Abstract
Clinically, Angiotensin II (Ang II) has been implicated in some forms of hypertension and linked to vascular injury. Experimentally, chronic Ang II infusion leads to an increase in blood pressure, resulting in impaired endothelial function and vascular hypertrophy. Ang II also upregulates the activity and expression of a number of inflammatory molecules, including nuclear factor kappa B (NFκB) and pro-inflammatory cytokines, such as interleukin-6 (IL-6). More recently, it has been reported that Ang II is associated with upregulation of toll-like receptor TLR expression, specifically TLR4. Classical TLR4 signaling is mediated in large part by the effector protein myeloid differentiation factor 88 (MyD88), with resultant activation of NFκB, a transcription factor that promotes expression of a number of inflammatory gene products, including IL-6. A role for IL-6 has been previously implicated in the vascular dysfunction associated with Ang II-dependent hypertension. It is not known whether the MyD88 signaling pathway represents a cellular mechanism by which Ang II promotes endothelial dysfunction via NFκB activation and increases in IL-6. Taken together, we propose to mechanistically elucidate the role of innate immune signaling in Ang II-dependent hypertension. We hypothesize MyD88-deficiency will prevent the activation and transcription of NFκB-related gene products, including IL-6, thereby limiting Ang II-dependent hypertension and vascular complications.
Keywords: inflammation, toll-like receptors, MyD88, endothelial dysfunction
Introduction
Hypertension is a major risk factor for cardiovascular disease affecting nearly one in three adults in the United States (1). Angiotensin II (Ang II), the main effector of the renin-angiotensin-system (RAS), is implicated in several forms of hypertension, including essential and renovascular hypertension (2, 3). Experimentally, chronic Ang II infusion results in a sustained increase in blood pressure, endothelial dysfunction, and vascular hypertrophy. Ang II mediates its cardiovascular effects primarily through angiotensin II receptors, type 1 (AT1). AT1-deficiency limits the increase in blood pressure and endothelial dysfunction in response to Ang II infusion (4). Moreover, AT1 receptor activation increases reactive oxygen species (ROS), such as superoxide via increased NADPH oxidase expression and activity (5–10). Functionally, increases in vascular superoxide are important for a number of reasons. For example, superoxide activates redox sensitive transcription factors, including NFκB (11, 12). ROS, including superoxide have been linked to activation of the innate immune response, and ROS scavengers limit the production of pro-inflammatory cytokines. Endothelial dysfunction is limited by the ROS scavengers Tiron and Tempol in mice infused with Ang II (13, 14). Similarly, transgenic expression of superoxide dismutase isoforms limits oxidative stress and endothelial dysfunction produced by Ang II (14). Taken together, there is strong pharmacological and genetic evidence linking AT1 activation with increases superoxide and vascular dysfunction.
In addition to promoting increases in ROS, Ang II increases expression of a number of inflammatory molecules, such as the pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IL-17 to name a few (15–18). Clinically, levels of IL-6 have been positively correlated with blood pressure (19). Furthermore, increased IL-6 levels are associated with end-organ damage in hypertensive subjects (20–22). Experimentally, IL-6 deficiency is protective against endothelial dysfunction as well as vascular hypertrophy produced by Ang II, suggesting a critical role for inflammation in vascular impairment (23–25). Thus, IL-6 appears to have an important role in the development of hypertension and endothelial dysfunction. Although immune signaling appears to be involved to varying degrees in the development and maintenance of hypertension, few interventions directed at the inflammatory response have been studied for the treatment of high blood pressure. We hypothesize MyD88 signaling plays an essential role in the vascular complications seen in Ang II-dependent hypertension. Therefore, we propose MyD88-deficiency will limit the activation and transcription of NFκB-related gene products and prevent Ang II-dependent hypertension and vascular complications.
The Hypothesis
Adaptive Immunity in Hypertension
Emerging evidence has begun to implicate a role for the adaptive immune system in the pathology of hypertension (26–31). The idea of the immune system playing a role in the development of hypertension first came from the observation that the thymus was necessary for the late salt-dependent phase of hypertension in a DOCA-salt model (26). The importance of the thymus in hypertension was later studied in the spontaneous hypertensive rat (SHR), which develops high blood pressure with age. The increase of blood pressure in the SHR parallels immunological depression and diminished T-cell function (27, 28). Furthermore, hypertension was prevented in the SHR that were injected by thymus grafts as neonates (29). In addition, mice lacking both B- and T-lymphocytes (RAG1−/−) do not develop hypertension or endothelial dysfunction and vascular hypertrophy following Ang II infusion (30). However, with the adoptive transfer of activated T-lymphocytes, but not B-lymphocytes, Ang II-dependent hypertension and vascular dysfunctions are restored (30). A large number of infiltrating T-lymphocytes and macrophages are found in the perivascular tissue of hypertensive animals, suggesting there is an initial innate immune response occurring locally in the vasculature. Classically, activation of the innate immune system is necessary for the stimulation of adaptive immunity. However, it is yet to be determined what specific innate immunity components are triggering T-lymphocytes in hypertension.
