Abstract
Selective suppression of effector CD4+ T cell functions is necessary to prevent immune cell-mediated damage to healthy tissues. This appears especially true during pregnancy or in individuals predisposed to autoimmunity. Foxp3+ regulatory T (Treg) cells and induction of anergy, an acquired state of T cell functional unresponsiveness in Foxp3− cells, have both been implicated as mechanisms to suppress dangerous immune responses to tissue-restricted self antigens. Anergic CD4+ T cells and Treg cells share a number of phenotypic and mechanistic traits—including the expression of CD73 and folate receptor 4 (FR4), and the epigenetic modification of Treg cell signature genes—and an interesting relationship between these two subsets has recently emerged. In this review, we will compare and contrast these two subsets as well as explore the role of anergy in the generation of peripheral Treg cells.
Introduction
T cell self-tolerance mechanisms can be broadly characterized as central or peripheral (Fig. 1). Central tolerance mechanisms destroy high affinity self-reactive T cells during thymic development, or else induce their differentiation into a regulatory T (Treg) cell lineage (1). Nonetheless, central tolerance appears insufficient to clear all self-reactive T cells and other peripheral tolerance mechanisms are necessary (2, 3). Peripheral tolerance may rely on 1) ignorance, wherein autoreactive T cells never encounter their cognate antigen, 2) deletion, whereby self-specific peripheral T cells are destroyed after TCR engagement, 3) anergy, which is a state of functional unresponsiveness induced upon self antigen recognition, and/or 4) Foxp3+ Treg cell-mediated suppression of dangerous T cell responses against self antigen. Each of these potential tolerance mechanisms has been clearly defined in numerous in vivo experimental systems using TCR-transgenic responder T cells—typically at abnormally high cell frequencies. However, much less is known about self-tolerance in the natural polyclonal CD4+ T cell repertoire.
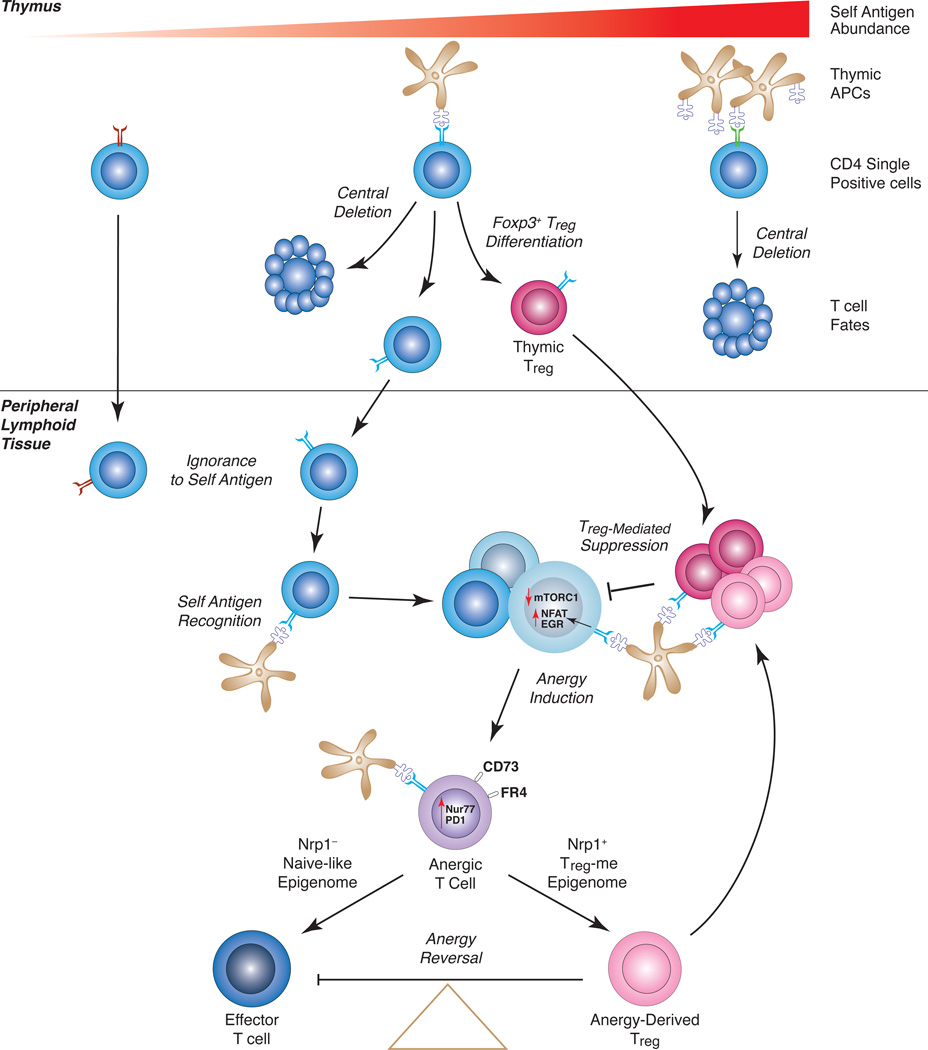
Figure 1. The Role of Anergy in pTreg Generation – A Model.
