Abstract
Background
The promising postsurgical weight loss and remission of type 2 diabetes (T2D) from bariatric surgery can be attributed to modified eating physiology after surgical procedures. We sought to investigate the changes in the parameters of consumption behaviors and appetite sensations induced by a mixed meal tolerance test, and to correlate these alterations with age, body mass index, C-peptide levels, and duration of T2D 1 year after bariatric surgery.
Methods
A total of 16 obese patients with T2D who underwent mini-gastric bypass (GB) and 16 patients who underwent sleeve gastrectomy (SG) were enrolled in this study and evaluated using a mixed meal tolerance test one year after surgery. A visual analogue scale was used for scoring appetite sensation at different time points. The area under the curve (AUC) and the incremental or decremental AUC (ΔAUC) were compared between the two groups.
Results
One year after surgery, a decreasing trend in the consumption time was observed in the GB group compared to the SG group, while the duration of T2D before surgery was negatively correlated with the post-operative consumed time in those after GB. Patients who underwent GB had significantly higher fasting scores for fullness and desire to eat, higher AUC0′–180′ of scores for desire to eat, as well as more effective post-meal suppression of hunger and desire to eat compared with those undergoing SG one year after surgery. Post-operative C-peptide levels were negatively correlated with ΔAUC0′–180′ for hunger and ΔAUC0′–180′ for desire to eat in the GB group, while negatively correlated with ΔAUC0′–180′ for fullness in the SG group.
Discussion
Patients with T2D after either GB or SG exhibit distinct nutrient-induced consumption behaviors and appetite sensations post-operatively, which may account for the differential effects on weight loss and glycemic control after different surgery.
Keywords: Appetite sensation, Bariatric surgery, C-peptide, Mixed meal tolerance test, Sleeve gastrectomy, Diabetes mellitus, Gastric bypass
Introduction
The rapidly increasing incidence of obesity is emerging as a worldwide public health problem. As the prevalence of obesity continues to increase, the incidence of diabetes is propelled to upsurge (Wild et al., 2004; Hossain, Kawar & El Nahas, 2007). The rate of attribution of type 2 diabetes (T2D) to obesity is approximately 90% (Isomaa et al., 2001). Bariatric surgery, including gastric bypass (GB) and sleeve gastrectomy (SG), is known as the most effective method of improving obesity, and it is a potential strategy for remission of T2D (Schauer et al., 2014). The promising postsurgical weight loss and the maintenance of weight loss might be a result of modified eating behavior (Saunders, 1999; Laurenius et al., 2012) or it may be directly due to the surgical procedure (Saunders, 2001). GB and SG have been shown to effectively increase satiety sensation (Valderas et al., 2010). Good control of satiety might be related to the attenuation of insulin response (Speechly & Buffenstein, 1999), while intranasal insulin has been shown to reduce the stimulation of brain activity caused by pictures of food (Guthoff et al., 2010). In addition, intracerebroventricular administration of insulin significantly inhibited food intake and functions as an appetite-suppressive peptide (Chen et al., 2012). Bariatric surgery has been demonstrated to reduce C-peptide levels and improve insulin resistance (Lee et al., 2013). The improvement of diabetes after bariatric surgery is due to the up-regulation of the insulin signaling pathway (Bonhomme et al., 2011).
C-peptide, a 31 amino-acid polypeptide, is considered to be a marker for pancreatic insulin secretion (Bonser & Garcia-Webb, 1984; Hovorka & Jones, 1994). Our previous studies proposed a diabetes surgery score, the ABCD score (age, body mass index, C-peptide and duration of T2D), which is a clinically applicable parameters used to predict the success of bariatric surgery in obese patients with T2D (Lee et al., 2013; Lee et al., 2015). Diabetic patients with higher ABCD score before surgery had a higher rate of T2D remission (Lee et al., 2013; Lee et al., 2015). However, the relationships between ABCD score with consumption behaviors and appetite sensations after bariatric surgery are still unknown. In this study, we used the mixed meal tolerance test (MMTT) to investigate the changes in consumption behaviors and appetite sensations, and we correlated these alterations with age, body mass index, C-peptide, and duration of T2D one year after bariatric surgery.
Methods
Patients and metabolic surgery
The patients were enrolled in a previous randomized trial, which included 32 eligible patients with T2D (laparoscopic mini-gastric bypass (GB), N = 16; SG, N = 16). The GB (Lee et al., 2011b) and SG surgery (Lee et al., 2011b) were performed as previously described. One year after bariatric surgery, all 32 patients agreed to undergo the MMTT. Informed consent was obtained before the study.
This study was conducted at the Department of Surgery of Min-Sheng General Hospital and at Taipei Veterans General Hospital, and was approved by the Ethics Committee of each hospital (the approval number: 201002056IC and 201002037IC).
Surgical technique
GB was performed as described in our previous studies (Lee et al., 2011b; Lee et al., 2011a). In brief, we used a standard 5-port laparoscopic technique to create a long-sleeve gastric tube by using the EndoGIA stapler (EndoGIA; Coviden, Norvalk, CT, USA); it was approximately 2.0 cm wide along with the lesser curvature from the antrum to the angle of His. We also use an EndoGIA stapler to create a Billroth II type loop gastroenterostomy with the small bowel about 120 cm distal to the ligament of Treitz. There was no drain tube left in place. By using the mesh plug technique with bio-absorbable hemostatic gauze (Cellulostat; Horng Tzer Medical Instruments, Kaohsiung, Taiwan) (Chiu et al., 2006), we closed all the trocar wounds.
For SG, we used a laparoscopic stapler (EndoGIA; Coviden, Norvalk, Connecticut) with 60-cm cartridges (3.5 mm stapler height, blue load) to resect the greater curvature from the distal antrum (4 cm proximal to the pylorus) to the angle of His, including the complete fundus (Lee et al., 2011b). We left the remnant stomach tube, which was approximately 2 cm wide along the less curved side. The extended periumbilical trocar site was used for the extraction of the resected stomach portion.
Mixed meal tolerance test
To compare the consumption behavior and appetite sensations among patients with T2D after metabolic surgery, we used an MMTT. After an overnight fast, patients ate ensure plus (4 oz; 175 kcal; 6.5 g protein; 5.5 g fat; 25 g carbohydrate; Abbott Nutrition, Zwolle, Netherlands) (Lee et al., 2011a). The volume consumed was recorded and the consumed energy was calculated based on the volume of the MMTT after digestion.
