Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease that typically leads to respiratory failure and death within 3–5 years of diagnosis. Sub-pleural pulmonary fibrosis is a pathological hallmark of IPF. Bleomycin treatment of mice is a an established pulmonary fibrosis model. We recently showed that bleomycin-induced epithelial-mesenchymal transition (EMT) contributes to pleural mesothelial cell (PMC) migration and sub-pleural pulmonary fibrosis. MicroRNA (miRNA) expression has recently been implicated in the pathogenesis of IPF. However, changes in miRNA expression in PMCs and sub-pleural fibrosis have not been reported. Using cultured PMCs and a pulmonary fibrosis animal model, we found that miR-18a-5p was reduced in PMCs treated with bleomycin and that downregulation of miR-18a-5p contributed to EMT of PMCs. Furthermore, we determined that miR-18a-5p binds to the 3′ UTR region of transforming growth factor β receptor II (TGF-βRII) mRNA, and this is associated with reduced TGF-βRII expression and suppression of TGF-β-Smad2/3 signaling. Overexpression of miR-18a-5p prevented bleomycin-induced EMT of PMC and inhibited bleomycin-induced sub-pleural fibrosis in mice. Taken together, our data indicate that downregulated miR-18a-5p mediates sub-pleural pulmonary fibrosis through upregulation of its target, TGF-βRII, and that overexpression of miR-18a-5p might therefore provide a novel approach to the treatment of IPF.
Keywords: pleural mesothelial cells, miR-18a, fibrosis, TGF-β1, epithelial-mesenchymal transition
Sub-pleural pulmonary fibrosis is a pathological hallmark of idiopathic pulmonary fibrosis (IPF). Zhang et al. found that downregulated miR-18a-5p mediates sub-pleural pulmonary fibrosis through upregulation of its target, TGF-βRII, suggesting that overexpression of miR-18a-5p might be an approach to the treatment of IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease that typically leads to respiratory failure and death within 3–5 years of diagnosis.1 The pathological features of IPF include fibroproliferative foci and over-deposition of extracellular matrix (ECM). Interestingly, these histopathological alterations are predominantly located in sub-pleural areas.2 The mechanism of sub-pleural localization remains unknown. Recently, Mubarak et al.3 reported that pleural mesothelial cell (PMC) trafficking into the lung parenchyma is involved in sub-pleural fibrotic foci in IPF, which suggests that PMCs might be involved in sub-pleural fibrosis. It is known that epithelial cells undergoing epithelial-mesenchymal transition (EMT) play an important role in fibrotic disease.4, 5, 6 As specific epithelial cells, PMCs can undergo EMT under several conditions.5, 7, 8 PMCs with EMT are considered a source of myofibroblasts, which are the key cells within fibroproliferative foci.3, 8, 9 Bleomycin is a classical inducer of pulmonary fibrosis model. In animal models, lung fibrosis induced by intraperitoneal bleomycin injection exhibits a sub-pleural fibrotic distribution similar to what is seen in human IPF.10 We recently demonstrated that bleomycin-induced EMT contributes to PMC migration and sub-pleural fibrosis in a mouse model of lung fibrosis.8 Transforming growth factor β1 (TGF-β1) is the key fibrotic factor and EMT inducer.6, 11 Several studies have revealed that TGF-β and Smad signaling is involved in EMT.12, 13 Our study showed that bleomycin induces EMT of PMCs through enhanced TGF-β1 and Smad2/3 signaling.8 However, it remains unknown whether blocking EMT of PMCs prevents sub-pleural pulmonary fibrosis.
MicroRNAs (miRNAs) are short non-coding single-stranded RNA molecules (∼22 nt in length) with evolutionarily conserved sequences.14, 15, 16 The role of miRNAs in the pathogenesis of IPF has recently received considerable attention. Approximately 10% of miRNAs expressed in the lungs of IPF patients show significant deregulation.17 It has been found that miRNA-326,18 let-7d, miRNA-29, miRNA-18a,17 miRNA-26a,18, 19, 20 and miRNA-20021 are downregulated and miRNA-21,22 miRNA-199a,23 and miRNA-14524 are upregulated in lungs of IPF patients. Alterations in these miRNAs have also been demonstrated in lung fibroblasts or alveolar epithelial cells from IPF patients and pulmonary fibrosis animal models.17, 18, 21, 22, 23, 24 However, changes in miRNAs in PMCs and their roles in PMC EMT and sub-pleural fibrosis have not been described. In the present study, we used cultured PMCs and a mouse pulmonary fibrosis animal model to explore the role of miRNA in PMC EMT and sub-pleural pulmonary fibrosis. We report, for the first time, that miRNA miR-18a-5p is reduced in bleomycin-treated PMCs and that downregulation of miR-18a-5p contributes to EMT of PMCs via upregulation of its target, TGF-β receptor II (TGF-βRII), which mediates signaling leading to sub-pleural pulmonary fibrosis. These observations argue that overexpression of miR-18a-5p might be a novel approach to the treatment of pulmonary fibrotic diseases, such as IPF.
Results
Bleomycin Induces Downregulation of miR-18a-5p in PMCs
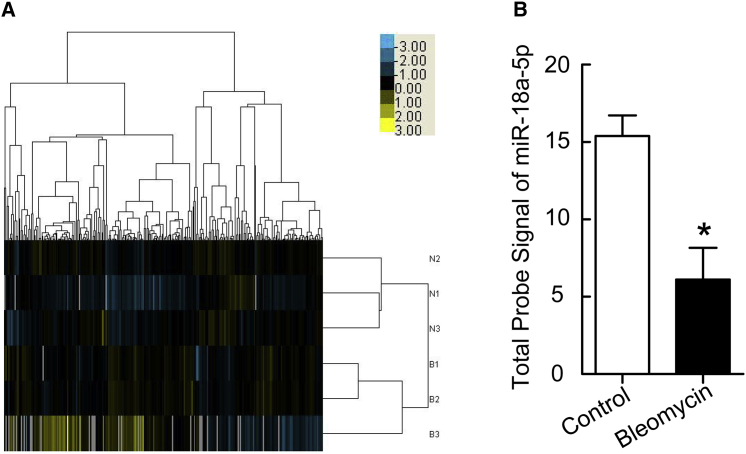
To investigate the effect of bleomycin on miRNA expression in PMCs, cells were treated with or without bleomycin (0.2 μg/mL) for 24 hr, RNA was isolated, and the miRNA expression profile was assessed by microarray analysis. Distinct changes were observed in the expression patterns of some miRNAs in bleomycin-treated PMCs compared to untreated control cells (Figure 1A). Of particular interest, bleomycin induced a 60.3% decrease in miR-18a-5p levels (Figure 1B).
Figure 1.
Bleomycin Induces Downregulation of miR-18a-5p in PMCs
PMCs were treated with or without bleomycin (0.2 μg/mL) for 24 hr, after which microarray-based miRNA expression analysis was performed. (A) The heatmap hierarchal clustering dendrogram analysis of miRNA expression in control (N) and bleomycin-treated (B) cells. (B) Changes in miR-18a-5p levels (n = 3). *p < 0.05 versus control.
