Abstract
Acquired pendular nystagmus is comprised of quasi-sinusoidal oscillations of the eyes significantly affecting gaze holding and clarity of vision. The most common causes of acquired pendular nystagmus include demyelinating disorders such as multiple sclerosis and the syndrome of ocular palatal tremor. However, several other deficits, such as pharmacological intoxication, metabolic and genetic disorders, and granulomatous disorders can lead to syndromes mimicking acquired pendular nystagmus. Study of the kinematic features of acquired pendular nystagmus has suggested a putative pathophysiology of an otherwise mysterious neurological disorder. Here we review clinical features of neurological deficits that co-occur with acquired pendular nystagmus. Subsequent discussion of the pathophysiology of individual forms of pendular nystagmus speculates on mechanisms of the underlying disease while providing insights into pharmacotherapy of nystagmus.
Keywords: saccade, nystagmus, integrator, cerebellum, midbrain, oscillopsia, tremor, demyelination disorder, multiple sclerosis, stroke, degenerative disorder, toxin exposure
1 Goal of the review and background
Pendular nystagmus is characterized by quasi-sinusoidal oscillations of the eyes disrupting the visual acuity and causing oscillopsia. This review will provide a comprehensive resource for the pathophysiology, differential diagnosis and pharmacotherapy of the pendular nystagmus due to acquired etiologies. We will first the physiology of gaze-holding pertinent to the acquired pendular nystagmus. Then we will discuss etiologies of acquired pendular nystagmus, pertinent pathophysiology and therapeutic strategies.
2 Applied physiology of gaze-holding and introduction to neural integration
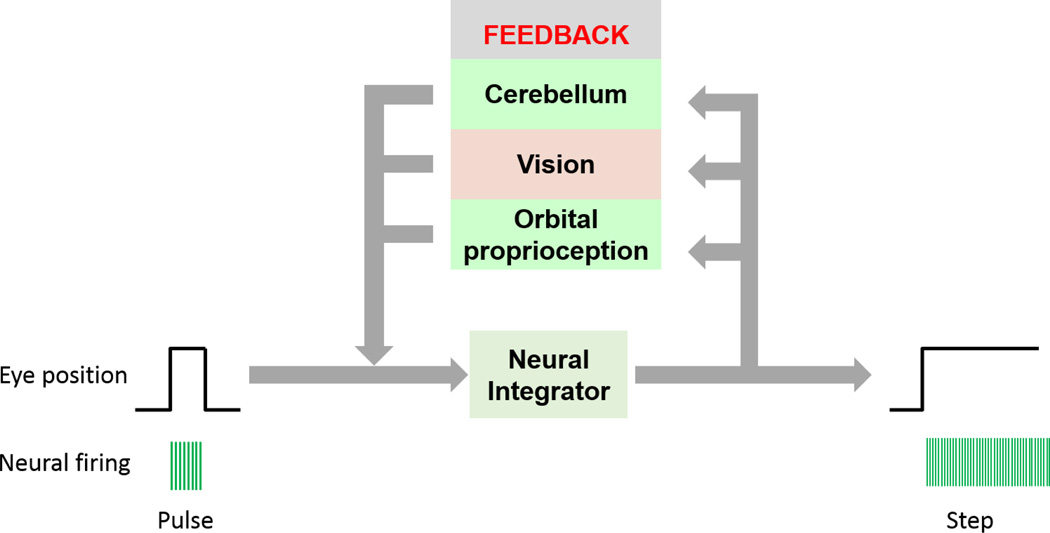
Normal gaze-holding requires the convergence of an accurate neural command, incorporation of visual, proprioceptive, and cerebellar feedback, and their implementation at the orbital muscles. Gaze has two fundamental requirements: an initial rapid eye movement (saccade) to the point of fixation, followed by maintenance of the eyes at the new position away from center. The saccade are encoded by the velocity command, or pulse, that results in phasic contraction of the extraocular muscles to rotate the eyes rapidly to the point of fixation. Since the elastic restoring forces of the eyes tend to return them to the central position, steady gaze-holding requires tonic contraction of the extraocular muscles to resist these restoring forces. The cellular network called neural integrator converts the velocity command to a position signal, hence integrating the eye movement commands.[1–4]
The neural network for integration is distributed across the brainstem and cerebellum. When the initial velocity command is sent to the extraocular muscles, the same signal is also sent to cells in the neural integrator, which then send out a position command, or step, to the extraocular muscles to hold them steady at their new position (Figure 1). The neural integrator is complemented by feedback loops in the cerebellum, orbital proprioception, and vision that help “boost” the position signal to compensate for insufficiencies or loss of fidelity of the neural integrator (Figure 1). Interference at any of these steps can lead to disorders of rapid, involuntary, and oscillating eye movements called nystagmus. These oscillations may be uncomfortable or disorienting.
Figure 1.
Schematic function of and organization of the neural integrator and critical feedback loops.
Many patterns of nystagmus have been characterized. If the eye positions are tracked in real time, the waveform of these oscillations may be sinusoidal, as in pendular nystagmus, or assume the shape of a saw tooth, as in jerk nystagmus. The waveform characteristics provide a helpful framework for distinguishing the underlying pathophysiology. Pendular nystagmus due to acquired etiology, the focus of current review, is described by its frequency, given in Hertz, and its amplitude, given in degrees. Other properties include the axis of movement, which may be horizontal, vertical or torsional, whether the movement is monocular or binocular, and conjugate (in which both eyes move in the same direction) or disconjugate (in which both eyes move in different directions).
2 Acquired pendular nystagmus
Many etiologies recognized to cause acquired pendular nystagmus are outlined in Table 1. Acquired pendular nysagmus may affect one or both eyes, and can occur in any axis or combination of axes. Although acquired pendular nystagmus may be idiopathic, the most common cause of secondary acquired pendular nystagmus is disorders of central myelin, namely multiple sclerosis (MS). Below we review the clinical characteristics of the acquired pendular nystagmus associated with each etiology, and outline the proposed pathophysiological mechanism for each.
Table 1.
