Abstract
Objective
To examine the association between HBV infection and liver fibrosis in HIV-infected patients in Zambia and Switzerland.
Design and Methods
We included HIV-infected adults starting antiretroviral therapy in two clinics in Zambia and Switzerland. Liver fibrosis was evaluated using the AST-to-Platelet-Ratio Index (APRI), with a ratio >1.5 defining significant fibrosis and >2.0 cirrhosis. The association between HBsAg-positivity, HBV replication and liver fibrosis was examined using logistic regression.
Results
In Zambia 96 (13.0%) of 739 patients were HBsAg-positive compared to 93 (4.5%) of 2,058 in Switzerland. HBsAg-positive patients were more likely to have significant liver fibrosis than HBsAg-negative ones: the adjusted odds ratio (aOR) was 3.25 (95% CI 1.44-7.33) in Zambia and 2.50 (1.19-5.25) in Switzerland. Patients with high HBV viral load (≥20,000 UI/mL) were more likely to have significant liver fibrosis compared to HBsAg-negative patients or patients with undetectable viral load: aOR 3.85 (1.29-11.44) in Zambia and 4.20 (1.64-10.76) in Switzerland. In both settings male sex was a strong risk factor for significant liver fibrosis.
Conclusions
Despite the differences in HBV natural history between sub-Saharan Africa and Europe, the degree of liver fibrosis and the association with important risk factors were similar.
Keywords: Hepatitis B infection, HIV, Liver fibrosis, Switzerland, Zambia
INTRODUCTION
The increasing availability of antiretroviral therapy (ART) for HIV-infected individuals has led to a dramatic reduction in AIDS-related mortality and to the emergence of liver-related complications of hepatitis B (HBV) and C (HCV) virus infections as a major cause of death (1). Worldwide, HBV infection is the single most important cause of liver cirrhosis and causes over 50% of cases of hepatocellular carcinomas (HCC) (2). HIV infection accelerates the progression of HBV-related liver fibrosis, especially if cellular immunity is impaired or in the absence of adequate treatment of HBV infection (3). A recent study from Nigeria showed that HIV/HBV-coinfected individuals were five times more likely to have advanced liver fibrosis compared to HIV-monoinfected people (4). Although the mechanisms of HBV-related fibrogenesis are not fully understood, recent results from prospective cohorts in Europe, North America and Asia have provided new insights to its main determinants (5). Several host and viral risk factors, including male sex, old age, high HBV viral load and specific HBV genotypes, have been associated with the development of liver fibrosis, cirrhosis and HCC. However, data from sub-Saharan Africa (SSA), where HBV prevalence is highest, are scarce. In SSA, most infections occur during early childhood, in contrast to Western Europe, where the majority of patients are infected as adults (6). As HBV transmission patterns and duration of infection may influence the development of liver fibrosis and HCC, HIV/HBV-coinfected patients in SSA could be at high risk of developing early liver disease (5). In addition, the burden of HBV-related complications might be increased by the presence of concurrent infections such as hepatitis delta virus (HDV) and schistosomal infections, as well as environmental exposures such as aflatoxins (7-9).
Several non-invasive measurements have been used for the staging of liver fibrosis in HIV-infected and HIV/HBV-coinfected individuals (10, 11). The AST-to-platelet ratio index (APRI), which has been associated with mortality in sub-Saharan Africa (12), is recommended by the World Health Organization (WHO) to assess the presence of liver fibrosis where liver biopsy is unavailable (13, 14). We compared stages of liver fibrosis using the APRI between HIV-infected and HIV/HBV-coinfected individuals in cohorts from Zambia and Switzerland. This provided a unique opportunity to assess the impact of HBV transmission patterns on the development of liver fibrosis and to evaluate its most important clinical and biological determinants in two distinct epidemiological contexts.
METHODS
HIV cohorts in Zambia and Switzerland
Analyses were based on two HIV cohorts in Zambia and Switzerland
We included HIV-infected adults receiving ART in two urban clinics in Lusaka, where care was provided to the standard of the national program (15). The routine baseline examination included a medical history, physical examination and laboratory measurements (CD4 cell count, full blood count, serum creatinine, and aminotransferases). In addition, we tested all patients for HBsAg, anti-HCV antibodies and performed HBV sequencing as well as viral load measurements in HBsAg-positive individuals within the framework of a sub-study of the International epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) (16). All data were entered into an electronic database for clinical care, monitoring, evaluation, and reporting purposes. Written informed consent was obtained from all patients. The Biomedical Research Ethics Committee of University of Zambia School of Medicine and the Institutional Review Board of University of North Carolina at Chapel Hill, USA, approved the study.