Inhibition of miR-18a-5p Induces EMT of PMCs and Promotes PMC Migration
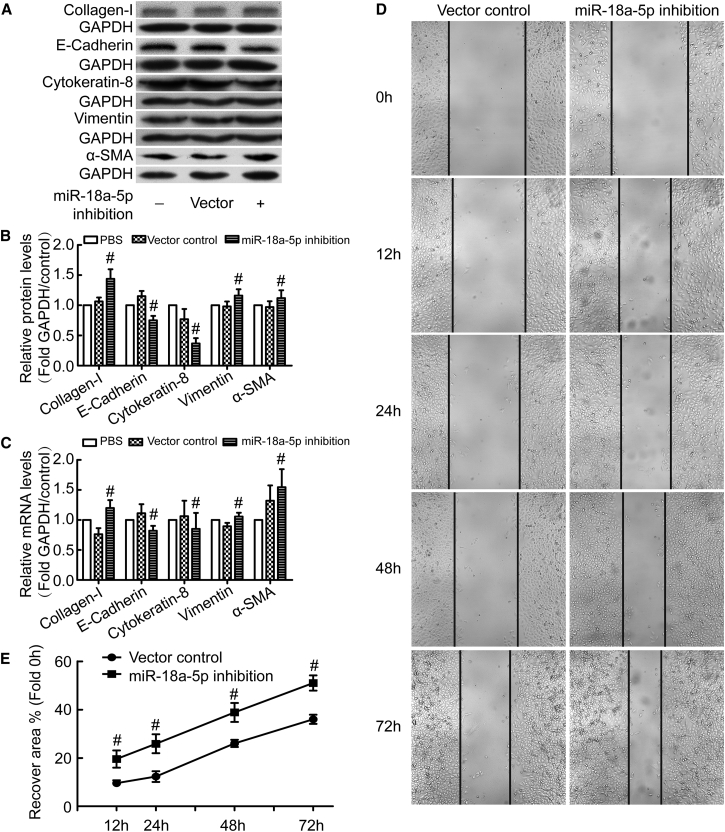
We previously reported that EMT of PMCs plays a pivotal role in sub-pleural pulmonary fibrosis.8 Here, we investigated the role of miR-18a-5p in EMT of PMCs. As shown in Figures 2A–2C, inhibition of miR-18a-5p using lentivirus-mediated expression of small interfering RNA (siRNA) against miR-18a-5p was associated with a decrease in the epithelial phenotypic markers E-cadherin and cytokeratin 8 and an increase in the mesenchymal phenotypic markers vimentin and α-smooth muscle actin (α-SMA). Moreover, inhibition of miR-18a-5p caused an increase in collagen I synthesis (Figures 2A–2C) and PMC migration (Figures 2D and 2E). These data argue that inhibition of miR-18a-5p induces EMT of PMCs and promotes PMC migration.
Figure 2.
miR-18a-5p Inhibition Induces EMT of PMCs and Promotes PMC Migration
PMCs were transduced with recombinant lentivirus encoding siRNA directed against miR-18a-5p (miR-18a-5p-inhibition) or scrambled negative controls (vector control). After 48 hr, protein and mRNA levels of E-cadherin, cytokeratin 8, vimentin, α-SMA, collagen I, and GAPDH were measured by western blot and qRT-PCR (A–C), and PMC migration was assessed in wound-healing assays (D and E). (A) Representative immunoblots. (B) Bar graphs depicting changes in relative density of E-cadherin (n = 5), cytokeratin 8 (n = 4), vimentin (n = 8), α-SMA (n = 10), and collagen I (n = 8). The density values of blots were normalized to the GAPDH and PBS controls. #p < 0.05 versus vector control. (C) Bar graphs depicting changes in relative mRNA levels of E-cadherin (n = 5), cytokeratin 8 (n = 6), vimentin (n = 7), α-SMA (n = 6), and collagen I (n = 5). #p < 0.05 versus vector control. (D) Representative image of a PMC wound-healing migration assay. (E) Line graph depicting changes in wound recovery area in percentage of the initial scratched area (n = 6). #p < 0.05 versus vector control.
Overexpression of miR-18a-5p Prevents Bleomycin-Induced EMT of PMCs
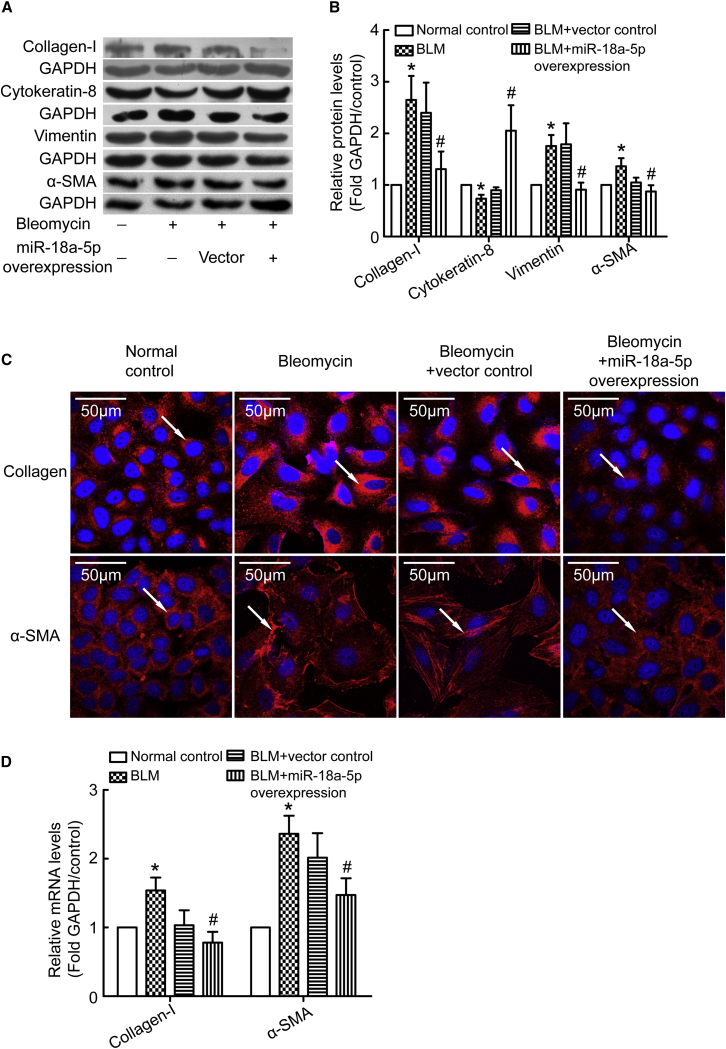
To further confirm the role of miR-18a-5p in bleomycin-induced EMT, we used recombinant lentivirus to overexpress miR-18a-5p in PMCs. Vector control or miR-18a-5p transduced PMCs were treated with bleomycin for 48 hr, and protein levels of cytokeratin 8, vimentin, α-SMA, and collagen I were measured. As shown in Figures 3A and 3B, bleomycin induced a decrease in cytokeratin 8 levels and an increase in vimentin, α-SMA, and collagen I levels. Overexpression of miR-18a-5p blocked the changes caused by bleomycin. Moreover, immunofluorescence staining and qRT-PCR determined that mRNA levels of α-SMA and collagen I correlated with western blotting analysis (Figures 3C and 3D). In addition, bleomycin promoted PMC migration (Figures S1A and S1B) and altered the typical spindle shape of mesenchymal cells (Figure S1C). Interestingly, overexpression of miR-18a-5p prevented bleomycin-induced increases in PMC migration and morphological changes (Figure S1). Using primary PMCs from rats, we also found that overexpression of miR-18a-5p prevented bleomycin-induced PMC EMT (Figure S2). These data provide evidence that overexpression of miR-18a-5p prevents bleomycin-induced EMT and cell migration of PMCs.