CD4 single-positive thymocytes specific for tissue-restricted self antigens can experience one of several fates. Abundant thymic self pMHCII presentation leads to central deletion. If self pMHCII abundance is very low, thymocytes escape to the periphery and often ignore the antigen. For the case of intermediate self pMHCII complex abundance, some thymocytes will die, some will differentiate to a Treg cell fate, and some will escape the thymus. Self antigen-specific CD4+ T cells that escape into the periphery can recognize peripheral self pMHCII, and through the suppressive actions of Treg cells become anergic cells that express CD73 and FR4. Persistent antigen encounter induces Nrp1 expression and partial de-methylation of the Treg-me in some of the anergic T cells. Nrp1+ Anergic T cells then become precursors for Treg cell differentiation. Upon conversion to a stable pTreg cell lineage, anergy-derived Treg cells join the tTreg cell pool to suppress immunopathology and reinforce anergy induction.
Treg cells control tolerance to some tissue-restricted self antigens
Two recent studies took advantage of experimental antigens (peptides derived from either the P1 bacteriophage Cre recombinase (Cre) or the Aequorea victoria enhanced green fluorescent protein (eGFP)) whose expression in mice was directed by transgenic tissue-specific promoters, and additionally made use of pMHCII tetramers to detect polyclonal CD4+ T cells that respond to these model self antigens (4, 5). Both experiments demonstrated that ubiquitous (both thymic and peripheral) expression of self antigen leads to deletion of 60 to 95% of the highest affinity self-specific CD4+ T cells, with the remaining low affinity cells left functionally unresponsive to antigen (Fig. 1). In contrast, restriction of self antigen expression exclusively to non-thymic tissues leads to a tolerance that appears to rely solely on ignorance by naive autoreactive T cells. The strongest evidence for an active peripheral self-tolerance mechanism in these studies came in the form of self antigens that were only weakly expressed in the thymus and, in particular, were otherwise restricted to mucosal tissues (e.g., Cre protein expression in the intestinal brush border driven by a Vil1-Cre transgene, or expression in airway Clara cells driven by a Scgb1a1-Cre transgene). These antigens induce little central deletion, and instead self-tolerance relies on the generation of self pMHCII-binding Foxp3+ Treg cells (5). Importantly, these Treg cells appear to suppress the conventional CD4+ T cell responses to self antigen, as systemic ablation of Foxp3+ Treg cells at the time of a self antigen immunization led to a restoration of the conventional T cell clonal expansion and cytokine production response to near control levels (5).
In addition to Treg cells, several other suppressor cells have been described such as the Tr1 cells, myeloid-derived suppressor cells (MDSCs), as well as the regulatory B cells. However, it is well established that Foxp3+ Treg cells maintain immune homeostasis and prevent adverse immune responses throughout the lifespan of an individual. Foxp3 is an essential lineage-defining transcription factor of Treg cells (6, 7), as mutations or deletion of Foxp3 gene lead to impaired generation of Treg cells and cause severe autoimmunity in humans and mice (6, 8). Furthermore, instability of Foxp3 expression allows for the trans-differentiation of Treg cells to T effector cell lineages capable of causing autoimmunity (9, 10). Although Foxp3 is considered to be the master regulator of Treg cell suppressive function, the expression of Foxp3 is not sufficient to maintain a stable Treg cell lineage (11, 12). Numerous Treg cell-specific genes are in fact expressed independently of Foxp3 protein, including Il2ra (the gene for CD25), Ctla4, Ikzf4 (Eos), and Nrp1 (neuropilin 1) (11, 13, 14). These data suggest that additional lineage-defining factors act with Foxp3 to ensure the generation of a stable, functional Treg cell compartment.
Most Foxp3+ Treg cells undergo their terminal differentiation in the thymus and are referred to as thymic Treg (tTreg) cells, whereas others originate in the periphery (particularly at mucosal barrier surfaces exposed to food antigens and commensal organisms) from conventional Foxp3− CD4+ T cells, and are consequently called peripheral Treg (pTreg) cells (15, 16). It is believed that the self pMHCII-specificity and suppressive functions of these two Treg subsets complement one another in preventing immunopathology (17). tTreg cells are primarily responsible for maintaining general T cell immune homeostasis, while pTregs control immunopathology that is directed against tissue-restricted antigens in mucosal tissues such as the lung and gut (17).
