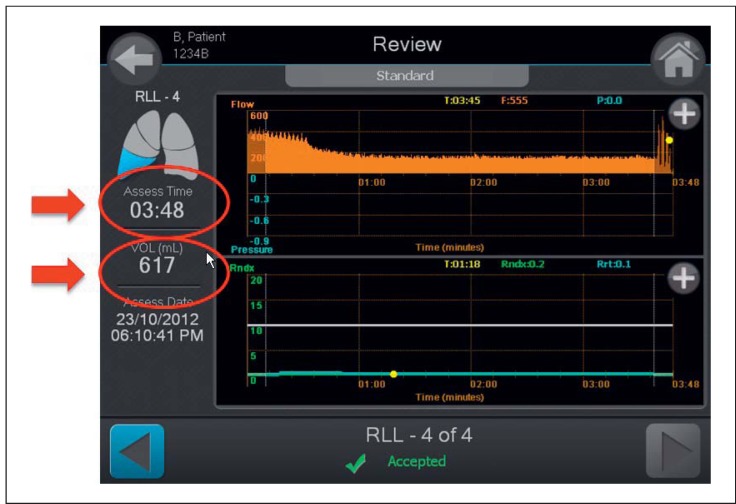
When using conscious sedation and once the stylet is removed, regular flow should be seen on the Chartis display prior to initiating the assessment. Be prepared to perform the assessment for up to 6 min, assuming there is good balloon contact. Care should be taken to closely monitor for catheter obstruction, as obstruction can cause an immediate drop of the flow curve and substantially extend the measurement. Once the flow is visualized and measured, the trend is typically quite evident. However, it can take some time to remove the volume of air from the target lobe in hyperinflated patients prior to seeing a downward trend in the flow reading. Therefore, it is important to consider the volume of exhaled air (which is displayed on the Chartis console) in addition to the time of assessment in order to conclude definitive collateral ventilation status of the target lobe. It is recommended that the operator observes both a significant volume of air recorded (800-1,000 mL) and a reasonable assessment time of at least 5-6 min to conclude a positive assessment of collateral ventilation (Fig. 5). Of note, the Chartis measurement is on average shorter when performed under general anesthesia.
Fig. 5.
Chartis system output screen demonstrating assessment time and volume of exhaled air from the target lobe during assessment. Example shows presence of collateral ventilation.
These parameters will help prevent a false CV-positive assessment. The slope of the flow decrement curve depends on the compliance and pressure of the lobe being measured; however, the decrease should be steady if the lobe is CV negative. The validity of measurement can be confirmed by continuing the measurement after stopping the assessment without deflating or moving the balloon; a notable spike in the expiratory flow once the assessment is stopped, confirms that the measurement was correct.
Occasionally, despite the best technique, testing for collateral ventilation can be inconclusive. For these situations, the ipsilateral lobe can be evaluated as a surrogate for CV negativity in the target lobe. This is straightforward for the left lower lobe, because there is only 1 fissure. To indirectly measure the right lower lobe, plug the right middle lobe (consider using a regular balloon catheter or Watanabe spigot) and use the Chartis balloon in the right upper lobe.
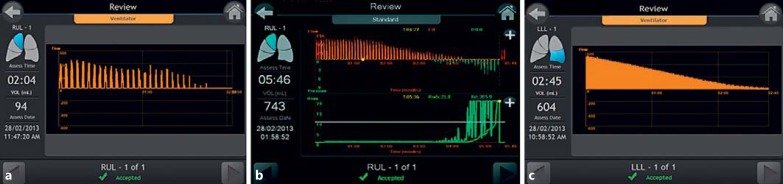
CV status is confirmed by a gradual decrease and eventual cessation of expiratory airway flow in addition to a corresponding increase in resistance. This will appear differently depending on the ventilation method used (Fig. 6) [16,17].
Fig. 6.
Chartis procedure showing absence of collateral flow performed under different anesthetic methods. a Positive pressure ventilation. b Conscious sedation. c Jet ventilation.
Balloon Placement for Right Lower Lobe Assessment
Due to the anatomy of the airway in the right lower lobe, ensure the balloon is inflated at the level of the B6 segment, blocking airflow into the segment but maintaining a patent right middle lobe for correct assessment (Fig. 7).
Fig. 7.