Figure 3.
Overexpression of miR-18a-5p Prevents Bleomycin-Induced EMT of PMCs
PMCs were transduced with recombinant lentivirus encoding miR-18a or a scrambled negative control mRNA (vector control). The cells were then treated with bleomycin (0.2 μg/mL) for 48 hr, after which protein levels, mRNA levels, and immunofluorescence staining were assessed. (A) Representative images of immunoblots of cytokeratin 8, vimentin, α-SMA, collagen I, and GAPDH. (B) Bar graphs depicting changes in relative density of cytokeratin 8 (n = 7), vimentin (n = 9), α-SMA (n = 10), and collagen I (n = 7). The density values of blots were normalized to the GAPDH and controls. *p < 0.05 versus control; #p < 0.05 versus vector control. (C) Representative images of immunofluorescence staining of collagen I and α-SMA. (D) Bar graphs depicting changes in relative mRNA levels of collagen I (n = 4) and α-SMA (n = 15), as determined by qRT-PCR analysis. *p < 0.05 versus control; #p < 0.05 versus vector control.
TGF-β1 Decreases miR-18a-5p Levels in PMCs
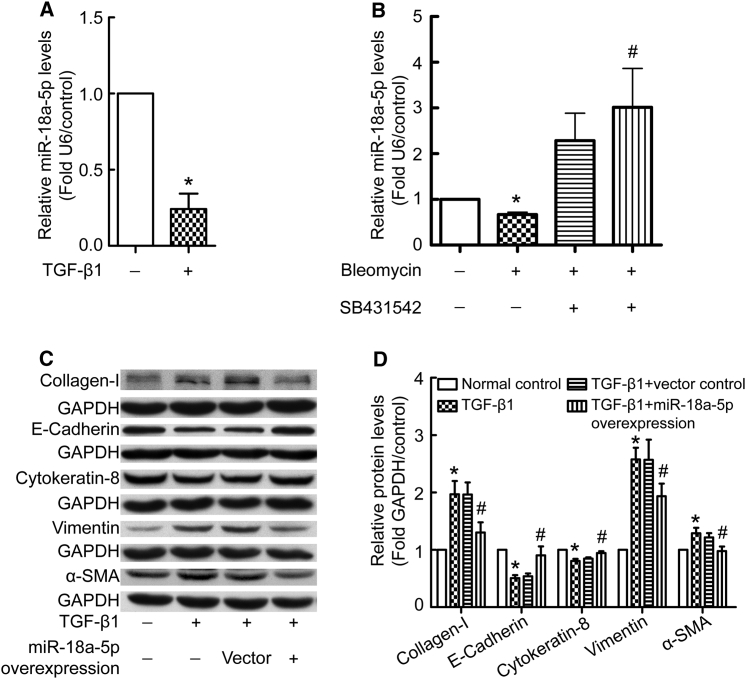
Because TGF-β1 is a master regulator of fibrogenesis, we evaluated the effect of TGF-β1 on miR-18a-5p expression. As shown in Figure 4A, incubation PMCs with TGF-β1 (5 ng/mL) for 24 hr resulted in a significant decrease in miR-18a-5p levels. Furthermore, blockage of TGF-β1 signaling using a TGF-β receptor inhibitor (SB431542, 10 μM) prevented bleomycin-induced reduction of miR-18a-5p levels (Figure 4B). These data suggest that bleomycin-induced downregulation of miR-18a-5p in PMCs might be mediated by TGF-β signaling. To further explore the relationship between TGF-β signaling and miR-18a-5p in the process of EMT, the effect of miR-18a-5p overexpression on TGF-β1 responses was assessed in PMCs. As shown in Figures 4C and 4D, TGF-β1 induced an increase in α-SMA, vimentin, and collagen I levels and a decrease in E-cadherin and cytokeratin 8 levels. However, overexpression of miR-18a-5p blocked these changes. These data provide evidence that TGF-β1 suppresses miR-18a-5p expression, while miR-18a-5p inhibits the EMT-promoting effects of TGF-β1.
Figure 4.
TGF-β1 Decreases miR-18a-5p Levels and Overexpression of miR-18a-5p Prevents TGF-β1-Induced EMT in PMCs
(A) PMCs were treated with or without TGF-β1 (5 ng/mL) for 24 hr, after which miR-18a-5p was analyzed using qRT-PCR (n = 9). *p < 0.05 versus control. (B) PMCs were treated by bleomycin (0.2 μg/mL) with or without TGF-β receptor inhibitor (SB431542, 10 μM) for 24 hr, after which miR-18a-5p was analyzed (n = 5). *p < 0.05 versus control; #p < 0.05 versus bleomycin group. (C and D) PMCs were transduced with recombinant lentivirus encoding miR-18a-5p or scrambled negative control mRNA (vector control). Cells were then treated with TGF-β1 (5 ng/mL) for 48 hr, after which protein levels were measured by western blotting. (C) Representative images of immunoblots. (D) Bar graphs depicting changes in the relative expression of E-cadherin (n = 5), cytokeratin 8 (n = 7), vimentin (n = 6), α-SMA (n = 5), and collagen I (n = 5). The density values of blots were normalized to the GAPDH and controls. *p < 0.05 versus control; #p < 0.05 versus vector control.
miR-18a-5p Regulates TGF-β-Smad2/3 Signaling in PMCs
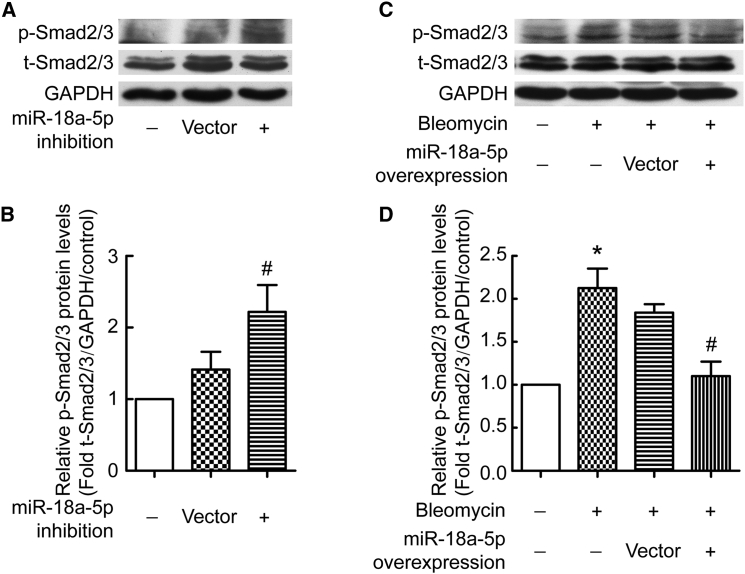
TGF-β-Smad signaling is the key pathway in EMT and fibrosis. To investigate whether miR-18a-5p is involved in TGF-β-Smad signaling to EMT in PMCs, we assessed the effects of manipulating miR-18a-5p expression on TGF-β-induced phospho-Smad (p-Smad). As shown in Figures 5A and 5B, lentivirus siRNA-mediated inhibition of miR-18a-5p was associated with an increase in p-Smad2/3 levels in PMCs. Moreover, lentivirus-mediated miR-18a-5p overexpression prevented bleomycin-induced increases in p-Smad2/3 levels in PMCs (Figures 5C and 5D). These data show that miR-18a-5p regulates the TGF-β-Smad2/3 signaling pathway in PMC EMT.