Established in 1988, the Swiss HIV Cohort Study (SHCS, www.shcs.ch) is a prospective nation-wide cohort study with on-going enrolment of HIV-infected adults. It covers approximately 50% of the cumulative number of HIV infections reported to the Swiss public health authorities and 75% of patients receiving ART in Switzerland(17). Detailed information on demographics, mode of HIV acquisition, risk behavior, clinical events, co-infections, and treatment is collected using a standard protocol at registration and then in 6-monthly intervals. All participants are screened for HBV infection at study entry. Positive HBsAg tests are confirmed with an HBV DNA measurement. Local ethical committees of all participating study sites approved the study and written consent was obtained from all participants.
Inclusion criteria and definitions
We included all adults with measurements of HBsAg, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and platelets before ART initiation. Patients who tested positive for anti-HCV antibodies were excluded. Liver fibrosis was evaluated using the AST-to-platelet ratio index (APRI), a non-invasive score originally validated in HIV/HCV-coinfected patients and previously used in studies of HIV-infected and HIV/HBV-coinfected individuals (11, 12, 18, 19). We used the following cut-offs: 0.5 and 1.5 to rule-out and confirm significant fibrosis (equivalent to METAVIR stages 2 and above), respectively, and 1.0 and 2.0 to rule-out and confirm cirrhosis (METAVIR stages 4), as recommended by the WHO (13). Grade 1 (1.25-2.5 × upper-limit norm [ULN]) ALT elevation was defined according to Division of AIDS criteria (20).
In the SHCS, alcohol intake has been routinely assessed at 6-monthly intervals since 2005 (21). In Zambia, alcohol consumption was investigated in all patients at ART initiation using the AUDIT-C tool (22). We defined at-risk alcohol intake according to the National Institute on Alcoholism and Alcohol Abuse (NIAAA) as an average daily consumption above one standard drink (10 g of pure alcohol) for women and above two standard drinks for men.
Laboratory analyses
HBsAg-positivity was assessed using the point-of-care Determine™ rapid test (Alere, Yavne, Israel) in Zambia and recommended commercial serological assays in Switzerland. HCV infection was evaluated using the anti-HCV antibody rapid tests OraquickTM (OraSure Technologies Inc., Bethlehem, USA) in Zambia and the ARCHITECT™ anti-HCV assay (Abbott Diagnostics, Wiesbaden, Germany) in Switzerland. HBV DNA testing was performed using real-time PCR (Roche COBAS AmpliPrep/Taqman HBV test) in both countries. All measurements were performed before the initiation or within the first month of ART.
Statistical analyses
We tested differences in baseline demographic, clinical and liver-related parameters at ART initiation between HBsAg-positive and HBsAg-negative individuals in the two cohorts using Chi-square and Mann-Whitney tests. We used logistic regression to evaluate the association between HBsAg-positivity and significant liver fibrosis (APRI≥1.5). The following potential confounders were adjusted for in multivariable models: sex, age (below vs. above 40 years old), country (Zambia vs. Switzerland) CD4 cell count (below vs. above 200 cells/μL), clinical stage of HIV disease (advanced vs. not advanced, with advanced defined as WHO stages III or IV for Zambia and CDC stage C for Switzerland) and alcohol consumption (at-risk consumption vs. no or not-at-risk consumption). The logistic regression analysis was repeated after stratification by country. As some of the comparisons of HBV-related determinants between patients from Zambia and Switzerland might have been biased by the presence of patients of African origin in the SHCS, we also repeated the main analyses after excluding the latter.
To evaluate the role of HBV replication on the degree of liver fibrosis, we repeated the logistic regression analyses including levels of HBV replication in three categories: 1) no HBV infection or HBV infection with an HBV VL<20 UI/mL 2) HBV VL between 20 and 19,999 UI/mL and 3) HBV VL ≥20,000 UI/mL. The association between HBV viral loads and significant fibrosis and cirrhosis was further explored by repeating the analyses after excluding HIV/HBV-coinfected patients with high viral loads but normal transaminases (as a proxy for the immune tolerant profile). All statistical analyses were performed using Stata 12.0 (Stata Corp, College Station, TX, USA).