Etiologies of Pendular Nystagmus
Disorders of central myelin
|
| Visual loss |
| Syndrome of oculopalatal tremor |
| Acute brainstem stroke |
| Alexander’s disease |
| Neurosarcoidosis |
| Whipple’s disease |
| Hypoxic encephalopathy |
| Sporadic cerebellar ataxia |
| Serotonin syndrome; serotonin reuptake inhibitors |
| Fosphenytoin toxicity |
3 Multiple Sclerosis
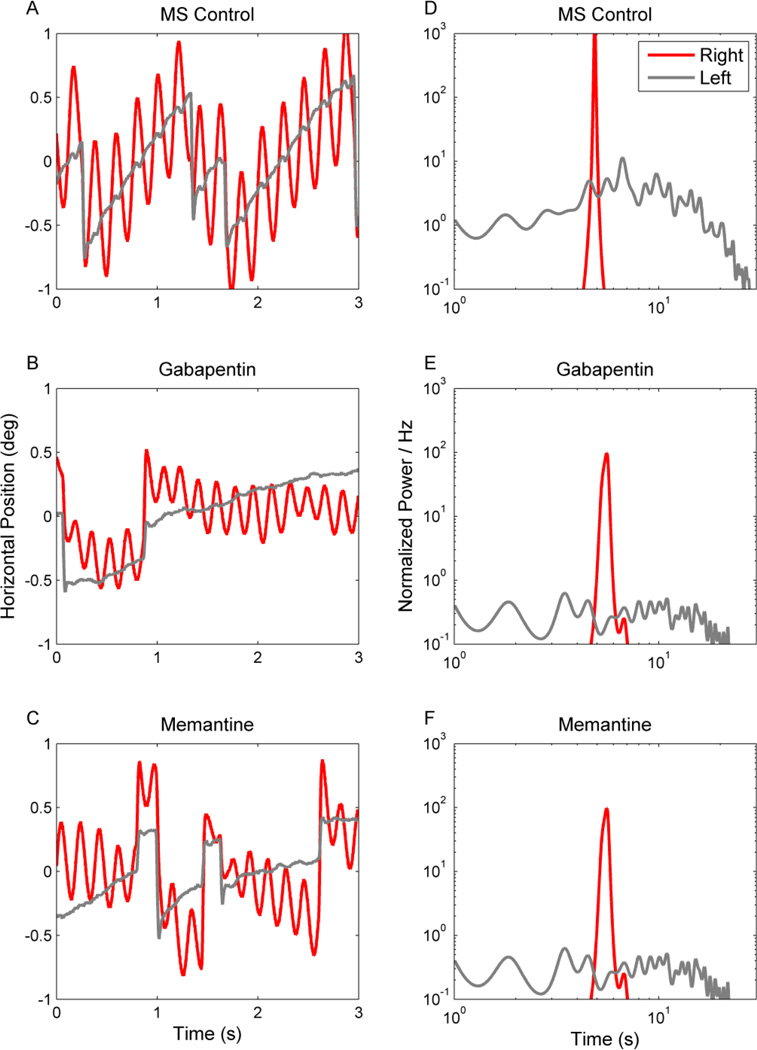
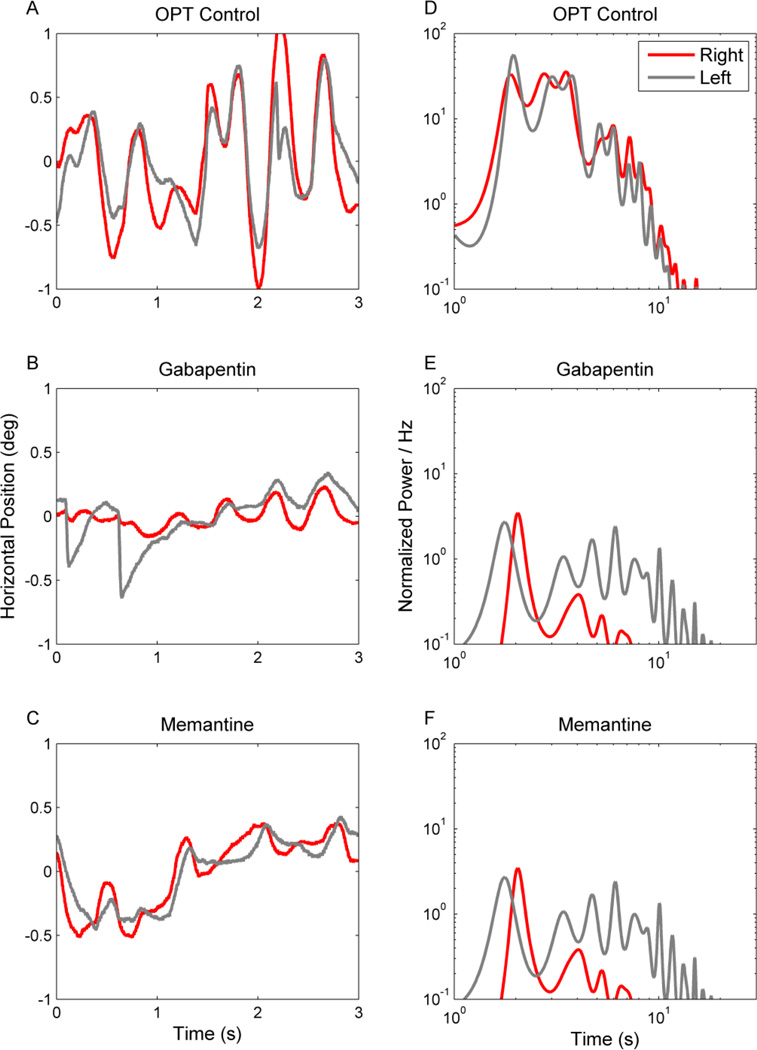
Multiple sclerosis (MS) is an immune-mediated inflammatory disease characterized by demyelination of the central nervous system. Eye movement disturbances are present in about 40–76% of MS patients.[5] Common abnormalities include internuclear opthalmoplegia, but disorders of saccades, vestibulo-ocular reflex, smooth pursuit, and gaze holding are also noteworthy.[5] Impaired gaze holding is often seen in MS patients, acquired pendular nystagmus is a classic cause of oscillopsia in these patients.[5–10] Figure 2A depicts an example of acquired pendular nystagmus in a patient with MS. In this figure eye position is plotted on the y-axis, while time is plotted on the x-axis. The red trace depicts eye position while attempting to hold gaze steady on a stationary target. Note that gaze holding of the right eye is characterized by sinusoidal oscillations but the left eye does not have such oscillations (Figure 2A). Indeed, asymmetric visual impairment, as was present in this subject, often results in asymmetric amplitude of acquired pendular nystagmus. The nystagmus illustrated in Figure 2A had a frequency of approximately 3 Hz, falling in the typical range of 2–6Hz[6] seen in MS patients.
Figure 2.
Horizontal acquired pendular nystagmus measured from one patient with MS. (A–C) Binocular eye positions are plotted against time. Red traces depict the right eye and grey traces the left. IN this example binocular jerk nystagmus superimposes upon monocular acquired pendular nystagmus in the right eye. There is a reduction in the amplitude of both types of nystagmus during treatment with gabapentin (1200 mg per day) (B) and memantine (10 mg per day) (C). Corresponding power spectra are plotted in D–F. The power spectrum of the pendular component is very sharp (D, red trace). The power spectrum of the jerk nystagmus is broader and lower (D, gray trace). Note that both gabapentin and memantine reduce the oscillation amplitude, but do not significantly affect its frequency distribution.
There are several hypotheses regarding the pathophysiology of acquired pendular nystagmus in MS patients. One hypothesis is that acquired pendular nystagmus in patients with MS arises due to a delay in transmission of visual information due to demyelination of the optic nerve. Delay in optic conductions could result in an abnormal feedback loop regardless of normal visual acuity or visual fixation.[11] This is supported by the fact that the oscillations are usually larger in the eye with more severe visual impairment.[12] However, there is evidence suggesting that optic feedback may not be the primary mechanism of acquired pendular nystagmus in patients with MS. The oscillations persist in darkness, that is, in the absence of visual input.[13] Ocular oscillations may be induced by experimentally delaying visual feedback, but the frequency of these oscillations does not match those of acquired pendular nystagmus due to MS (2 Hz as opposed to 2–6 Hz, respectively).[11] When this is done to patients with existing acquired pendular nystagmus, their nystagmus is not changed but rather another, low-frequency oscillation is imposed on top of it.[11] Thus, this mechanism is not likely to be the primary one responsible for acquired pendular nystagmus in patients with MS.
Blinks and saccades transiently stop or “reset” the nystagmus, resulting in a phase shift and gradual buildup of the nystagmus amplitude after the “reset”.[13] Therefore another proposed mechanism for the pathogenesis is that the oscillations are due to the instability of the neural integrator. These cells, which are distributed across the brainstem and cerebellum, function to integrate eye movement commands from various inputs and mathematically integrate them into position commands. This is especially important in the maintenance of gaze, which involves tonic contraction of the extraocular muscles to hold the eye steady. Thus, if the fidelity of the neural integrator is compromised, the ability of the eyes to maintain eccentric gaze will also be affected. If the integrator is “leaky,” the eye position signal will decay over time, and the corrective mechanism (saccade) will reposition the eyes back to the desired position.[14] A “leaky” neural integrator due to impaired cerebellar feedback leads to gaze-evoked nystagmus.[15] In contrast, excessive compensation by feedback circuits will lead to an unstable neural integrator and cause movement of the eyes away from central position, followed by decay of the position signal, resulting in oscillating eye movement as seen in acquired pendular nystagmus. This hypothesis is supported by the fact that larger amplitude saccades result in a larger “reset” or phase shift, and the size of the phase shift is directly proportional to the duration of the neural pulse.[13] It is thought that the burst of neural activity generated by the saccade transiently silences neurons that periodically fire and cause the nystagmus. Further consistent with the prediction, patients with acquired pendular nystagmus associated with MS have demyelinating plaques in the region of the paramedian tracts,[6, 13] an important area associated with neural integration of eye velocity signals. Other sites where lesions have been found include the dorsal pontine tegmentum in the brainstem and the anterolateral pons and midbrain.[6, 13, 16]
Pharmacological tests further tested the mechanism of pathogenesis and explored possible treatment options for acquired pendular nystagmus in MS. Treatment with gabapentin or memantine, putative blockers of alpha-2-delta calcium channels and glutamate receptors respectively, reduces acquired pendular nystagmus amplitude in MS patients (Figure 2B,C,E,F). The subjective reduction in the oscillation amplitude (comparison of Figure 2A with Figure 2B,C) is further quantified in the power-spectrum in Figure 2D,E,F. It is noteworthy, however, that gabapentin and memantine reduce the acquired pendular nystagmus amplitude but not frequency.[17, 18]
4 Cockayne Syndrome
Cockayne syndrome type B is an autosomal recessive disorder caused by a mutation in the human DNA repair gene ERCC6, resulting in white matter demyelination of the central nervous system. The disease usually presents in early childhood with symptoms including dwarfism, mental retardation, microcephaly, and a sunken eye appearance.[19] Common neurological findings include sensorineural deafness, tremor, ataxia, spasticity, and normal-pressure hydrocephalus. Ocular findings include enophthalmos, hyperopia, poor pupillary dilation, and retinal dystrophy. Cataract extraction is often required at an early age.[20]
The presence of congenital cataracts and retinal dystrophy in many Cockayne syndrome patients makes it difficult to isolate the mechanism of pathogenesis responsible for coexistent nystagmus. There are at least two possible mechanisms. One, given that it is a demyelinating disorder, it is likely that it shares pathophysiological mechanisms with acquired pendular nystagmus in other demyelinating disorders.[21] Impaired visual function due to cataracts and retinal dystrophy might further impair gaze-holding function that is already abnormal in Cockayne syndrome due to demyelinating etiology.