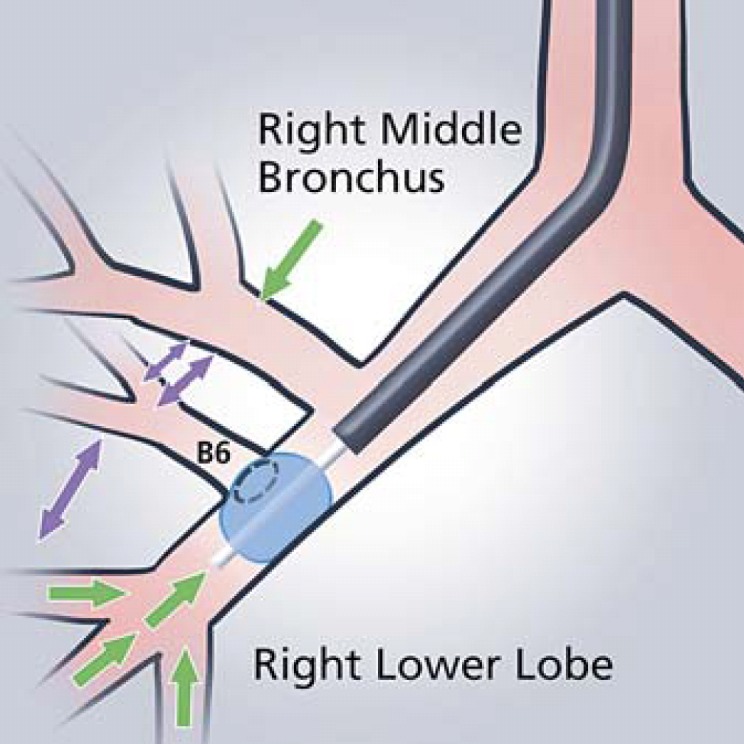
Chartis balloon placement occluding the RB6 segment in order to achieve appropriate measurement in the right lower lobe.
An important consideration that may affect patient outcome is the effect of oxygen saturations during the assessment procedure: If it decreases significantly during assessment, abort and avoid treating that lobe, as it may indicate wrong assessment or important contribution of the lobe to total V/Q [Slebos, pers. commun.]. Proceed to assess a secondary target lobe if available.
Key Messages
• Using general anesthesia with conventional mechanical positive pressure ventilation is the recommended approach to perform the Chartis assessment and valve placement; both Chartis assessment and valve placement can be successfully performed under conscious sedation also, but this method is technically more challenging
• Perform Chartis and EBV placement in 1 procedure where possible (2 procedures may increase the risk of bronchoscopy induced exacerbations)
• If Chartis measurements are inconclusive even after evaluating the ipsilateral (nontarget) lobe or the secondary target lobe, there are 2 options:
- Study CT scan for fissure completeness; retrospective data suggest a high proportion of patients with fissure completeness above 80% may be responders [3]; this may be further supported if the trend for Chartis suggests no significant collateral ventilation
- Alternatively, repeat the Chartis assessment after converting the patient to general anesthesia with positive pressure ventilation
EBV Placement
Before placing the valves (Fig. 8), a clear treatment plan should be developed, starting with the selection of the target lobe and then considering the anatomy of the airways leading to that lobe. If possible, identify a secondary target based on the distribution of disease and/or perfusion and negative collateral ventilation status.
Fig. 8.
Currently available sizes of Zephyr® endobrochial valves (EBV, Pulmonx) designed to occlude varying bronchial airway lengths, with diameters between 4.0 and 8.5 mm. a EBV-TS-4.0-LP. b EBV-TS-4.0. c EBV-TS-5.5.
The target lobe should be selected combining absence of collateral ventilation, greater emphysema tissue destruction on CT, and confirmation of low lobar perfusion using perfusion scintigraphy. Also in patients where multiple target lobes are identified, perfusion scintigraphy, especially in homogeneous emphysema, may be helpful to identify the target lobe (low perfusion in the target, with high perfusion in the ipsilateral, not to be treated lobe) (Fig. 9) [17,18,19,20]. Furthermore, absence of large bulla adjacent to the target lobe, paraseptal emphysema, as well as absence of severe scarring, fibrotic lesions, and significant pleural adhesions is critical.