RESULTS
Demographic and clinical characteristics
In Zambia 96 (13.0%) of 739 HIV-infected patients were HBsAg-positive compared to 93 (4.5%) of 2,058 patients in Switzerland (P<0.001). Age and sex distribution were similar between HBsAg-positive and negative patients (Table 1). The prevalence of advanced disease and median CD4 counts were also similar in HBsAg-positive and HBsAg-negative patients in the two cohorts, but patients in Zambia had more advanced disease and lower CD4 cell counts. Both in Zambia and Switzerland the prevalence of at-risk alcohol use was somewhat higher in HBsAg-positive patients, but differences failed to reach statistical significance (P>0.20). At-risk alcohol intake was more common in Zambia than in Switzerland (24.9% vs. 9.9%, P<0.001). In Switzerland, 26.9% of HIV/HBV-coinfected individuals were of African origin whereas this group represented only 15.6% of HBsAg-negative patients.
Table 1.
Baseline characteristics of patients, by cohort
| Zambia |
Switzerland |
|||||
|---|---|---|---|---|---|---|
| HIV (n=643) | HIV/HBV (n=96) | P | HIV (n=1,965) | HIV/HBV (n=93) | P | |
| General characteristics | ||||||
| Female (%) | 346 (53.8) | 42 (43.8) | 0.07 | 447 (22.8) | 21 (22.6) | 0.97 |
| African origin (%) | 643 (100) | 96 (100) | 0.99 | 306 (15.6) | 25 (26.9) | 0.004 |
| Median age in years (IQR) | 34 (29-41) | 34 (29-39) | 0.30 | 39 (32-46) | 38 (31-45) | 0.38 |
| At-risk alcohol intake (%) | 155 (24.1) | 29 (30.2) | 0.20 | 191 (10.7) | 12 (14.6) | 0.26 |
| Advanced HIV disease (%) | 288 (45.4) | 38 (40.0) | 0.32 | 216 (11.0) | 8 (8.6) | 0.47 |
| Median CD4 in cells/μL (IQR) | 226 (121-330) | 233 (114-361) | 0.59 | 277 (178-381) | 293 (170-413) | 0.64 |
| Median creatinine in μmol/L (IQR) | 80 (70-92) | 79 (70-91) | 0.72 | 76 (66-87) | 75 (64-88) | 0.84 |
| Median platelets in G/L (IQR) | 250 (197-316) | 246 (190-303) | 0.35 | 205 (168-252) | 199 (165-242) | 0.41 |
| Liver-related characteristics | ||||||
| Grade 1 ALT elevation or above (%) | 49 (7.6) | 14 (14.6) | 0.02 | 215 (10.9) | 23 (24.7) | <0.001 |
| Median APRI (IQR) | 0.36 (0.26-0.53) | 0.44 (0.29-0.76) | 0.01 | 0.39 (0.29-0.59) | 0.50 (0.37-0.89) | <0.001 |
| HBV viral load category (%) | ||||||
| <20 | NA | 11 (12.2) | NA | NA | 12 (16.2) | NA |