Questionnaire on hunger, fullness, desire to eat, satiation, and prospective consumption
A visual analogue scale (VAS) of 100-mm lines was designed for patients to score their appetite sensations after a test meal with good reliability and validity (Parker et al., 2004). Similar to our previous study (Chen et al., 2013; Zachariah et al., 2016), the participants were familiarized with assessing the VAS and scoring their appetite sensations, such as hunger, fullness, desire to eat, satiation, and prospective consumption before food intake (0 min) and at 30, 60, 90, 120, 150 and 180 min after a mixed meal. The area under the curve (AUC) and the incremental or decremental area under the curve (ΔAUC) of various appetite VAS scores during the MMTT were measured by the trapezoidal method (He et al., 2011; Lee et al., 2011a). The VAS score for peak-0′ was calculated by subtracting the score at 0 min (before food intake) from the highest score recorded during the MMTT. The VAS score for peak-nadir was calculated by subtracting the lowest score from the highest score recorded during the MMTT.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences, version 12.01 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as the mean ± SD. The chi-square test or Fisher’s exact test was used to compare categorical variables, while the Mann–Whitney U test was used to compare continuous variables. The Wilcoxon signed-rank test was used to compare between baseline and post-operative variables. Friedman’s one-way analysis of variance followed by a post hoc test was used to analyze the differences among VAS scores for different appetite sensations at 0, 30, 60, 90, 120, 150, and 180 min after intake of a mixed meal. Correlations between the two groups were examined using Spearman’s correlation method. A P value less than 0.05 was considered statistically significant.
Results
Treatment effect one year after bariatric surgery
In total, 16 patients undergoing GB and 16 patients undergoing SG were enrolled. Before surgery, there are no static significant differences between GB and SG in age, BMI, C-peptide, the duration of T2D, and homeostasis model assessment-insulin resistance (HOMA-IR) (Table 1). One year after bariatric surgery, the BMI in the GB and SG groups were 22.6 ± 2.5 and 24.4 ± 2.5 kg/m2 (P < 0.05), respectively, which was the only statistically significant difference between the two groups. Although the BMI was lower in patients after GB than those after SG, but the ratio of BMI after/before either GB or SG had no significant difference (P > 0.05). Post-operative levels of C-peptide and HOMA-IR were comparable between the GB and SG groups one year after surgery (Table 1).
Table 1. ABCD scale (age, body mass index, C-peptide, and duration of T2D) before and 1 year after bariatric surgery.
| GB (n = 16) | SG (n = 16) | P value | |
|---|---|---|---|
| Age before surgery | 44.3 ± 8.6 | 46.3 ± 8.0 | NS |
| BMI before surgery | 29.1 ± 3.1 | 31.0 ± 2.9 | NS |
| BMI one year after surgery | 22.6 ± 2.5 | 24.4 ± 2.5 | <0.05 |
| Ratio of BMI after/before surgery | 0.78 | 0.79 | NS |
| C-peptide before surgery | 2.5 ± 1.1 | 3.3 ± 1.4 | NS |
| C-peptide one year after surgery | 1.6 ± 1.1 | 1.7 ± 0.5 | NS |
| Duration of T2D before surgery | 6.0 ± 5.5 | 6.9 ± 5.4 | NS |
| HOMA-IR before surgery | 9.3 ± 23.2 | 8.5 ± 6.5 | NS |
| HOMA-IR one year after surgery | 1.2 ± 1.2 | 2.4 ± 3.5 | NS |
Notes.
Data is expressed as the mean ± SD.
- BMI
- body mass index
- HOMA-IR
- homeostasis model assessment-insulin resistance
- GB
- gastric bypass
- SG
- sleeve gastrectomy
- T2D
- type 2 diabetes
The volume, energy, and time of consumption after bariatric surgery
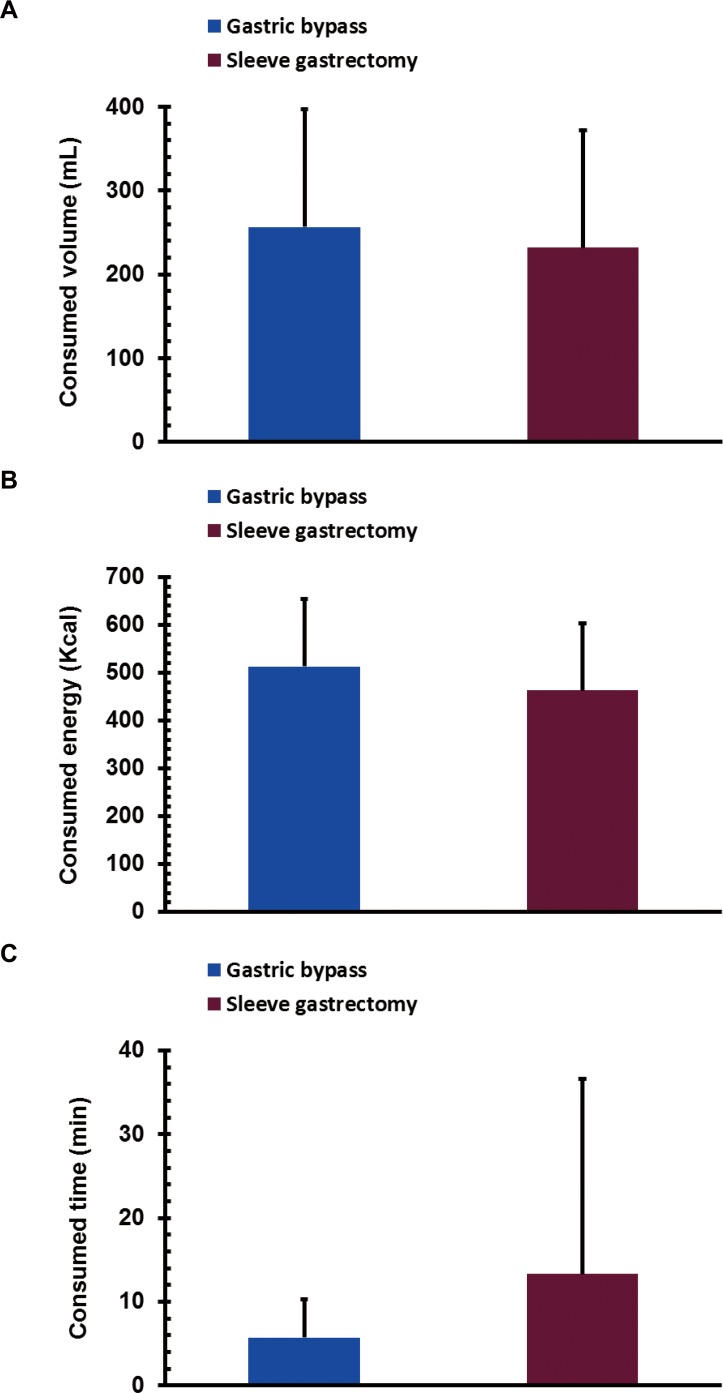
One year after surgery, there were no significant differences in the volume of consumption between the GB and SG groups (256.6 ± 142.3 mL vs. 231.8 ± 140.3 mL, P > 0.05; Fig. 1A). The total energy consumed in the GB and SG groups was comparable (513.1 ± 284.6 Kcal vs. 463.5 ± 280.5 Kcal, P > 0.05; Fig. 1B). The time of consumption in the GB had a decreasing trend compared to that in SG (5.7 ± 4.6 min vs. 13.3 ± 23.3 min, P > 0.05; Fig. 1C).