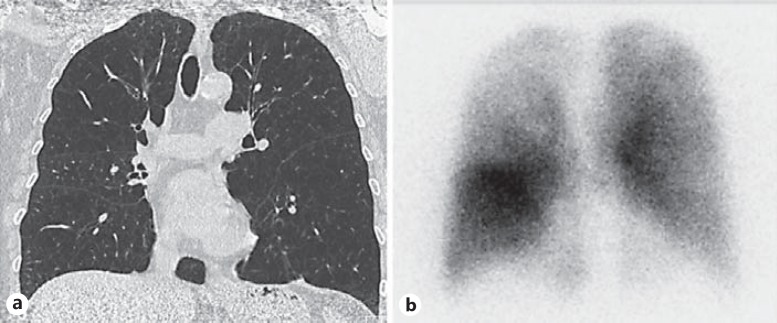
Fig. 9.
An HRCT scan with homogeneous emphysema distribution (a) with an accompanying perfusion scintigraphy scan indicating areas of lower perfusion especially in the right upper zone (b).
Efficient placement of the valves requires careful airway evaluation and systematic and thoughtful placement. It is important to review the patient's CT scan prior to the procedure to evaluate the airway anatomy and consider the depth and size of each target segment. This will help define the order of valve placement and prevent overlap of the proximal ends of the valves, which can sometimes obstruct access to subsequent airways where valves are to be placed. Consideration should therefore be given to first placing the valves in the more distal and least accessible airways, prior to the easier more accessible proximal airways. The depth marker on the endobronchial delivery catheter can be used to help determine the distance that the valve will protrude from the landing zone and validate the optimal order of placement. It is very important to ensure the target segment has enough length between the distal bifurcating carina and ostium to land the body of the valve. This can be accomplished by ensuring the length of the airway segment is greater than the distance from the tip of the delivery catheter to the blue marker (Fig. 10). This distance represents the length of the valve housing that should be seated behind the ostium to ensure correct placement.
Fig. 10.
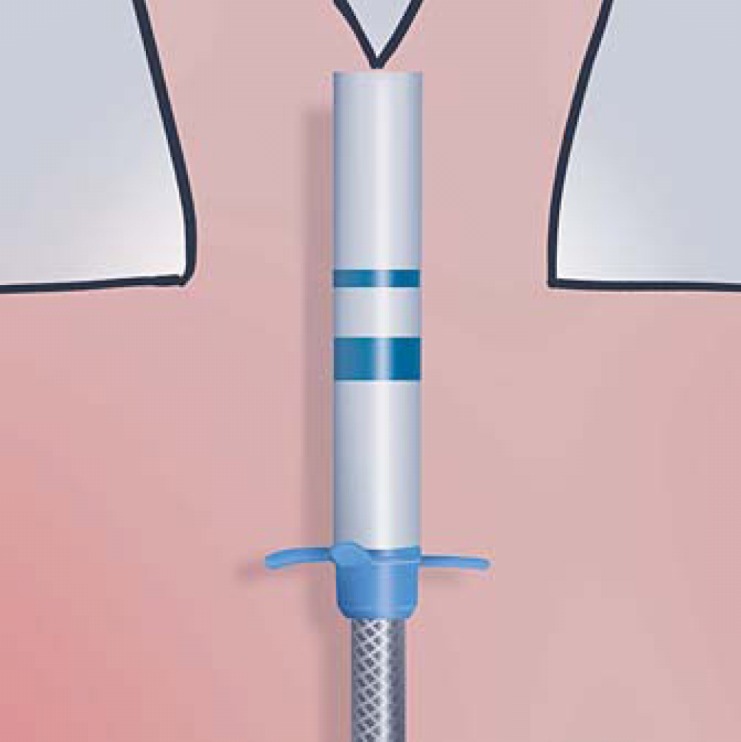
EBV 4.0 EDC delivery catheter with depth markers illustrating the appropriate length of the valve body for the EBV-TS-4.0-LP (thin blue line) and EBV-TS-4.0 valves (thick blue line).
In addition, the wings on the catheter should be used to identify the minimum and maximum diameter of the valve (Fig. 11). The longer wings, representing the maximum diameter of the valve, should touch the airway walls at the widest point of the lumen. This can be accomplished by rotating the catheter by turning the handle when the catheter is in position. The 2 shorter wings indicate the smallest size of the bronchial segment that can be treated. When in doubt, physicians should oversize the diameter of the valve if they have the airway depth to do so, as this will provide a tighter seal.
Fig. 11.