Natural Treg (nTreg) cells are defined as all Treg cells generated in vivo and thus include tTreg and some pTreg cells (16). For the case of most nTreg cells, epigenetic changes associated with the development of a unique Treg cell methylome (Treg-me) are thought to be necessary for stable Foxp3 expression. The Treg-me consists of de-methylated DNA CpG motifs at four Treg cell-related gene loci, including Tnfrsf18 (the gene for GITR), Ctla4, Ikzf4, and the Foxp3 conserved non-coding DNA sequence 2 (CNS2) (18). Therefore, the expression of Foxp3 and the development of the Treg-me are independent and complementary events. TCR signaling is required for Treg-me induction, which in turn helps maintain the function and stability of Treg cells (12, 18). It has been proposed that within the thymus, the strength of TCR signaling controls Foxp3 expression, whereas the duration of signaling establishes the de-methylations that are associated with the Treg-me (12, 18). In contrast, quantitative de-methylation of the Treg-me loci (including Foxp3 CNS2) appears unnecessary for some pTreg cell differentiation. Rather, Foxp3 transcription in mucosal tissue Treg cells can be stabilized by Tgfb1- and Smad3-dependent activation of the conserved non-coding DNA sequence 1 (CNS1) enhancer element (19, 20).
Even though pTreg cells have been found to play an essential role in the suppression of tissue-specific immunopathology in adoptive transfer experiments (17), the experimental data of Malhotra et al. (4) and Legoux et al. (5) have seemed to point toward a thymic origin for the increased self antigen-specific Treg cells observed when tissue-restricted antigen expression in the thymus is only modest. Vil1 promoter-driven Cre-specific Treg cells express high levels of Helios and Nrp1, both markers compatible with a thymic Treg cell origin (5). Additionally, genetic disruption of the CNS1 sequence from the Foxp3 locus fails to inhibit the generation of Cre-specific Foxp3+ Treg cells or restore the functional responsiveness of the conventional Cre-reactive CD4+ T cell population in Vil1-Cre transgenic mice (5). Interestingly, Foxp3+ Treg cells specific for eGFP are more frequently detected in the thymus of Ins2 promoter-driven eGFP transgenic mice (4), whereas the number of Cre-specific Treg cells in Vil1-Cre and Scgb1a1-Cre transgenic mice is similar to the wild type (5). Taken together, the experiments suggest that weak expression of self antigen in the thymus (presumably as a consequence of Aire-mediated transcription in medullary thymic epithelial cells) is insufficient to elicit central deletion of all high affinity autoreactive CD4+ T cells, yet facilitates the maintenance of self-tolerance by inducing the differentiation of highly functional self pMHCII-specific Treg cells that suppress peripheral responses. In contrast, CNS1-dependent pTreg cells may be more important to suppress mucosal T cell reactivity to food and commensal antigens introduced only after thymic development has completed.
Anergy induction versus pTreg cell generation – differences and similarities
Notably, neither of these elegant studies suggested a role for anergy in the development of tolerance to peripherally expressed self antigens. Should one conclude, therefore, that the induction and maintenance of anergy are unimportant to natural peripheral self-tolerance? Or is it possible that anergy plays some role in the establishment of self-tolerance in concert with antigen-specific Foxp3+ Treg cells? Historically, anergy has been defined as state of functional inactivation wherein CD4+ T cells lose the capacity to produce growth factors and proliferate in response to pMHCII recognition (21–23). But it has been unclear how long this state lasts and/or whether it is an intermediate state in vivo. Anergy can be induced in CD4+ T cells when TCR signaling is unaccompanied by strong CD28 co-stimulatory receptor ligation (24–26). The binding of co-inhibitory receptors such as CTLA4 and PD-1 reinforces the induction and/or maintenance of anergy (27, 28). In vivo, anergy is observed in CD4+ T cells following systemic, repeated exposure to a soluble antigen or superantigen in the absence of infection or adjuvant (29). Likewise, the adoptive transfer of naive self-antigen specific CD4+ T cells to normal mice bearing relevant self pMHCII complexes leads to the development of functional unresponsiveness (30, 31). This is in contrast to the adoptive transfer of self-reactive CD4+ T cells into lymphopenic hosts lacking a population of Foxp3+ Treg cells, whereby a failure of anergy induction typically leads to dangerous T effector cell clonal expansion and differentiation, with consequent severe immunopathology (31, 32). It is important to note that there are many independent paths to anergy, which result in different types “functional unresponsiveness” or anergic states. The different ways that assays are performed lead to varied readouts because the measurement of anergy has not been standardized. We define anergy here as defective proliferation in response to pMHCII recognition by CD4 T cells.
Multiple biochemical signaling defects have been ascribed to the CD4+ T cell anergic state, including blocked signaling to Ras and mitogen-activated protein kinases 1 and 8, and impaired expression of functional activator protein 1 (AP-1) transcription factor complexes (21, 33, 34). Up-regulation of counter-regulatory gene products such as Cblb, Dgka, Rap1, Rnf128, Itch, Dtx1, and Ndrg1 (which in most cases lie downstream of NFAT- and Egr2/Egr3-dependent transactivation) are understood to underlie this development of functional unresponsiveness (35–39). Despite the presence of multiple counter-regulatory molecules and signal transduction defects, anergic CD4+ T cells retain their capacity to recognize pMHCII and undergo anergy reversal in its absence (29).