Figure 1. The volume of food consumed (A), energy consumed (B), and consumption time (C) during a mixed meal tolerance test in gastric bypass (n = 16) and sleeve gastrectomy (n = 16) patients one year after surgery.
Data is expressed as the mean ± SD.
Hunger sensation during the MMTT after bariatric surgery
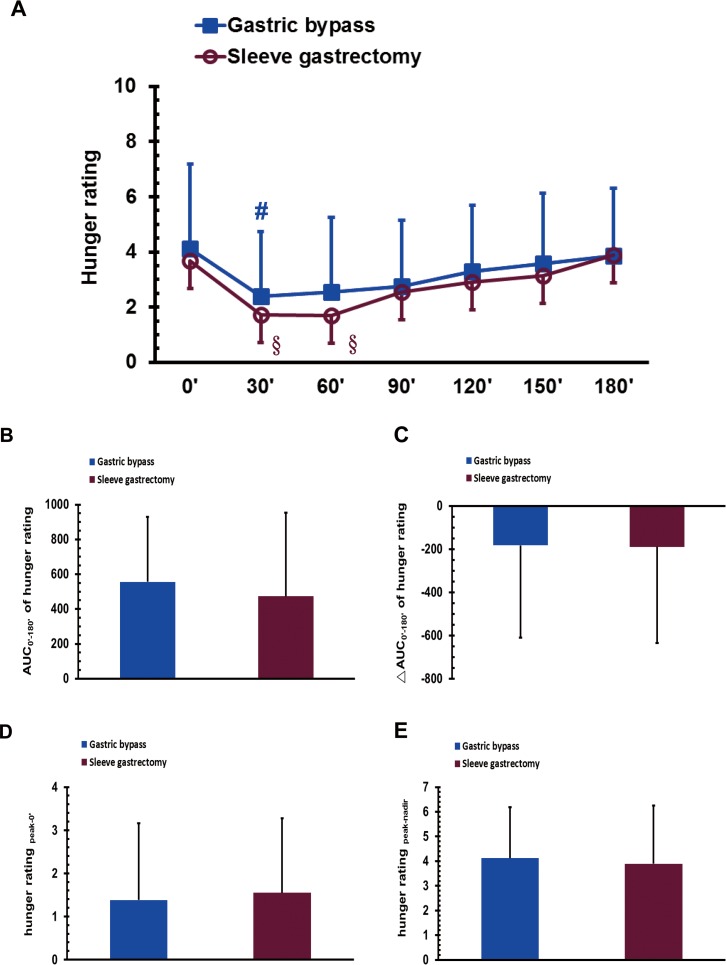
The VAS score of hunger sensation in the GB group, but not in the SG group, at 30 min was significantly lower than that at 0 min in the MMTT (P < 0.05; Fig. 2A). The scores for hunger sensation in the SG group at 30 and 60 min were significantly lower than that at 180 min (P < 0.05 and 0.05; Fig. 2A). No significant difference was found between the GB and SG groups for the AUC0′–180′ of the hunger score, the ΔAUC0′–180′ of the hunger score, the hunger score for peak-0′ or the hunger score for peak-nadir (Figs. 2B–2E).
Figure 2. The visual analogue scale scores of hunger sensation in gastric bypass and sleeve gastrectomy patients at 0, 30, 60, 90, 120, 150 and 180 min (A), as well as the AUC0′–180′ of the hunger rating (B), the ΔAUC0′–180′ of the hunger rating (C), the hunger rating peak-0′ (D), and the hunger rating peak-nadir (E) in a mixed meal tolerance test.
# P < 0.05 compared to 0 min, §P < 0.05 compared to 180 min.
Fullness sensation in MMTT after bariatric surgery
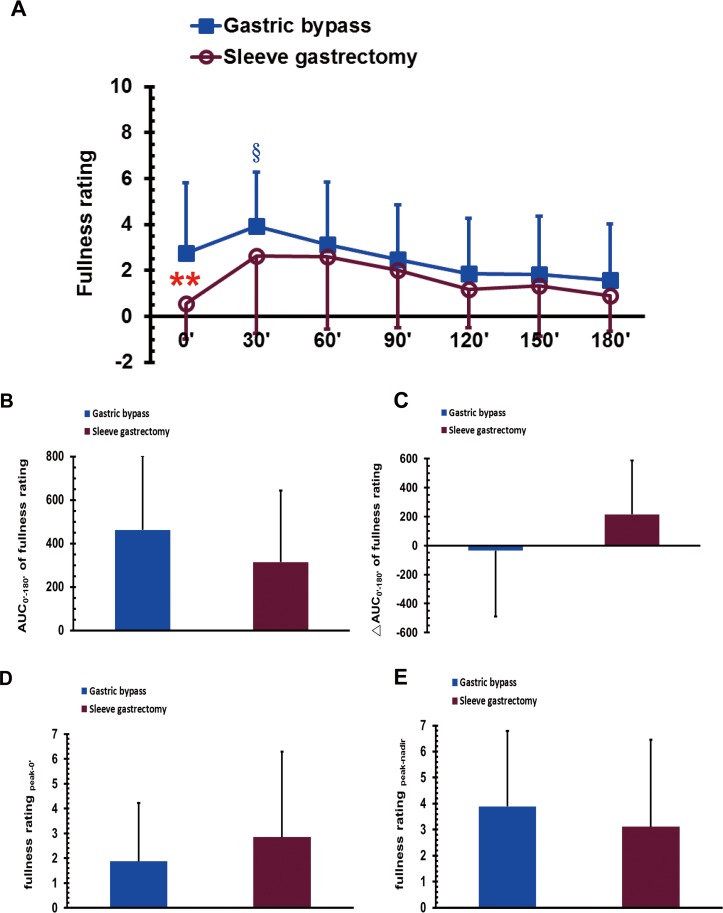
The VAS score of postoperative fasting fullness sensation was significantly higher in the GB group than in the SG group (P < 0.01; Fig. 3A). The score for fullness sensation in the GB group at 30 min was significantly higher than that at 120, 150, and 180 min in the MMTT (P < 0.05; Fig. 3A). No significant difference was found between the GB and SG groups in either the AUC0′–180′ of the fullness rating, the ΔAUC0′–180′ of the fullness rating, the fullness score for peak-0′ or the fullness score for peak-nadir (Figs. 3B–3E).