Pharmacotherapy with memantine or gabapentin might be an attractive treatment option since one possible etiology of acquired pendular nystagmus in these patients is the disruption of the neural integrator due to demyelination, as proposed for MS. However, comorbid visual deficits and resultant abnormality in the gaze holding might pose substantial limitation to successful pharmacological intervention.
5 Pelizaeus-Merzbacher Disease
Pelizaeus-Merzbacher disease (PMD) is an X-linked recessive disorder resulting from a mutation in PLP1, a transmembrane proteolipid protein that is an important component of myelin. As opposed to a demyelinating disorder, in which normal myelin is laid down and later destroyed, PMD is considered a dysmyelinating or hypomyelinating disorder, in which patients are incapable of forming normal myelin in the first place.[22, 23] The symptoms and neurological findings can range widely in severity, but classical findings include pendular nystagmus, hypotonia, spastic quadriparesis, ataxia titubation, and cognitive impairment.[24–27] [28, 29]
A case report of a 49-year-old woman with hereditary spastic paraplegia, age 12 at onset, revealed conjugate horizontal pendular nystagmus that changed to jerk nystagmus on lateral gaze. The eye movement recordings showed a regular sinusoidal pattern with a mean frequency of 4.3 Hz and the presence of a small vertical component.[30] Although this patient did not have typical PMD, she met criteria for hypomyelination on MRI which demonstrated hypointensity of the white matter in two separate controls 4 years apart.[30] The pathophysiology of the nystagmus in this patient could be due to dysfunction of the neural integrator and internal feedback loops due to dysmyelination, similar to the proposed pathophysiology for MS. The change of pendular nystagmus to jerk nystagmus in lateral gaze suggests additional cerebellar involvement leading to gaze-evoked nystagmus. Again, gabapentin and memantine could be potentially used for the treatment of acquired pendular nystagmus; however, added pharmacotherapy might be justified for superimposed cerebellar nystagmus. Latter can be treated by addition of baclofen or aminopyridines.
6 Peroxisomal Disorders
Peroxisomal disorders include a range of autosomal recessive mutations that affect peroxisome function. Affected individuals experience build-up of very long chain fatty acids and branched chain fatty acids, with neurological symptoms including cerebellar signs such as gait ataxia, dysartria, and dysmetria.[31, 32] Pendular nystagmus has been well-documented in Zellweger spectrum disorders, a subset of inherited peroxisomal disorders that typically present in newborns or early childhood and can range from mild to severe phenotype.[33]
In a case study of three siblings with perioxisomal disorder two had pendular nystagmus. All had bilateral optic atrophy; one had diffuse demyelination of the subcortical white matter on brain MRI.[34] It is certainly difficult to elucidate the mechanism of pathogenesis of the pendular nystagmus in the presence of such varied neurological effects, but the combination of demyelination and visual deficits due to optic atrophy are likely to have important role in the instability of the neural integrator.[11, 34] Due to multifactorial etiology there is less likelihood of satisfactory resolution of the pendular nystagmus with gabapentin or memantine but the therapeutic trial is justified.
7 Toluene Abuse
Toluene is an organic solvent commonly used in industrial glues, paints, and paint thinner. Due to its presence in many commercial products, accidental overexposure is not uncommon, but intentional abuse through inhalation has been observed due to toluene’s ability to create acute euphoria and altered consciousness. However, toluene is highly damaging to the central nervous system. It is highly lipophilic, adhering readily to myelin and cell membranes. In patients with chronic toluene abuse, abnormal MRI findings include generalized atrophy of the cerebrum, cerebellum, brainstem, and corpus callosum, loss of gray/white matter discrimination, and high signal intensity in the cerebral white matter.[35, 36] These deficits may result in ataxia, tremor, anosmia, sensorineural hearing loss, dementia, and seizures.[37] Neuro-opthalmologic findings may include pendular nystagmus, ocular flutter, opsoclonus, optic neuropathy, and internuclear ophthalmoplegia.[29, 38] The demyelinating effects of toluene are thought to be responsible for acquired pendular nystagmus in these patients, following a similar mechanism like that of MS. However, superimposed cerebellar nystagmus, instability in gaze due to visual deficits secondary to optic neuropathy, and ophthlamoplegia might complicate the presentation and therapeutic interventions. Depending on the extent of clinical presentation, it is reasonable to consider baclofen in addition to gabapentine and/or memantine for the therapeutic intervention.
8 Visual Loss
Ocular drifts in the form of pendular or jerk nystagmus have been observed in individuals with vision loss. For example, study of patients with acquired monocular vision loss revealed small, low-frequency, irregular oscillations with a predominantly vertical component in the affected eye.[39] Study of nystagmus in these patients offers insight into the role that visual feedback plays in the maintenance of steady gaze in the absence of cerebellar or other central nervous system abnormalities.
It is hypothesized that the ocular drift occurring in patients with vision loss is due to loss of calibration of the neural integrator.[39] This predicts that loss of vision in one eye will affect the gaze stability of both eyes, with greater instability in the affected eye. Additionally, this predicts that movements that do not require the neural integrator, such as saccade, will be more conjugate than movements that require the neural integrator, such as the maintenance of steady gaze.
Glioma of the optic pathway can lead to acquired pendular nystagmus. In a study of 22 patients nystagmus was present in 10. Once present the nystagmus did not resolve and continued till the treatment was initiated. Therapy consisted of resection or chemotherapy and/or radiation. About one third remained stable after treatment, third had radiographic response, while third continued to progress.[40]
9 Alexander’s Disease
Alexander’s disease is a rare, autosomal dominant disorder most often caused by de novo mutations in the GFAP gene, which encodes for glial fibrillary acidic protein, an important structural component of glial cells. Mutations in this protein result in the deterioration of myelin and the buildup of abnormal protein deposits known as Rosenthal fibers.[41] Neonatal, juvenile, and adult-onset variations have all been described. The classic juvenile presentation includes spasticity, mental retardation, megalencepathy, and seizures.[41] Adult-onset forms, on the other hand, usually display ataxia and/or spasticity without cognitive impairment or optic atrophy.[42]
Eye movement deficits typically develop later in the course of Alexander’s disease.[43] Nystagmus is the most common eye movement deficit, pendular and gaze evoked are common subtypes of nystagmus in these patients.[42] Syndrome of ocular palatal tremor is also commonly present in conjunction with Alexander’s disease.[43, 44] It is therefore possible that the pathophysiology of pendular nystagmus in Alexander’s disease can be attributed to the mechanisms leading to ocular palatal tremor. Therapeutic options are however the same. The ocular palatal tremor can be successfully treated with gabapentin, memantine, or even baclofen. Latter can be even more useful, as an adjunct therapy to gabapentin and/or memantine for the treatment of combination of ocular palatal tremor and coexisting cerebellar nystagmus. Quinine or tonic water blocks connexin gap junctions, and could be used as putative therapy of ocular palatal tremor. Benzodiazpine can affect any form of hyperkinetic movement, including the acquired pendular nystagmus; however, it is often associated with side effects such as drowsiness.
