Abstract
Cystic fibrosis (CF) is a fatal recessive genetic disorder caused by a mutation in the gene encoding CF transmembrane conductance regulator (CFTR) protein. Alteration in CFTR leads to thick airway mucus and bacterial infection. Cell therapy has been proposed for CFTR restoration, but efficacy has been limited by low engraftment levels. In our previous studies, we have shown that using a pre-conditioning regimen in combination with optimization of cell number and time of delivery, we could obtain greater bone marrow cell (BMC) retention in the lung. Here, we found that optimized delivery of wild-type (WT) BMC contributed to apical CFTR expression in airway epithelium and restoration of select ceramide species and fatty acids in CFTR−/− mice. Importantly, WT BMC delivery delayed Pseudomonas aeruginosa lung infection and increased survival of CFTR−/− recipients. Only WT BMCs had a beneficial effect beyond 6 months, suggesting a dual mechanism of BMC benefit: a non-specific effect early after cell delivery, possibly due to the recruitment of macrophages and neutrophils, and a late beneficial effect dependent on long-term CFTR expression. Taken together, our results suggest that BMC can improve overall lung function and may have potential therapeutic benefit for the treatment of CF.
Keywords: cell therapy, lung regeneration, CFTR
Cell and gene therapies are commonly used to treat various conditions and improve a patient’s daily life. In this issue of Molecular Therapy, Duchesneau et al. propose a method for lung epithelial replacement leading to wild-type CFTR expression and improved overall lung function.
Introduction
Cystic fibrosis (CF) is the most prevalent lethal genetic disorder among the Caucasian population and is caused by a mutation in the CF transmembrane receptor (CFTR) gene.1 Alteration in this cyclic-AMP-activated chloride transport channel affects the lung, digestive tract, reproductive system, and other organs. In the lung, thick mucus accumulates in the airways, hindering normal clearance and promoting pathogen growth. This leads to chronic infection and respiratory complications, which are the main causes of mortality and morbidity. Pseudomonas aeruginosa is a common pathogen infecting CF patients and has been shown to be a marker of poor survival.2, 3
After the cloning of the CFTR gene in 1989, animal models were developed, but in CFTR−/− mice, the typical CF lung phenotype, such as airway mucus plugging, was not observed.4, 5, 6 This was believed to be the result of a redundant chloride transport channel in murine lungs. After further investigations, a few subtle differences between wild-type (WT) and CFTR−/− mice were reported. These include congenital tracheal malformation, a thick layer of material covering the epithelium observed by electron microscopy, and increased non-ciliated airway cells.7, 8 Sphingolipids and fatty acid imbalance have also been observed in CF patients and CFTR−/− mice.9, 10, 11 Ceramides are a sphingolipid part of the plasma membrane and have been reported to mediate inflammation in the airways. Ceramide alterations have been shown in CFTR−/− mice, although no consensus has been reached as to which direction. Some groups showed an accumulation of ceramide in the airways by antibody staining, while others showed decreased ceramide levels by mass spectrometry (MS).12, 13 Alterations of fatty acids, such as increases in phospholipid-bound arachidonic acid (AA) and decreases in phospholipid-bound docosahexaenoic acid (DHA) have also been well described.9 Restoration of lipid balance in CFTR−/− mice has a beneficial effect on the overall inflammatory process.11, 13, 14
While lung transplantation remains the most viable treatment for end-stage lung disease, every year, patients die waiting for suitable donors.15 Early genetic screening for CF means that the structure of the lungs could be relatively intact at the time of diagnosis, making cell replacement therapy and correction of the underlying genetic defect possible.16 Cell therapy has made great progress in recent years, using organ preconditioning regimens to enhance cell engraftment.17, 18, 19 In the long term, it could be a less invasive alternative to transplantation, while in the short term, it could be an interim treatment option, easing the organ supply shortage. A variety of exogenous cells could be used for treatment; however, gene-corrected autologous cells are ideal to minimize rejection by the host immune system.20 Even partial replacement of the CF airway epithelium would be greatly beneficial, since in vitro studies showed improved chloride transport with as little as 6%–10% restoration of CFTR−/− cells.21 Other groups attempted to correct CFTR deficiency in vivo, using various cell types and delivery methods, but the low engraftment levels were not sufficient to substantially impact CFTR function.22, 23
We have previously shown that a heterogeneous bone marrow cell (BMC) population increases its club cell secretory protein (CCSP) expression while in culture for 7 days. These BMCs were capable of enhancing regeneration of naphthalene-damaged airways.24, 25 Here, we show that a similar non-fractionated, plastic-adherent BMC population can repopulate the lung over a sustained period and result in CFTR expression in airway epithelium. In addition, WT BMCs can restore select ceramide species and fatty acids, improve bacterial clearance, and increase the survival of CFTR−/− recipient mice when assessed as much as 6 months after treatment of the lungs. Delivery of CFTR−/− BMCs only had beneficial effects on bacterial clearance for a short period, which did not last 6 months. These findings suggest that BMCs may regenerate the airway epithelium in CFTR−/− mice, partially restore the CF genotype, and significantly impact the CF phenotype and lung function.
Results
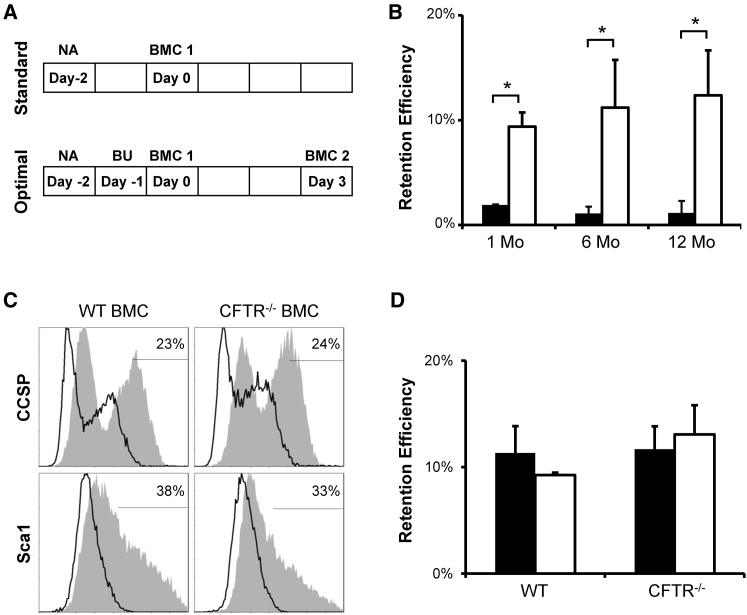
Optimal BMC Delivery Leads to Improved Long-Term Cell Retention in the Lung
We previously optimized various parameters of our cell-therapy regimen, such as cell number, route of delivery, and details of the conditioning regimen, such as naphthalene and busulfan dosage, which resulted in improved cell retention in the lung.24, 25 To determine whether improved retention of BMCs in the lung could be long lasting, we compared our standard and optimized delivery protocols over a period of 1 year. As described previously,25 C57BL/6 female mice were preconditioned with naphthalene (200 mg/kg) alone to deplete CCSP cells (standard),24 or in combination with busulfan (30 mg/kg) to suppress endogenous BMC recruitment (optimal),26 before receiving 1 million male BMCs transtracheally once (standard) or twice (optimal) (Figure 1A). BMC retention efficiency was significantly increased following our optimal protocol at 1 month and persisted at 6 and 12 months (Figure 1B). Experimental samples were evaluated by Y-chromosome quantitative real-time PCR (YqRT-PCR) compared to standard samples containing known amounts of male cells mixed with female lung tissue. Retention efficiency is represented as the percentage of total cells delivered (1 million) compared to known standards. To determine whether BMCs could be used for CF correction in the lung, we adapted our optimal protocol to the CFTR−/− genetically modified animal model (a mixed background containing C57Bl6, 129, and FVB/N). CFTR−/− BMCs, cultured on plastic for 7 days, and those from their WT littermate controls both had similar expression of the epithelial and progenitor markers, CCSP and Sca1 (Figure 1C). Due to the increased mortality of the CFTR−/− homozygous mice following naphthalene and busulfan preconditioning, the optimal dosage for busulfan was reduced to 20 mg/kg for this strain. Retention efficiency analysis using YqRT-PCR showed sustained donor retention at 1 and 6 months after cell delivery of WT or CFTR−/− BMCs in optimally preconditioned CFTR−/− female recipients (Figure 1D). Retention efficiencies obtained with CFTR−/− mice were similar to that seen in the C57BL/6 strain (Figure 1B), and CFTR correction was investigated using this model.