Figure 3. The visual analogue scale scores of fullness sensation in gastric bypass and sleeve gastrectomy patients at 0, 30, 60, 90, 120, 150 and 180 min (A), as well as the AUC0′–180′ of the fullness rating (B), the ΔAUC0′–180′ of the fullness rating (C), the fullness rating peak-0′ (D), and the fullness rating peak-nadir (E) in a mixed meal tolerance test.
** P < 0.01 compared between gastric bypass and sleeve gastrectomy groups, § P < 0.05 compared to 120, 150, and 180 min.
Desire to eat sensation in the MMTT after bariatric surgery
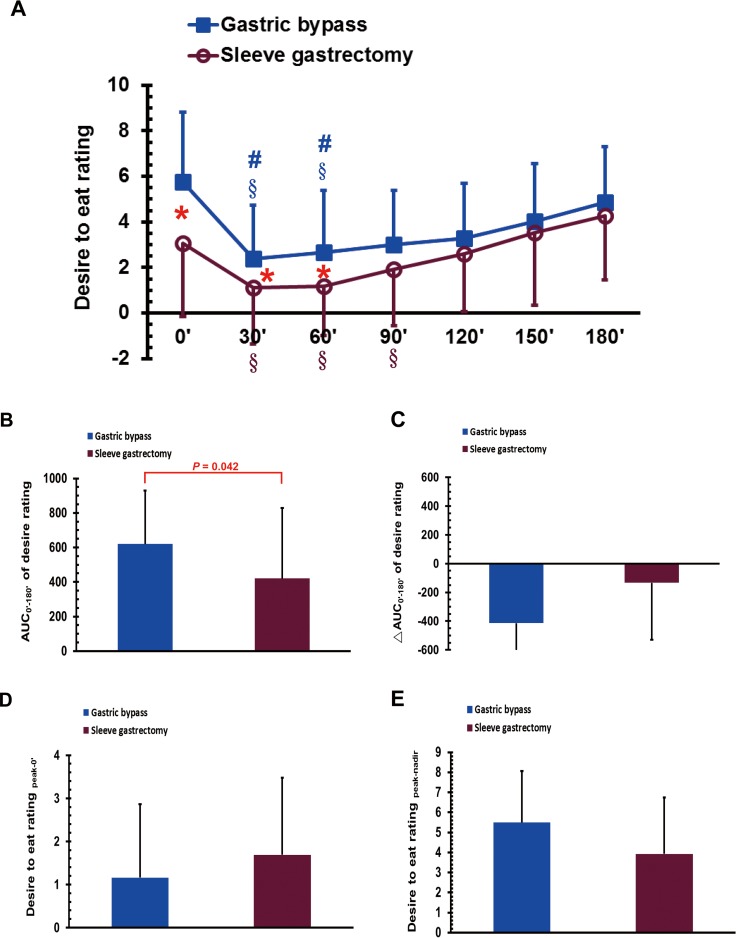
The VAS scores for postoperative desire to eat were significantly higher in the GB group than in the SG group at 0, 30, and 60 min (P < 0.05; Fig. 4A). The scores for desire to eat in the GB group at 30 and 60 min were significantly lower than that at 0 and 180 min during the MMTT (P < 0.05; Fig. 4A). The scores for desire to eat in the SG group at 30, 60, and 90 min were significantly lower than that at 180 min during the MMTT (P < 0.05; Fig. 4A). The AUC0′–180′ of the desire to eat was higher in the GB group than in the SG group (P < 0.05; Fig. 4B). No significant differences were observed between the GB and SG groups in either the ΔAUC0′–180′ of the desire to eat rating, the desire to eat rating for peak-0′, or the desire to eat rating for peak-nadir (Figs. 4C–4E).
Figure 4. The visual analogue scale scores of desire to eat in gastric bypass and sleeve gastrectomy patients at 0, 30, 60, 90, 120, 150 and 180 min (A), as well as the AUC0′–180′ of the desire to eat (B), the ΔAUC0′–180′ of the desire to eat (C), the desire to eatpeak-0′ (D) and the desire to eat peak-nadir (E) in a mixed meal tolerance test.
* P < 0.05 compared between gastric bypass and sleeve gastrectomy groups, # P < 0.05 compared to 0 min, §P < 0.05 compared to 180 min.
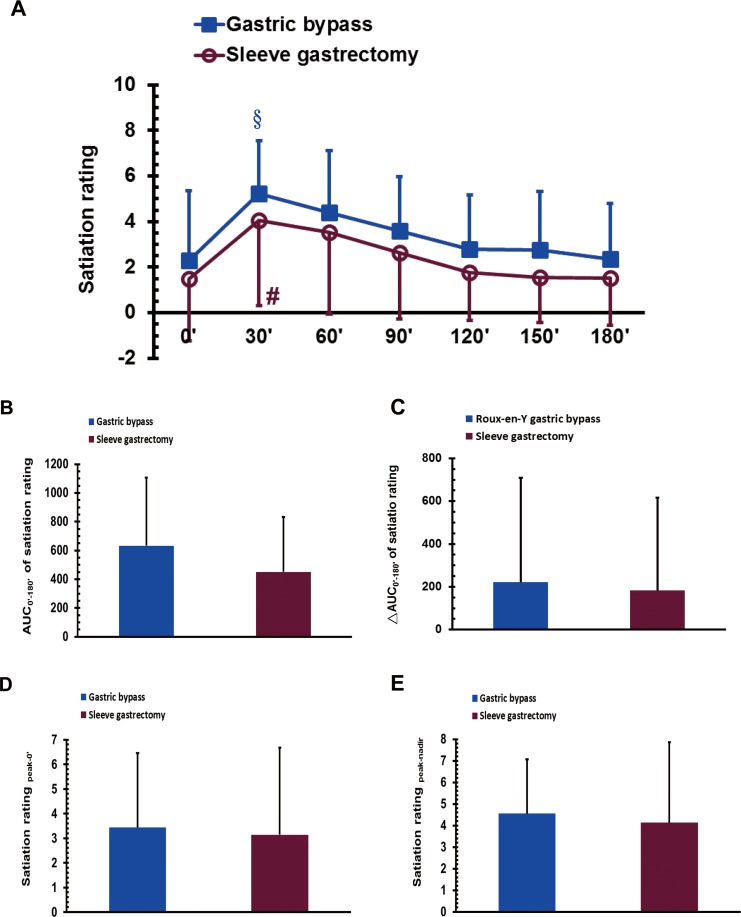
Satiation sensation during the MMTT after bariatric surgery
The VAS score for satiation sensation in the GB group at 30 min was significantly higher than that at 150 and 180 min during the MMTT (P < 0.05; Fig. 5A). The VAS score for satiation in the SG at 30 min was significantly higher than that at 0 min during the MMTT (P < 0.05; Fig. 5A). No significant difference was observed between the GB and SG groups for either the AUC0′–180′ of satiation, the ΔAUC0′–180′ of satiation, the satiation score for peak-0′ or the satiation score for peak-nadir (Figs. 5B–5E).