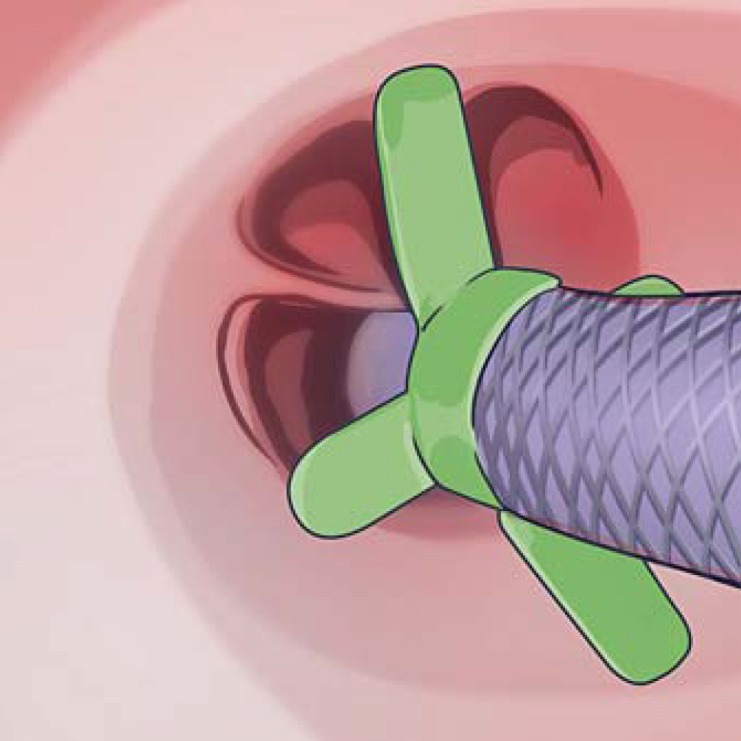
EBV delivery catheter width sizing wings used to determine the minimum and maximum diameter of the valve.
During the placement of a valve, it can be helpful to locate the tip of the delivery catheter just outside of the bronchoscope and then maneuver as close as possible to the target area. After valve sizing measurements are made, advance the catheter so that the marker on the tip of the catheter is visible and then slowly, partially release the valve proximal to the bifurcating carina (Fig. 12). The entire unit should be then advanced to the carina and then fully deployed; this ensures the valve blocks all airways distal to the target bronchus, i.e. that the valve is not inadvertently deployed down a subsegmental airway. Note that during valve deployment, the catheter will automatically retract into the bronchoscope. Accidental or manual retraction of the delivery catheter during valve deployment is a common cause of valve misplacement.
Fig. 12.
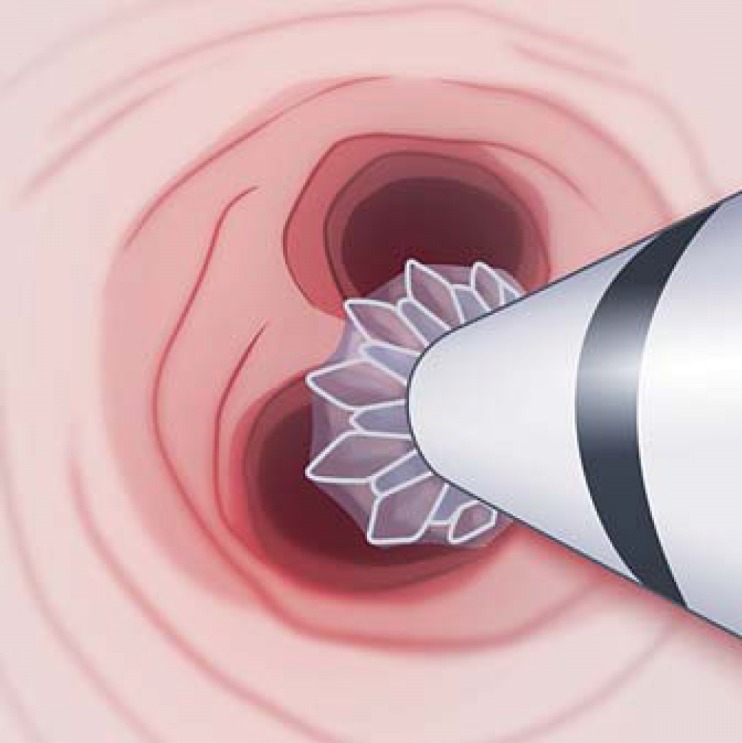
Partial deployment technique illustrating EBV placed directly onto the next distal carina.
