Treatment of Type 2 diabetes with glucagon‐like peptide (GLP)‐1 receptor agonists leads to a modest increase in fasting plasma pancreatic enzyme levels, i.e. lipase and amylase 1, 2. The relevance of this observation is currently unknown, although GLP‐1 receptor agonists have been linked to the development of pancreatitis 1. The rate by which these enzymes increase remains largely unstudied. To date, the earliest observed enzyme increment is 4 weeks after drug initiation, while elevated levels are sustained during prolonged treatment 1, 2. In vitro, native GLP‐1 increases amylase secretion from isolated pancreatic acinar cells within 30 min 3, suggesting that the effect is immediate; however, in a recent study by Sonne et al. 4, liquid meal‐stimulated endogenous GLP‐1 (reaching two to three times fasting GLP‐1 levels) did not raise plasma lipase or amylase levels in people with Type 2 diabetes within 4 h. Whether plasma concentrations of therapeutic GLP‐1 receptor agonist increase pancreatic enzyme levels acutely in the clinical setting remains unclear. In the present study, we measured plasma lipase and amylase during i.v. administration of the GLP‐1 receptor agonist exenatide in people with Type 2 diabetes.
A detailed description of the design of this double‐blind, placebo‐controlled trial has been reported previously 5. Briefly, 57 people with Type 2 diabetes (mean ± sd age 62.8 ± 6.9 years, BMI 31.8 ± 4.1 kg/m2, HbA1c 56 ± 7 mmol/mol (7.3 ± 0.6%), diabetes duration 7.8 ± 4.9 years) were randomized to exenatide (AstraZeneca, London, UK) or placebo (isotonic saline). Exenatide was administered i.v., with a loading dose of 50 ng/min in 30 min, followed by continuous infusion of 25 ng/min, which is known to achieve steady‐state plasma concentrations within the therapeutically relevant range (130–150 pg/ml) 6. Plasma lipase and amylase were measured at baseline (~150 min before the start of intervention), and repeatedly during intervention in both the fasting state and after a high‐fat mixed meal (905.7 kCal; 50 g fat, 36.8 g protein and 75 g carbohydrates), using enzymatic colorimetric assays (Modular Analytics; Roche Diagnostics GmbH, Mannheim, Germany). Statistical analyses were performed using linear mixed models, which inherently correct for the multiple time points tested.
Lipase and amylase levels were in the normal range at baseline (mean ± sem lipase 44.6 ± 3.3 U/l and amylase 50.7 ± 2.4 U/l) and showed a similar initial decrease in both intervention groups (between‐group difference P > 0.05; Fig. 1). Thereafter, plasma amylase levels showed an increase during exenatide infusion, but not during placebo infusion. After 280 min infusion, amylase was significantly higher with exenatide compared with placebo (4.7 ± 1.4 U/l; P = 0.001). Amylase levels in individual participants did not exceed 3 × the upper limit of normal (maximum value was 110 U/l). Neither exenatide nor placebo had an effect on plasma lipase levels.
Figure 1.
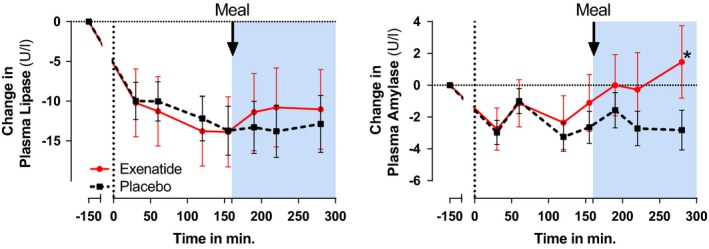
Pre‐ and postprandial effects of exenatide (circles, red solid line) and placebo (squares, black dashed line) on changes in plasma lipase and amylase concentrations. The high‐fat mixed meal was given 155 min after start of intervention. Asterisk indicates a statistically significant difference (P < 0.05) between the treatment groups at that time point.