Figure 5. The visual analogue scale scores of satiation in gastric bypass and sleeve gastrectomy patients at 0, 30, 60, 90, 120, 150 and 180 min (A), as well as the AUC0′–180′ of satiation (B), the ΔAUC0′–180′ of satiation (C), the satiation peak-0′ (D), and the satiation peak-nadir (E) in a mixed meal tolerance test.
# P < 0.05 compared to 0 min, § P < 0.05 compared to 150 and 180 min.
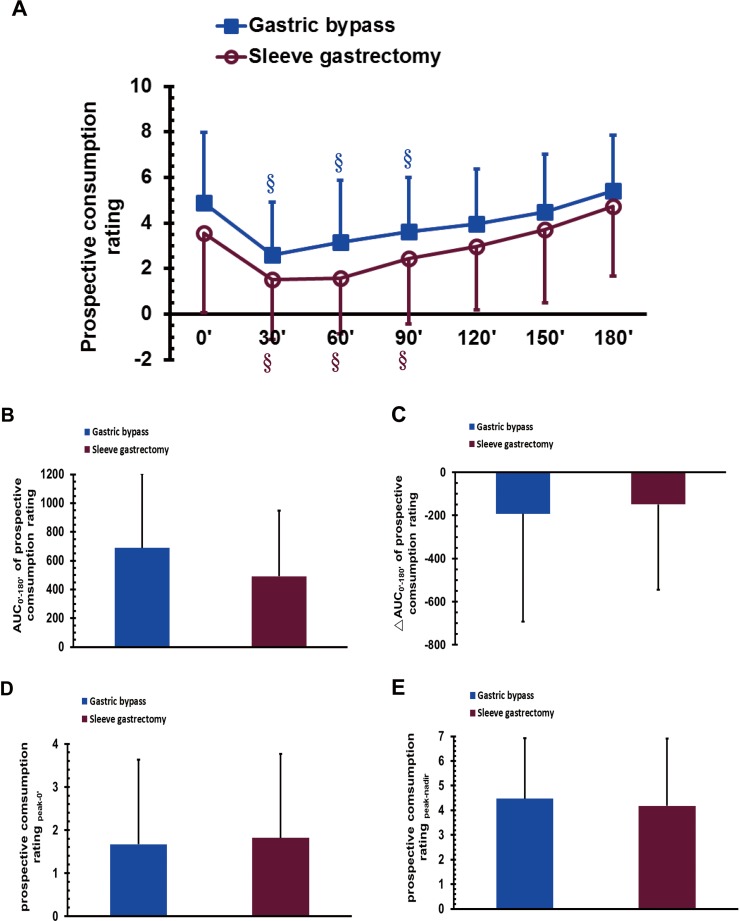
Prospective consumption sensations during the MMTT after bariatric surgery
The VAS scores of prospective consumption in the GB group at 30, 60, and 90 min were significantly lower than that at 180 min during the MMTT (P < 0.05; Fig. 6A); a similar trend was observed for the SG group (P < 0.05; Fig. 6A). No significant difference was observed between the GB and SG groups in either the AUC0′–180′ of prospective consumption, the ΔAUC0′–180′ of prospective consumption, the prospective consumption score for peak-0′, or the prospective consumption score for peak-nadir (Figs. 6B–6E).
Figure 6. The visual analogue scale scores of prospective consumption in gastric bypass and sleeve gastrectomy patients at 0, 30, 60, 90, 120, 150 and 180 min (A), as well as the AUC0′–180′ of prospective consumption (B), the ΔAUC0′–180′ of prospective consumption (C), the prospective consumption peak-0′ (D), and the prospective consumption peak-nadir (E) in a mixed meal tolerance test.
§P < 0.05 compared to 180 min.
The correlations between ABCD score and consumption behaviors
In the GB group, the duration of T2D before surgery was negatively correlated with the post-operative consumed time during the MMTT (ρ = − 0.617, P = 0.0105). The other parameters of ABCD score was not correlated with any consumption behavior.
In the SG group, there was no any correlation between any parameter of ABCD score and any consumption behavior.
The correlations between ABCD score and appetite sensations
The correlations between the age or duration of T2D before surgery with appetite sensations
In the GB group, there was no any correlation between the age or duration of T2D before surgery, with any appetite sensations.
In the SG group, the age was negative correlated with the post-operative ΔAUC0′–180′ of the desire to eat rating (ρ = − 0.569, P = 0.021), and the post-operative fasting satiation rating (ρ = − 0.554, P = 0.026). In addition, the duration of T2D before surgery was positively correlated with the post-operative fasting satiation rating (ρ = 0.501, P = 0.048).
The correlations between BMI and appetite sensations
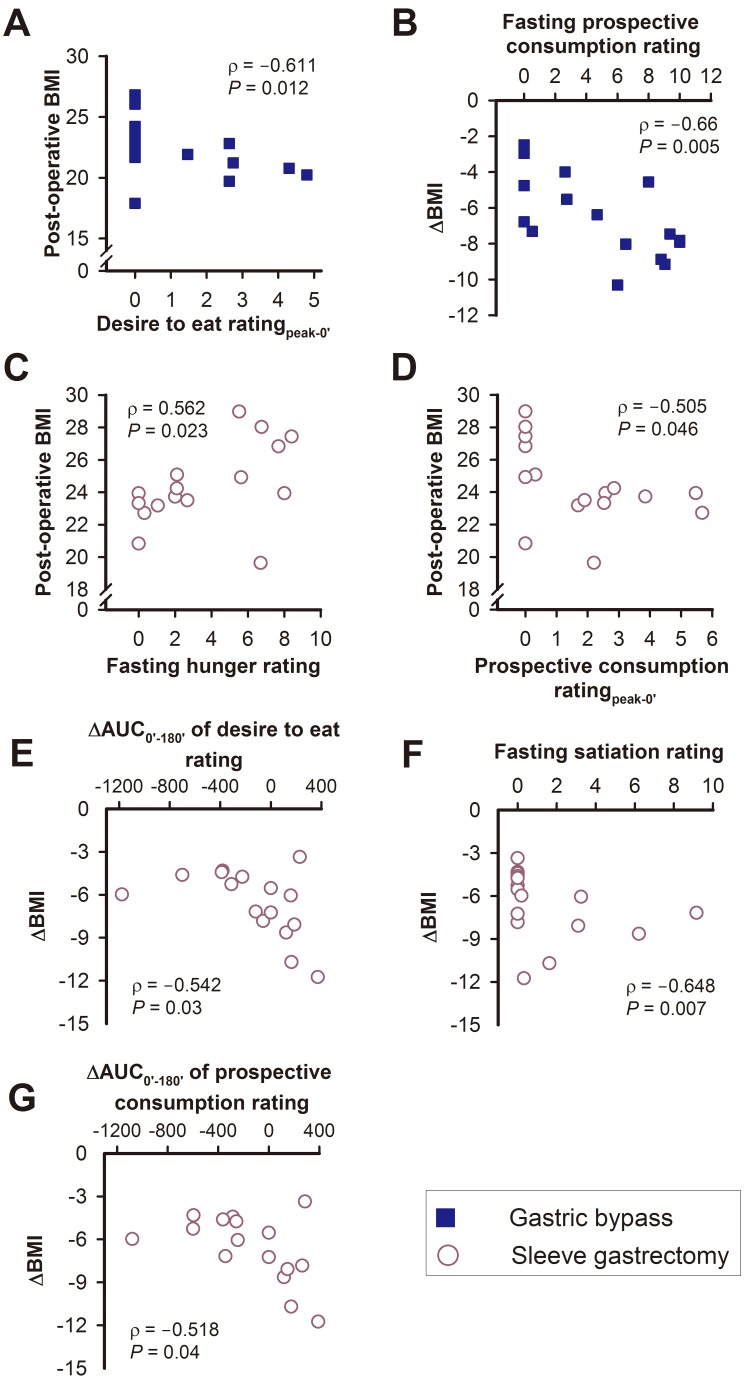
In the GB group, post-operative BMI was negatively correlated with the desire to eat rating for peak-0′, while the ΔBMI was negatively correlated with post-operative fasting VAS scores for prospective consumption one year after surgery (Figs. 7A–7B).