| 20-19,999 | NA | 40 (44.4) | NA | NA | 29 (39.2) | NA |
| ≥20,000 | NA | 39 (43.3) | NA | NA | 33 (44.6) | NA |
NA: not applicable
Liver-related parameters
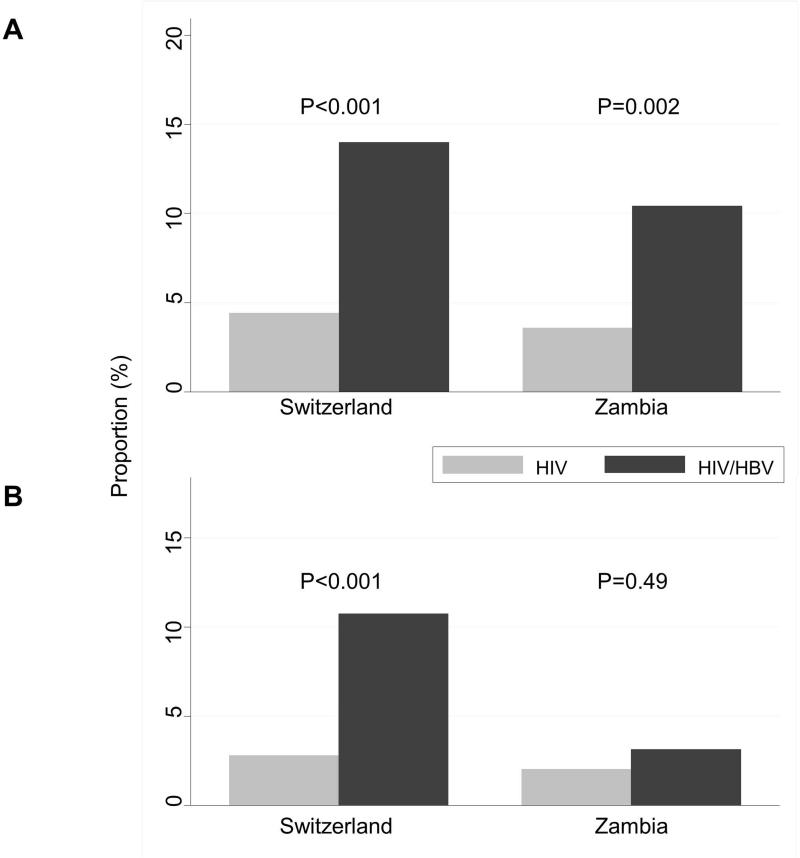
In both countries, HIV/HBV-coinfected individuals were approximately twice as likely to have a grade 1 ALT elevation or higher as HIV-monoinfected patients (Table 1). As shown in Figure 1, the proportion of patients with significant liver fibrosis (APRI>1.5) was higher in HIV/HBV-coinfected patients compared to HIV-monoinfected patients (10.4% vs. 3.6% in Zambia, 14.0% vs. 4.4% in Switzerland). The proportion of individuals with liver cirrhosis was below 3% in all groups except in HIV/HBV-coinfected patients in Switzerland, in whom the prevalence was 10.8% (Figure 1). Based on the lower APRI cut-off for cirrhosis (APRI>1.0), cirrhosis could be excluded in over 90% of HIV-monoinfected patients, as well as in 84.4% and 76.3% of HIV/HBV-coinfected patients in Zambia and Switzerland, respectively.
Figure 1.
Proportion of patients with significant fibrosis (A) and cirrhosis (B), by country and HBV status
Determinants of significant liver fibrosis
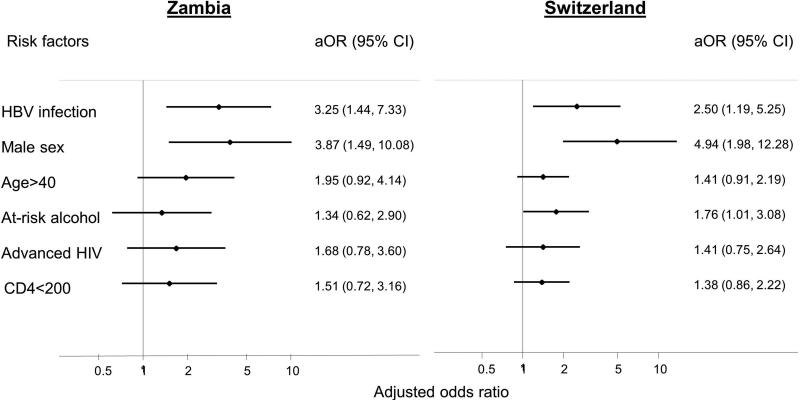
In adjusted analyses, HBsAg-positive patients were over approximately three times more likely to have significant liver fibrosis compared to HBsAg-negative individuals (adjusted odds ratio (aOR) 2.75, 95% confidence interval (CI) 1.61-4.74, Table 2). The estimates were similar when each cohort was analyzed separately (3.25, 1.44-7.33 in Zambia and 2.50, 1.19-5.25 in Switzerland, Figure 2). Male sex was another strong predictor of significant liver fibrosis in pooled (OR 4.38, 95% CI 2.30-8.32) and stratified analyses (3.87, 1.49-10.08 in Zambia and 4.94, 1.98-12.28 in Switzerland). Associations with age above 40 years, at-risk alcohol intake, advanced HIV disease, and a CD4 count <200 cells/μL were weaker and further attenuated in multivariable analysis (Table 2). At-risk alcohol consumption predicted significant liver fibrosis in Switzerland (OR 1.78, 95% CI 1.01-3.08) but not in Zambia (OR 1.34, 95% CI 0.62-2.90). The results of the analysis in the SHCS after the exclusion of patients from African origin (n=331) remained similar, including the association between HBV and liver fibrosis (OR 2.64, 95% CI 1.20-5.82).