Anergy is also reversed in the setting of T cell lymphopenia, regardless of the continued presence of antigen (40). This may relate to Treg cell deficiency as well as to increased availability of homeostatic cytokines in lymphopenic hosts. Consistent with this, anergy induction and maintenance are strongly antagonized by IL-2R signaling that leads to downstream PI-3K, Akt, and mTORC1 activation, and that promotes a shift in metabolism away from oxidative phosphorylation and toward aerobic glycolysis (41, 42). The activation, clonal expansion, and differentiation of aggressive CD4+ T effector cells is associated with unique metabolic demands that require mTORC1 activity to integrate environmental, nutrient, and intracellular signaling cues (43). If activated CD4+ T cells fail to increase their glycolytic metabolism due to inefficient mTORC1 activation, they can become anergic (44).
It is worth noting that inhibition of mTORC1 signaling not only induces anergy, but also promotes the differentiation of Foxp3+ Treg cells from conventional precursors and stabilizes the expression of Foxp3 (45–47). Not surprisingly, Treg cells themselves appear to be anergic, as they do not produce IL-2 or proliferate when stimulated unless exogenesis IL-2 is provided. Nonetheless, mTORC1 activity is also necessary for proliferation by Treg cells. In fact, it has been suggested that alternate activation and subsequent inhibition of mTORC1 facilitate optimal Treg cell function and lineage stabilization (48).
Our own investigations have led to the discovery of two additional anergy factors: ecto 5’-nucleotidase (Nt5e; hereafter referred to as CD73) and folate receptor 4 (Izumo1r, FR4). Both molecules are found expressed at moderate levels on Foxp3+ Treg cells (as well as at lower levels on conventional CD4+ naive T cells and T follicular helper cells), but CD73 and FR4 are most highly expressed on conventional antigen-experienced CD44hi CD4+ T cells following the induction of anergy (31, 49, 50). Little is understood about the function of FR4 on T cells; however, the gene encoding FR4 (Izumo1r) was recently shown to encode Juno, the receptor for Izumo1 (51). In reproductive biology, Izumo1 expression on sperm and Juno on eggs guide normal sperm-egg fusion during fertilization (51). It remains unclear how the expression of FR4 relates to anergy.
CD73 is an ecto-enzyme that normally acts in tandem with CD39 to convert extracellular ATP to adenosine (52). Extracellular ATP is secreted by activated T cells and also accumulates at sites of tissue ischemia and necrosis (53–56). Although the exact mechanisms remain unclear, it is thought that the CD39/CD73-mediated depletion of extracellular ATP limits the triggering of purinogenic receptors such as P2X7. Activation of P2X7 in some systems stabilizes the expression of the glycolytic gene positive regulator Hif1α (57). CD73-dependent production of extracellular adenosine may also serve to resist glycolytic reprogramming through the suppressive effects of the adenosine A2a receptor and its intracellular second messenger cAMP on mTORC1 activity (54). Note that CD39 is not expressed on anergic conventional CD4+ T cells, and the role that CD73 plays in the induction or maintenance of anergy remains uncertain. Nevertheless, these findings may indicate one mechanism by which CD39+ Treg cells facilitate the induction of anergy through their conversion of extracellular ATP to the more tolerogenic nucleotide adenosine (58, 59).
An anergic polyclonal CD4+ T cell compartment in healthy mice
Tissue-restricted expression of Aire-regulated transgenic model antigens has clearly defined self pMHCII-specific Foxp3+ Treg cells as an important barrier to peripheral self-reactivity by CD4+ T cells, whereas no evidence for peripheral anergy induction was obtained. Nonetheless, extrathymic Aire-regulated expression of self antigens within secondary lymphoid organs has also been shown to functionally inactivate naïve conventional autoreactive CD4+ TCR-transgenic T cells, as well as promote the accumulation of self antigen-specific Treg cells (60). We have questioned whether the detection of naturally occurring anergic CD4+ T cells has been hindered by the lack of sensitive and specific markers for detection of anergic T cells in the polyclonal repertoire.
Our previous discovery of CD73 and FR4 as reliable surface markers of anergic conventional T cells, together with the development of antigen tetramer technologies by our collaborators, offered us the opportunity to thoroughly investigate tolerant polyclonal CD4+ T cells (31, 49, 50, 61). In healthy B6.g7 mice, insulin B chain (InsB) pMHCII tetramer-binding conventional polyclonal CD4+ T cells were found to be naive in phenotype and generally ignorant of self antigen (49, 50), consistent with the results of Legoux et al. (5), and Malhotra et al. (4). On the other hand, conventional InsB/I-Ag7 tetramer-binding polyclonal T cells isolated from the pancreas-draining lymph node of non-diabetic NOD mice demonstrated 1) evidence of previous antigen-recognition (increased CD44 expression), 2) up-regulation of FR4 and CD73, and 3) defective IFNγ production, all consistent with the induction of anergy (49, 50). Notably, InsB/I-Ag7–specific Foxp3+ Treg cells were also identified and found to be similar in number in both syngeneic strains. These data, therefore, generally supported the model that normal naive CD4+ T cells often ignore tissue-restricted self antigens in the presence of a stable self antigen-specific Treg cell compartment. Nonetheless, NOD mice predisposed to autoimmune disease development apparently reveal InsB/I-Ag7 complexes to the naive peripheral T cell repertoire, and then the anergy mechanism becomes available to maintain self-tolerance.