Figure 7. The relationships between post-operative BMI or ΔBMI with various visual analogue scale scores of appetite sensations in the gastric bypass (A, B) and sleeve gastrectomy (C–G) group.
In the SG group, post-operative BMI was positively correlated with the post-operative fasting score for hunger one year after surgery, and negatively correlated with the prospective consumption rating for peak-0′ (Figs. 7C–7D). The ΔBMI was negatively correlated with the post-operative ΔAUC0′–180′ of the desire to eat rating, the post-operative fasting score for satiation, and the post-operative ΔAUC0′–180′ of the prospective consumption (Figs. 7E–7G).
The correlations between C-peptide levels and appetite sensations
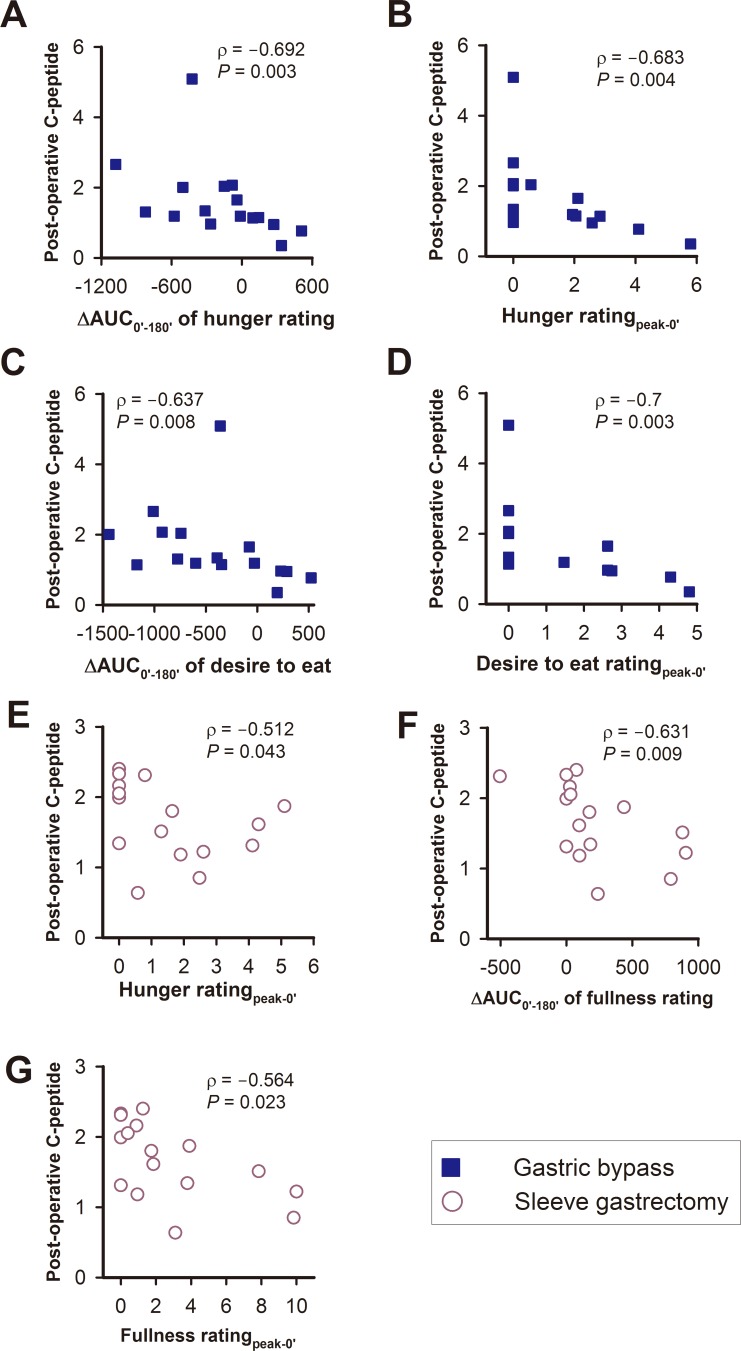
In the GB group, post-operative C-peptide levels were negatively correlated with the ΔAUC0′–180′ of the hunger rating, the hunger rating for peak-0′, ΔAUC0′–180′ of the desire to eat, and the desire to eat rating for peak-0′ (Figs. 8A–8D).
Figure 8. The relationships between post-operative C-peptide levels with various visual analogue scale scores of appetite sensations in the gastric bypass (A–D) and sleeve gastrectomy (E–G) group.
In the SG patients, post-operative C-peptide levels were negatively correlated with the hunger rating for peak-0′, ΔAUC0′–180′ of the fullness rating and the fullness rating score for peak-0′ (Figs. 8G–8H).
Discussion
Bariatric surgery is a promising method for the treatment of obesity by maintaining long-term weight loss, improving risk factors, and decreasing overall mortality (Sjöström, 2008). After bariatric surgery, patients have lower energy intake and higher physical activity levels than obese patients without surgery (Sjöström et al., 2004). These changes might be attributed to modified eating behavior (Saunders, 1999; Laurenius et al., 2012) or bariatric surgical procedures (Saunders, 2001).