Table 2.
Risk factors for significant liver fibrosis
| No. (%) with significant fibrosis | Univariable analysis (95% CI) | Multivariable analysis (95% CI) | |
|---|---|---|---|
| HBV-coinfection | |||
| No | 110 (4.2) | 1 | 1 |
| Yes | 23 (12.2) | 3.15 (1.95-5.06) | 2.75 (1.61-4.74) |
| Sex | |||
| Female | 12 (1.4) | 1 | 1 |
| Male | 121 (6.3) | 4.68 (2.57-8.51) | 4.38 (2.30-8.32) |
| Age, y | |||
| 16-40 | 65 (3.9) | 1 | 1 |
| >40 | 68 (6.0) | 1.57 (1.11-2.23) | 1.54 (1.05-2.25) |
| At-risk alcohol intake | |||
| No | 93 (4.2) | 1 | 1 |
| Yes | 30 (7.8) | 1.93 (1.26-2.95) | 1.55 (0.98-2.44) |
| Advanced HIV disease | |||
| No | 95 (4.3) | 1 | 1 |
| Yes | 38 (6.9) | 1.67 (1.13-2.47) | 1.48 (0.92-2.38) |
| CD4+ count, cells/μL | |||
| >=200 | 75 (4.0) | 1 | 1 |
| <200 | 58 (6.2) | 1.56 (1.10-2.21) | 1.40 (0.94-2.08) |
| Country | |||
| Zambia | 33 (4.5) | 1 | 1 |
| Switzerland | 100 (4.9) | 1.09 (0.73-1.63) | 1.10 (0.66-1.81) |
Figure 2.
Risk factors for significant liver fibrosis (APRI>=1.5) from multivariable logistic regression, by cohort
HBV viral load and liver fibrosis
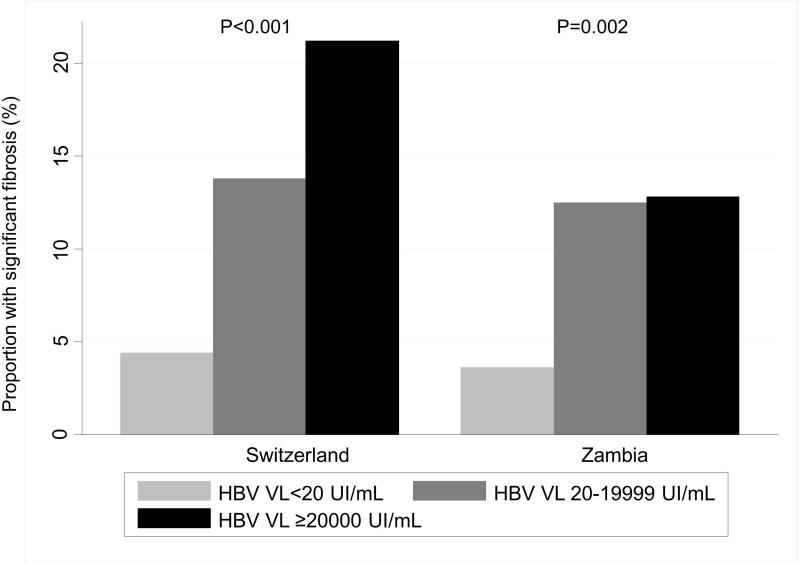
Of 96 HIV/HBV-coinfected individuals in Zambia, 90 (94%) had an available HBV VL before the initiation of ART and the median HBV VL was 1,186 (interquartile range (IQR) 53-1.94e+06) UI/mL. Among the 93 HIV/HBV-coinfected individuals in Switzerland, 74 (80.0%) had an available measurement and the median HBV VL was 7,299 (90-1.62e+08) UI/mL (P-value for the comparison between the two countries: 0.07). A similar proportion of HIV/HBV-coinfected patients had a high baseline HBV VL (≥20,000 UI/mL) in both countries: 43.3% in Zambia and 44.6% in Switzerland (Table 1). The proportion of patients with significant liver fibrosis increased with HBV VL: from 3.7% in HBsAg-negative or HBsAg-positive with HBV VL<20 UI/mL to 12.8% in those with a HBV VL≥20,000 UI/mL in Zambia, and from 4.4% to 21.2% in Switzerland (Figure 3). In multivariable analyses, individuals with high HBV VL were more likely to have significant liver fibrosis compared to HBsAg-negative or HBsAg-positive patients with undetectable HBV VL: aOR 3.85 (95% CI 1.29-11.44) in Zambia and 4.20 (1.64-10.76) in Switzerland (Supplementary table). Of note, there was no clear difference in the proportion of patients with significant liver fibrosis between HIV/HBV-coinfected patients with moderate and those with high viral loads in Zambia (Figure 3). Among patients with high HBV viral loads, 72% in Zambia and 58% in Switzerland had normal transaminases and only one of these 47 patients had a significant fibrosis. After exclusion of these potentially immune-tolerant patients, the proportion of individuals with a significant fibrosis among those with high viral loads raised from 13% to 36% Zambia and from 21% to 50% in Switzerland.