Figure 1.
Optimal BMC Delivery Leads to Improved Long-Term Cell Retention in the Lung
(A) Standard and optimal protocol schematics for transtracheal delivery of BMC to naphthalene (NA) and busulfan (BU) preconditioned female recipient. (B) Y-chromosome quantitative real-time PCR analysis of C57BL/6 female recipient lungs shows increased donor BMC retention at 1, 6, and 12 months (Mo) in mice preconditioned by optimal protocol (white) when compared to standard protocol (black). (C) Flow cytometry histogram of CCSP and Sca1 expression from 7-day-cultured WT or CFTR−/− BMCs shows a similarity between the two cultures. (D) Optimal preconditioning of female CFTR−/− recipients resulted in WT or CFTR−/− male BMC retention in recipient lungs at 1 month (black) and 6 months (white) in the same range as in C57BL/6 recipients. Retention efficiency is represented as the percentage of total cells delivered (1 million) compared to known standards. The error bars represent SEM (n ≥ 4 mice per group). *p < 0.05, by one-way ANOVA and Tukey post-test.
CFTR Is Detected in CFTR−/− Lungs after WT BMC Delivery
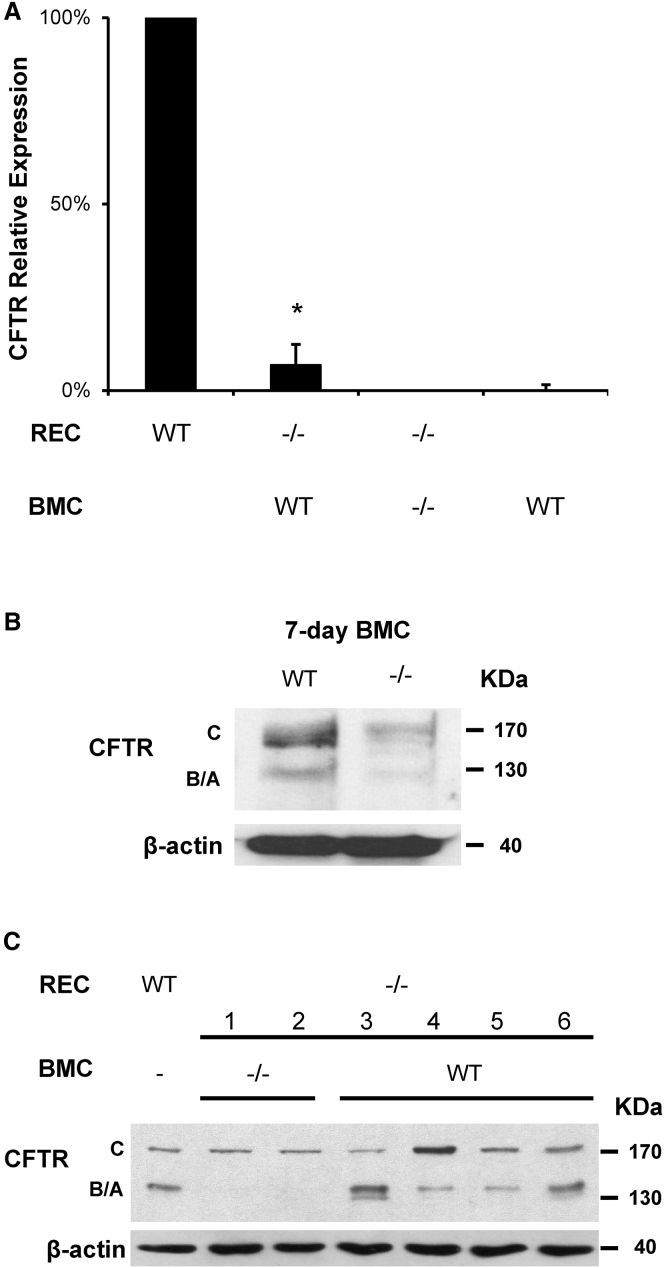
To determine whether WT BMCs could restore function in CFTR−/− mice, WT or CFTR−/− BMCs were cultured for 7 days and delivered transtracheally to naphthalene- and busulfan-preconditioned CFTR−/− recipients. After 1 month, the lungs were harvested and assayed for CFTR mRNA and protein. CFTR−/− recipients treated with WT BMCs expressed CFTR at 6.9% ± 5.5% of the WT level, while those treated with CFTR−/− BMCs had no detectable mRNA (Figure 2A). BMCs cultured for 7 days had a relative CFTR mRNA expression of ∼0.1%. Western blot analysis of 7-day-cultured BMCs revealed a significantly lower detection of CFTR bands (C, B, and A) in CFTR−/− compared to WT cells (Figure 2B). An analysis of lung lysates also showed three CFTR bands in WT lungs (Figure 2C). Only band C was detected in CFTR−/− mice that received CFTR−/− BMCs (mice 1 and 2), as well as in CFTR−/− mice that did not receive any BMCs (data not shown). CFTR−/− recipients that received WT BMCs transtracheally 1 month earlier (mice 3–6) showed restoration of the three bands, although band A appeared weak in some samples.
Figure 2.
CFTR Is Detected in CFTR−/− Lungs after BMC Delivery
Transtracheal delivery of 7-day-cultured WT male BMCs was performed in CFTR−/− female recipients (REC) following our optimal conditioning protocol. One month later, CFTR mRNA was detected in lung tissue by real-time qPCR using TaqMan probes. (A) CFTR−/− mice treated with WT BMCs expressed CFTR mRNA at ∼7% of WT lungs normalized to GAPDH, while BMCs cultured for 7 days had ∼0.1% expression. CFTR mRNA was not detected in CFTR−/− recipients treated with CFTR−/− BMCs. (B) CFTR protein detection was significantly decreased in 7-day-cultured BMCs from CFTR−/− mice compared to WT. (C) CFTR protein was also detected by western blot in CFTR−/− recipient mice treated with WT BMCs (mice 3–6) but not in those treated with CFTR−/− BMC (mice 1 and 2) where only band C can be seen. The error bar represents SEM (n ≥ 4 mice per group). *p < 0.05, by one-way ANOVA and Tukey post-test.