If there is a longer segment where placement of valves directly on the distal carina is not easily achievable, position the depth marker on the delivery catheter slightly distal (1-2 mm) to the ostium of the target segment and deploy the valve (Fig. 13). It is important to note that a valve should never be deployed before visualizing the location of the distal carina to ensure the valve is correctly positioned to block all the airways of the target segment.
Fig. 13.

The EBV delivery catheter with a depth marker distal to the ostium ensuring valve housing sits within the target segmental airway.
It is often tempting to place a single 5.5 valve in the lower lobes for the basal pyramid; however, this can often result in valve movement (possible dislodgement or loss of volume reduction). Instead, multiple valves should be used at the next bifurcation. In airway targets where the valve protector region is in contact with the proximal airway, irritation and/or formation of granulation tissue could occur [9,11]. Therefore, shorter valves or deeper placement may be optimal. The “low profile” EBV-TS-4.0-LP valve is often useful for placement in short segments.
After valve placement, chest X-ray evaluation is recommended immediately and again after 4 h. Bed rest and cough suppression can be helpful for patient comfort. Significant volume reduction or atelectasis of the treated lobe may be observed within the first few days, although in some patients it may take up to a month. If there is no visible volume reduction on X-ray at 1 month, the Panel recommends performing a low dose CT scan to study valve positioning and consider replacing any valve(s) that do not appear to be correctly positioned relative to the anatomy, which may result in a leak into the target lobe (Fig. 14).
Fig. 14.
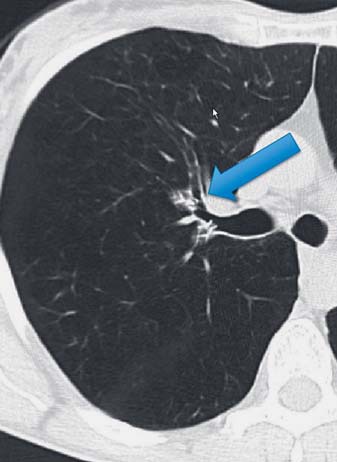
Axial reconstruction of a CT image demonstrating poor valve misplacement and subsegmental airflow preventing lobar atelectasis.
Key Messages
The sequence of valve placement should be in an order so that they do not obstruct the deployment of subsequent valves
Always ensure a distal bifurcating carina is visualized prior to deploying the valve to ensure the valve is deployed proximal to the carina
When in doubt, oversize the valves and treat lower lobes more distally
If atelectasis has not occurred by 1 month after treatment in a lobe that has been confirmed as CV negative, evaluate valve position on CT and consider replacing the incorrectly positioned valves
Management of Acute Complications
The most common complications of EBV placement include pneumothorax, pneumonia, respiratory exacerbations, and valve migrations [2,11,21]. All of these require immediate attention, and the medical staff should be instructed to anticipate, recognize and treat these complications. The currently available data suggest that the pneumothorax rate is approximately 20-30% in experienced centers [9]. Because this is relatively common, we recommend vigilance to proactively detect and treat these events. Skilled and aggressive pneumothorax management is warranted in this patient population as every pneumothorax, in particular a tension pneumothorax, can be life threatening. A management algorithm for pneumothorax has been previously published [22] and should be adhered to. There are not a lot of published data regarding the time of occurrence of pneumothorax after treatment. Approximately 80% of pneumothoraces occur in the first 48 h, 10% within days 3-5, and 10% after day 6 [23]. Hence, current practice is to admit patients to hospital for 3-5 days following insertion of EBV. Maintain an emergency pneumothorax kit at the patient's bedside for rapid decompression.
Patients that demonstrate significant volume reduction on postprocedure chest X-rays may present a higher risk of pneumothorax due to movement in the pleural cavity and should be closely monitored. Patients and staff should be educated on the signs and symptoms of pneumothorax, and patients' discharge instructions should include clear steps to take if pneumothorax symptoms occur.
After valve placement, up to 20% of patients can manifest acute bronchitis, pneumonia and/or lung infections within the first 3 months of the procedure [11,21]. Acute bronchitis often occurs following routine bronchoscopy and is independent of valve placement [24]. To decrease the incidence of bronchitis and respiratory exacerbations, many centers prescribe prophylactic antibiotics and a short course of oral steroids. If a bacterial pneumonia occurs, we suggest prescription of a broad spectrum oral antibiotic initially. If pneumonia does not clear with initial course of antibiotic, we suggest removal of valves and prescription of a second course of IV or oral antibiotics. We would then consider replacing valves 6 weeks after the pneumonia has cleared.