10 Neurosarcoidosis
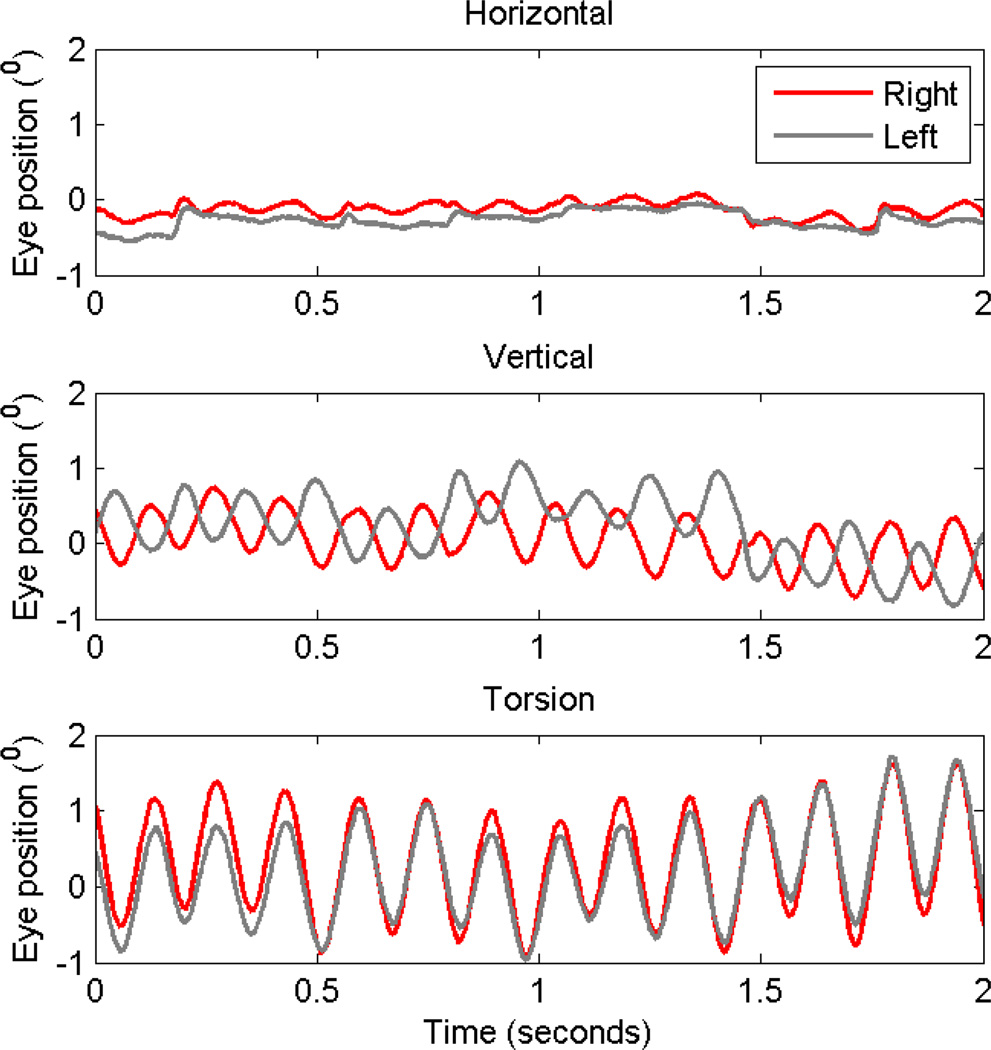
Sarcoidosis is a granulomatous disorder most commonly affecting the lungs, but it can involve any organ in the body, including the nervous system. The pathophysiology of sarcoidosis is unclear, however, one of the recognized theories is that an inappropriate Th1 immune response to an unknown extrinsic antigen leads to excess inflammation and granuloma formation. Characteristic histological findings include immune cell aggregations in the form of non-caseating granulomas, asteroid bodies, Schaumann bodies, and crystalline inclusions.[45] Typical nervous system manifestations of sarcoidosis include neuroendocrine dysfunction, seizures, spinal cord lesions, hydrocephalus, and peripheral or multiple cranial neuropathy.[45–47] Central nervous system sarcoidosis rarely involves the ocular motor system. The manifestations are secondary to involvement of the afferent visual pathway. We hypothesize that involvement of the optic chiasm and accessory optic system carrying signals to the inferior olive results in pendular oscillations in subjects with chiasmal involvement from sarcoidosis. Figure 3 depicts an example of eye movements measured from a patient with sarcoidosis and pendular nystagmus. The oscillations are distinct from several standpoints. Their frequency is relatively higher (6–7Hz) compared to acquired pendular from other etiologies. Horizontal oscillations were minimal, while torsional and vertical oscillations were robust. Torsional oscillations were conjugate while vertical oscillations were disconjugate. There was an attenuation of oscillation amplitude with eye closure, and they did not reset after a visually guided saccade. These characteristics suggest that the physiological basis of acquired pendular nystagmus in sarcoidosis does not involve the neural integrator, but has a novel pathophysiology. It is predicted that instability in the visuo-vestibular system could lead to acquired pendular nystagmus in subjects with sarcoidosis where the visual system is substantially impaired.[48] We have successfully used levetiracetam to attenuate high-frequency pendular oscillations in one subject with sarcoidosis. Banzodiazepines can be efficaciously used, but they are frequently associated with drowsiness.
Figure 3.
This is an example of acquired pendular nystagmus in a subject with sarcoidosis. Horizontal (A), vertical (B) and torsional (C) eye positions are plotted against time. Red traces are right eye positions, while grey traces are left. Horizontal oscillations have minimal amplitude, while the amplitude increased in vertical and then in torsional direction. Torsional oscillations are conjugate, while vertical oscillations are disconjugate as depicted by 180° phase shift.
11 Whipple’s disease
Whipple’s disease is a rare infection of the small intestine caused by the bacterium T. whipplei. The bacterium is ubiquitous in the environment, but not all that encounter the pathogen become symptomatic. Whipple’s disease classically presents with abdominal pain, diarrhea, weight loss, and arthritic joint pain.[49] Untreated, the infection may progress to involve the nervous system. Central nervous system involvement is usually asymptomatic, but 10–40% of Whipple’s patients manifest cognitive impairment, Parkinsonism, ataxia, and motor disturbances of the orbital and facial muscles.[50, 51] Oculomasticatory myorythmia, a specific pattern of movement in which rhythmic movement of the eyes occurs in conjunction with contractions of the masticatory muscles and convergent-divergent nystagmus is considered pathognomonic for Whipple’s disease.[49] The frequency and waveform pattern of eye movement trajectories in the patients with ocular masticatory myorythmia however does not follow that of typical (sinusoidal) acquired pendular nystagmus; it often has irregular waveform trajectories.
The pathophysiology of Whipple’s disease as far as its neurological effects is unclear. Focal lesions have been observed on MRI in patients with central effects from Whipple’s disease, suggesting that the infection may anatomically disrupt pathways in the brain important for regulation of orbital and facial muscles.[52] The antibiotic therapy is the mainstay of treatment of Whipple’s disease and associated neurological complications.