Evolutionarily, the innate immune system represents the body’s first line of defense against infection. Once activated by a foreign pathogen, antigen-presenting cells bind and activate leukocytes of the adaptive immune system to initiate an antigen-specific response. Phagocytes are the major cells to initiate this process. These include macrophages, neutrophils, and dendritic cells, all of which possess TLR’s. Initially, these cells will activate intracellular processes resulting in the transcription of pro-inflammatory cytokines. Exogenous and endogenous molecules, including Ang II, have been shown to activate TLR’s through binding recognition patterns.
Ang II and Inflammation
TLR’s are an essential part of the mammalian inflammatory response and are able to recognize and respond to foreign pathogens. Though most often considered the lipopolysaccharide (LPS) receptor, TLR4 can also be activated by endogenous molecules, such as heat shock proteins and fibrinogen (32). TLR4 is located on the cell surface of myeloid dendritic cells as well as peripheral and central macrophages. More recently, it has been shown TLR4 is present in atherosclerotic legions, suggesting a role in cardiovascular disease (33–35). In vitro experiments utilizing vascular smooth muscle cells demonstrate Ang II increases TLR4 expression (36). Clinically, TLR4 gene Asp299Gly polymorphisms decrease inflammatory responses and are associated with decreased vascular inflammation and a reduced risk for coronary artery disease (37–39). Our laboratory has preliminary data showing that the loss-of-function point mutation in TLR4 limits endothelial dysfunction after chronic Ang II infusion (40). Taken together, this suggests detrimentally high levels of IL-6 in hypertensive patients may be derived through TLR4 signaling.
Several cytokines, including IL-6, are responsible for the development and progression of cardiovascular diseases, such as heart failure, atherosclerosis, and hypertension (20, 41–43). IL-6 production is not limited to macrophages but can be produced locally by endothelial cells and vascular smooth muscle cells (44, 45). Ang II increases expression of IL-6 in the vasculature in-vitro. Our laboratory has shown IL-6 deficient mice limited endothelial dysfunction and vascular hypertrophy in mice chronically infused with Ang II (23). Additionally, we have shown that an increase in vascular superoxide, in particular NOX2- derived superoxide, contributes to the endothelial dysfunction produced by IL-6 in Ang II-dependent hypertension (13, 46). Taken together, these data suggest a role for inflammation in vascular pathophysiology in hypertension.
MyD88 Signaling
TLR’s, with the exception of TLR3, signal primarily through the MyD88-dependent signaling pathway (47, 48). As an adaptor protein, MyD88 plays a crucial role in the innate immune system and in inflammatory responses, through activation of NFkB. Upon TLR4 activation, the receptor’s intracellular domain binds with the homologous TIR domain of MyD88, initiating the recruitment cascade of IRAK’s and TRAF-6 (49, 50). This complex dissociates from MyD88 and causes the dissociation of IkB from NFkB to allow translocation to the nucleus and transcription of pro-inflammatory cytokines (51, 52). MyD88-deficient mice have little macrophage production or leukocyte proliferation in response to LPS (53). However, NFkB activation in MyD88-deficient mice occurs but with delayed kinetics (53). In addition to a suspended inflammatory response, there is evidence suggesting MyD88 deficiency protects against cardiovascular disease.
MyD88 deficiency limits vascular hypertrophy and the frequency of ascending aortic aneurysms in mice treated with Ang II (54). Though little has been done to relate hypertension to TLR4 signaling, we hypothesize MyD88- deficiency will limit endothelial dysfunction and vascular hypertrophy after chronic Ang II infusion.