WT BMC Delivery Leads to Apical Airway Epithelium Expression of CFTR in CFTR−/− Mice
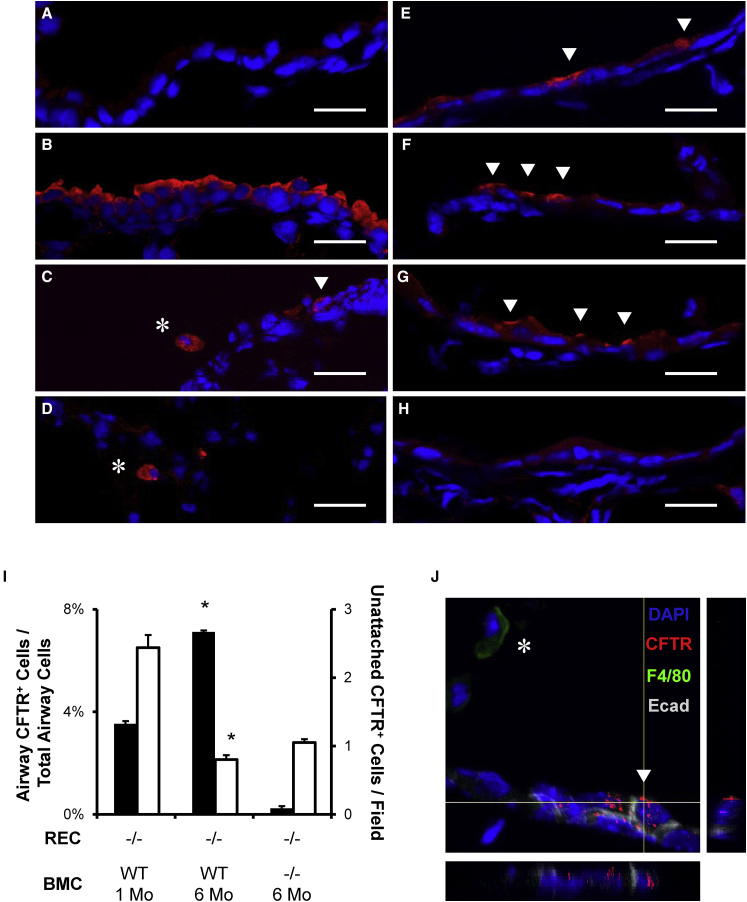
To determine the localization of donor CFTR in CFTR−/− recipient lungs after BMC delivery, immunofluorescence confocal microscopy was performed on lung sections and compared to WT lungs. Our results showed that CFTR was evenly distributed across the apical portion of the airway epithelial cells of WT lungs (Figure 3B), while isotype control was negative (Figure 3A). In CFTR−/− mice that received WT BMC delivery 1 month (Figures 3C and 3D) or 6 months (Figures 3E–3G) earlier, we found sporadic cells with an intense CFTR signal in the alveolar space and in airway epithelium. CFTR-positive airway cells were not found in untreated CFTR−/− mice or those treated with CFTR−/− BMCs at either time point (Figure 3H). A blind count of CFTR-positive alveolar and airway cells was performed from at least two different sections per mouse and four different mice per group. We found increased apical CFTR-positive cells at 6 months compared to 1 month, while alveolar and unattached-airway CFTR-positive cells decreased with time after BMC delivery (Figure 3I). Z projection of multiple confocal microscopy slices showed CFTR proximity with cell nuclei and E-cadherin in the airway (Figure 3J). The F4/80 macrophage marker was observed in unattached cells but not in CFTR-expressing cells attached in the airway.
Figure 3.
WT BMC Delivery Leads to Apical Airway Epithelial Expression of CFTR in CFTR−/− Mice
(A–J) WT or CFTR−/− BMCs were transtracheally delivered to optimally preconditioned CFTR−/− recipient mice. After 1 or 6 months, the lungs were frozen in OCT and sectioned for immunofluorescence. CFTR was uniformly detected in the apical portion of airway epithelial cells WT (B) lungs and sporadically in WT BMC-treated mice at 1 month (C and D) or 6 months (E–G). There was no intense apical CFTR observed in isotype control (A) or CFTR−/− BMC-treated CFTR−/− recipients (H). Arrows point to airway-attached CFTR-positive cells, and asterisks point to unattached alveolar or airway cells. (I) Cell counts of CFTR-positive cells showed increased airway cells (black) with time after BMC delivery while alveolar and unattached cells (white) decreased. *p < 0.05, by one-way ANOVA and Tukey post-test. (J) The z stack projection confocal microscopy shows the proximity of CFTR with E-cadherin and nuclear DAPI, while the macrophage marker F4/80 was not observed near CFTR-positive airway cells. Scale bars, 20 μm. The error bars represent SEM (n ≥ 4 mice per group).
WT BMC Delivery Partially Restores Select Lung Ceramides and Fatty Acids
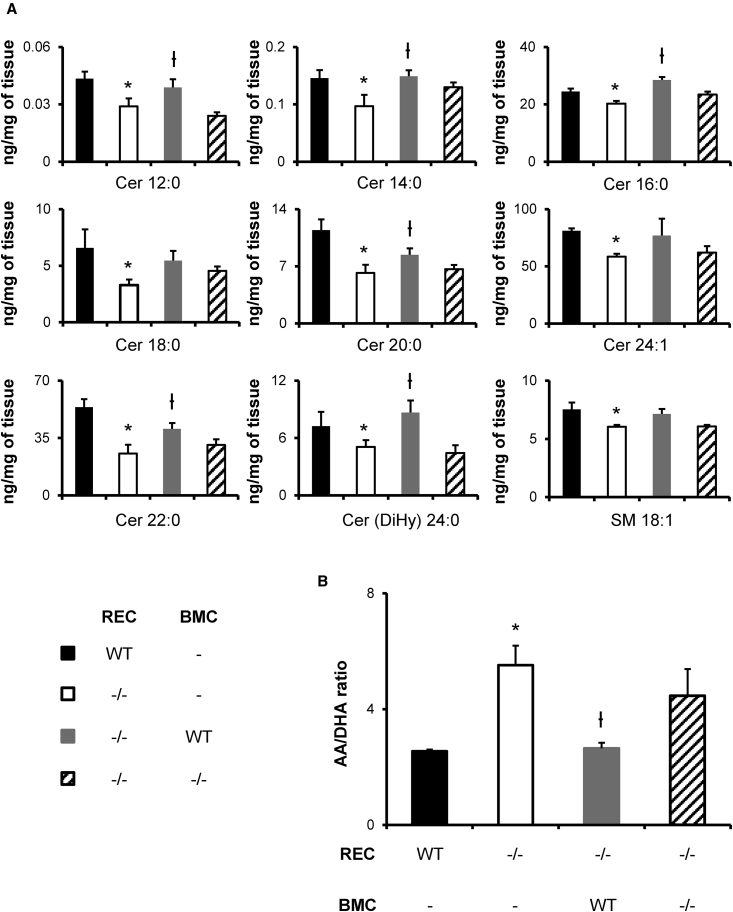
Since ceramide imbalance has been linked to CFTR pathophysiology, we investigated total lung lipids after BMC delivery by MS.13 Eight-month-old CFTR−/− mice had lower levels of ceramide and sphingomyelin when compared to age-matched WT littermates (Figure 4A). After 6 months, among significantly altered lipids in CFTR−/− mice, ceramides Cer 12:0, Cer 14:0, Cer 16:0, Cer 20:0, Cer 22:0, and Cer(DiHy) 24:0 were restored by WT BMCs but not by CFTR−/− BMCs. Ceramides Cer 18:0 and Cer 24:1 and sphingomyelin SM 18:1 were significantly decreased in CFTR−/− mice but not restored by WT BMC delivery. Previous reports also showed fatty acid imbalance; specifically, increasing levels of arachidonic acid (AA) and decreasing levels of docosahexaenoic acid (DHA) associated with CFTR deficiency.9, 27, 28 Using MS, we evaluated and found the AA/DHA ratio to be elevated in CFTR−/− mice as expected (Figure 4B). This altered AA/DHA ration was restored by WT but not by CFTR−/− BMC delivery.
Figure 4.
WT BMC Delivery Partially Restores Select Lung Ceramides and Fatty Acids
BMCs were transtracheally delivered to CFTR−/− mice between 15 and 20 weeks of age. After 6 months, the lungs were collected for MS analysis. (A) Few ceramide species and other sphingolipids were significantly decreased in old CFTR−/− mice (white) when compared to age-matched WT (black). Ceramides Cer 12:0, Cer 14:0, Cer 16:0, Cer 20:0, Cer 22:0, and Cer(DiHy) 24:0 were restored in CFTR−/− mice treated with WT BMCs (gray) but not CFTR−/− BMCs (stripes). Cer 18:0, Cer 24:1, and SM 18:1 were significantly decreased in CFTR−/− mice but were not restored by any BMC delivery. (B) In a similar way, the arachidonic acid (AA) and docosahexaenoic acid (DHA) ratio was increased in CFTR−/− mice compared to WT (WT) and was restored to a normal level by WT BMCs only (CFTR−/− + WT). CFTR−/− BMC delivery (CFTR−/− + CFTR−/−) did not induce significant changes. Data are expressed in nanograms per 2.5 mg of tissue for sphingolipids. The error bars represent SEM (n ≥ 4 mice per group). *p < 0.05, by one-way ANOVA and Tukey post-test compared to WT. ϯp < 0.05, by one-way ANOVA and Tukey post-test compared to knockout.