12 Oculopalatal Tremor
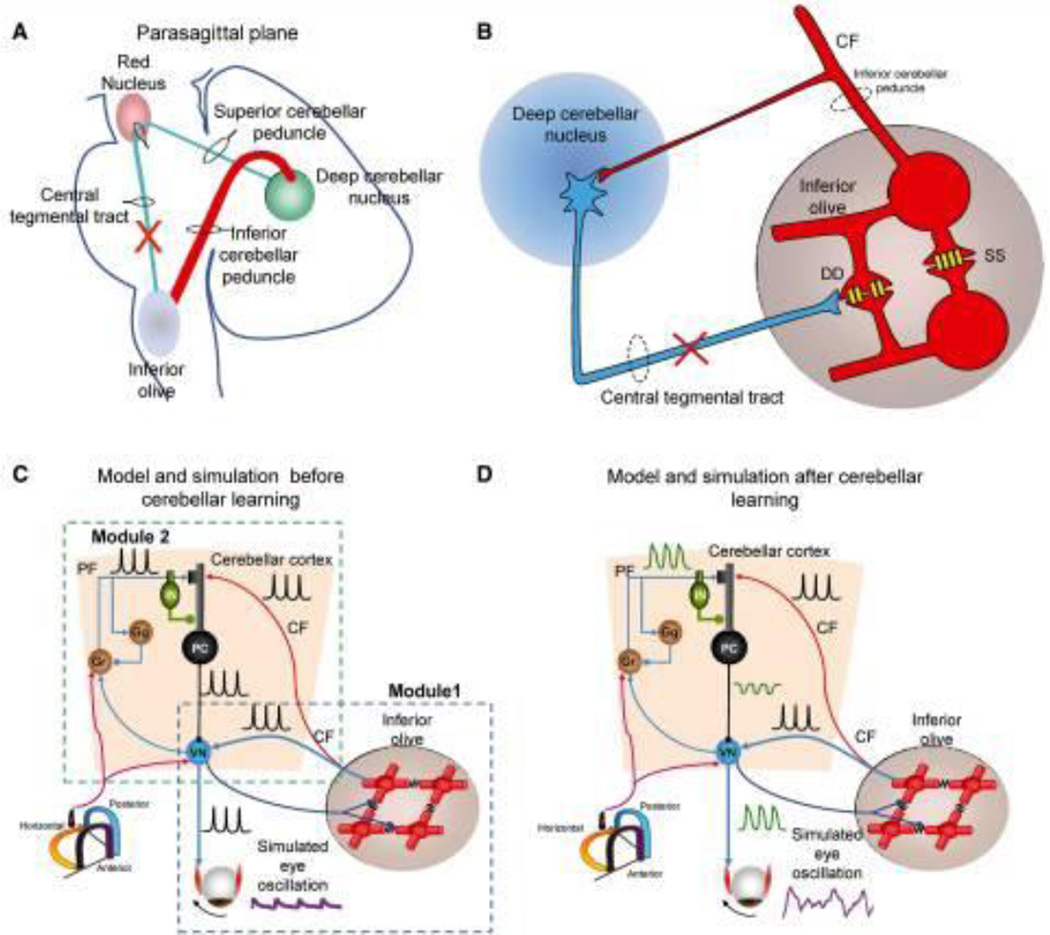
As the name suggests, the syndrome of oculopalatal tremor (OPT) is characterized by oscillations of the eyes and palate. The syndrome can also affect the facial muscles, pharynx, tongue, larynx, diaphragm, neck, and trunk. It may develop weeks to months following a brainstem or cerebellar lesion such as infarction or hemorrhage leading to impairment in the Guillain-Mollaret Triangle (Figure 4A), but is also associated with degenerative conditions such as Alexander’s disease.[42] We recently described a novel variant of OPT with dystonia (OPTd). In this variant, among six OPT patients two had focal, three had multifocal, and one had generalized dystonia. Three out of four patients who had cervical dystonia also had head tremor, and two patients had speech involvement.
Figure 4.
Schematic representation of the Guillain–Mollaret triangle that is comprised of fibers connecting the deep cerebellar nuclei and contralateral inferior olive. These fibers pass near the red nucleus without any rubral relay (A). The coupling strength through the connexn gap junctions (schematized with yellow channels; DD) between neghboring inferior olivary neurons are inhibited by projections from the deep cerebellar nuclei (blue projection) (B). Lesions in the Guillain–Mollaret triangle (red X in A and B) leads to hypertrophy of the inferior olive and subsequently increased soma-somatic gap junction. Schematic representation of a “dual-mechanism model” (C and D). Model and traces from simulations after inferior olive hypertrophy but before maladaptive cerebellar modulation (C). Inferior olive and cerebellar modules after hypertrophy and maladaptation of the cerebellum module (D). Lower left corner shows icon for semicircular canals (C and D). Simulated membrane potentials (black), eye oscillations (magenta). CF = climbing fibres; PF = parallel fibres; DD = dendro-dendritic gap junction; SS = soma-somatic gap junction; Gr = granule cell layer; IN = interneurons; PC = Purkinje neurons.
A variant of OPT is called progressive ataxia palatal tremor (PAPT), where OPT is present without a focal brainstem lesion, but in the presence of progressive cerebellar degeneration.[53] Superficial siderosis was reported in five patients who had ocular palatal tremor along with progressive ataxia.[54] The etiology of hemosiderin deposition was likely due to trauma in two patients, while in three it was from brainstem vascular malformation.[54] OPT and progressive cerebellar ataxia, chin tremor, frequent blinks, and dysarthria was noted in a patient who had adult-onset GM2 gangliosidosis type II (Sandhoff disease) resulting from HEXB mutations causing deficiency of beta-hexosaminidase A and B.[55] There was absence of cerebellar or pontine atrophy or pseudohypertrophy of inferior olive (unlike typical OPT). The OPT was associated with horizontal and vertical saccade hypometria. In addition there was slowing of vertical saccades suggesting supranuclear vertical gaze palsy.[55] Pure progressive ataxia and palatal tremor along with pendular nystagmus was noted in a patient who was heterozygote for a known pathogenic (W748S) and novel POLG variant (I1185N).[56]
OPT was noted in a patient with Behcet disease who also had pseudohypertrophy of the inferior olive. The authors initiated treatment with gabapentin.[57] Progressive supranuclear palsy was once reported to cause acquired pendular nystagmus and inferior olive hypertrophy.[58]
Histologically, OPT is typically associated with pseudohypertrophy of the inferior olivary nucleus. The cells of the inferior olive appear to be enlarged, vacuolated neurons with enlarged astrocytes.[59] Cells in the inferior olive communicate via connexins, a special type of gap junction. Therefore, deafferentation of the inferior olive and subsequent hypertrophic degeneration results in the development of excessive soma-somatic connexins as schematized in Figure 4B. The consequence is spontaneous discharge of patches of olivary neurons at a frequency of 1–2 Hz.[17, 59]
While spontaneous discharge and increased soma-somatic coupling from the inferior olive account for the oscillation frequency, they do not describe the coarse amplitude and irregularity of the oscillations. Instead, it predicts a small, jerky, and regular waveform. Figure 5A depicts an example of “pendular” nystagmus in OPT. Eye position is plotted on the y-axis, while the corresponding time is on the x-axis. The red traces depict the right eye, while grey traces depict the left eye. The red and grey traces are not symmetric; the oscillations have a coarse and irregular waveform. We propose a “dual mechanism model” to explain the coarse and irregular features of the “pendular” nystagmus in OPT. This model has two mechanisms that interact to create the features of OPT. The inferior olive contains the primary oscillator, and the cerebellum modulates the oscillations generated at the inferior olive. This dual mechanism model is schematized in Figure 4C, D. The proof of this concept was provided in the neuromimetic mathematical model of OPT that depicted that synchronized inferior olive output was too small to induce noticeable ocular oscillations, requiring amplification by the cerebellar cortex. Reducing the influence of the cerebellar cortex in the computational model reduced the amplitude of ocular tremor and made it more periodic and pulse-like, however its frequency remained unchanged.[59] In contrast, reducing the coupling among the inferior olive neurons decreased the oscillation amplitude until they stopped at approximately one fifth of full coupling strength. The dual mechanism model accounts for several features of OPT. Further it suggests that drug therapies designed to reduced coupling within the inferior olive or reduce the disinhibition of the cerebellar cortex could treat pendular nystagmus in OPT.[59]
Figure 5.
This is an example of the attenuation of horizontal acquired pendular nystagmus by gabapentin and memantine in an OPT patient. (A–C) Binocular eye positions are plotted against time. Red traces represent the right eye positions while grey lines are left. The oscillations are irregular and disconjugate. The amplitude is reduced during treatment with both gabapentin (B) and memantine (C).
Corresponding power spectra are plotted in D–F. The power spectrum is very broad, with a large peak around 2 Hz. Gabapentin and memantine not only reduce the oscillation amplitude but they also reduce the irregularity of the waveform as depicted by the reduction of the range of its frequency distribution.