We show for the first time that the GLP‐1 receptor agonist exenatide increases amylase levels within hours after treatment initiation. The exact mechanisms by which exenatide increased amylase cannot be concluded from the present study; however, increased plasma pancreatic enzyme levels can be caused by acinar secretion or leakage. Because in vitro data show that GLP‐1 induces amylase secretion 3, it is likely that the increase in amylase in the present study was caused by augmented secretion. Also, in case of acute cellular damage with exenatide‐infusion, a combined increase in amylase and lipase would be expected 7, arguing against damage in the present study.
An initial decrease in both lipase and amylase levels was observed. This could be explained by circadian rhythm, because the baseline measurement and the first intervention measurement were separated by 3 h. Intraday variability has been shown in a canine study 8, and importantly, is not affected by feeding status. The fact that meal ingestion, and release of endogenous GLP‐1, has no effect on pancreatic plasma enzymes underlines our findings and those of Sonne et al. 4. However, the exenatide‐induced increase in amylase occurred after the test meal. Whether this increase would have occurred without a test‐meal remains speculative, although levels tended to rise before meal ingestion. Further studies are needed to determine the clinical relevance of these modest pancreatic enzyme elevations, and whether these increases are modulated by food intake.
Funding sources
This research received funding from the European Community's Seventh Framework Programme (FP7/2007‐2013) under grant agreement no. 282521: the SAFEGUARD project.
Competing interests
Before her death on 9 April 2014, M. Diamant received research grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Medtronic, Merck Sharp & Dohme, Novo Nordisk and Sanofi. Through M.H.H. Kramer, the VU University Medical Center received research grants from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi. Dr van Raalte received research funding from AstraZeneca. The remaining authors have no competing interests to declare.
Acknowledgements
We thank all the study participants for their time and commitment to the research protocol. Furthermore, we would like to thank Jeannette Boerop for her excellent practical assistance during the study.
Diabet. Med. 34, 591–592 (2017)
References
- 1. Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T et al Pancreatic safety of incretin‐based drugs–FDA and EMA assessment. N Engl J Med 2014; 370: 794–797. [DOI] [PubMed] [Google Scholar]
- 2. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV et al Efficacy of liraglutide for weight loss among patients with type 2 diabetes. JAMA 2015; 314: 687. [DOI] [PubMed] [Google Scholar]
- 3. Hou Y, Ernst SA, Heidenreich K, Williams JA. The glucagon‐like peptide‐1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cyclic AMP. Am J Physiol Gastrointest Liver Physiol 2016; 310: G26–G33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sonne DP, Vilsbøll T, Knop FK. Pancreatic amylase and lipase plasma concentrations are unaffected by increments in endogenous GLP‐1 levels following liquid meal tests. Diabetes Care 2015; 38: e71–e72. [DOI] [PubMed] [Google Scholar]
- 5. Smits MM, Tonneijck L, Muskiet MHA, Hoekstra T, Kramer MHH, Pieters IC et al Cardiovascular, renal and gastrointestinal effects of incretin‐based therapies: an acute and 12‐week randomised, double‐blind, placebo‐controlled, mechanistic intervention trial in type 2 diabetes. BMJ Open 2015; 5: e009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL et al Exenatide augments first‐ and second‐phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005; 90: 5991–5997. [DOI] [PubMed] [Google Scholar]
- 7. Mayerle J, Sendler M, Lerch MM. Secretagogue (Caerulein) induced pancreatitis in rodents. Pancreapedia Exocrine Pancreas Knowl Base. 2013; DOI: 10.3998/panc.2013.2.
- 8. Piccione G, Giannetto C, Fazio F, Giudice E. Daily rhythm of serum lipase and alpha‐amylase activity in fed and fasted dogs. J Vet Diagn Invest 2008; 20: 795–799. [DOI] [PubMed] [Google Scholar]