VAS scores for appetite are significantly attenuated after GB and SG (Karamanakos et al., 2008), and the volume of consumed food is consistently decreased after bariatric surgery (le Roux et al., 2006). Patients who underwent GB had decreased AUC0′–180′ of the scores for hunger sensation (Borg et al., 2006; Valderas et al., 2010) and increased AUC0′–180′ of the scores for satiety sensation (Korner et al., 2005; Borg et al., 2006; Valderas et al., 2010) as compared with obese patients who did not undergo surgery or with the preoperative condition. GB patients also had a greater increase in satiety sensations than gastric banding patients (Korner et al., 2006). In SG patients, satiety sensation was increased (Valderas et al., 2010), but the results for hunger sensation were inconsistent (Himpens, Dapri & Cadière, 2006; Valderas et al., 2010). Our previous study showed no significant difference in appetite sensation between patients treated with SG and those who underwent duodenal-jejunal bypass with SG (Zachariah et al., 2016).
In this study, we compared the consumption behaviors and appetite sensations of GB and SG patients one year after surgery. The ratio of BMI after/before surgery had no significant difference between GB and SG, which is comparable with our previous study (Lee et al., 2011a). The effect of GB on BMI is similar to that of SG. Interestingly, T2D patients after GB had a decreasing trend of length of time of food consumption compared to those after SG. Because ABCD score is a validated, simple multidimensional grading system to predict successful diabetes remission after GB (Lee et al., 2013) and SG (Lee et al., 2015), our further analysis revealed that the duration of T2D was negatively correlated with the post-operative consumed time during the MMTT in the GB but not in the SG group. This novel finding highlights the importance of the duration of T2D in determining consumption behaviors after GB, in addition to its prediction value in diabetes remission.
Patients who underwent GB had significantly higher fasting scores for fullness and desire to eat, higher AUC0′–180′ of desire to eat, as well as more effective post-meal suppression of hunger and desire to eat compared with those undergoing SG one year after surgery. Taken together, T2D patients after GB might initially have a higher desire to eat with an opposing pre-meal fullness, as well as more significant post-meal suppression of hunger at 30 min and desire to eat at 30 and 60 min during the MMTT compared with those after SG, resulting in a trend toward a shorter length of time of food consumption in patients receiving GB one year after surgery. These differences in appetite sensations between T2D patients after either GB or SG may result from the different surgical procedures. Further studies are required for a detailed explanation of this interesting phenomenon.
T2D remission rates of 93% and 47% can be achieved in GB and SG patients, respectively, one year after surgery (Lee et al., 2011b). Diabetes remission corresponds with the significantly decreased homeostasis model of assessment-insulin resistance and C-peptide levels in obese patients with T2D after bariatric surgery (Lee et al., 2013; Chen et al., 2016). C-peptide is considered as a marker for pancreatic insulin secretion (Bonser & Garcia-Webb, 1984; Hovorka & Jones, 1994), and it is typically used as an index of beta-cell function (Berger, Stenström & Sundkvist, 2000). C-peptide is also one part of the ABCD score, which predicts the success of bariatric surgery for diabetes remission (Lee et al., 2013). Previous studies have been shown that insulin in the brain suppresses food intake in rats (Chen et al., 2012), and intranasal insulin administration reduces the human brain activity stimulated by pictures of food (Guthoff et al., 2010), intensifies satiety and reduces the intake of palatable snacks in women (Hallschmid et al., 2012), and enhances postprandial thermogenesis in healthy men (Benedict et al., 2011). In addition, incremental increases in the AUC for plasma insulin during an oral glucose tolerance test have been shown to predict lower food intake, lower carbohydrate consumption, and reduced weight gain in healthy Pima Indians (He et al., 2011). Collectively, the evidence indicates a role for insulin as a negative feedback signal in the regulation of energy intake and body weight. In our current study, post-operative C-peptide levels in patients after GB were negatively correlated with hunger and desire to eat. Moreover, post-operative C-peptide levels in the SG group were negatively correlated with hunger and fullness.
Lastly, a pleiotropic endocrine response and newly established jejunal nutrient-sensing may contribute to appetite reduction, as well as to long-term improvement in body weight and glycemic control after bariatric surgery. Altered secretions of gut hormones caused by anatomic rearrangement of the gastrointestinal tract after bariatric surgery have been proposed as one of the mechanisms underlying this phenomenon (Chen et al., 2009; Chen et al., 2010; Lee et al., 2011a; Ting et al., 2016). Recently, alteration of gut nutrient-sensing has been considered a potential therapeutic strategy to improve insulin sensitivity and glycemic control in diabetic rats after surgery (Breen et al., 2012; Ren et al., 2015). Collective evidence with our current results corroborates the concept that eating center controlling the consumption behaviors and appetite sensations is structured in a complex manner in the brain. However, these mechanisms require further investigation.
Conclusions
In summary, T2D patients after either GB or SG exhibit distinct nutrient-induced consumption behaviors and appetite sensations post-operatively, which may account for the differential effects on weight loss and glycemic control after different surgery. Post-operative C-peptide levels have differential correlations with various appetite sensations in T2D patients after either GB or SG.
Supplemental Information
Funding Statement
This work was supported by grants from Min-Sheng General Hospital, Taoyuan, and Cheng Hsin General Hospital—National Yang-Ming University Joint Research Program (CY10517 to C Yeh and C-Y Chen), Taipei, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Chun Yeh conceived and designed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Hsien-Hao Huang performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Shu-Chun Chen performed the experiments, follow up all patients.
Tung-Fang Chen performed the experiments.
Kong-Han Ser conceived and designed the experiments, performed the experiments, follow up all patients.
Chih-Yen Chen conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Institutional Review Board of Taipei Veterans General Hospital (the approval number: 201002056IC and 201002037IC).
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Data S1.