Pharmacological tests further assessed the validity of the dual-mechanism model and its application in the treatment of OPT.[17] We analyzed the effects of gabapentin, memantine, and baclofen on “pendular” nystagmus in OPT. No drug changed the frequency of oscillations, but the amplitude was reduced with gabapentin and memantine. Such effects of pharmacotherapy on oscillations are objectively depicted in form of powerspectra in Figure 5D,E,F. Analyzing the effects of drug therapies on ocular oscillations provided a novel approach to test models of pendular nystagmus and confirm that modulation of cerebellar influence by alpha-2-delta calcium channel blockers and glutamate receptor antagonists could attenuate “pendular” nystagmus in OPT. In typical forms of OPT the oscillations usually persist for several years, sometimes decades, but may occasionally resolve spontaneously.[59]
13 Hypoxic Encephalopathy
Hypoxic encephalopathy describes a subset of encephalopathy caused by lack of oxygen to the brain. Although hypoxic or ischemic events can occur at any age or stage of development, there are major differences in etiology and prognosis between events that occur in utero as opposed to after birth and into adulthood. In infants, the effects of hypoxic encephalopathy on vision and ocular motor processes at various developmental stages offer insight into the anatomical regions involved in the pathophysiology of nystagmus. Effects include visual loss due to optic atrophy, optic nerve hypoplasia, and damage to other parts of the visual pathway.[60]
Strabismus, nystagmus, and saccadic abnormalities have also been observed in children who suffered hypoxic insult in utero. Infants who are born preterm are more likely to suffer damage to the subcortical white matter, including the optic radiations, which develop between the 27th-34th week of gestation.[61] Optic nerve hypoplasia is often seen as a result of early prenatal damage. In contrast, full-term infants are more likely to suffer damage to the striate and peristriate cortex, resulting in cortical visual impairment.[61] Nystagmus, either latent or manifest, is common in infants who have suffered damage to the subcortical white matter. However, most children with cortical visual impairment do not have nystagmus.[60] It was hypothesized that an intact geniculostriate pathway is a prerequisite for the development of nystagmus, which is absent in posterior visual pathway disease.[62] Individuals with cortical visual impairment and nystagmus may have a mixed mechanism of visual loss involving both anterior and posterior visual pathways. Latent nystagmus is commonly observed in children with subcortical damage, and is thought to be mediated subcortically by the nucleus of the optic tract within the midbrain.[61, 63]
14 Fosphenytoin Toxicity
Fosphenytoin, the prodrug of phenytoin, is a standard treatment for breakthrough seizures. Phenytoin’s mechanism of action is that it blocks sodium conductance across cell membranes, thus stabilizing neuronal membranes and reducing the velocity of signal conductance. The delay in conduction is directly proportional to the baseline rate of spontaneous discharge, where a higher baseline frequency of discharge results in greater conduction delay. Common side effects include encephalopathy, chorea, athetosis, dyskinesia, ataxia, jerk nystagmus, and opsoclonus.[64]
Case study of an epileptic who received intravenous fosphenytoin for a breakthrough seizure revealed transient pendular nystagmus which began 45 minutes after administration of the fosphenytoin bolus and lasted approximately 12 hours and then spontaneously resolved.[65] The characteristics of the pendular nystagmus were similar to that seen in demyelinating disorders: the oscillations were quasi-sinusoidal, with a mean frequency of 3–4 Hz and transient resets following blinks.
The mode of action for phenytoin in patients with acute seizures describes the pathophysiology of transient pendular nystagmus. Phenytoin blocks sodium conductance responsible for the generation of action potentials and reduces the neuronal conduction velocity.[66] Such effects are dependent upon the membrane threshold, which is lower in hyperexcitable neurons immediately after a seizure.[67] The membrane threshold dependent effects, particularly the conduction delay, if severe, may lead to conduction block.[67, 68] It is proposed that phenytoin-induced decrease in conduction velocity had increased signal delay in the feedback pathway between the cerebellum and the neural integrator. Such delay leading to the network instability can result in pendular nystagmus.[65] The proof of this concept is resolution of pendular nystagmus several hours after the seizure, as the neural excitability reverts to normal and the efficacy of phenytoin to induce conduction delay gets weaker.
16 Ocular Tremor in Parkinson’s Disease
Recent controversy has arisen over the report of ocular tremor in Parkinson’s disease (PD) patients by Gitchel et al.[69] They report a pervasive ocular tremor with an average fundamental frequency of 5–6 Hz and mean horizontal amplitude of 0.27 degrees. This tremor was reported in all 112 patients in the study, hence it was deemed pervasive, and it was suggested that further investigations could lead to use of this ocular tremor as an early diagnostic marker for PD. However, such a tremor has not previously been reported in Parkinson’s patients, and clinical wisdom suggests that eye movement abnormalities are usually minor in Parkinson’s and not ubiquitous. A response published by Kaski et al[70] investigated the possible origin of this reported ocular tremor and concluded that it is the result of an intact vestibulo-ocular reflex response to head tremor. That is, tremor of the head causes compensatory eye movements that allow the eye to maintain a steady fixed gaze. This appears as though the eyes are moving in relation to the head, although in reality the eyes are not moving in relation to space. To further support this point, Kaski et al studied eye and head movements in 2 patients with PD. They recorded an ocular tremor similar to what Gitchel et al reported, but also recorded a head tremor of the same fundamental frequency but in opposite phase to the ocular oscillations.[70] This observation supports the hypothesis that the ocular tremor is the result of vestibulo-ocular reflex to compensate for the head tremor. Furthermore, when the patient’s head was fixed, the amplitude of both the ocular and head oscillations decreased, suggesting that the ocular movements are linked to the head movement.
Although further studies may be conducted to reevaluate the characteristics of head tremor in PD and their possible link to this apparent ocular tremor, the findings of Kaski et al along with the history of clinical observations of PD do not support that this is a newly identified ocular tremor pervasive in PD patients. Rather, the apparent “tremor” is an eye movement evoked by transmitted tremor of the head and subsequent vestibulo-ocular reflex.
Highlights.
Acquired pendular nystagmus are quasi-sinusoidal eye oscillations.
Demyelinating disorders, visual deficits or disorders of inferior olive is common cause.
Pathophysiology, differential diagnosis, and therapy of acquired pendular nystagmus is reviewed.