Evaluation of the Hypothesis
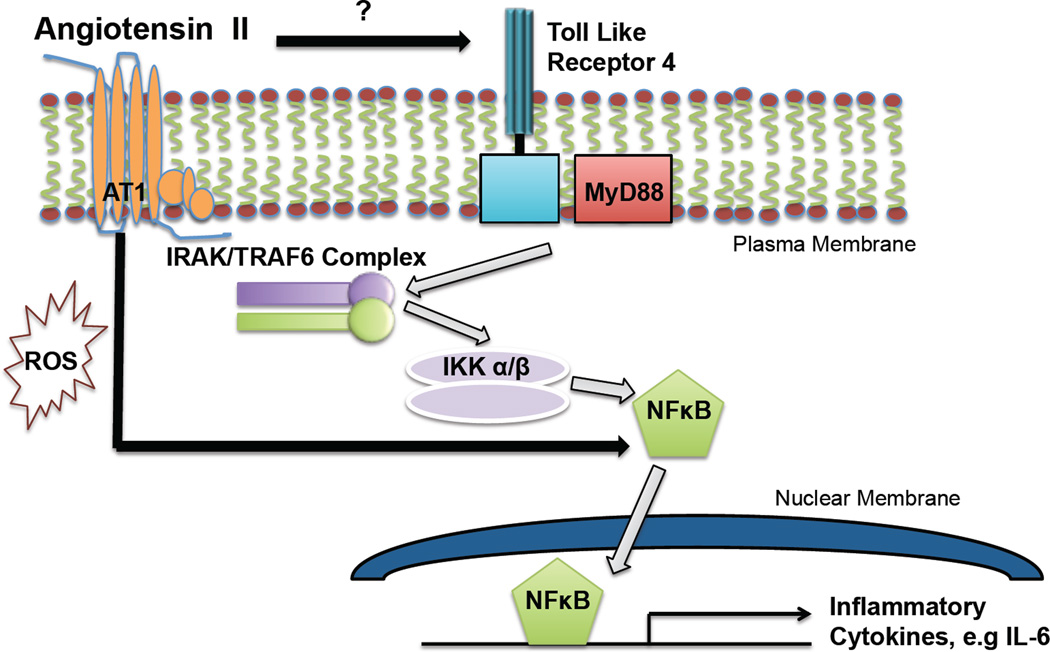
Although initial evidence, including our own, has demonstrated TLR4 is expressed in vascular cells, no studies to our knowledge have examined the in vivo contribution of vascular TLR4 and MyD88 expression in the development of hypertension and related vascular sequale. Thus, we hypothesize that as a part of the innate immune response, MyD88 signaling contributes to the hypertension and endothelial dysfunction produced by Ang II (Figure).
Figure 1.
Hypothetical scheme in which MyD88 plays an essential role in NFκB-related gene products, which results in endothelial dysfunction and vascular hypertrophy in Ang II-dependent hypertension. Ang II increases TLR4 expression, however the mechanism of which is not clear. In addition, the AT1 receptor via Ang II increases NFκB activation.
We will test this hypothesis in TLR4- and MyD88-deficient mice made hypertensive by chronic Ang II infusion. We will assess blood pressure and vascular function as well as the ROS and inflammatory profiles of these mice. If our hypothesis is true, we predict that TLR4 and MyD88 deficiency will limit the development of hypertension and related endothelial dysfunction produced by Ang II. We predict NFkB translocation and levels of IL-6 will be decreased in TLR4- and MyD88-deficient mice relative to their respective controls after Ang II infusion.
If our hypothesis is correct, we predict that the reductions in blood pressure, endothelial dysfunction, and vascular hypertrophy through the loss of MyD88, would correlate with reductions in superoxide levels and, therefore improve endothelial function. Furthermore, we expect TLR4 expression and IL-6 levels will be the decreased in MyD88-deficient mice following Ang II infusion relative to wild-type mice. We hypothesize this decreased degree of inflammation will limit the vascular impairment seen in Ang II-dependent hypertension. Taken together, these results could lead to potential therapeutic approaches in the treatment of hypertension and its associated vascular dysfunction.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants NIH HL-089884, HL-107632 and T32 HL-105324.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare they have no conflicts of interest.