Of course, these observations in disease-prone NOD mice begged the question of whether anergy develops only when other immune tolerance mechanisms fail. This point was addressed in a series of experiments that examined fetal tolerance in healthy B6 pregnant mice, following mating to syngeneic B6 males made transgenic for a ubiquitously expressed 2W self antigen (62, 63). Clonal expansion of 2W pMHCII-specific Foxp3+ Treg cells is known to be necessary for fetal success in this system (64), with Foxp3 expression apparently stabilized by the CNS1 enhancer element (65). Given that the niche for Treg cells having any particular antigen specificity appears limited (66, 67), and the observations of similar metabolic programming for both the anergic and Treg fates (48), we hypothesized that during pregnancy both anergic T cells and Foxp3+ Treg cells will be present among the 2W-specific T cells. 2W/I-Ab tetramer-binding CD4+ T cells were observed to undergo a 5–fold clonal expansion during pregnancy that resulted in approximately equal numbers of unresponsive CD44hi FR4hi CD73hi anergic T cells and Foxp3+ Treg cells (49). Interestingly, most of the anergic compartment disappeared during the postpartum period, perhaps reflecting a requirement for continuous TCR recognition of fetus-derived 2W/I-Ab complexes to maintain anergy or cell survival. Therefore, normal pregnancy is associated with CD4+ T cell anergy to fetal antigens.
The discovery of functionally unresponsive CD44hi FR4hi CD73hi CD4+ T cells specific for self InsB/I-Ag7 or fetal 2W/I-Ab complexes lends support to the notion that peripheral self-tolerance can rely on CD4+ T cell anergy. To investigate the generality of these observations, we characterized the natural repertoire of polyclonal conventional CD4+ T cells that express these anergy markers in combination. A subpopulation of CD44hi FR4hi CD73hi Foxp3− cells was found to make up 2–5% of the polyclonal CD4+ T cell repertoire in the secondary lymphoid organs (but not thymus) of multiple normal mouse strains, and this subpopulation increased with age (49). Furthermore, expression of these markers was found to strongly correlate with proliferative arrest and defective cytokine production, the two hallmarks of anergy. Finally, loss of Aire-dependent gene expression and central deletion in the thymus of mutant Aire−/− mice led to an increase in the proportion of peripheral CD4+ T cells that have this anergic phenotype (49).
Evidence of anergy in this polyclonal CD44hi FR4hi CD73hi CD4+ T cell compartment cannot be taken as proof of self antigen-reactivity. However, loss of the anergic phenotype in fetal antigen-specific T cells postpartum following the expulsion of fetal tissues did suggest a requirement for continuous TCR engagement to maintain the anergic state (49). Consistent with this, steady state polyclonal anergic T cells were shown to express high levels of PD-1, CTLA4, CD69, and Nrp1––all molecules whose expression can be induced and/or maintained by persistent TCR engagement. Uniformly increased levels of CD5 and a Nur77 reporter gene in anergic T cells also suggested that these cells have high affinity TCRs specific for available self pMHCII complexes, similar to bona fide self antigen-specific Treg cells (68–70). Additionally, the frequency and number of anergic cells was not different between germ-free mice and specific pathogen free mice from our colony, suggesting that the functional inactivation observed here was not solely in response to commensal antigens (unpublished data). Therefore, we now hypothesize that many or all anergic phenotype CD4+ T cells in secondary lymphoid organs have recently recognized self pMHCII.
Anergy reversal can result in immunopathology or alternatively lead to protective Treg cell differentiation
Additional experiments were designed to formally test the self-reactivity of anergic phenotype CD4+ T cells by transferring them into autoimmune disease-prone Tcra−/− mice and observing for the development of immunopathology. Initial experiments failed to demonstrate reliable autoimmune disease development, but instead led to the discovery that polyclonal anergic T cells can trans-differentiate into Foxp3+ Treg cells (49). Following the adoptive transfer of highly purified Foxp3− CD44hi FR4hi CD73hi CD4+ T cells into lymphopenic Tcra−/− mice, as many as 25% of the resulting peripheral CD4+ T cells expressed Foxp3. Analogous to tTreg cells that experience persistent high affinity TCR engagements during their differentiation in the thymus, most of these anergy-derived Treg cells also expressed Nrp1 and demonstrated a fully de-methylated Treg-me (including the Foxp3 CNS2).