WT BMC Delivery Improved Bacterial Clearance and Survival
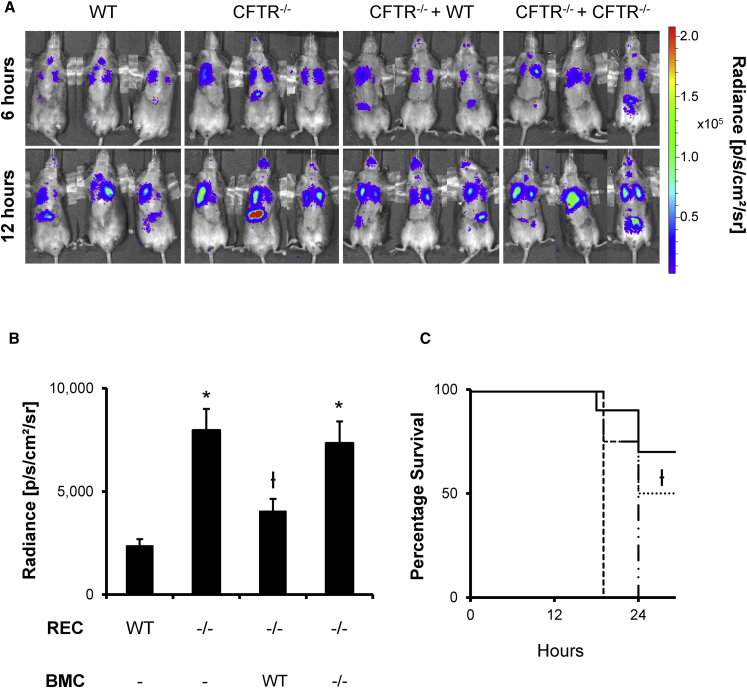
To determine whether cell replacement therapy had any effect on bacterial clearance, we used a luxCDABE-expressing Pseudomonas aeruginosa Xen 41, which emits light spontaneously,29 in an infection model 2 months after BMC delivery. Pseudomonas entrapped in agar beads (5 million colony-forming units [CFUs]) were inoculated transtracheally to induce lung infection, and luminescence was monitored over time in vivo using a Xenogen IVIS imaging system. Untreated CFTR−/− mice had earlier onset and stronger bacterial infection than age-matched WT littermates, as measured by luminescence intensity (Figures 5A and 5B), as well as an accelerated mortality (Figure 5C). WT BMC treatment in CFTR−/− mice 2 months prior to bacterial exposure led to weaker infection and improved survival when compared to untreated CFTR−/− mice at 24 hr. CFTR−/− mice treated with CFTR−/− BMCs displayed no significant reduction of infection and had similar mortality rates as those of untreated CFTR−/− mice.
Figure 5.
WT BMC Delivery Improved Bacterial Clearance and Survival
BMCs cultured for 7 days were delivered transtracheally into 15- to 20-week-old preconditioned mice. After allowing donor BMCs to reside in the recipient lung for 2 months, the mice were transtracheally inoculated with 5 million Pseudomonas aeruginosa Xen 41 cells entrapped in agar beads. Lung infection development was observed using a Xenogen IVIS imaging system at regular intervals. (A) CFTR−/− mice had stronger bacterial infection than WT at 6 hr, and this difference persisted to 12 hr. CFTR−/− mice pretreated with WT BMCs (CFTR−/− + WT) had improved bacterial clearance compared to mice pretreated with CFTR−/− BMCs (CFTR−/− + CFTR−/−). (B) Average radiance intensity of the lung area was calculated using ImageJ and a region of interest that excluded the gut infection observed in some mice. Donor WT BMC treatment significantly improved bacterial clearance in CFTR−/− recipients. (C) Infection intensity correlated with mortality where WT mice (solid line) and CFTR−/− mice treated with WT BMCs (dotted line) both had significant improved survivability compared to CFTR−/− mice (dashed line) and CFTR−/− mice pretreated with knockout BMCs (dashed-dotted line). The error bars represent SEM (n ≥ 4 mice per group). *p < 0.05, by one-way ANOVA and Tukey post-test compared to WT. ϯp < 0.05, by one-way ANOVA and Tukey post-test compared to knockout.
Mechanism of Improved Bacterial Clearance by WT BMC Delivery
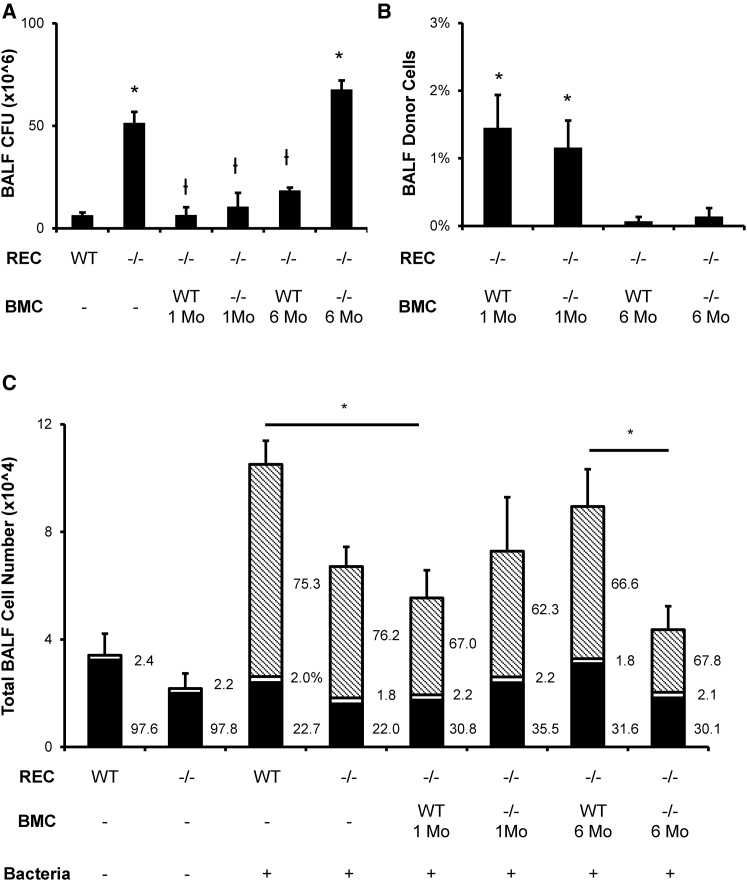
To explore possible mechanisms of improved bacterial clearance, we delivered WT or CFTR−/− BMCs to CFTR−/− recipients 1 or 6 months before bacterial infection induced by Pseudomonas aeruginosa beads. Mice were sacrificed 8 hr later to collect bronchoalveolar lavage fluid (BALF) and tissue. An analysis of BALF CFUs showed that CFTR−/− mice had a stronger bacterial load than their WT littermates, which suggests impaired bacterial clearance (Figure 6A). WT or CFTR−/− BMC treatment 1 month earlier both resulted in a decreased CFU count, while only WT BMCs had a beneficial effect on CFU count after 6 months. Donor cells in BALF and lung tissue were then detected by YqRT-PCR relative to male controls. We detected approximately ten times more donor cells in BALF from recipient mice treated with BMCs 1 month earlier, compared to those treated 6 months earlier (Figure 6B), despite having similar amounts of male cells in lung tissue (Figure 1D). An analysis of total and differential cells from BALF 8 hr post-infection showed a significant reduction of total cells in CFTR−/− mice compared to their WT littermates (Figure 6C). Inflammatory cell recruitment was restored to almost WT levels in mice treated with WT BMCs 6 months earlier. Cytokine profiling at 6 months revealed elevated levels of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-12 (IL-12), and interleukin-4 (IL-4) in CFTR−/− mice treated with CFTR−/− BMCs (Table 1). Mice treated with WT BMCs for the same period had lower cytokine levels, consistent with reduced severity of infection. Interestingly, CFTR−/− mice treated with either BMC genotype for 1 month had lower levels of IL-2 and IL-4 than CFTR−/− control mice.
Figure 6.