References
- 1.CDC. Vital signs: prevalence, treatment, and control of hypertension— United States, 1999–2002 and 2005–2008. MMWR. 2011;60(4):103–108. [PubMed] [Google Scholar]
- 2.Catt KJ, Cain MD, Zimmet PZ, Cran MB. Blood angiotensin II levels of normal and hypertensive subjects. Brit Med J. 1969;1:819–821. doi: 10.1136/bmj.1.5647.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catt KJ, Cran E, Zimmet PZ, Best JB, Cain MD, Coghlan JP. Angiotensin II blood-levels in human hypertension. Lancet. 1971;1:459–464. doi: 10.1016/s0140-6736(71)91085-3. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 6.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capters Q, 4th, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 8.Lassègue B, Sorescu D, Szöcs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells nox 1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 9.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox 1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 10.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satriano J, Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-alpha, immunoglobulin G, and adenosine 3’:5’-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Invest. 1994;94:1629–1636. doi: 10.1172/JCI117505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kB. Cell. 1995;2:13. doi: 10.1016/1074-5521(95)90076-4. 22. [DOI] [PubMed] [Google Scholar]
- 13.Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–2042. doi: 10.1161/01.STR.0000081225.46324.AA. [DOI] [PubMed] [Google Scholar]
- 14.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 15.Ferreri N, Zhao Y, Takizawa H, McGiff JC. Tumor necrosis factor-α- angiotensin interactions and regulation in blood pressure. Hypertension. 1997;15:1481–1484. doi: 10.1097/00004872-199715120-00016. [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension. 1999;34:118–125. doi: 10.1161/01.hyp.34.1.118. [DOI] [PubMed] [Google Scholar]
- 17.Dörffel Y, Lätsch C, Stuhlmüller B, Schreiber S, Scholze S, Burmester GR, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 18.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of IL-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule I and risk of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 23.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II-induced endothelial dysfunction and hypertrophy. Arterioslcer Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 24.Lee DL, Strugis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 25.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O’Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A. 1976;84 doi: 10.1111/j.1699-0463.1976.tb00150.x. 532-528. [DOI] [PubMed] [Google Scholar]
- 27.Takeichi N, Suzuki T, Okayasu T, Kobayashi H. Immunological depression in spontaneously hypertensive rats. Clin Exp Immunol. 1980;40:120–126. [PMC free article] [PubMed] [Google Scholar]
- 28.Takeichi N, Ba D, Suzuki K, Kobayashi H. The arrest of T-cell maturation in spontaneously hypertensive rats with a deficient production of thymic factors. Cell Immunol. 1982;128:1211–1216. doi: 10.1111/j.1440-1827.1985.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 29.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T Cell Depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immuno. 1982;128:1211–1216. [PubMed] [Google Scholar]
- 30.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. JEM. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowly SD, Frey CW, Gould SK, Griffins R, Ruiz P, Burchette JL, Howell DW, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/ 10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 33.Xu XH, Shah PK, Faure E, Equills O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 34.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 35.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2009;23:265–276. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 36.Wolf G, Bohlender J, Bondeva T, Roger T, Thaiss F, Wenzel UO. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006;17:1585–1593. doi: 10.1681/ASN.2005070699. [DOI] [PubMed] [Google Scholar]
- 37.Kolek MJ, Carlquist JF, Muhlestein JB, Whiting BM, Horne BD, Bair TL, Anderson JL. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am Heart J. 2004;148:1034–1040. doi: 10.1016/j.ahj.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 38.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 39.Ameziane N, Beillat T, Verpillat P, Chollet-Martin S, Aumont MC, Seknadji P, Lamotte M, Lebret D, Ollivier V, de Prost D. Association of the toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol. 2003;23:e61–e64. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 40.Palvia V, Pyle C, Chen X, Didion SP. Toll-like receptor 4 contributes to hypertension and endothelial dysfunction produced by angiotensin II. FASEB. 2012;26:875–879. [Google Scholar]
- 41.Ikeda U, Ohkawa F, Seino Y, Yamamoto K, Hidaka Y, Kasahara T, Kawai T, Shimada K. Serum interleukin 6 levels become elevated in acute myocardial infarction. J Mol Cell Cardiol. 1992;24:579–584. doi: 10.1016/0022-2828(92)91042-4. [DOI] [PubMed] [Google Scholar]
- 42.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 43.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 44.Loppnow H, Libby P. Adult human vascular endothelial cells express the IL-6 gene differentially in response to LPS to IL1. Cell Immunol. 1989;122:493–503. doi: 10.1016/0008-8749(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 45.Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990;85:731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girouard H, Park L, Anrather J, Zhuo P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88- dependent signaling cascades. Int Immunol. 2000;12:113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Takeuchi O, Fujita T, Inoue JI, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and expression of a subset of lipopolysaccharide-inducible genes. J Immuno. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 49.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 50.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A. TRAF6 deficiency results in osteoporosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng L, Wang C, Spencer E, Yang L, Braun A, You J. Activation of IkB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 52.Wang C, Deng L, Hong M, Akkaraju GR, Inoue JI, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 53.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 54.Owens AP, Rateri DL, Howatt DA, Moore KJ, Tobias PS, Curtiss LK, Lu H, Cassis LA, Daughtery A. MyD88 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation independent of signaling through Toll-like receptors 2 and 4. Arterioscler Thromb Vasc Biol. 2011;31:2813–2819. doi: 10.1161/ATVBAHA.111.238642. [DOI] [PMC free article] [PubMed] [Google Scholar]