Figure 3.
Proportion of patients with significant fibrosis, by country and HBV replication status
DISCUSSION
In this cross-sectional study of HIV-infected, ART-naïve adults in Zambia and Switzerland, we showed that HBV-coinfection was strongly associated with liver fibrosis. HIV/HBV-coinfected patients were at least three times more likely to have significant fibrosis compared to HIV-monoinfected individuals. This association was similar in Zambia and Switzerland despite differences in the natural history of HBV infection and the genetic background of study populations, and seemed to be driven by HBV viral load. Liver cirrhosis was rare in Zambia but present in over 10% of HIV/HBV-coinfected individuals in Switzerland. Our analysis also showed differences in alcohol intake and impact on liver disease between the two regions, underlining the need to better characterize alcohol consumption and its health-related effects in sub-Saharan Africa.
Few studies have evaluated the impact of HBV-coinfection on the development of liver fibrosis in HIV-infected individuals using non-invasive methods. The Multicenter AIDS Cohort Study (MACS) showed a strong association between viral hepatitis coinfection and significant liver fibrosis using the APRI score, but the number of HBV-infected patients analyzed was small (19). In SSA, two studies based on transient elastography measurements showed conflicting results: Hawkins et al. reported a strong association between HBV-coinfection and liver fibrosis in HIV-infected individuals in Nigeria, whereas Stabinski et al. found no such association in a large cohort in Uganda (4, 23).
Our analysis of two large HIV cohorts provides further evidence in favor of a link between HBV-coinfection and the development of liver fibrosis. Although non-invasive markers of liver fibrosis have mostly been validated and widely used in studies of HCV infection, they will be increasingly used in the management of HBV-infection, especially in the context of improving coverage of ART in resource-limited settings. Recently, Stockdale et al. showed that the APRI score had a good negative predictive value for excluding cirrhosis and significant fibrosis among HIV/HBV-coinfected individuals in West Africa, but had a poor overall diagnostic performance (24). As APRI is now recommended by the WHO to evaluate treatment eligibility for chronic HBV infection in the absence of liver biopsy or transient elastography (13, 14, 25), there is an urgent need for validation studies of this marker in other contexts, ideally in comparison with results of liver biopsies.