Figure 5.
miR-18a-5p Regulates Smad2/3 Phosphorylation in PMCs
(A and B) PMCs were transduced with recombinant lentivirus encoding siRNA directed against miR-18a-5p or scrambled negative control (vector control). After 48 hr, protein levels of p-Smad2/3, t-Smad2/3, and GAPDH were measured by western blotting. (C and D) PMCs were transduced with recombinant lentivirus encoding miR-18a-5p or scrambled negative control (vector control). The cells were treated with or without bleomycin (0.2 μg/mL) for 48 hr, after which protein levels of p-Smad2/3, t-Smad2/3, and GAPDH were measured by western blotting. (A and C) Representative images of immunoblots. (B and D) Bar graphs depicting changes in relative density according to (A) and (C). The density values of blots were normalized to the control (n = 9 in B; n = 7 in D). *p < 0.05 versus control; #p < 0.05 versus vector control.
miR-18a-5p Targets TGF-βRII
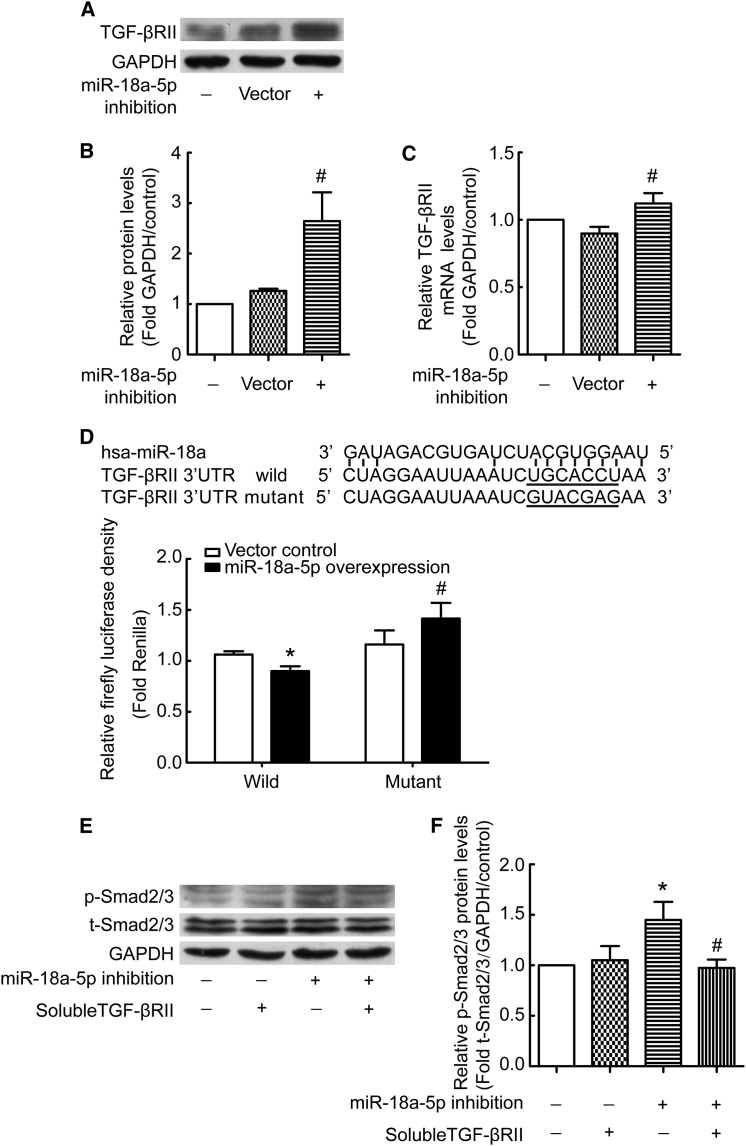
We made an effort to identify the target molecules of miR-18a-5p using TargetScan prediction software. The results indicated that TGF-βRII is highly likely to be a target gene. To confirm this result, we measured TGF-βRII expression in PMCs transduced with recombinant lentivirus encoding siRNA against miR-18a-5p or a scrambled negative control. The results showed that TGF-βRII protein and mRNA levels were higher in PMCs with miR-18a-5p inhibition than in controls (Figures 6A–6C). To determine whether miR-18a-5p directly regulates TGF-βRII gene expression, binding of miR-18a-5p to the 3′ UTR of the TGF-βRII gene was evaluated. Overexpression of miR-18a-5p was associated with significantly decreased luciferase activity when using a reporter vector containing wild-type TGF-βRII 3′ UTR sequences (Figure 6d). Mutation of the putative miR-18a-5p binding sequence in the TGF-βRII 3′ UTR of the reporter vector abolished the miR-18a-5p-associated decrease in luciferase activity (Figure 6D). Moreover, to validate that the observed increase in p-Smad2/3 levels was mediated by TGF-β-induced TGF-βRII signaling, exogenous soluble TGF-βRII was used to sequester TGF-β and thus compete with TGF-βRII in the cell membrane. Soluble TGF-βRII prevented the increase in p-Smad2/3 levels induced by miR-18a-5p inhibition (Figures 6E and 6F). These observations indicate that miR-18a-5p binds to the 3′ UTR of TGF-βRII mRNA and inhibits TGF-βRII gene expression.
Figure 6.
miR-18a-5p Targets TGF-βRII-Mediated Signaling to p-Smad2/3
(A–C) PMCs were transduced with recombinant lentivirus encoding siRNA directed against miR-18a-5p (inhibition) or scrambled negative control (vector). After 48 hr, protein and mRNA levels of TGF-β RII and GAPDH were measured by western blotting and qRT-PCR. (A) Representative images of immunoblots. (B) Bar graphs depicting changes in relative TGF-βRII levels according to (A) (n = 6). #p < 0.05 versus vector control. (C) Bar graphs depicting changes in relative TGF-βRII mRNA levels (n = 5). #p < 0.05 versus vector control. (D) Sequences of miR-18a-5p and the putative target sequence in the TGF-β RII mRNA (wild-type) or an engineered mutant of this sequence (mutant) for the luciferase activity assay (n = 3). *p < 0.05 versus wild-type control; #p < 0.05 versus mutant control. (E and F) PMCs were transduced with recombinant lentivirus encoding siRNA directed against miR-18a-5p (inhibition) or a scrambled negative control. Cells were then treated with or without soluble TGF-βRII for 24 hr, and protein levels of p-Smad2/3, t-Smad2/3, and GAPDH were measured by western blotting. (E) Representative images of immunoblots. (F) Bar graphs depicting changes in relative p-Smad2/3 levels according to (D). The density values of blots were normalized to the control (n = 7). *p < 0.05 versus control; #p < 0.05 versus miR-18a-5p inhibition group.