Mechanism of Improved Bacterial Clearance by WT Donor BMCs
BMC cultured for 7 days were delivered transtracheally into 15- to 20-week-old preconditioned mice following our optimal protocol. After allowing donor BMCs to reside in a recipient (REC) lung for 1 month (Mo.) or 6 months, the mice were transtracheally inoculated with 5 million Pseudomonas aeruginosa cells entrapped in agar beads. (A) CFU count from BALF collected 8 hr after bacterial inoculation shows that either WT or CFTR−/− BMC treatment 1 month earlier improved bacterial clearance in the lung, while only WT BMCs were beneficial after 6 months. (B) Y-chromosome real-time PCR shows that BALF donor cells decrease with time after BMC delivery. (C) Total and differential cell counts showed lower total cell and reduced neutrophil (pattern) recruitment after bacterial infection in CFTR−/− mice, which is restored only by 6-month WT BMC treatment. Macrophages (black) and lymphocytes (white) were not significantly affected. The error bars represent SEM (n ≥ 4 mice per group). *p < 0.05, by one-way ANOVA and Tukey post-test compared to WT. ϯp < 0.05, by one-way ANOVA and Tukey post-test compared to CFTR−/− mice.
Table 1.
BALF Cytokine Concentrations
| REC | BMCs | TNF-α | IL-1β | IL-2 | IL-6 | IL-12 | IL-4 | GM-CSF | IL-10 | IFN-γ | IL-5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | – | 127 ± 27 | 15 ± 4.9 | – | 73 ± 16 | 14 ± 3.5 | – | – | – | – | – |
| CFTR−/− | – | 134 ± 25 | 41 ± 5.7b | 5.8 ± 2.4a | 135 ± 14b | 18 ± 2.8 | 52 ± 14a | 1.0 ± 0.5 | – | – | – |
| CFTR−/− | WT 1 month | 268 ± 52b | 76 ± 13b | 2.2 ± 1.0b | 268 ± 45b | 23 ± 2.9b | 19 ± 7.0b | 4.2 ± 2.9 | 0.7 ± 0.4 | – | – |
| CFTR−/− | CFTR−/− 1 month | 260 ± 47b | 82 ± 13b | 3.8 ± 2.2b | 272 ± 44b | 21 ± 3.2b | 25 ± 11b | 7.5 ± 2.3b | – | – | – |
| CFTR−/− | WT 6 months | 146 ± 29 | 61 ± 10b | 4.6 ± 1.9a | 86 ± 25 | 18 ± 1.4 | 30 ± 10b | – | – | – | – |
| CFTR−/− | CFTR−/− 6 months | 313 ± 47a | 186 ± 31a | 7.9 ± 4.8b | 529 ± 96a | 28 ± 4.5b | 181 ± 73a | 38 ± 33 | 5.2 ± 4.5 | 1.9 ± 1.62 | – |
Concentrations are given as mean picograms per milliliter ± SEM. Each group represents BALF samples from n = 4–7 mice taken 8 hr after Pseudomonas infection. REC, recipient; GM-CSF, granulocyte-macrophage colony-stimulating factor.
p ≤ 0.05.
p ≤ 0.08 compared to WT infection control.
Discussion
Earlier attempts at restoring CFTR in CFTR−/− mouse lung used systemic BMC transplantation as opposed to organ-targeted delivery in combination with an optimized conditioning regimen.22, 23 The resulting number of chimeric cells appeared to be rare and was speculated to unlikely have any effect in one case, while in the other case, it was shown to have modest physiological effects on the overall lung that were still far from those for WT. The data presented here are novel in showing that, by improving cell retention with an optimized conditioning regimen and optimal route of delivery, more donor cells can be found in the recipient lungs over a long period, hence, resulting in a near-WT physiological role for CFTR attributed to donor cells. Our results suggest that donor BMCs can localize to and reside in the lung over long time periods and have beneficial effects in their new environment. Expression of CFTR mRNA was detected in CFTR−/− lungs after delivery of WT BMCs but not CFTR−/− BMCs. This is supported by western blot analysis, where CFTR protein expression was detected in CFTR−/− mice treated with WT BMCs and not with CFTR−/− BMCs. Bands A and B appeared in the lung only after WT BMC delivery and not after CFTR−/− BMC delivery. In some samples, band C was stronger after delivery of WT BMCs compared to CFTR−/− BMCs. These results suggest that 7-day-cultured BMCs are capable of synthesizing CFTR protein and maintain expression in the lung over a long period. The presence of band C in CFTR−/− mice was previously reported and is believed to result from the binding of a non-CFTR homologous protein by commercially available antibodies.30, 31 The authors eliminated this band from CFTR−/− mice by generating a murine mono-specific antibody clone, which is not commercially available. In our case, since we are using a gut-corrected CFTR−/− mouse model, this band C could also result from residual human CFTR transgene used to correct intestinal defect.32 In this case, it is clear that at least bands A and B are specific to the WT BMCs.
Our confocal microscopy results also showed CFTR protein expression, which appeared as sporadic intense fluorescence in an apical location in the airway epithelium of CFTR−/− mice treated with WT BMCs and not in those treated with CFTR−/− BMCs. Short-term BMC delivery (1 month) resulted in more unattached CFTR-positive cells in the airways or alveolar spaces, possibly representing alveolar macrophages. These unattached decreased at 6 months, while the number of epithelial cells with apical CFTR increased. A diffuse faint signal was observed in CFTR−/− mice with or without CFTR−/− BMCs when compared to isotype control, but no strong apical signal was observed in any of the CFTR−/− samples. This faint signal could be due to a truncated form of CFTR present in the cell, as predicted by the group who made this deletion.32 In co-staining experiments, we observed E-cadherin expression surrounding CFTR-positive cells lining the epithelium, while F4/80 was negative for those attached cells. Taken together, the RNA and protein results (Figures 2 and 3) suggest restoration of CFTR after WT BMC delivery and that it could persist in airway epithelium over a prolonged period.
Based on the lipid composition study, we believe that resident BMCs partially impact their new lung environment by restoring select ceramides and fatty acids. Since CFTR−/−-resident BMCs failed to restore most lipids, it appeared that the effects are CFTR dependent, although it could be due to paracrine factors or the effects of non-epithelial cells such as macrophages, which also express CFTR.33 Further investigations could explore possible the paracrine effects of WT or CFTR−/− BMC culture supernatant. Although conflicting results have been reported in regard to ceramide alterations in CF using different techniques,11, 34 the main purpose of the present study was to restore the altered lipids by BMC treatment and not to resolve the controversy surrounding this issue. It is possible that ceramide migrates and accumulates in a certain area of the lung without new synthesis and that the total concentration of select species decreases in the lysate. That said, our results for total lung MS of ceramides appear consistent with those published by the Radzioch group using the same method.35 Together with AA and DHA fatty acids, where alterations in CF are well described and consistent, these results suggest that donor BMCs can alter lung lipid composition after delivery and potentially affect overall lung health by affecting inflammation and bacterial clearance.9, 11, 12
To study bacterial infection in real time, we used a luciferase-expressing Pseudomonas aeruginosa, which allows the visualization of infection in progress while maintaining the animal alive. One disadvantage of this model is reduced in vivo sensitivity so that a higher lethal bacterial dose had to be used, making the assay useful for short-term observations only. Our results showed that WT, but not CFTR−/−, BMCs improved the reduced Pseudomonas aeruginosa clearance seen in CFTR−/− mice. To further explore possible mechanisms involved in this improvement by BMCs, we compared BMC treatment at 1 month and 6 months before infection with Pseudomonas (Figure 6; Table 1). Interestingly, we observed that either BMC genotype was beneficial for bacterial clearance at 1 month, while only WT BMCs improved clearance at 6 months. This could be due to functional expression of CFTR by epithelial cells36 or to mesenchymal-stem-cell (MSC)-induced expression of CFTR,37 but since non-epithelial cells such as macrophages and neutrophils can also express CFTR at low levels,38, 39 there could be other explanations, such as different bactericidal activity of macrophages40 or anti-inflammatory and anti-microbial properties of BMCs.41 Since there were more donor cells in BALF from mice treated with BMCs 1 month earlier compared to 6 months earlier, it is possible that macrophage and other hematopoietic cells are present in the BMC population and have a temporary effect on bacterial clearance.42 Over time, unattached cells may be cleared or reach the end of their life cycle, ending their beneficial effect in the lung, while putative BMC epithelial progenitors could engraft, regenerate, and improve overall lung function.43, 44, 45, 46