Valve migration is rare, but should be suspected when a patient experiences increased coughing or sudden perceived loss of efficacy. A chest X-ray should be performed to exclude a pneumothorax. If this is unremarkable, we recommend conducting a CT scan and/or bronchoscopy to inspect for valve migration and misplacement. During the bronchoscopy, the displaced valve should be removed and replaced immediately. Valve migration predominantly occurs if the initial valve has been seated incorrectly or undersized.
COPD exacerbations are expected but rarely seen during hospitalization as activity is limited. If they occur, standard treatment with bronchodilators, antibiotics, and corticosteroid are recommended. The GOLD guidelines outline this management extensively [25].
Key Messages
Pneumothorax is a common complication (20% of cases) and typically occurs in the 2 days following treatment, but can occur after discharge
Signs of significant volume reduction on postprocedure chest X-rays may indicate a higher risk of pneumothorax; patients should be closely monitored
Patients and staff should be trained to recognize symptoms of pneumothorax
A management algorithm for pneumothorax has been previously published and should be adhered to
Infections, pneumonia, and COPD exacerbations should be treated according to standard of care
Long-Term Follow-Up and Management of Complications
Long-term complications do occur after EBV placement; however, the overall risk to benefit ratio favors the use of EBV therapy [26,27,28]. In most cases, the complications can be managed by the patient's primary pulmonologist, but coordinated and collaborative communication between the primary pulmonologist and treating center is essential and strongly recommended. The most common long-term complications include pneumonia, COPD exacerbations, granulation tissue formation and valve migrations/loss of efficacy [11,21,29]. Because the patient will often be back under the care of their primary physician, in order to ensure a smooth transition back to their care, it is critical to keep the patient's referring team apprised of the planned procedure, outcomes, and potential complications.
We recommend following up with the patient's referring physician at 1, 3, and 6 months, and yearly after the procedure to maintain surveillance of all patient outcomes and possible complications. The patient and family should be sufficiently informed and must understand when to alert their treating physician. A 24-h hotline is also recommended so that patients can contact an on call provider if questions or emergencies arise.
In the long term, we re-evaluate patients if there is a loss of effect, no effect, or other complications. If the patient's breathing deteriorates or there is no improvement, we recommend an additional low-dose CT scan to assess valve positioning as appropriate. If the patient has any of the following, we recommend the addition of bronchoscopic evaluation and valve adjustment or replacement:
No volume reduction (at scheduled 30-45-day check, or symptom triggering study) on CT scan
Sudden loss of benefit/loss of volume reduction on CT
Persistent cough
Persistent hemoptysis
Obstruction pneumonia
Pneumothorax management
If the patient is having an increased frequency of respiratory exacerbations, but no changes are seen on the CT scan, we will first assess exacerbation frequency prior to EBV treatment to determine if this is the likely natural course of the disease or due to valves. If it is the natural course of the disease, then we would recommend that the primary pulmonologist consider increasing doses of inhaled bronchodilators and other therapies as outlined in the GOLD guidelines. If the patient has had a loss of effect, we would recommend direct bronchoscopy in order to evaluate for abnormalities such as migration or granulation tissue formation. Granulation tissue formation has been noted in some cases [9,11] and can present asymptomatically, or with persistent cough, hemoptysis or loss of volume reduction after initial success.
Sometimes, it may not be due to the direct interaction of the valve with the target bronchus itself but due to an interaction of the valve housing with the adjacent bronchial wall or if an edge of an eccentrically placed valve scratches over the mucosa during a cough. In either case, the management is to remove the valve and if needed address any granulation tissue with cryoablation if available. Replace the valve either with one more distally positioned or with a larger-sized valve 6 weeks later, assuming granulation tissue has disappeared (depending on bronchus). Care must be taken to place the valve as centrally as possible to avoid wall irritation.
Airway remodeling or bronchial torsion has been observed on rare occasions [9]. A tightly fitted valve may leak if the bronchus reshapes elliptically. Changing the valve is recommended in such a situation.
Long-term infection is rare, and the recommendation is to follow the standard course of 7-14 days of oral antibiotics. If pneumonia does not clear after appropriate antibiotic therapy, consider removing the valves and prescribing a 2nd course of antibiotic therapy.