TGF-βRI and TGF-βRIII protein levels were also detected in PMCs, and bleomycin treatment induced increases in their expression. However, miR-18a-5p did not modulate their expression (Figure S3).
miR-18a-5p Inhibition Results in Murine Pulmonary Fibrosis
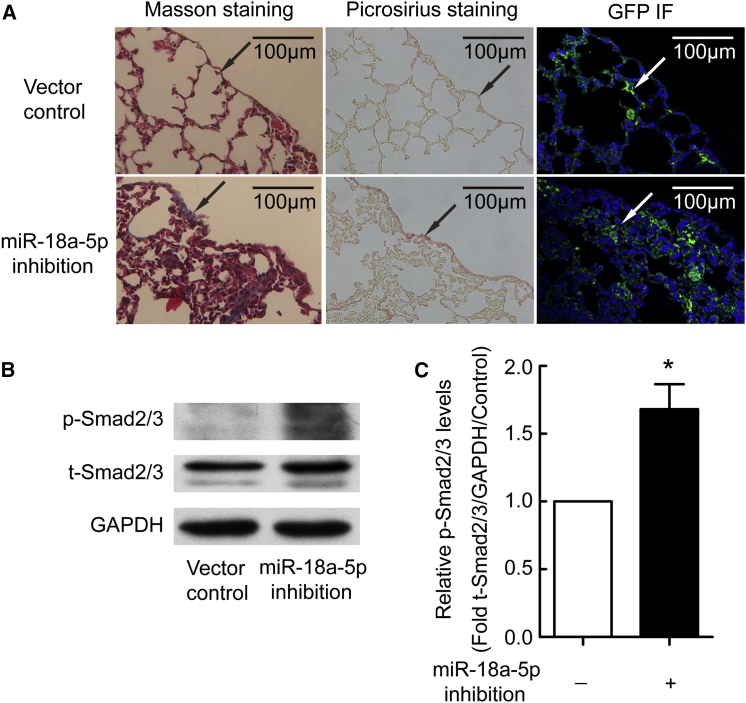
To determine the potential role of miR-18a-5p in the development of experimental sub-pleural pulmonary fibrosis, mice were treated by intraperitoneal injection of a lentivirus encoding siRNA directed against miR-18a-5p or scrambled negative control. As shown in Figure 7A, lungs of mice that received lentiviral vectors showed strong expression of the GFP reported gene, indicating that the siRNAs were efficiently expressed. Masson’s trichrome and picrosirius staining revealed that treatment with the miR-18a-5p inhibition vector was associated with induced sub-pleural fibrosis in mice (Figure 7A). Moreover, p-Smad2/3 levels were increased in lung tissue from miR-18a-5p siRNA-treated mice compared with control mice (Figure 7B). These data provide evidence that miR-18a-5p inhibition induces sub-pleural fibrosis and that p-Smad2/3 signaling was involved in this process.
Figure 7.
miR-18a-5p Inhibition Induces Murine Pulmonary Fibrosis
C57BL/6 mice were treated with lentivirus encoding siRNA directed against miR-18a-5p (inhibition) or vector control lentivirus by intraperitoneal injection at days 1, 3, 7, 10, 14, 21, 28, and 35. All mice were euthanized at day 40, and then lung tissues were taken for histological staining and western blot analysis. (A) Masson’s trichrome staining, picrosirius staining, and GFP immunofluorescence staining of lung tissues. (B and C) Western blotting images of p-Smad3 proteins and bar graphs depicting changes in relative expression levels. The density values of blots were normalized to the control (n = 4). *p < 0.05 versus control.
miR-18a-5p Overexpression Prevents Bleomycin-Induced Sub-pleural Fibrosis in Mice
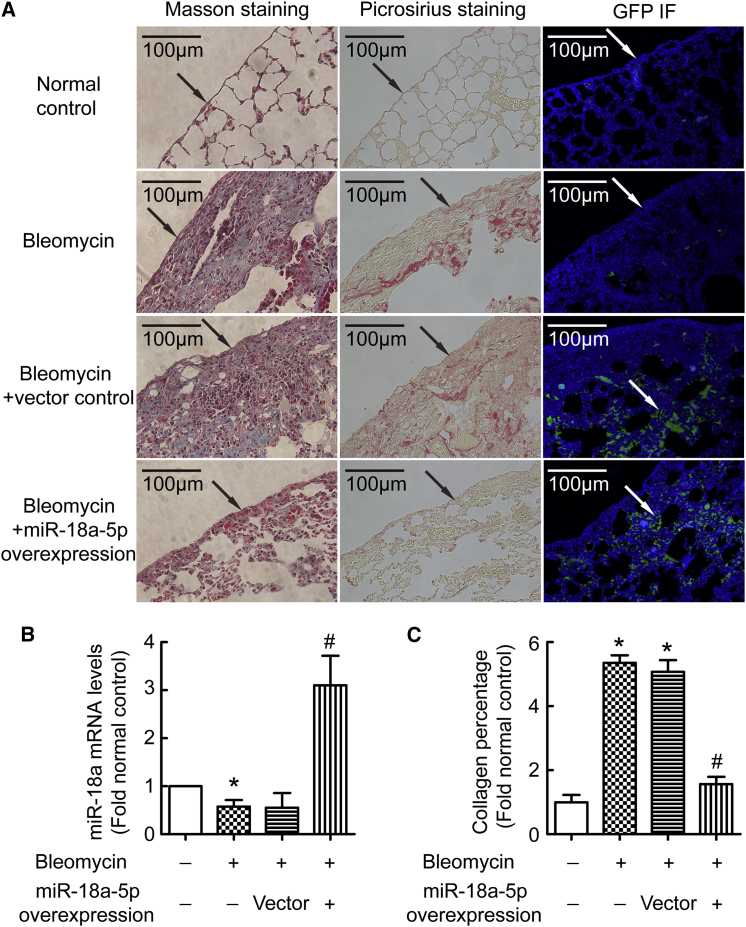
To further confirm the role of miR-18a-5p in pulmonary fibrosis in vivo, lentivirus expressing miR-18a-5p or scrambled negative control was injected intraperitoneally into mice at days 24, 26, and 28 after the first bleomycin administration. As shown in Figure 8A, lungs of mice receiving lentiviral vectors showed strong expression of the GFP reporter gene as assessed by immunofluorescence, indicating that miR-18a-5p or the scrambled sequence was efficiently expressed. Similar to the results in cultured cells, bleomycin induced downregulation of miR-18a-5p in vivo (Figure 8B). Expression of TGF-βRII and p-Smad2/3 levels were increased in fibrotic lung tissues, and these were inhibited by overexpression of miR-18a-5p (Figure S4). Masson’s trichrome and picrosirius staining showed that bleomycin induced pulmonary fibrosis, especially sub-pleural fibrosis, in mice. More importantly, miR-18a-5p overexpression attenuated pulmonary fibrosis and sub-pleural fibrosis in bleomycin-injected mice (Figures 8A and 8C).
Figure 8.
miR-18a-5p Overexpression Prevents Bleomycin-Induced Sub-pleural Fibrosis in Mice
C57BL/6 mice were treated with bleomycin (50 mg/kg) by intraperitoneal injection at days 1, 5, 8, 11, and 15. miR-18a-5p overexpressing or control lentivirus were intraperitoneal injected at a dose of 2 × 106 TU on days 24, 26, and 28. All mice were euthanized at day 40, and then lung tissues were taken for histological staining or immunostaining and miR-18a-5p qRT-PCR. (A) Masson’s trichrome staining, picrosirius staining, and GFP immunofluorescence staining of lung tissues. (B) miR-18a-5p levels were detected by qRT-PCR. (C) Changes in collagen percentages in the lung tissues according to (A) (n = 4). *p < 0.05 versus control; #p < 0.05 versus vector control.