Further studies will be needed to confirm the involvement of both inflammatory and epithelial subsets. However, Bonfield et al., using a conditional CFTR knockout in myeloid cells, showed partial involvement of macrophages and neutrophils in the late phase (10 days) of bacterial clearance, and no effect in the acute phase (3 days), but did not report on the 8-hr time point that we used.47 CFTR deficiency in myeloid cells resulted in intermediate survival and inflammatory profile compared to mice with CF. These data suggest that a functional CFTR-expressing epithelium is essential for complete bacterial clearance. In our study, infection was terminated at 8 hr to correspond with bioluminescence live imaging. Since our bacterial dosage was higher, it is possible that this time point corresponds to the late inflammation stage seen in studies using a lower bacterial dose or a different strain. Lower amounts of BALF cells in CFTR−/− mice treated 6 months earlier could be responsible for decreased bacterial clearance, although bacterial clearance was equivalent at 1 month after BMC treatment despite lower BALF cell numbers in recipients of CFTR−/− cells. The cytokine profile is also intriguing, since mice treated with CFTR−/− BMCs for 6 months had the highest level for most cytokines, including those that are pro-inflammatory, yet had reduced cell counts. This could result from persisting recruitment signals during ineffective clearance and massive bacterial load while leukocyte recruitment to BALF is impaired. WT BMC treatment for 6 months appeared to restore the levels of TNF-α, IL-6, and IL-12 close to WT levels in CFTR−/− mice. It is also possible that this apparent discrepancy between leukocytes in BALF and cytokine profile is due to an alteration in the overall time course of infection in mice treated with WT versus CFTR−/− BMCs. That is, those treated with WT are already responding (with leukocyte recruitment), and recruitment signals (cytokines) are quickly returning to normal, compared to those treated with CFTR−/− cells, where bacterial clearance and leukocyte recruitment are delayed or impaired, leading to even higher cytokine signals. Treatment with either BMC genotype for 1 month maintained cytokines at intermediate levels between CFTR−/− infection control and CFTR−/− 6-month BMCs. Surprisingly, IL-2 and IL-4 were lower in mice treated 1 month earlier, compared to CFTR−/− controls. This may indicate differences in T cell activity induced by short-term resident BMCs. Increased cytokine levels at 6 months in CFTR−/− BMC-treated mice could result from some degree of chronic inflammatory state or low-level infection, perhaps initiated by the transtracheal delivery of cells 6 months earlier, since it was not seen in the untreated CFTR−/− animals. An in-depth analysis of the WT or CFTR−/− alveolar macrophages and BMC anti-microbial mechanisms will further clarify these studies.
In the CF pig model, transgenic gene correction studies have recently made substantial progress,48, 49 but viral treatment still faces efficacy and safety considerations.50, 51 Cell-based therapeutic options may be a safer alternative. Cell therapy could be advantageous compared to gene therapy where repeated administration of the virus may be needed. In addition, cell therapy with autologous cells is without any significant risk of mutagenesis, while allogeneic cells, corrected in vitro, could be subject to extensive testing prior to administration. However, there are also potential challenges with cell replacement therapy. A more suitable conditioning regimen for human application will require additional studies. Unlike CFTR−/− mice, CF patients have thick airway mucus, which could prevent the delivered cells from reaching the epithelium. Moreover, there will be a risk of rejection faced with any allogenic transplant. This may be surmountable by combining cell and gene therapies in which autologous CF patient BMCs would be gene corrected ex vivo by improved, safer techniques, screened, and delivered locally to the airways. In conclusion, we believe that CFTR expression may be restored by BMC delivery, resulting in long-lasting benefits in bacterial clearance and resistance to infection.
Materials and Methods
Mice
C57BL/6 and CFTR−/− (Cftrtm1Unc Tg(FABPCFTR)1Jaw/J) mice were from The Jackson Laboratory. CFTR−/− mice were generally smaller than their WT littermates and had chalky white incisors as opposed to orange pigment.52 For confirmation, CFTR−/− mice were genotyped according to the supplier’s protocol. All mice were used between 15 and 20 weeks of age for cell delivery and used for experimental procedures between 1 and 12 months later. Animal procedures were all approved by the University Health Network Animal Care Committee. Mice received care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Experimental Animals formulated by the Canadian Council on Animal Care.
BMCs
BMC isolation was described in detail previously.25 Briefly, BMCs were harvested aseptically by flushing the femur and tibiae from donor mice using a 25G needle. BMCs were plated onto plastic tissue-culture flasks at a density of ∼1 × 106/cm2 in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Life Technologies). The medium was replaced every 2 to 3 days, gradually washing away non-adherent. The remaining plastic-adherent BMCs were cultured for a total of 7 days before being used for in vivo delivery.
Lung Injury and BMC Delivery
Mice were given 200 mg/kg naphthalene (Sigma-Aldrich) 2 days prior to cell delivery by intraperitoneal injection, as previously described.25 One day later, busulfan (Otsuka America Pharmaceutical) was given by intraperitoneal injection on the opposite side at either 20 or 30 mg/kg. The next day, the mice were anesthetized with 5% isoflurane, and 2 million BMCs in 50 μL PBS were delivered through the trachea using a gel-loading tip and pipette while holding the tongue to prevent swallowing. The mice were then left hanging vertically for an extra minute to help the dispersion of BMCs. For the optimal protocol, a second dose of BMCs was given 3 days after the first one.
Flow Cytometry
Cells were blocked by incubating 2 × 105 BMCs in PBS + 0.5% BSA + 10% normal goat serum on ice for 30 min. After PBS washes, BMCs were incubated in the blocking buffer with anti-CCSP (EMD Millipore) or with anti-CFTR peptide around serine 737 (Abcam) primary rabbit antibody (1:500) for 30 min on ice followed by a 30-min incubation with Alexa Fluor 488 anti-rabbit secondary immunoglobulin G (IgG) (1:500; Thermo Fisher Scientific). Data were analyzed using FlowJo software (Tree Star). Isotype-matched antibodies were used for controls. The positive gate was set to include <1% of the distribution peak obtained with isotype control antibody.
Real-Time PCR
Genomic DNA (gDNA) was isolated with the DNeasy Blood & Tissue Kit (QIAGEN). Male BMCs in female lungs were quantified using YqRT-PCR (SRY primers; forward: 5′-GGGATGCAGGTGGAAAAGC-3′ and reverse: 5′-GTGACACTTTAGCCCTCCGAT-3′) and the LightCycler 480 SYBR Green 1 Master Mix (Roche). BMC numbers in experimental mice were extrapolated from the standard curve, made by isolating gDNA from the known amount of cells mixed with lung homogenate, and normalized to GAPDH (forward primer: 5′- TGTGTCCGTCGTGGATCTGA-3′ and reverse primer: 5′-GATGCCTGCTTCACCACCTT-3′). The PCR conditions were as follows: 95°C for 5 min; 40 cycles at 95°C for 15 s; 62°C for 20 s, and 72°C for 20 s, followed by the dissociation curve. Total RNA was isolated from experimental lungs using the RNeasy Mini Kit, followed by cDNA preparation using Maxima H Minus Reverse Transcriptase (Thermo Scientific). To evaluate CFTR mRNA expression in CFTR−/− mice, a murine-specific TaqMan primer probe located on exons 10 and 11 was used (assay ID Mm01156902_m1) in combination with GAPDH control (Life Technologies). Samples were processed with the LightCycler 480 Probes Master (Roche) under the following PCR conditions: 50°C for 2 min; 95°C for 5 min; 50 cycles at 95°C for 15 s; 60°C for 30 s; and they were expressed as relative to normal WT lung. This probe was chosen since CFTR−/− mice were made by inserting a STOP codon in exon 10 and by adding human CFTR transgene under the control of rat Fabp2 promoter.32 Murine gDNA, BMC mRNA, and human mRNA were all negative.