Key Messages
Long-term sequelae from EBV treatment do occur but are generally manageable
Ongoing communication between the patient's physician and treating physician is important to evaluate symptoms and decide next steps together
A repeat bronchoscopy may be necessary to restore loss of initial benefit due to valve dislocation, expectoration, or granulation tissue formation
Conclusion
EBV treatment for emphysema is an efficacious therapy that is proven to improve lung function, exercise capacity, and quality of life in patients afflicted with emphysema and absence of collateral ventilation in the target lobe. These clinical best practice recommendations should aid physicians in maximizing the response rate and patient outcomes with EBV treatment.
Financial Disclosure and Conflicts of Interest
D.-J.S., P.L.S., F.J.F.H. and A.V. are advisors to PulmonX Corp. and all participated in clinical trials funded by PulmonX Corp.
Acknowledgements
The authors are grateful to Richard Sue, MD, for helping with the preparation of the manuscript and for the critical review of the final document.
References
- 1.Herth FJ, Slebos DJ, Rabe KF, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation. Respiration. 2016;91:241–250. doi: 10.1159/000444090. [DOI] [PubMed] [Google Scholar]
- 2.Herth FJ, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, Schmidt B, Slebos DJ. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J. 2013;41:302–308. doi: 10.1183/09031936.00015312. [DOI] [PubMed] [Google Scholar]
- 3.Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, Hansell DM, Rubens MB, Banya W, Polkey MI, Shah PL, Hopkinson NS. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386:1066–1073. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 4.Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, Petermann C, Hubner RH, Stanzel F, Eberhardt R, IMPACT Study Team Endobronchial valve therapy in patients with homogeneous emphysema: results from the IMPACT study. Am J Respir Crit Care Med. 2016;194:1073–1082. doi: 10.1164/rccm.201607-1383OC. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Xu M, Xie Y, Gao J, Ni S. Efficacy and safety of endobronchial valves for advanced emphysema: a meta analysis. J Thorac Dis. 2015;7:320–328. doi: 10.3978/j.issn.2072-1439.2014.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah PL, Herth FJ. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax. 2014;69:280–286. doi: 10.1136/thoraxjnl-2013-203743. [DOI] [PubMed] [Google Scholar]
- 7.Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, Hornberg D, Heussel CP, Wood S, Herth FJ. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med. 2015;191:767–774. doi: 10.1164/rccm.201407-1205OC. [DOI] [PubMed] [Google Scholar]
- 8.Koster TD, van Rikxoort EM, Huebner RH, Doellinger F, Klooster K, Charbonnier JP, Radhakrishnan S, Herth FJ, Slebos DJ. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration. 2016;92:150–157. doi: 10.1159/000448849. [DOI] [PubMed] [Google Scholar]
- 9.Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373:2325–2335. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen C, Theilig D, Herzog D, Poellinger A, Doellinger F, Schreiter N, Schreiter V, Schürmann D, Temmesfeld-Wollbrueck B, Hippenstiel S, Suttorp N, Hubner R. Lung perfusion and emphysema distribution affect the outcome of endobronchial valve therapy. Int J Chron Obstruct Pulmon Dis. 2016;11:1245–1259. doi: 10.2147/COPD.S101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G, VENT Study Research Group A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 12.Ninane V, Geltner C, Bezzi M, Foccoli P, Gottlieb J, Welte T, Seijo L, Zulueta JJ, Munavvar M, Rosell A, Lopez M, Jones PW, Coxson HO, Springmeyer SC, Gonzalez X. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J. 2012;39:1319–1325. doi: 10.1183/09031936.00019711. [DOI] [PubMed] [Google Scholar]
- 13.Hillerdal G, Mindus S. One- to four-year follow-up of endobronchial lung volume reduction in alpha-1-antitrypsin deficiency patients: a case series. Respiration. 2014;88:320–328. doi: 10.1159/000365662. [DOI] [PubMed] [Google Scholar]