Acknowledgments
AS was supported by career development grant from Dystonia Medical Research Foundation and Dystonia Coalition (NIH U54TR001456)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cannon SC, Robinson DA. An improved neural-network model for the neural integrator of the oculomotor system: more realistic neuron behavior. Biological cybernetics. 1985;53:93–108. doi: 10.1007/BF00337026. [DOI] [PubMed] [Google Scholar]
- 2.Cannon SC, Robinson DA. Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. Journal of neurophysiology. 1987;57:1383–1409. doi: 10.1152/jn.1987.57.5.1383. [DOI] [PubMed] [Google Scholar]
- 3.Cannon SC, Robinson DA, Shamma S. A proposed neural network for the integrator of the oculomotor system. Biological cybernetics. 1983;49:127–136. doi: 10.1007/BF00320393. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DA. The effect of cerebellectomy on the cat’s bestibulo-ocular integrator. Brain research. 1974;71:195–207. doi: 10.1016/0006-8993(74)90961-5. [DOI] [PubMed] [Google Scholar]
- 5.Frohman EM, Frohman TC, Zee DS, McColl R, Galetta S. The neuro-ophthalmology of multiple sclerosis. The Lancet Neurology. 2005;4:111–121. doi: 10.1016/S1474-4422(05)00992-0. [DOI] [PubMed] [Google Scholar]
- 6.Gresty MA, Ell JJ, Findley LJ. Acquired pendular nystagmus: its characteristics, localising value and pathophysiology. Journal of neurology, neurosurgery, and psychiatry. 1982;45:431–439. doi: 10.1136/jnnp.45.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serra A, Derwenskus J, Downey DL, Leigh RJ. Role of eye movement examination and subjective visual vertical in clinical evaluation of multiple sclerosis. Journal of neurology. 2003;250:569–575. doi: 10.1007/s00415-003-1038-8. [DOI] [PubMed] [Google Scholar]
- 8.Tilikete C, Jasse L, Vukusic S, Durand-Dubief F, Vardanian C, Pelisson D, et al. Persistent ocular motor manifestations and related visual consequences in multiple sclerosis. Annals of the New York Academy of Sciences. 2011;1233:327–334. doi: 10.1111/j.1749-6632.2011.06116.x. [DOI] [PubMed] [Google Scholar]
- 9.Tilikete C, Jasse L, Pelisson D, Vukusic S, Durand-Dubief F, Urquizar C, et al. Acquired pendular nystagmus in multiple sclerosis and oculopalatal tremor. Neurology. 2011;76:1650–1657. doi: 10.1212/WNL.0b013e318219fa9c. [DOI] [PubMed] [Google Scholar]
- 10.Jasse L, Vighetto A, Vukusic S, Pelisson D, Tilikete C. Unusual monocular pendular nystagmus in multiple sclerosis. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2011;31:38–41. doi: 10.1097/WNO.0b013e3181f8dc23. [DOI] [PubMed] [Google Scholar]
- 11.Averbuch-Heller L, Zivotofsky AZ, Das VE, DiScenna AO, Leigh RJ. Investigations of the pathogenesis of acquired pendular nystagmus. Brain : a journal of neurology. 1995;118(Pt 2):369–378. doi: 10.1093/brain/118.2.369. [DOI] [PubMed] [Google Scholar]
- 12.Barton JJ, Cox TA. Acquired pendular nystagmus in multiple sclerosis: clinical observations and the role of optic neuropathy. Journal of neurology, neurosurgery, and psychiatry. 1993;56:262–267. doi: 10.1136/jnnp.56.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das VE, Oruganti P, Kramer PD, Leigh RJ. Experimental tests of a neural-network model for ocular oscillations caused by disease of central myelin. Experimental brain research. 2000;133:189–197. doi: 10.1007/s002210000367. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. Journal of neurophysiology. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- 15.Zee DS, Yee RD, Cogan DG, Robinson DA, Engel WK. Ocular motor abnormalities in hereditary cerebellar ataxia. Brain : a journal of neurology. 1976;99:207–234. doi: 10.1093/brain/99.2.207. [DOI] [PubMed] [Google Scholar]
- 16.Lopez LI, Bronstein AM, Gresty MA, Du Boulay EP, Rudge P. Clinical and MRI correlates in 27 patients with acquired pendular nystagmus. Brain : a journal of neurology. 1996;119(Pt 2):465–472. doi: 10.1093/brain/119.2.465. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh AG, Thurtell MJ, Optican LM, Leigh RJ. Pharmacological tests of hypotheses for acquired pendular nystagmus. Annals of the New York Academy of Sciences. 2011;1233:320–326. doi: 10.1111/j.1749-6632.2011.06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurtell MJ, Joshi AC, Leone AC, Tomsak RL, Kosmorsky GS, Stahl JS, et al. Crossover trial of gabapentin and memantine as treatment for acquired nystagmus. Annals of neurology. 2010;67:676–680. doi: 10.1002/ana.21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nance MA, Berry SA. Cockayne syndrome: review of 140 cases. American journal of medical genetics. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- 20.McElvanney AM, Wooldridge WJ, Khan AA, Ansons AM. Ophthalmic management of Cockayne’s syndrome. Eye (London, England) 1996;10(Pt 1):61–64. doi: 10.1038/eye.1996.9. [DOI] [PubMed] [Google Scholar]
- 21.Harbord MG, Finn JP, Hall-Craggs MA, Brett EM, Baraitser M. Early onset leukodystrophy with distinct facial features in 2 siblings. Neuropediatrics. 1989;20:154–157. doi: 10.1055/s-2008-1071282. [DOI] [PubMed] [Google Scholar]
- 22.Gencic S, Abuelo D, Ambler M, Hudson LD. Pelizaeus-Merzbacher disease: an X-linked neurologic disorder of myelin metabolism with a novel mutation in the gene encoding proteolipid protein. American journal of human genetics. 1989;45:435–442. [PMC free article] [PubMed] [Google Scholar]
- 23.Takanashi J, Sugita K, Tanabe Y, Nagasawa K, Inoue K, Osaka H, et al. MR-revealed myelination in the cerebral corticospinal tract as a marker for Pelizaeus-Merzbacher’s disease with proteolipid protein gene duplication. AJNR American journal of neuroradiology. 1999;20:1822–1828. [PMC free article] [PubMed] [Google Scholar]
- 24.Cailloux F, Gauthier-Barichard F, Mimault C, Isabelle V, Courtois V, Giraud G, et al. Genotype-phenotype correlation in inherited brain myelination defects due to proteolipid protein gene mutations. Clinical European Network on Brain Dysmyelinating Disease. European journal of human genetics : EJHG. 2000;8:837–845. doi: 10.1038/sj.ejhg.5200537. [DOI] [PubMed] [Google Scholar]
- 25.Inoue K, Osaka H, Imaizumi K, Nezu A, Takanashi J, Arii J, et al. Proteolipid protein gene duplications causing Pelizaeus-Merzbacher disease: molecular mechanism and phenotypic manifestations. Annals of neurology. 1999;45:624–632. [PubMed] [Google Scholar]
- 26.Nezu A. Neurophysiological study in Pelizaeus-Merzbacher disease. Brain Dev. 1995;17:175–181. doi: 10.1016/0387-7604(95)00028-a. [DOI] [PubMed] [Google Scholar]
- 27.Huygen PL, Verhagen WI, Renier WO. Oculomotor and vestibular anomalies in Pelizaeus-Merzbacher disease: a study on a kindred with 2 affected and 3 normal males, 3 obligate and 8 possible carriers. Journal of the neurological sciences. 1992;113:17–25. doi: 10.1016/0022-510x(92)90259-n. [DOI] [PubMed] [Google Scholar]
- 28.Trobe JD, Sharpe JA, Hirsh DK, Gebarski SS. Nystagmus of Pelizaeus-Merzbacher disease. A magnetic search-coil study. Archives of neurology. 1991;48:87–91. doi: 10.1001/archneur.1991.00530130099026. [DOI] [PubMed] [Google Scholar]
- 29.Maas EF, Ashe J, Spiegel P, Zee DS, Leigh RJ. Acquired pendular nystagmus in toluene addiction. Neurology. 1991;41:282–285. doi: 10.1212/wnl.41.2_part_1.282. [DOI] [PubMed] [Google Scholar]
- 30.Bassani R, Pareyson D, D’Incerti L, Di Bella D, Taroni F, Salsano E. Pendular nystagmus in hypomyelinating leukodystrophy. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2013;20:1443–1445. doi: 10.1016/j.jocn.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner MR, Poll-The BT, Verhoeven NM, Jakobs C, Espeel M, Roels F, et al. Clinical approach to inherited peroxisomal disorders: a series of 27 patients. Annals of neurology. 1998;44:720–730. doi: 10.1002/ana.410440505. [DOI] [PubMed] [Google Scholar]
- 32.Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annual review of biochemistry. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 33.Rosini F, Vinciguerra C, Mignarri A, Di Giovanni M, Federico A, Rufa A. Eye movement abnormalities in a patient with Zellweger spectrum disorder. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2016;37:1013–1015. doi: 10.1007/s10072-016-2499-8. [DOI] [PubMed] [Google Scholar]
- 34.Kori AA, Robin NH, Jacobs JB, Erchul DM, Zaidat OO, Remler BF, et al. Pendular nystagmus in patients with peroxisomal assembly disorder. Archives of neurology. 1998;55:554–558. doi: 10.1001/archneur.55.4.554. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, Dreisbach JN, Hormes JT, Filley CM. Toluene abuse causes diffuse central nervous system white matter changes. Annals of neurology. 1988;23:611–614. doi: 10.1002/ana.410230614. [DOI] [PubMed] [Google Scholar]