Nrp1 was originally thought to distinguish tTreg cells from peripherally differentiated pTreg cells (71, 72). However, this notion has since been challenged because activated Treg cells (which include both tTreg cells and pTreg cells) as well as inducible Treg cells generated in vitro from naive CD4+ T cells can express Nrp1 (73, 74). Similar to natural polyclonal Treg cells, anergy-derived polyclonal Treg cells demonstrated an ability to protect lymphopenic Tcra−/− mice from inflammatory bowel disease (49). Anergy-derived Treg cells also suppressed the development of autoimmune arthritis, and demonstrated a capacity to induce anergy in other self antigen-specific CD4+ T cells (49).
Interestingly, the treatment of Tcra−/− recipients of Foxp3DTR anergic T cells with diphtheria toxin to destroy any developing Foxp3-expressing cells not only prevented the accumulation of anergy-derived Treg cells, but also led to the development of severe wasting disease and the generation of tissue-specific autoantibodies, further demonstrating the self-reactivity of naturally anergic polyclonal CD4+ T cells (49). Taken together, the data suggest that anergic phenotype polyclonal CD4+ T cells have potentially dangerous TCRs that are specific for peripheral self pMHCII complexes, but these TCRs also make them ideal progenitor cells for the peripheral differentiation of Foxp3+ Treg cells. Furthermore, these experiments indicate that anergy-derived Treg cells cannot be readily distinguished from tTreg cells based on phenotype or Treg-me. Therefore, it is conceivable that some of the Nrp1+ Foxp3+ Treg cells that preferentially expanded during the course of self antigen immunization by Malhotra et al. (4) and Legoux et al. (5) were originally conventional CD4+ T cells that had undergone anergy induction following peripheral recognition of the same tissue-restricted self antigen.
Role of Neuropilin-1 in the generation of anergy-derived Treg cell progenitors
Nrp1 is a transmembrane glycoprotein on the surface of many cell types, including Treg cells, dendritic cells (DCs), NKT cells, neurons, and endothelial cells (71, 72, 77) and its function is important for axonal guidance in the developing nervous system (78). Nrp1 is a co-receptor for the soluble class 3 semaphorins (79), but can also promote angiogenesis by binding to vascular endothelial growth factor (VEGF) (77). More recently, Nrp1+ Treg cells have been shown to bind to semaphorin-4a on plasmacytoid DC, with subsequent recruitment of PTEN and inhibition of downstream Akt and mTORC1 signaling pathways (75, 80). The stability of the Treg cell lineage is maintained by a Nrp1:semaphorin-4a axis, and Nrp1 can induce the expression of Treg cell lineage-related genes independently of Foxp3 (75, 81).
As described above, a majority of polyclonal anergic CD4+ T cells express high levels of Nrp1 and demonstrate a unique pattern of partial Treg-me DNA de-methylation. Consistent with this coordinate expression of Nrp1 and de-methylation of the Treg-me, FR4+ CD73+ anergic CD4+ T cells sorted for high Nrp1 expression and transferred to lymphopenic Tcra−/− hosts were found to be most efficient for the generation of Foxp3+ Treg cells (49). Conversely, the Nrp1− fraction of anergic T cells was found to be a poor source of Treg cell progenitors, and preferentially differentiated toward a TH17 lineage that caused wasting disease following adoptive transfer to Tcra−/− mice ((49) and unpublished data). A previous study similarly showed that autoreactive CD4+ T cells lacking Nrp1 would induce a more severe form of experimental autoimmune encephalitis (EAE), with their Nrp1− CD4+ T cells also appearing biased towards the TH17 lineage (80). Loss of Nrp1 expression on Treg cells limits the nuclear localization of Foxo1/3a, leads to a failure of Foxp3 expression, and allows for the up-regulation of transcription factors such as RORγt that facilitate TH17 differentiation (75). Thus, the skewing of Nrp1− polyclonal anergic CD4+ T cells towards a TH17 fate suggests that a similar Nrp1− directed genetic program may govern lineage selection in both anergic T cells and Treg cells.
It remains unclear whether Nrp1 expression is essential for the induction and/or the maintenance of the anergic phenotype. Nrp1 was previously implicated in immunological synapse formation, and on Treg cells Nrp1 enhances the duration of the Treg interactions with dendritic cells (DCs) resulting in higher sensitivity to small amounts of antigen (82). How the TCR repertoire and self pMHCII-specificity of anergic T cells relates to this expression of Nrp1 remains uncertain. Nrp1 has also been shown to directly induce the expression of CD73 in Treg cells (75). Nevertheless, our analysis of fetal antigen-specific anergic T cells suggested that Nrp1 is expressed only after the anergic phenotype (CD73hi FR4hi) is established. Only half of fetal antigen-specific FR4hi CD73hi CD4+ T cells expressed Nrp1 at day 10 of gestation (unpublished data), whereas Nrp1 expression on anergic phenotype T cells increased to 80–90% by day 18 ((49) and unpublished data). Thus, the level of Nrp1 on anergic T cells may simply indicate the degree of unresponsiveness. Alternatively, Nrp1 expression on anergic T cells may reinforce longer interactions with self pMHCII on DCs to promote quantitative de-methylation of Treg-me genes and induce the expression of Foxp3.