- 14.Eberhardt R, Herth FJ, Radhakrishnan S, Gompelmann D. Comparing clinical outcomes in upper versus lower lobe endobronchial valve treatment in severe emphysema. Respiration. 2015;90:314–320. doi: 10.1159/000437358. [DOI] [PubMed] [Google Scholar]
- 15.Eberhardt R, Gerovasili V, Kontogianni K, Gompelmann D, Ehlken N, Herth FJ, Grunig E, Nagel C. Endoscopic lung volume reduction with endobronchial valves in patients with severe emphysema and established pulmonary hypertension. Respiration. 2015;89:41–48. doi: 10.1159/000368369. [DOI] [PubMed] [Google Scholar]
- 16.Gesierich W, Samitas K, Reichenberger F, Behr J. Collapse phenomenon during Chartis collateral ventilation assessment. Eur Respir J. 2016;47:1657–1667. doi: 10.1183/13993003.01973-2015. [DOI] [PubMed] [Google Scholar]
- 17.Herzog D, Poellinger A, Doellinger F, Schuermann D, Temmesfeld-Wollbrueck B, Froeling V, Schreiter NF, Neumann K, Hippenstiel S, Suttorp N, Hubner RH. Modifying post-operative medical care after EBV implant may reduce pneumothorax incidence. PLoS One. 2015;10:e0128097. doi: 10.1371/journal.pone.0128097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SC, Peters MJ, Chen S, Emmett L, Ing AJ. Effect of unilateral endobronchial valve insertion on pulmonary ventilation and perfusion: a pilot study. Respirology. 2010;15:1079–1083. doi: 10.1111/j.1440-1843.2010.01815.x. [DOI] [PubMed] [Google Scholar]
- 19.Pizarro C, Ahmadzadehfar H, Essler M, Fimmers R, Nickenig G, Skowasch D. Volumetric and scintigraphic changes following endoscopic lung volume reduction. Eur Respir J. 2015;45:262–265. doi: 10.1183/09031936.00108914. [DOI] [PubMed] [Google Scholar]
- 20.Strange C, Herth FJ, Kovitz KL, McLennan G, Ernst A, Goldin J, Noppen M, Criner GJ, Sciurba FC, VENT Study Group Design of the Endobronchial Valve for Emphysema Palliation Trial (VENT): a non-surgical method of lung volume reduction. BMC Pulm Med. 2007;7:10. doi: 10.1186/1471-2466-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, Egan JJ, Gasparini S, Agusti C, Holmes-Higgin D, Ernst A, International VENT Study Group Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39:1334–1342. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 22.Valipour A, Slebos DJ, de Oliveira HG, Eberhardt R, Freitag L, Criner GJ, Herth FJ. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema – potential mechanisms, treatment algorithm, and case examples. Respiration. 2014;87:513–521. doi: 10.1159/000360642. [DOI] [PubMed] [Google Scholar]
- 23.Skowasch D, Fertl A, Schwick B, Schafer H, Hellmann A, Herth FJ, LIVE Study Investigators A long-term follow-up investigation of endobronchial valves in emphysema (the LIVE Study): study protocol and six-month interim analysis results of a prospective five-year observational study. Respiration. 2016;92:118–126. doi: 10.1159/000448119. [DOI] [PubMed] [Google Scholar]
- 24.Sharif-Kashani B, Shahabi P, Behzadnia N, Mohammad-Taheri Z, Mansouri D, Masjedi MR, Zargari L, Salimi Negad L. Incidence of fever and bacteriemia following flexible fiberoptic bronchoscopy: a prospective study. Acta Med Iran. 2010;48:385–388. [PubMed] [Google Scholar]
- 25.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 26.Hopkinson NS, Kemp SV, Toma TP, Hansell DM, Geddes DM, Shah PL, Polkey MI. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J. 2011;37:1346–1351. doi: 10.1183/09031936.00100110. [DOI] [PubMed] [Google Scholar]
- 27.Venuta F, Anile M, Diso D, Carillo C, De Giacomo T, D'Andrilli A, Fraioli F, Rendina EA, Coloni GF. Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J. 2012;39:1084–1089. doi: 10.1183/09031936.00071311. [DOI] [PubMed] [Google Scholar]
- 28.Garner J, Kemp SV, Toma TP, Hansell DM, Polkey MI, Shah PL, Hopkinson NS. Survival after endobronchial valve placement for emphysema: a 10-year follow-up study. Am J Respir Crit Care Med. 2016;194:519–521. doi: 10.1164/rccm.201604-0852LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klooster K, Hartman J, Ten Hacken N, Slebos DJ. One year follow-up after endobronchial valve treatment in patients with emphysema without interlobar collateral ventilation (abstract A7910). Eur Respir Soc Int Congr, London, September 3-7. 2016 [Google Scholar]