The association between HBV infection and liver fibrosis depended on the level of HBV viral replication. In both countries, the proportion of patients with significant liver fibrosis was similar in HBV-uninfected and HBV-coinfected patients with a VL<20 UI/mL, whereas the prevalence of liver disease was much higher in those with high viral loads. These results are in line with prospective studies of viral replication in HBV-monoinfected individuals and the risk of liver cirrhosis and HCC (26, 27). HBV replication is influenced by the natural history of HBV infection: patients infected during early childhood are likely to undergo HBeAg seroconversion early in life and to remain in a non-replicating phase for many years, whereas those acquiring chronic HBV infection in adulthood often experience high-level replication, hepatic inflammation and progressive development liver fibrosis (5). Compared to HIV/HBV-coinfected patients in Switzerland, liver cirrhosis was less common among Zambians, despite what were likely longer durations of HBV infection and similar HBV viral loads at ART initiation. Host genetic factors could partially explain the lower prevalence of cirrhosis in Zambia, analogous to the protective effect of black ethnicity on the development of HCV-related complications (28). Other potential explanations include differences in viral factors (HBV genotypes and HBe-Ag positivity), as well as environmental factors unaccounted for in our analyses. For instance, insulin resistance, a known risk factor for the development of liver fibrosis and HCC, might have been more prevalent in the Swiss population (29). Although our results confirm previous evidence on the impact of replicating HBV infection on liver disease in treatment-naïve populations, it is not yet known to what extent HBV-active therapy reduces the excess liver-related morbidity seen in HIV/HBV-coinfected patients.
Another important difference between patients in Zambia and Switzerland was the difference in the association between alcohol consumption and liver disease. At-risk alcohol consumption did not seem to be a strong risk factor for hepatic damage in Zambia whereas there was a clear association with significant liver fibrosis in Switzerland. Due to the paucity of data published on alcohol consumption and related health consequences in HIV-infected individuals in SSA, it was difficult to evaluate the generalizability and robustness of our data. However, besides the potential explanations mentioned above, the nature of the alcohol assessment could have influenced our results. Self-reported alcohol intake is subject to several biases and socio-cultural differences between the two settings studied make the comparison difficult. More research on alcohol-related and metabolic-induced liver complications is urgently needed to improve our understanding of liver disease progression in SSA, especially in HIV-infected populations, in which alcohol consumption is high. This assessment of liver-related complications using detailed assessments of HBV virological determinants as well as other risk factors for liver disease, including alcohol consumption and HIV stage of disease is a unique strength of our study. The impact of HCV-coinfection on liver fibrosis could be excluded as all patients were tested for HCV infection, including in the Zambian cohort. We could test the association between HBV replication and liver fibrosis as we had viral load measurements for >80% of our HBV-coinfected participants. However, due to missing HBeAg data in a large sample of our patients, we could not evaluate the influence of HBeAg-positivity. As the majority of adult patients with HBV infection in SSA are HBeAg-negative, these data could have explained the differences in the prevalence of liver cirrhosis between the two settings. Similarly, we did not have data on hepatitis delta infection, a well-known risk factor of liver fibrosis in HIV/HBV-coinfected individuals. Another limitation of our study was the reliance on the APRI score to measure liver fibrosis and cirrhosis. As this test has not been properly validated in HBV-infected populations in SSA, we had to apply cut-offs generally used for HCV infection. Although this score has a good negative predictive value to rule out liver cirrhosis (APRI<1.0), more data are needed from SSA to better define its accuracy and results need to be compared with data from HBV-monoinfected populations. To expand the results of our study, similar comparative analyses should be repeated in cohorts with access to more detailed data on liver-related events, including hepatocellular carcinoma and liver decompensation. Finally, we were unable to determine when our coinfected participants acquired HBV infection. Although we assumed that most patients in Zambia were infected during early childhood and most in Switzerland during adulthood, individual-level data on the time of infection would have allowed us to better define the role of duration of infection.
CONCLUSION
As HBV-active antiretroviral agents such as tenofovir become increasingly available, it is essential to improve our knowledge on the prevalence and main determinants of liver disease among those infected with HIV. Our results show that the prevalence and determinants of HBV-related liver fibrosis in Zambia and Switzerland are largely comparable. Further research is needed to better understand the complex relationship between duration of HBV infection, natural history, viral replication and liver disease in different parts of the world.
Supplementary Material
HIGHLIGHTS.