Conclusions
Despite recent advances in biological therapy, the treatment for autoimmune diseases remains problematic. For instance, the treatment of rheumatoid arthritis (RA) with drugs that suppress aberrant immune responses to self antigens still poses risk for infection, as these drugs can have off-target effects leading to the suppression of T cells that recognize and destroy pathogens (2). A better approach for the treatment of autoimmune disorders would be to reinforce a stable T cell tolerance to self-antigens, while maintaining full responsiveness to non self-antigens.
Anergy, a state of long-term functional unresponsiveness, is one such peripheral tolerance mechanism that has been studied extensively. Nevertheless, until recently it has never been shown that anergy can be induced in self antigen-reactive CD4+ T cells that escape negative selection in the thymus, mainly because of a lack of identifying markers specific for anergy development. It has also remained uncertain as to why the immune system would maintain viable anergic T cells long-term. Our experiments have made use of a panel of predictive markers for anergy development in the natural polyclonal CD4+ T cell repertoire—CD44 expression to identify antigen-experienced T cells, the absence of Foxp3 expression to exclude Treg cells, and a combination of elevated CD73 and FR4. The results now suggest that CD4+ T cells that have persistently recognized peripheral self pMHCII enter a CD44hi CD73hi FR4hi unresponsive state. Moreover, anergy cannot be sustained in the absence of self antigen recognition or in the setting of Treg deficiency. Anergy reversal can lead to the differentiation of functional Treg cells that suppress autoimmune disease or, alternatively, potentially pathogenic Teff-mem cells. Finally, the up-regulation of Nrp1 expression on anergic T cells is predictive of a partially de-methylated Treg-me and serves as a marker for Treg cell progenitors (Fig. 1).
Self antigen-specific Treg cell generation from anergic T cells is now reminiscent of the in vivo “infectious tolerance” model previously proposed by Kendal and Waldmann (75). Infectious tolerance is described as a process during which a “tolerant” state is passed on from one group of lymphocytes to another. Newly tolerant T cells would then reprogram, survey the immune system, and pass on their tolerant state to other T cell populations to continuously maintain self-tolerance. Since Treg cells are important for inducing and/or maintaining anergy (31) and anergic T cells in turn can alter their epigenetic and transcriptional programs to become Treg cells (49), anergic T cells may represent the intermediate reprogramming stage before themselves becoming surveying Treg cells that maintain self-tolerance.
Acknowledgments
Funding sources: This work was supported by a Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign grant from the Rheumatology Research Foundation, and by the National Institutes of Health grant P01 AI35296.
References
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat. Rev. Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 3.Davis MM. Not-So-Negative Selection. Immunity. 2015;43:833–835. doi: 10.1016/j.immuni.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, Nelson RW, Fife BT, Orr HT, Anderson MS, Hogquist KA, Jenkins MK. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol. 2016;17:187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legoux FP, Lim J-B, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, Sparwasser T, Way SS, Moon JJ. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. 2015;43:896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 8.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 11.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 15.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu. Rev. Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DAA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 17.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li S-H, Relland LM, Wise PM, Chen A, Zheng Y-Q, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J. Exp. Med. 2012;209:1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 24.Mueller DL, Jenkins MK, Schwartz RH. An accessory cell-derived costimulatory signal acts independently of protein kinase C activation to allow T cell proliferation and prevent the induction of unresponsiveness. J. Immunol. 1989;142:2617–2628. [PubMed] [Google Scholar]
- 25.Harding FA, Harding AF, McArthur JG, McArthur JG, Gross JA, Gross JA, Raulet DH, Raulet DH, Allison JP, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 26.Mondino A, Whaley CD, DeSilva DR, Li W, Jenkins MK, Mueller DL. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. J. Immunol. 1996;157:2048–2057. [PubMed] [Google Scholar]
- 27.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 28.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 30.Adler AJ, Marsh DW, Yochum GS, Guzzo JL, Nigam A, Nelson WG, Pardoll DM. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, Mueller DL. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J. Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: roles of endogenous T and B lymphocytes in tolerance. J. Immunol. 2005;175:21–26. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Li W, Whaley CD, Mueller A, Mondino DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 34.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 35.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 36.Bachmaier K, Penninger JM, Bachmaier K, Krawczyk C, Krawczyk C, Kozieradzki I, Kozieradzki I, Kong YY, Sasaki T, Kong Y-Y, Oliveira-dos-Santos A, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Mariathasan S, Bouchard D, Wakeham A, Bouchard D, Wakeham A, Itie A, Le J, Itie A, Le J, Ohashi PS, Sarosi I, Ohashi PS, Sarosi I, Nishina H, Nishina H, Lipkowitz S, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 37.Heissmeyer V, Macián F, Im S-H, Varma R, Feske S, Venuprasad K, Gu H, Liu Y-C, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 38.Seroogy CM, Soares L, Ranheim EA, Su L, Holness C, Bloom D, Fathman CG. The gene related to anergy in lymphocytes, an E3 ubiquitin ligase, is necessary for anergy induction in CD4 T cells. J. Immunol. 2004;173:79–85. doi: 10.4049/jimmunol.173.1.79. [DOI] [PubMed] [Google Scholar]