References
- Benedict et al. (2011).Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, Hallschmid M. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes. 2011;60(1):114–118. doi: 10.2337/db10-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, Stenström & Sundkvist (2000).Berger B, Stenström G, Sundkvist G. Random C-peptide in the classification of diabetes. Scandinavian Journal of Clinical and Laboratory Investigation. 2000;60(8):687–693. doi: 10.1080/00365510050216411. [DOI] [PubMed] [Google Scholar]
- Bonhomme et al. (2011).Bonhomme S, Guijarro A, Keslacy S, Goncalves CG, Suzuki S, Chen C, Meguid MM. Gastric bypass up-regulates insulin signaling pathway. Nutrition. 2011;27(1):73–80. doi: 10.1016/j.nut.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Bonser & Garcia-Webb (1984).Bonser AM, Garcia-Webb P. C-peptide measurement: methods and clinical utility. CRC Critical Reviews in Clinical Laboratory Sciences. 1984;19(4):297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- Borg et al. (2006).Borg CM, Le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. British Journal of Surgery. 2006;93(2):210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- Breen et al. (2012).Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nature Medicine. 2012;18(6):950–955. doi: 10.1038/nm.2745. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2009).Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacological Reviews. 2009;61(4):430–481. doi: 10.1124/pr.109.001958. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2010).Chen CY, Fujimiya M, Laviano A, Chang FY, Lin HC, Lee SD. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. Journal of the Chinese Medical Association. 2010;73(5):225–229. doi: 10.1016/S1726-4901(10)70048-4. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2016).Chen YC, Inui A, Chang ES, Chen SC, Lee WJ, Chen CY. Comparison of gut hormones and adipokines stimulated by glucagon test among patients with type II diabetes mellitus after metabolic surgery. Neuropeptides. 2016;55:39–45. doi: 10.1016/j.npep.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2013).Chen CY, Tsai CY, Lee PC, Lee SD. Long-term etanercept therapy favors weight gain and ameliorates cachexia in rheumatoid arthritis patients: roles of gut hormones and leptin. Current Pharmaceutical Design. 2013;19(10):1956–1964. doi: 10.2174/1381612811319100014. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2012).Chen CY, Tsai CY, Lee WJ, Liaw WJ, Chiang CH, Ho ST, Lee SD. Intracerebroventricular O-n-octanoylated ghrelin and its splice variant-induced feeding is blocked by insulin, independent of obestatin or CRF receptor, in satiated rats. Nutrition. 2012;28(7–8):812–820. doi: 10.1016/j.nut.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Chiu et al. (2006).Chiu CC, Lee WJ, Wang W, Wei PL, Huang MT. Prevention of trocar-wound hernia in laparoscopic bariatric operations. Obesity Surgery. 2006;16(7):913–918. doi: 10.1381/096089206777822269. [DOI] [PubMed] [Google Scholar]
- Guthoff et al. (2010).Guthoff M, Grichisch Y, Canova C, Tschritter O, Veit R, Hallschmid M, Häring HU, Preissl H, Hennige AM, Fritsche A. Insulin modulates food-related activity in the central nervous system. The Journal of Clinical Endocrinology & Metabolism. 2010;95(2):748–755. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- Hallschmid et al. (2012).Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes. 2012;61(4):782–789. doi: 10.2337/db11-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2011).He J, Votruba S, Venti C, Krakoff J. Higher incremental insulin area under the curve during oral glucose tolerance test predicts less food intake and weight gain. International Journal of Obesity. 2011;35(12):1495–1501. doi: 10.1038/ijo.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens, Dapri & Cadière (2006).Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obesity Surgery. 2006;16(11):1450–1456. doi: 10.1381/096089206778869933. [DOI] [PubMed] [Google Scholar]
- Hossain, Kawar & El Nahas (2007).Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. New England Journal of Medicine. 2007;356(3):213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Hovorka & Jones (1994).Hovorka R, Jones RH. How to measure insulin secretion. Diabetes/Metabolism Reviews. 1994;10(2):91–117. doi: 10.1002/dmr.5610100204. [DOI] [PubMed] [Google Scholar]
- Isomaa et al. (2001).Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44(9):1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- Karamanakos et al. (2008).Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Annals of Surgery. 2008;247(3):401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- Korner et al. (2005).Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- Korner et al. (2006).Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14(9):1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- Laurenius et al. (2012).Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lönroth H, Fändriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. International Journal of Obesity. 2012;36(3):348–355. doi: 10.1038/ijo.2011.217. [DOI] [PubMed] [Google Scholar]
- Le Roux et al. (2006).Le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Annals of Surgery. 2006;243(1):108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2015).Lee WJ, Almulaifi A, Tsou JJ, Ser KH, Lee YC, Chen SC. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surgery for Obesity and Related Diseases. 2015;11(5):991–996. doi: 10.1016/j.soard.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2011a).Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surgery for Obesity and Related Diseases. 2011a;7(6):683–690. doi: 10.1016/j.soard.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2011b).Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Archives of Surgery. 2011b;146(2):143–148. doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2013).Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC, Lee YC, Ser KH. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surgery for Obesity and Related Diseases. 2013;9(3):379–384. doi: 10.1016/j.soard.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Parker et al. (2004).Parker BA, Sturm K, Macintosh CG, Feinle C, Horowitz M, Chapman IM. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. European Journal of Clinical Nutrition. 2004;58(2):212–218. doi: 10.1038/sj.ejcn.1601768. [DOI] [PubMed] [Google Scholar]
- Ren et al. (2015).Ren ZQ, Zhang PB, Zhang XZ, Chen SK, Zhang H, Lv DT, Zhuang BQ, Wen YQ, Hu HH, Ding WC, Zhang C. Duodenal-jejunal exclusion improves insulin resistance in type 2 diabetic rats by upregulating the hepatic insulin signaling pathway. Nutrition. 2015;31(5):733–739. doi: 10.1016/j.nut.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Saunders (1999).Saunders R. Binge eating in gastric bypass patients before surgery. Obesity Surgery. 1999;9(1):72–76. doi: 10.1381/096089299765553845. [DOI] [PubMed] [Google Scholar]
- Saunders (2001).Saunders R. Compulsive eating and gastric bypass surgery: what does hunger have to do with it? Obesity Surgery. 2001;11(6):757–761. doi: 10.1381/09608920160558731. [DOI] [PubMed] [Google Scholar]
- Schauer et al. (2014).Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR, STAMPEDE Investigators Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. New England Journal of Medicine. 2014;370(21):2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström (2008).Sjöström L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. International Journal of Obesity. 2008;32:S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- Sjöström et al. (2004).Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H, Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New England Journal of Medicine. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- Speechly & Buffenstein (1999).Speechly DP, Buffenstein R. Greater appetite control associated with an increased frequency of eating in lean males. Appetite. 1999;33(3):285–297. doi: 10.1006/appe.1999.0265. [DOI] [PubMed] [Google Scholar]
- Ting et al. (2016).Ting CH, Syu YF, Chen LY, Lee FY, Lee SD, Lee WJ, Chen CY. Perspectives in interventional diabetology: duodenal exclusion is promising for human type 2 diabetes mellitus remission. Nutrition. 2016;32(1):141–145. doi: 10.1016/j.nut.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Valderas et al. (2010).Valderas JP, Irribarra V, Boza C, De la Cruz R, Liberona Y, Acosta AM, Yolito M, Maiz A. Medical and surgical treatments for obesity have opposite effects on peptide YY and appetite: a prospective study controlled for weight loss. The Journal of Clinical Endocrinology & Metabolism. 2010;95(3):1069–1075. doi: 10.1210/jc.2009-0983. [DOI] [PubMed] [Google Scholar]
- Wild et al. (2004).Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Zachariah et al. (2016).Zachariah PJ, Chen CY, Lee WJ, Chen SC, Ser KH, Chen JC, Lee YC. Compared to sleeve gastrectomy, duodenal-jejunal bypass with sleeve gastrectomy gives better glycemic control in T2DM patients, with a lower β-cell response and similar appetite sensations: mixed-meal study. Obesity Surgery. 2016;26(12):2862–2872. doi: 10.1007/s11695-016-2205-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Data S1.