Discussion
In this study, we found that bleomycin induced downregulation of miR-18a-5p in PMCs. Inhibition of miR-18a-5p induced PMC EMT, while overexpression of miR-18a-5p prevented bleomycin-induced PMC EMT. We also provide compelling evidence that miR-18a-5p regulated the TGF-β1-Smad2/3 EMT signaling pathway in PMCs by targeting TGF-βRII gene expression. Furthermore, miR-18a-5p inhibition caused sub-pleural fibrosis in vivo, and overexpression of miR-18a-5p attenuated bleomycin-induced sub-pleural fibrosis in mice. These data represent the first evidence that miR-18a-5p is reduced in bleomycin-treated PMCs and that the downregulation of miR-18a-5p contributes to PMC EMT, thereby mediating sub-pleural pulmonary fibrosis.
The role of PMCs in the pathogenesis of pulmonary fibrosis has recently received a great deal of attention. Studies indicate that PMC trafficking into lung parenchyma is involved in IPF sub-pleural fibrosis.3 Nasreen et al.9 reported that TGF-β1 induces PMC EMT, and PMCs are a source of myofibroblasts in IPF. PMCs undergoing EMT produce large amount of collagen and contribute to the over-deposition of ECM in sub-pleural fibrosis.8 Our previous study revealed that bleomycin induces EMT through TGF-β1-Smad2/3 signaling in PMCs, contributing to sub-pleural fibrosis.8 Thus, these studies from both IPF patients and pulmonary fibrosis animal models indicate that PMCs undergoing EMT contribute to sub-pleural fibrosis. The results of the present study show that miR-18a-5p inhibition mediates bleomycin-induced PMC EMT. To the best of our knowledge, this is the first study to report that miR-18a-5p is a regulator of PMC EMT.
miRNAs play an important role in the posttranscriptional control of gene expression that is dysregulated in different physiological pathophysiological processes, including metabolism, growth, cell differentiation and development, apoptosis, inflammation, and cell signaling.25 The role of miRNAs in the pathogenesis of IPF has recently been recognized. Dakhlallah et al.26 reported that downregulation of miR-17∼92 contributes to the pathogenesis of pulmonary fibrosis. Notably, miR-18a levels are extraordinarily decreased in the lung of human IPF patients.17, 26 However, the mechanisms involved in the regulatory effects of miRNA in pulmonary fibrosis have not been revealed. Here, we show that bleomycin induced downregulation of miR-18a-5p and that this causes activation of Smad2/3 in both lung tissues of animal models and cultured PMCs. Overexpression of miR-18a-5p blocks phosphorylation of Smad2/3 induced by bleomycin. Thus, the TGF-β-Smad2/3 pathway is implicated in PMC EMT induced by miR-18a-5p inhibition. Overexpression of miR-18a-5p prevents activation of TGF-β-Smad2/3 signaling and inhibits pulmonary fibrosis. Xiao and colleagues reported that miR-29 was a downstream target gene of Smad3 and was negatively regulated by TGF-β-Smad signaling in bleomycin-induced lung fibrosis.27 Their data, in addition to our own, indicate that there is crosstalk between specific miRNAs and TGF-β-Smad signaling in fibrosis.
TGF-βRII is a transmembrane serine-threonine kinase receptor necessary for TGF-β1 signal transduction,28 and it has been implicated in lung development and pulmonary fibrotic disease.29 It has been shown that activation of TGF-β-Smad signaling induces the expression of specific miRNAs that, in turn, may modulate TGF-β-Smad signaling in a feedback manor.30, 31, 32 For example, TGF-β1 induces miR-9-5p expression, while overexpression of miR-9-5p in lung fibroblasts inhibits TGF-βRII expression and prevents myofibroblast differentiation, ECM deposition, and organ fibrogenesis.31 Here, we found that TGF-β1 induces a decrease in miR-18a-5p. In order to clarify how miR-18a-5p regulates TGF-β-Smad signaling, we first measured TGF-β1. We found that miR-18a-5p did not affect TGF-β1 expression (data not shown). Nevertheless, we found that TGF-βRII expression is much higher in PMCs after miR-18a-5p inhibition and that miR-18a-5p targets the 3′ UTR of TGF-βRII mRNA and inhibits its expression. Thus, our results indicate that when miR-18a-5p was suppressed by bleomycin, the inhibitory effect of miR-18a-5p on TGF-βRII expression was decreased, TGF-βRII was upregulated, and TGF-β-Smad2/3 signaling was enhanced.
Finally, we determined a role for miR-18a-5p in a bleomycin-induced pulmonary fibrosis animal model. Consistent with in vitro data, miR-18a-5p was suppressed in fibrotic lungs, and TGF-βRII upregulation and enhanced TGF-β-Smad2/3 signaling were also confirmed in this mouse model. We also show that miR-18a-5p inhibition caused sub-pleural fibrosis in the absence of bleomycin challenge and that overexpression of miR-18a-5p attenuated bleomycin-induced sub-pleural fibrosis in vivo.
In summary, our findings provide evidence that bleomycin reduces miR-18a-5p expression in PMCs. miR-18a-5p regulates the TGF-β1-Smad2/3 signaling pathway by suppressing TGF-βRII expression in PMC EMT and sub-pleural pulmonary fibrosis. This provides proof of concept that therapeutic overexpression of miR-18a-5p might be a novel approach in the treatment of pulmonary fibrotic diseases, such as IPF.
Materials and Methods
Cell Culture
The human PMC line MeT-5A was purchased from the American Type Culture Collection (ATCC). PMCs were cultured in RPMI 1640 medium (Hyclone) supplemented with 20% fetal bovine serum (FBS) and 5% CO2, 95% air at 37°C. The cells were sub-cultured at 1:3 ratios, and culture medium was changed every 2 days. Cells equilibrated overnight in medium containing 2% FBS were used for all experiments.
Isolation and Primary Culture of Rat PMCs
Primary PMCs were isolated from rat pleura with pronase E digestion as described previously.33 In brief, the whole thorax was isolated under sterile conditions after 1% pronase E in RPMI-1640 medium injected into thoracic cavity and then digested at 4°C overnight. PMCs were harvested and centrifuged at 1,000 rpm for 5 min. The cells were resuspended in culture medium (Cell Biologics) and then incubated and treated in the same way as Met-5A cells.
miRNA Expression Analysis
miRNA microarray analysis was performed by the Bioassay Laboratory of CapitalBio Corporation. Total RNA of PMCs treated with and without bleomycin for 24 hr was extracted using TRIzol Reagent (Ambion, Life Technologies). miRNA was then enriched by using the mirVana miRNA Isolation Kit (Sigma-Aldrich). A miRNA Complete Labeling and Hyb Kit (Agilent Technologies) was used for sample labeling and hybridization. Data were processed using Agilent Feature Extraction software (Agilent) and analyzed using Agilent GeneSpring software (Agilent).
Quantification of miR-18a-5p
miR-cDNAs were synthesized from total RNA by reverse transcription using a One Step PrimeScript miRNA cDNA Synthesis Kit (Takara Bio) according to the manufacturer’s instructions. miR-cDNAs were then used for amplification by RT-PCR in a 25-μL reaction using SYBR Premix EX Taq II (Takara Bio). miR-18a-5p expression was normalized with U6. All primers were obtained from Takara Bio.
miRNA-18a-5p Inhibition and Overexpression
Inhibition of miR-18a-5p in PMCs and animal models was achieved using recombinant lentivirus encoding an siRNA directed against miR-18a-5p (siRNA sequence 5′-CTATCTGCACTAGATGCACCTTA-3′). Overexpression was achieved using a recombinant lentivirus encoding miR-18a-5p. Control lentiviruses expressed scrambled sequence RNAs. All recombinant lentivirus vectors were made by Genechem.