- 39.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong X-P. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 40.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, Zhang L, Gajewski TF. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin. Cancer. Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 41.DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J. Immunol. 1991;147:3261–3267. [PubMed] [Google Scholar]
- 42.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J. Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 43.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr. Opin. Immunol. 2010;22:552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Y, Zheng Y, Delgoffe GM, Delgoffe GM, Meyer CF, Meyer CF, Chan W, Chan W, Powell JD, Powell JD. Anergic T cells are metabolically anergic. J. Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalekar LA, Schmiel SE, Nandiwada SL, Lam WY, Barsness LO, Zhang N, Stritesky GL, Malhotra D, Pauken KE, Linehan JL, O’Sullivan MG, Fife BT, Hogquist KA, Jenkins MK, Mueller DL. CD4(+) T cell anergy prevents autoimmunity and generates regulatory T cell precursors. Nat. Immunol. 2016;17:304–314. doi: 10.1038/ni.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pauken KE, Linehan JL, Spanier JA, Sahli NL, Kalekar LA, Binstadt BA, Moon JJ, Mueller DL, Jenkins MK, Fife BT. Cutting Edge: Type 1 diabetes occurs despite robust anergy among endogenous insulin-specific CD4 T cells in NOD mice. J. Immunol. 2013;191:4913–4917. doi: 10.4049/jimmunol.1301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin. Exp. Immunol. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 54.Ohtsuka T, Changelian PS, Bouïs D, Noon K, Harada H, Lama VN, Pinsky DJ. Ecto-5’-Nucleotidase (CD73) Attenuates Allograft Airway Rejection through Adenosine 2A Receptor Stimulation. J. Immunol. 2010;185:1321–1329. doi: 10.4049/jimmunol.0901847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Müller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 57.Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 2012;3:e370. doi: 10.1038/cddis.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, Linden J, Crowe SE, Ernst PB. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J. Infect. Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner JM, Metzger TC, McMahon EJ, Au-Yeung BB, Krawisz AK, Lu W, Price JD, Johannes KP, Satpathy AT, Murphy KM, Tarbell KV, Weiss A, Anderson MS. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4+ T cells. Immunity. 2013;39:560–572. doi: 10.1016/j.immuni.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, Jameson SC, Hogquist KA. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moon JJ, Dash P, Oguin TH, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsieh C-S, Lee H-M, Lio C-WJ. Selection of regulatory T cells in the thymus. Nat. Rev. Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 67.Lee H-M, Bautista JL, Scott-Browne J, Mohan JF, Hsieh C-S. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37:475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yadav M, Louvet C, Davini D, Gardner JM, Martínez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P, Ignatowicz L. Differences in expression level of Helios and Neuropilin-1 do not distinguish thymus-derived from extrathymically-induced CD4+Foxp3+ regulatory T cells. PLoS ONE. 2015;10:e0141161. doi: 10.1371/journal.pone.0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schliesser U, Chopra M, Beilhack A, Appelt C, Vogel S, Schumann J, Panov I, Vogt K, Schlickeiser S, Olek S, Wood K, Brandt C, Volk H-D, Sawitzki B. Generation of highly effective and stable murine alloreactive Treg cells by combined anti-CD4 mAb, TGF-β, and RA treatment. Eur. J. Immunol. 2013;43:3291–3305. doi: 10.1002/eji.201243292. [DOI] [PubMed] [Google Scholar]
- 75.Delgoffe GM, Woo S-R, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DAA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milpied P, Massot B, Renand A, Diem S, Herbelin A, Leite-de-Moraes M, Rubio M-T, Hermine O. IL-17-producing invariant NKT cells in lymphoid organs are recent thymic emigrants identified by neuropilin-1 expression. Blood. 2011;118:2993–3002. doi: 10.1182/blood-2011-01-329268. [DOI] [PubMed] [Google Scholar]
- 77.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 78.Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J. Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 79.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 80.Solomon BD, Mueller C, Chae W-J, Alabanza LM, Bynoe MS. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2040–2045. doi: 10.1073/pnas.1008721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delgoffe GM, Vignali DAA. A Fox of a different color: FoxA1 programs a new regulatory T cell subset. Nat. Med. 2014;20:236–237. doi: 10.1038/nm.3493. [DOI] [PubMed] [Google Scholar]
- 82.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 Expression on Regulatory T Cells Enhances Their Interactions with Dendritic Cells during Antigen Recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kendal AR, Waldmann H. Infectious tolerance: therapeutic potential. Cur. Opin. Immunol. 2010;22:560–565. doi: 10.1016/j.coi.2010.08.002. [DOI] [PubMed] [Google Scholar]