Western Blotting
Lung tissue lysis was performed in cold RIPA buffer (1× PBS, 1% NP-40 substitute, 0.5% SDS) containing a complete, Mini, EDTA-free protease inhibitor tablet (Roche) for 1 hr on ice. The supernatant was resolved by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and blocked with 5% non-fat milk. Rabbit polyclonal antibodies raised against a region near serine 737 CFTR and β-actin (Cell Signaling Technology) were used for detection.
Immunofluorescence Imaging
The lungs were inflated with O.C.T. (optimal cutting temperature) Compound (Sakura Finetek) by intra-tracheal instillation and quickly frozen. Sections 5 μm thick were cut, fixed in cold acetone, and blocked with 5% goat serum in PBS. Rabbit polyclonal CFTR antibody (Abcam) and its isotype were fluorescently labeled with the Zenon Alexa Fluor 546 Labeling Kit (Thermo Fisher Scientific) and then incubated with the sections in PBS 5% goat serum overnight at 4°C in a humidified chamber. Anti-CD324 Alexa Fluor 647 (E-cadherin) and anti-F4/80 Alexa Fluor 488 primary antibodies were used in co-staining experiments. After washes, the slides were counterstained with DAPI (1:5,000, Thermo Fisher Scientific) and mounted for imaging. Images were acquired by confocal microscopy using an Olympus FluoView 1000 Confocal Imaging System). Cell counts based upon fields imaged at 40× were performed by two different individuals and averaged. At least four different mice per group and two sections per mouse were counted.
Lipid Chromatography, Gas Chromatography, and MS
Ceramide, sphingosine, and sphingomyelin were extracted and analyzed by lipid chromatography/tandem MS (LC-MS-MS), while fatty acids were analyzed by gas chromatography (GC)-MS. All procedures were performed by the Analytical Facility for Bioactive Molecules of the Centre for the Study of Complex Childhood Diseases, The Hospital for Sick Children, Toronto, ON.
Bioluminescent Bacterial Clearance
Bacteria contained within agar beads were made as previously described by others.53 Basically, Pseudomonas aeruginosa Xen 41 (PerkinElmer: Waltham), which possesses a single stable copy of the P. luminescens luxCDABE operon on the bacterial chromosome, was grown in lysogeny broth (LB) containing 60 μg/mL tetracycline overnight at 37°C. The next day, the bacteria pellet was collected by centrifugation and grown for 3 hr in fresh LB media before measuring optical density at 600 nm (OD600). 2 × 1010 bacteria were suspended in 1 mL of PBS and then added to 9 mL of pre-warmed Trypticase soy agar (∼50°C). The mixture was mixed with 50 mL of pre-warmed heavy mineral oil (∼50°C), with vigorous stirring for 10 min at room temperature. The mixture was allowed to cool down for an extra 10 min on ice. The beads containing bacteria were collected by centrifugation at 5,000 × g for 30 min and washed twice in PBS. Dilutions were plated overnight to obtain an accurate CFU count, and 5 million bacteria were used for transtracheal delivery. For detection in vivo, mice were shaved 1 day before bacterial delivery, using hair removal cream. The next day, bacteria-containing beads were delivered, and mice were anesthetized with isoflurane (PPC) for image acquisition using the Xenogen IVIS Imaging System at different time intervals.
Broncho Alveolar Lavage
Mice were sacrificed by CO2 asphyxiation, and the trachea-lung block was removed. A 23G cannula was inserted in the trachea, and the lungs were flushed with 1 mL of PBS three times. The BALF was evaluated for total cell count, differential cell count, bacterial count, and the presence of proinflammatory cytokines. Differential cell count was done by Shandon Kwik-Diff staining (Thermo Fisher Scientific). Cytokine analysis was performed by the Princess Margaret Genomic Center (Toronto, ON), using the mouse cytokine 10-Plex Panel for Luminex Platform (Thermo Fisher Scientific).
Statistical Analysis
Data are presented as mean ± SEM; one-way ANOVAs followed by Tukey’s (to compare pairs of groups) or Dunnett’s (to compare each group to a control group) post hoc tests (using GraphPad Prism 4.0) were performed as appropriate. Statistical significance was defined as p < 0.05 and p < 0.01.
Author Contributions
Conceptualization, P.D., G.K., A.P.W., and T.K.W.; Methodology, P.D. and T.K.W.; Investigation, P.D., R.B., M.F.D., L.G., and A.S.; Writing – Original Draft, P.D., G.K., and T.K.W.; Writing – Review and Editing, P.D., G.K., and T.K.W.; Supervision, T.K.W.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The present study was supported by Cystic Fibrosis Canada to T.K.W. and a CIHR Emerging Team grant (P.I., M. Bhatia).
References
- 1.Davies J.C., Alton E.W., Bush A. Cystic fibrosis. BMJ. 2007;335:1255–1259. doi: 10.1136/bmj.39391.713229.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry R.L., Mellis C.M., Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr. Pulmonol. 1992;12:158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 3.Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 4.Riordan J.R., Rommens J.M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 5.Snouwaert J.N., Brigman K.K., Latour A.M., Malouf N.N., Boucher R.C., Smithies O., Koller B.H. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 6.Guilbault C., Novak J.P., Martin P., Boghdady M.-L., Saeed Z., Guiot M.-C., Hudson T.J., Radzioch D. Distinct pattern of lung gene expression in the Cftr-KO mice developing spontaneous lung disease compared with their littermate controls. Physiol. Genomics. 2006;25:179–193. doi: 10.1152/physiolgenomics.00206.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bonvin E., Le Rouzic P., Bernaudin J.-F., Cottart C.-H., Vandebrouck C., Crié A., Leal T., Clement A., Bonora M. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J. Physiol. 2008;586:3231–3243. doi: 10.1113/jphysiol.2008.150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent G., Iles R., Bear C.E., Huan L.J., Griesenbach U., McKerlie C., Frndova H., Ackerley C., Gosselin D., Radzioch D. Lung disease in mice with cystic fibrosis. J. Clin. Invest. 1997;100:3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman S.D., Katz M.H., Parker E.M., Laposata M., Urman M.Y., Alvarez J.G. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr(-/-) mice. Proc. Natl. Acad. Sci. USA. 1999;96:13995–14000. doi: 10.1073/pnas.96.24.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Heeckeren A.M., Scaria A., Schluchter M.D., Ferkol T.W., Wadsworth S., Davis P.B. Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L717–L726. doi: 10.1152/ajplung.00227.2003. [DOI] [PubMed] [Google Scholar]
- 11.Guilbault C., Wojewodka G., Saeed Z., Hajduch M., Matouk E., De Sanctis J.B., Radzioch D. Cystic fibrosis fatty acid imbalance is linked to ceramide deficiency and corrected by fenretinide. Am. J. Respir. Cell Mol. Biol. 2009;41:100–106. doi: 10.1165/rcmb.2008-0279OC. [DOI] [PubMed] [Google Scholar]
- 12.Becker K.A., Tümmler B., Gulbins E., Grassmé H. Accumulation of ceramide in the trachea and intestine of cystic fibrosis mice causes inflammation and cell death. Biochem. Biophys. Res. Commun. 2010;403:368–374. doi: 10.1016/j.bbrc.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Guilbault C., De Sanctis J.B., Wojewodka G., Saeed Z., Lachance C., Skinner T.A.A., Vilela R.M., Kubow S., Lands L.C., Hajduch M. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2008;38:47–56. doi: 10.1165/rcmb.2007-0036OC. [DOI] [PubMed] [Google Scholar]
- 14.Zaman M.M., Martin C.R., Andersson C., Bhutta A.Q., Cluette-Brown J.E., Laposata M., Freedman S.D. Linoleic acid supplementation results in increased arachidonic acid and eicosanoid production in CF airway cells and in cftr-/- transgenic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L599–L606. doi: 10.1152/ajplung.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett D., Fossi A., Bargagli E., Refini R.M., Pieroni M., Luzzi L., Ghiribelli C., Paladini P., Voltolini L., Rottoli P. Mortality on the waiting list for lung transplantation in patients with idiopathic pulmonary fibrosis: a single-centre experience. Lung. 2015;193:677–681. doi: 10.1007/s00408-015-9767-x. [DOI] [PubMed] [Google Scholar]