Transduction of cells with lentiviruses was performed according to the manufacturers’ instructions. PMCs were seeded into 60-mm dishes at 20%–30% confluence and transduced with lentivirus (1 × 108 TU/mL) to achieve an MOI of 5. Cells were incubated in CO2 incubator for 12 hr, and the medium was then replaced with fresh RPMI-1640 containing 20% FBS. Transduction efficiency was monitored by detecting expression of the GFP reporter gene by fluorescence microscopy. Cells were used for experiments after 3 days.
Western Blot Analysis
Cell lysates or lung tissue lysates (10–20 μg protein) were denatured and electrophoresed on SDS-PAGE gel. Separated proteins were electro-transferred to nitrocellulose membranes. The membranes were incubated with 5% nonfat milk for 1 hr; incubated with antibodies against collagen I, E-cadherin, cytokeratin 8, vimentin, α-SMA, p-Smad, total-Smad (t-Smad), TGFβRII, or GAPDH overnight at 4°C; and then washed with 0.1% Tween-20, 20 mM Tris-HCl (pH 7.5), and 150 mM NaCl (TTBS) three times for 10 min. Secondary antibody immunoglobulin G (IgG) conjugated to alkaline phosphatase was diluted in TTBS plus 5% nonfat milk and incubated with the membranes at room temperature for 1 hr. The membranes were washed with TTBS and then developed with Supersignal West Pico (Pierce) for 3 min before exposure to film (Kodak). Films were developed using Kodak Medical X-ray processor 102 (Kodak) to visualize the reactive proteins followed by densitometric quantification using Image-Pro Plus software (Media Cybernetics).
Real-Time qRT-PCR
After treatment, total cellular RNA was extracted using TRIzol reagent. Levels of collagen I, E-cadherin, cytokeratin 8, vimentin, α-SMA, and TGF-βRII mRNA were determined by real-time qRT-PCR. RNA in 1 μg of each sample was reverse transcribed, and real-time RT-PCR was performed under the following conditions: 95°C for 30 s, 40 cycles of 95°C for 10 s, and 60°C for 20 s. The following PCR primers were used: COL1A1 forward, 5′-TAGGGTCTAGACATGTTCAGCTTTGT-3′; COL1A1 reverse, 5′-GT GATTGGTGGGATGTCTTCGT-3′; E-cadherin forward, 5′-CGAGAGCTACACGTTCACGG-3′; E-cadherin reverse, 5′-GGGTGTCGAGGGAAAAATAGG-3′; cytokeratin 8 forward, 5′-CAGAAGTCCTACAAGGTGTCCA-3′; cytokeratin 8 reverse, 5′-CTCTGGTTGACCGTAACTGC G-3′; vimentin forward, 5′-GACGCCATCAACACCGAGTT-3′; vimentin reverse, 5′-CTTTGTCGTTGGTTAGCTGGT-3′; α-SMA forward, 5′-GTGTTGCCCCTGAAGAGCAT-3′; α-SMA reverse, 5′-GCTGGGACATTGAAAGTCTCA-3′; TGF-βRII forward, 5′-GTAGCTCTGATGAGTGCAATGAC-3′; TGF-βRII reverse, 5′-CAGATATGGCAACTCCCAGTG-3′; GAPDH forward, 5′-GGAGTCCACTGGCGTCTTCA-3′; and GAPDH reverse, 5′-GTCATGAGTCCTTCCACGATACC-3′. The results were expressed as 2−ΔΔCT using GAPDH as a reference.
Immunofluorescence Staining of PMCs
To determine the intracellular localization of and changes in collagen I and α-SMA, PMCs were incubated with bleomycin (0.2 μg/mL) for 48 hr. Then the cells were stained using antibodies against collagen I or α-SMA at 4°C overnight and then with tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit antibody for 30 min. The nuclei were stained for DAPI for 10 min in the dark. Labeled cells were examined using a fluorescence microscope (Olympus FV500, Olympus).
In Vitro Wound-Healing Assay of PMC Migration
Confluent monolayers of PMCs in six-well plates were scratched using the tip of a p-200 pipette to create a uniform cell-free zone in each well. Cellular debris was removed by washing with PBS. Wounded monolayers were then incubated in the presence or absence of bleomycin. Microscopic images were taken with a digital camera at different time points after wounding. The recovered area was measured with a computer-assisted image analysis system (Image-Pro Plus software) and expressed as a percentage of the initial scratched area. At the same time, morphologic changes in PMCs were observed by microscopy.
Bleomycin-Induced Murine Pulmonary Fibrosis Model
This protocol was approved by the Institutional Animal Care and Use Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Pulmonary fibrosis was induced by bleomycin as described previously by us.8, 11 Briefly, C57BL/6 mice (6–8 weeks of age) were housed under standard conditions with free access to water and rodent laboratory food. The mice were divided into four groups (normal control, bleomycin, bleomycin plus vector control, and bleomycin plus miR-18a-5p overexpression). Bleomycin dissolved in saline solution (5 mg/mL) was administrated by intraperitoneal injection at a dose of 50 mg/kg at days 1, 5, 8, 11, and 15. Lentivirus encoding miR-18a-5p was administered by intraperitoneal injection at a dose of 2 × 106 transducing units (TU) on days 24, 26, and 28. All mice were euthanized after 40 days, and then lung tissues were taken for RNA and histological analysis.
miR-18a-5p Inhibition-Induced Murine Pulmonary Fibrosis Model
C57BL/6 mice (age 6–8 weeks) were divided into two groups (vector control and miR-18a-5p inhibition). Lentivirus expressing an siRNA directed against miR-18a-5p was administrated by intraperitoneal injection at a dose of 2 × 106 TU on days 1, 3, 7, 10, 14, 21, 28 and 35. All mice were euthanized after 40 days, and then lung tissues were taken for protein and histological analysis.
Dual Luciferase Assay
The 206-nt sequence of the wild-type TGF-βRII (NM_003242) 3′ UTR containing the putative seed binding sequence for miR-18a-5p (TGCACCT) was synthesized and sub-cloned into a GV306 dual-luciferase miRNA-target expression vector (Genechem) between the firefly and Renilla luciferase coding sequences. A control was generated with a mutation of the miR-18a-5p binding sequence in the TGFβ−RII 3′ UTR that changed the seed binding sequence to GTACGAG (Genechem). We first established stably overexpressed miR-18a-5p cell line by transducing HEK293T cells with lentivirus expressing mir-18a-5p and vector control. Cells were then seeded in 24-well plates for 80% confluence and transfected with 1 μg per well of GV306-wt-TGFBR2-3′UTR or GV306-mut-TGFβRII-3′UTR vectors using lipofectamine 2000. Cells were harvested after 24 hr, and luciferase activity was measured using a dual-luciferase reporter assay system (Promega) with a Synergy 2 Multi-Mode microplate reader (BioTek Instruments). Relative expression of firefly and Renilla luciferase was determined.
Statistical Analysis
Results are shown as the mean ± SEM. Control and experimental cells were matched for cell line, age, seeding density, number of passages, and number of post-confluence days. Differences between groups were analyzed using unpaired t tests or two-way analysis of variance. A p value less than 0.05 was considered to be statistically significant.