- 36.Xiong L, Matthes JD, Li J, Jinkins JR. MR imaging of “spray heads”: toluene abuse via aerosol paint inhalation. AJNR American journal of neuroradiology. 1993;14:1195–1199. [PMC free article] [PubMed] [Google Scholar]
- 37.Hormes JT, Filley CM, Rosenberg NL. Neurologic sequelae of chronic solvent vapor abuse. Neurology. 1986;36:698–702. doi: 10.1212/wnl.36.5.698. [DOI] [PubMed] [Google Scholar]
- 38.Hunnewell J, Miller NR. Bilateral internuclear ophthalmoplegia related to chronic toluene abuse. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 1998;18:277–280. [PubMed] [Google Scholar]
- 39.Schneider RM, Thurtell MJ, Eisele S, Lincoff N, Bala E, Leigh RJ. Neurological basis for eye movements of the blind. PloS one. 2013;8:e56556. doi: 10.1371/journal.pone.0056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toledano H, Muhsinoglu O, Luckman J, Goldenberg-Cohen N, Michowiz S. Acquired nystagmus as the initial presenting sign of chiasmal glioma in young children. Eur J Paediatr Neurol. 2015;19:694–700. doi: 10.1016/j.ejpn.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Martidis A, Yee RD, Azzarelli B, Biller J. Neuro-ophthalmic, radiographic, and pathologic manifestations of adult-onset Alexander disease. Archives of ophthalmology (Chicago, Ill : 1960) 1999;117:265–267. doi: 10.1001/archopht.117.2.265. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer G, Abegg M, Vertinsky AT, Ceccherini I, Caroli F, Barton JJ. The ocular motor features of adult-onset alexander disease: a case and review of the literature. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2011;31:155–159. doi: 10.1097/WNO.0b013e31820ecb28. [DOI] [PubMed] [Google Scholar]
- 43.Hirayama T, Fukae J, Noda K, Fujishima K, Yamamoto T, Mori K, et al. Adult-onset Alexander disease with palatal myoclonus and intraventricular tumour. European journal of neurology. 2008;15:e16–e17. doi: 10.1111/j.1468-1331.2007.02031.x. [DOI] [PubMed] [Google Scholar]
- 44.Howard KL, Hall DA, Moon M, Agarwal P, Newman E, Brenner M. Adult-onset Alexander disease with progressive ataxia and palatal tremor. Movement disorders : official journal of the Movement Disorder Society. 2008;23:118–122. doi: 10.1002/mds.21774. [DOI] [PubMed] [Google Scholar]
- 45.Stern BJ, Krumholz A, Johns C, Scott P, Nissim J. Sarcoidosis and its neurological manifestations. Archives of neurology. 1985;42:909–917. doi: 10.1001/archneur.1985.04060080095022. [DOI] [PubMed] [Google Scholar]
- 46.Joseph FG, Scolding NJ. Neurosarcoidosis: a study of 30 new cases. Journal of neurology, neurosurgery, and psychiatry. 2009;80:297–304. doi: 10.1136/jnnp.2008.151977. [DOI] [PubMed] [Google Scholar]
- 47.Pawate S, Moses H, Sriram S. Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM : monthly journal of the Association of Physicians. 2009;102:449–460. doi: 10.1093/qjmed/hcp042. [DOI] [PubMed] [Google Scholar]
- 48.Nakada T, Kwee IL. Role of visuo-vestibular interaction in pathological ocular oscillation: new model and control system analysis. Medical hypotheses. 1992;38:261–269. doi: 10.1016/0306-9877(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 49.Simpson DA, Wishnow R, Gargulinski RB, Pawlak AM. Oculofacial-skeletal myorhythmia in central nervous system Whipple’s disease: additional case and review of the literature. Movement disorders : official journal of the Movement Disorder Society. 1995;10:195–200. doi: 10.1002/mds.870100210. [DOI] [PubMed] [Google Scholar]
- 50.Gerard A, Sarrot-Reynauld F, Liozon E, Cathebras P, Besson G, Robin C, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine. 2002;81:443–457. doi: 10.1097/00005792-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Louis ED, Lynch T, Kaufmann P, Fahn S, Odel J. Diagnostic guidelines in central nervous system Whipple’s disease. Annals of neurology. 1996;40:561–568. doi: 10.1002/ana.410400404. [DOI] [PubMed] [Google Scholar]
- 52.Mohamed W, Neil E, Kupsky WJ, Juhasz C, Mittal S, Santhakumar S. Isolated intracranial Whipple’s disease--report of a rare case and review of the literature. Journal of the neurological sciences. 2011;308:1–8. doi: 10.1016/j.jns.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 53.Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE. Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain : a journal of neurology. 2004;127:1252–1268. doi: 10.1093/brain/awh137. [DOI] [PubMed] [Google Scholar]
- 54.Kumar N, Eggers SD, Milone M, Keegan BM. Acquired progressive ataxia and palatal tremor: importance of MRI evidence of hemosiderin deposition and vascular malformations. Parkinsonism & related disorders. 2011;17:565–568. doi: 10.1016/j.parkreldis.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 55.Pretegiani E, Rosini F, Federighi P, Cerase A, Dotti MT, Rufa A. Pendular nystagmus, palatal tremor and progressive ataxia in GM2-gangliosidosis. European journal of neurology. 2015;22:e67–e69. doi: 10.1111/ene.12661. [DOI] [PubMed] [Google Scholar]
- 56.Nicastro N, Ranza E, Antonarakis SE, Horvath J. Pure Progressive Ataxia and Palatal Tremor (PAPT) Associated with a New Polymerase Gamma (POLG) Mutation. Cerebellum. 2016;15:829–831. doi: 10.1007/s12311-015-0749-6. [DOI] [PubMed] [Google Scholar]
- 57.Morgan ML, Espino Barros Palau A, Lee AG, Foroozan R. Neuro-Behcet disease presenting with oculopalatal tremor. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2015;35:51–53. doi: 10.1097/WNO.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 58.von der Gablentz J, Sprenger A, Heldmann M, Munte TF, Helmchen C. Acquired pendular nystagmus and its therapy in progressive supranuclear palsy (PSP) due to inferior olivary hypertrophy. Journal of neurology. 2013;260:2424–2426. doi: 10.1007/s00415-013-7059-z. [DOI] [PubMed] [Google Scholar]
- 59.Shaikh AG, Hong S, Liao K, Tian J, Solomon D, Zee DS, et al. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain : a journal of neurology. 2010;133:923–940. doi: 10.1093/brain/awp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chhablani PP, Kekunnaya R. Neuro-ophthalmic manifestations of prematurity. Indian journal of ophthalmology. 2014;62:992–995. doi: 10.4103/0301-4738.145990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brodsky MC, Fray KJ, Glasier CM. Perinatal cortical and subcortical visual loss: mechanisms of injury and associated ophthalmologic signs. Ophthalmology. 2002;109:85–94. doi: 10.1016/s0161-6420(01)00849-1. [DOI] [PubMed] [Google Scholar]
- 62.Fielder AR, Evans NM. Is the geniculostriate system a prerequisite for nystagmus? Eye (London, England) 1988;2(Pt 6):628–635. doi: 10.1038/eye.1988.116. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Survey of ophthalmology. 2000;45:1–13. doi: 10.1016/s0039-6257(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 64.Boucher BA. Fosphenytoin: a novel phenytoin prodrug. Pharmacotherapy. 1996;16:777–791. [PubMed] [Google Scholar]
- 65.Shaikh AG. Fosphenytoin induced transient pendular nystagmus. Journal of the neurological sciences. 2013;330:121–122. doi: 10.1016/j.jns.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Lipicky RJ, Gilbert DL, Stillman IM. Diphenylhydantoin inhibition of sodium conductance in squid giant axon. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:1758–1760. doi: 10.1073/pnas.69.7.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adler EM, Yaari Y, David G, Selzer ME. Frequency-dependent action of phenytoin on lamprey spinal axons. Brain research. 1986;362:271–280. doi: 10.1016/0006-8993(86)90451-8. [DOI] [PubMed] [Google Scholar]
- 68.Le Quesne PM, Goldberg V, Vajda F. Acute conduction velocity changes in guinea-pigs after administration of diphenylhydantoin. Journal of neurology, neurosurgery, and psychiatry. 1976;39:995–1000. doi: 10.1136/jnnp.39.10.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gitchel GT, Wetzel PA, Baron MS. Pervasive ocular tremor in patients with Parkinson disease. Archives of neurology. 2012;69:1011–1017. doi: 10.1001/archneurol.2012.70. [DOI] [PubMed] [Google Scholar]
- 70.Kaski D, Saifee TA, Buckwell D, Bronstein AM. Ocular tremor in Parkinson’s disease is due to head oscillation. Movement disorders : official journal of the Movement Disorder Society. 2013;28:534–537. doi: 10.1002/mds.25342. [DOI] [PubMed] [Google Scholar]