- 16.Sly P.D., Brennan S., Gangell C., de Klerk N., Murray C., Mott L., Stick S.M., Robinson P.J., Robertson C.F., Ranganathan S.C., Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am. J. Respir. Crit. Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 17.Rosen C., Shezen E., Aronovich A., Klionsky Y.Z., Yaakov Y., Assayag M., Biton I.E., Tal O., Shakhar G., Ben-Hur H. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat. Med. 2015;21:869–879. doi: 10.1038/nm.3889. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson F.L., Sergijenko A., Langford-Smith K.J., Malinowska M., Wynn R.F., Bigger B.W. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol. Ther. 2013;21:868–876. doi: 10.1038/mt.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa J., Hsieh M.M., Uchida N., Phang O., Tisdale J.F. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells. 2009;27:175–182. doi: 10.1634/stemcells.2008-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firth A.L., Menon T., Parker G.S., Qualls S.J., Lewis B.M., Ke E., Dargitz C.T., Wright R., Khanna A., Gage F.H., Verma I.M. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep. 2015;12:1385–1390. doi: 10.1016/j.celrep.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson L.G., Olsen J.C., Sarkadi B., Moore K.L., Swanstrom R., Boucher R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 22.Bruscia E.M., Price J.E., Cheng E.-C., Weiner S., Caputo C., Ferreira E.C., Egan M.E., Krause D.S. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc. Natl. Acad. Sci. USA. 2006;103:2965–2970. doi: 10.1073/pnas.0510758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loi R., Beckett T., Goncz K.K., Suratt B.T., Weiss D.J. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am. J. Respir. Crit. Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong A.P., Dutly A.E., Sacher A., Lee H., Hwang D.M., Liu M., Keshavjee S., Hu J., Waddell T.K. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L740–L752. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- 25.Duchesneau P., Wong A.P., Waddell T.K. Optimization of targeted cell replacement therapy: a new approach for lung disease. Mol. Ther. 2010;18:1830–1836. doi: 10.1038/mt.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas M., Xu J., Woods C.R., Mora A.L., Spears W., Roman J., Brigham K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman S.D., Blanco P.G., Zaman M.M., Shea J.C., Ollero M., Hopper I.K., Weed D.A., Gelrud A., Regan M.M., Laposata M. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 28.Njoroge S.W., Laposata M., Katrangi W., Seegmiller A.C. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. J. Lipid Res. 2012;53:257–265. doi: 10.1194/jlr.M018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craney A., Hohenauer T., Xu Y., Navani N.K., Li Y., Nodwell J. A synthetic luxCDABE gene cluster optimized for expression in high-GC bacteria. Nucleic Acids Res. 2007;35:e46. doi: 10.1093/nar/gkm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouret F., Bernard A., Hermans C., Dom G., Terryn S., Leal T., Lebecque P., Cassiman J.J., Scholte B.J., de Jonge H.R. Cystic fibrosis is associated with a defect in apical receptor-mediated endocytosis in mouse and human kidney. J. Am. Soc. Nephrol. 2007;18:707–718. doi: 10.1681/ASN.2006030269. [DOI] [PubMed] [Google Scholar]
- 31.Bronckers A., Kalogeraki L., Jorna H.J.N., Wilke M., Bervoets T.J., Lyaruu D.M., Zandieh-Doulabi B., Denbesten P., de Jonge H. The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells. Bone. 2010;46:1188–1196. doi: 10.1016/j.bone.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L., Dey C.R., Wert S.E., DuVall M.D., Frizzell R.A., Whitsett J.A. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Li X., Grassmé H., Döring G., Gulbins E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by Cftr-deficient macrophages on infection. J. Immunol. 2010;184:5104–5111. doi: 10.4049/jimmunol.0902851. [DOI] [PubMed] [Google Scholar]
- 34.Teichgräber V., Ulrich M., Endlich N., Riethmüller J., Wilker B., De Oliveira-Munding C.C., van Heeckeren A.M., Barr M.L., von Kürthy G., Schmid K.W. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat. Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 35.Wojewodka G., De Sanctis J.B., Radzioch D. Ceramide in cystic fibrosis: a potential new target for therapeutic intervention. J. Lipids. 2011;2011:674968. doi: 10.1155/2011/674968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pier G.B. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. Proc. Natl. Acad. Sci. USA. 2000;97:8822–8828. doi: 10.1073/pnas.97.16.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paracchini V., Carbone A., Colombo F., Castellani S., Mazzucchelli S., Gioia S.D., Degiorgio D., Seia M., Porretti L., Colombo C., Conese M. Amniotic mesenchymal stem cells: a new source for hepatocyte-like cells and induction of CFTR expression by coculture with cystic fibrosis airway epithelial cells. J. Biomed. Biotechnol. 2012;2012:575471. doi: 10.1155/2012/575471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di A., Brown M.E., Deriy L.V., Li C., Szeto F.L., Chen Y., Huang P., Tong J., Naren A.P., Bindokas V. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 39.Gao Z., Su X. CFTR regulates acute inflammatory responses in macrophages. QJM. 2015;108:951–958. doi: 10.1093/qjmed/hcv067. [DOI] [PubMed] [Google Scholar]
- 40.Del Porto P., Cifani N., Guarnieri S., Di Domenico E.G., Mariggiò M.A., Spadaro F., Guglietta S., Anile M., Venuta F., Quattrucci S., Ascenzioni F. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS ONE. 2011;6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonfield T.L., Lennon D., Ghosh S.K., DiMarino A.M., Weinberg A., Caplan A.I. Cell based therapy aides in infection and inflammation resolution in the murine model of cystic fibrosis lung disease. Stem Cell Discovery. 2013;3:139–153. [Google Scholar]
- 42.Parihar A., Eubank T.D., Doseff A.I. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2010;2:204–215. doi: 10.1159/000296507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bustos M.L., Mura M., Marcus P., Hwang D., Ludkovski O., Wong A.P., Waddell T.K. Bone marrow cells expressing Clara cell secretory protein increase epithelial repair after ablation of pulmonary Clara cells. Mol. Ther. 2013;21:1251–1258. doi: 10.1038/mt.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bustos M.L., Mura M., Hwang D., Ludkovski O., Wong A.P., Keating A., Waddell T.K. Depletion of bone marrow CCSP-expressing cells delays airway regeneration. Mol. Ther. 2015;23:561–569. doi: 10.1038/mt.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendt C., Tram K., Price A., England K., Stiehm A., Panoskaltsis-Mortari A. Club cell secretory protein improves survival in a murine obliterative bronchiolitis model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;305:L642–L650. doi: 10.1152/ajplung.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong A.P., Keating A., Lu W.-Y., Duchesneau P., Wang X., Sacher A., Hu J., Waddell T.K. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J. Clin. Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonfield T.L., Hodges C.A., Cotton C.U., Drumm M.L. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J. Leukoc. Biol. 2012;92:1111–1122. doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potash A.E., Wallen T.J., Karp P.H., Ernst S., Moninger T.O., Gansemer N.D., Stoltz D.A., Zabner J., Chang E.H. Adenoviral gene transfer corrects the ion transport defect in the sinus epithelia of a porcine CF model. Mol. Ther. 2013;21:947–953. doi: 10.1038/mt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoltz D.A., Rokhlina T., Ernst S.E., Pezzulo A.A., Ostedgaard L.S., Karp P.H., Samuel M.S., Reznikov L.R., Rector M.V., Gansemer N.D. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J. Clin. Invest. 2013;123:2685–2693. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson W.F. Human gene therapy: scientific and ethical considerations. J. Med. Philos. 1985;10:275–291. doi: 10.1093/jmp/10.3.275. [DOI] [PubMed] [Google Scholar]
- 51.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright J.T., Kiefer C.L., Hall K.I., Grubb B.R. Abnormal enamel development in a cystic fibrosis transgenic mouse model. J. Dent. Res. 1996;75:966–973. doi: 10.1177/00220345960750041101. [DOI] [PubMed] [Google Scholar]
- 53.Facchini M., De Fino I., Riva C., Bragonzi A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 2014;85:51019. doi: 10.3791/51019. [DOI] [PMC free article] [PubMed] [Google Scholar]