Author Contributions
W.-L.M., H.Y., J.-B.X., and Q.Z. conceived and designed the experiments. Q.Z., H.Y., F.X., L.-J.S., L.-L.Z., and P.-C.C. performed the experiments. H.Y., J.-C.Z., F.Y., H.-Z.S., and Y.S. analyzed the data. W.-L.M., Y.S., and J.-B.X. wrote the paper. The authors thank Professor Peter A. Greer at Queen’s University (Cancer Research Institute, Kingston, Ontario, Canada) for his help in editing the revision of this manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (grants 81300047, 81370186, 81573485, 91643101, 31271490, 81570087, 81200020, and 81470257) and the 12th Five-Year National Science and Technology Program of Social Development, Ministry of Science and Technology of China (grant 2012BAI05B02).
Footnotes
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.12.017.
Contributor Information
Jian-Bao Xin, Email: 814643835@qq.com.
Wan-Li Ma, Email: whmawl@aliyun.com.
Supplemental Information
References
- 1.King T.E., Jr., Brown K.K., Raghu G., du Bois R.M., Lynch D.A., Martinez F., Valeyre D., Leconte I., Morganti A., Roux S., Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G., Collard H.R., Egan J.J., Martinez F.J., Behr J., Brown K.K., Colby T.V., Cordier J.F., Flaherty K.R., Lasky J.A., ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mubarak K.K., Montes-Worboys A., Regev D., Nasreen N., Mohammed K.A., Faruqi I., Hensel E., Baz M.A., Akindipe O.A., Fernandez-Bussy S. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur. Respir. J. 2012;39:133–140. doi: 10.1183/09031936.00141010. [DOI] [PubMed] [Google Scholar]
- 4.Bartis D., Mise N., Mahida R.Y., Eickelberg O., Thickett D.R. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 5.Batra H., Antony V.B. Pleural mesothelial cells in pleural and lung diseases. J. Thorac. Dis. 2015;7:964–980. doi: 10.3978/j.issn.2072-1439.2015.02.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis B.C., Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 7.Batra H., Antony V.B. The pleural mesothelium in development and disease. Front. Physiol. 2014;5:284. doi: 10.3389/fphys.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L.J., Ye H., Zhang Q., Li F.Z., Song L.J., Yang J., Mu Q., Rao S.S., Cai P.C., Xiang F. Bleomycin induced epithelial-mesenchymal transition (EMT) in pleural mesothelial cells. Toxicol. Appl. Pharmacol. 2015;283:75–82. doi: 10.1016/j.taap.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Nasreen N., Mohammed K.A., Mubarak K.K., Baz M.A., Akindipe O.A., Fernandez-Bussy S., Antony V.B. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L115–L124. doi: 10.1152/ajplung.90587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouratis M.A., Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr. Opin. Pulm. Med. 2011;17:355–361. doi: 10.1097/MCP.0b013e328349ac2b. [DOI] [PubMed] [Google Scholar]
- 11.Li F.Z., Cai P.C., Song L.J., Zhou L.L., Zhang Q., Rao S.S., Xia Y., Xiang F., Xin J.B., Greer P.A. Crosstalk between calpain activation and TGF-β1 augments collagen-I synthesis in pulmonary fibrosis. Biochim. Biophys. Acta. 2015;1852:1796–1804. doi: 10.1016/j.bbadis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R., Muthusamy B.P., Saeteurn K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuxe J., Vincent T., Garcia de Herreros A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 15.Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 16.Lee R.C., Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 17.Pandit K.V., Corcoran D., Yousef H., Yarlagadda M., Tzouvelekis A., Gibson K.F., Konishi K., Yousem S.A., Singh M., Handley D. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das S., Kumar M., Negi V., Pattnaik B., Prakash Y.S., Agrawal A., Ghosh B. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2014;50:882–892. doi: 10.1165/rcmb.2013-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang H., Gu Y., Li T., Zhang Y., Huangfu L., Hu M., Zhao D., Chen Y., Liu S., Dong Y. Integrated analyses identify the involvement of microRNA-26a in epithelial-mesenchymal transition during idiopathic pulmonary fibrosis. Cell Death Dis. 2014;5:e1238. doi: 10.1038/cddis.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang H., Xu C., Pan Z., Zhang Y., Xu Z., Chen Y., Li T., Li X., Liu Y., Huangfu L. The antifibrotic effects and mechanisms of microRNA-26a action in idiopathic pulmonary fibrosis. Mol. Ther. 2014;22:1122–1133. doi: 10.1038/mt.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S., Banerjee S., de Freitas A., Sanders Y.Y., Ding Q., Matalon S., Thannickal V.J., Abraham E., Liu G. Participation of miR-200 in pulmonary fibrosis. Am. J. Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J., Kaminski N., Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lino Cardenas C.L., Henaoui I.S., Courcot E., Roderburg C., Cauffiez C., Aubert S., Copin M.C., Wallaert B., Glowacki F., Dewaeles E. miR-199a-5p Is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet. 2013;9:e1003291. doi: 10.1371/journal.pgen.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang S., Cui H., Xie N., Icyuz M., Banerjee S., Antony V.B., Abraham E., Thannickal V.J., Liu G. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–2391. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimi A., Sadroddiny E. MicroRNAs in lung diseases: Recent findings and their pathophysiological implications. Pulm. Pharmacol. Ther. 2015;34:55–63. doi: 10.1016/j.pupt.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Dakhlallah D., Batte K., Wang Y., Cantemir-Stone C.Z., Yan P., Nuovo G., Mikhail A., Hitchcock C.L., Wright V.P., Nana-Sinkam S.P. Epigenetic regulation of miR-17∼92 contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013;187:397–405. doi: 10.1164/rccm.201205-0888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao J., Meng X.M., Huang X.R., Chung A.C., Feng Y.L., Hui D.S., Yu C.M., Sung J.J., Lan H.Y. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol. Ther. 2012;20:1251–1260. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 29.Li M., Krishnaveni M.S., Li C., Zhou B., Xing Y., Banfalvi A., Li A., Lombardi V., Akbari O., Borok Z., Minoo P. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J. Clin. Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abonnenc M., Nabeebaccus A.A., Mayr U., Barallobre-Barreiro J., Dong X., Cuello F., Sur S., Drozdov I., Langley S.R., Lu R. Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c. Circ. Res. 2013;113:1138–1147. doi: 10.1161/CIRCRESAHA.113.302400. [DOI] [PubMed] [Google Scholar]
- 31.Fierro-Fernández M., Busnadiego Ó., Sandoval P., Espinosa-Díez C., Blanco-Ruiz E., Rodríguez M., Pian H., Ramos R., López-Cabrera M., García-Bermejo M.L., Lamas S. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 2015;16:1358–1377. doi: 10.15252/embr.201540750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García R., Nistal J.F., Merino D., Price N.L., Fernández-Hernando C., Beaumont J., González A., Hurlé M.A., Villar A.V. p-SMAD2/3 and DICER promote pre-miR-21 processing during pressure overload-associated myocardial remodeling. Biochim. Biophys. Acta. 2015;1852:1520–1530. doi: 10.1016/j.bbadis.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Yang J., Xiang F., Cai P.C., Lu Y.Z., Xu X.X., Yu F., Li F.Z., Greer P.A., Shi H.Z., Zhou Q. Activation of calpain by renin-angiotensin system in pleural mesothelial cells mediates tuberculous pleural fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;311:L145–L153. doi: 10.1152/ajplung.00348.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.