Cross-sectional study of HIV-infected, ART-naïve adults in Zambia and Switzerland
HIV/HBV-coinfected patients are at least three times more likely to have significant liver fibrosis compared to HIV-monoinfected individuals
The association between HBV and liver fibrosis seems to be driven by HBV viral load
The main risk factors for liver fibrosis are similar in Switzerland and Zambia
Acknowledgements
We thank all patients, doctors and nurses associated with the Centre for Infectious Disease Research in Zambia, Lusaka, Zambia and the Swiss HIV Cohort Study (SHCS). The members of the Swiss HIV Cohort Study are: Aubert V, Battegay M, Bernasconi E, Böni J, Bucher HC, Burton-Jeangros C, Calmy A, Cavassini M, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Gorgievski M, Günthard H (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos R, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Metzner K, Müller N, Nadal D, Nicca D, Pantaleo G, Rauch A (Chairman of the Scientific Board), Regenass S, Rickenbach M (Head of Data Center), Rudin C (Chairman of the Mother & Child Substudy), Schöni-Affolter F, Schmid P, Schüpbach J, Speck R, Tarr P, Trkola A, Vernazza P, Weber R, Yerly S.
Funding/support
This study was supported by the Swiss National Science Foundation (SNF grant number 148522, SHCS project number 592) within the framework of the SHCS. The Zambian cohort was supported by the National Institutes of Health (IeDEA-Southern Africa, grant U01AI069924). G.W. was supported by an Ambizione-PROSPER fellowship from the Swiss National Science Foundation (PZ00P3_154730). M.J.V. was supported by the Fogarty International Center of the National Institutes of Health (K01TW009998). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
References
- 1.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL, Seaberg EC, Skolasky R, Jr., Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. 2002;360(9349):1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins C, Agbaji O, Ugoagwu P, Thio CL, Auwal M, Ani C, et al. Assessment of Liver Fibrosis by Transient Elastography in Patients with HIV and Hepatitis B Virus (HBV) Co-infection in Nigeria. Clin Infect Dis. 2013 doi: 10.1093/cid/cit564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Martinson FE, Weigle KA, Royce RA, Weber DJ, Suchindran CM, Lemon SM. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. American journal of epidemiology. 1998;147(5):478–87. doi: 10.1093/oxfordjournals.aje.a009474. [DOI] [PubMed] [Google Scholar]
- 7.Honge BL, Jespersen S, Medina C, Te Dda S, da Silva ZJ, Lewin S, et al. Hepatitis B and Delta Virus Are Prevalent but Often Subclinical Co-Infections among HIV Infected Patients in Guinea-Bissau, West Africa: A Cross-Sectional Study. PLoS One. 2014;9(6):e99971. doi: 10.1371/journal.pone.0099971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berhe N, Myrvang B, Gundersen SG. Intensity of Schistosoma mansoni, hepatitis B, age, and sex predict levels of hepatic periportal thickening/fibrosis (PPT/F): a large-scale community-based study in Ethiopia. The American journal of tropical medicine and hygiene. 2007;77(6):1079–86. [PubMed] [Google Scholar]
- 9.Kuniholm MH, Lesi OA, Mendy M, Akano AO, Sam O, Hall AJ, et al. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in the Gambia, West Africa. Environmental health perspectives. 2008;116(11):1553–7. doi: 10.1289/ehp.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottero J, Lacombe K, Guechot J, Serfaty L, Miailhes P, Bonnard P, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV co-infected patients. J Hepatol. 2009;50(6):1074–83. doi: 10.1016/j.jhep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 11.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC infectious diseases. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinikoor MJ, Sinkala E, Mweemba A, Zanolini A, Mulenga L, Sikazwe I, et al. Elevated AST-to-platelet ratio index is associated with increased all-cause mortality among HIV-infected adults in Zambia. Liver Int. 2015 doi: 10.1111/liv.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Guidelines for the screening, care and treatment of persons with hepatitis C infection. 2014. [PubMed] [Google Scholar]
- 14.World Health Organization . Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015. [PubMed] [Google Scholar]
- 15.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2011 doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol. 2010;39(5):1179–89. doi: 10.1093/ije/dyp321. [DOI] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Price JC, Seaberg EC, Badri S, Witt MD, D'Acunto K, Thio CL. HIV monoinfection is associated with increased aspartate aminotransferase-to-platelet ratio index, a surrogate marker for hepatic fibrosis. J Infect Dis. 2012;205(6):1005–13. doi: 10.1093/infdis/jir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AIDS Clinical Trials Group . Division of AIDS table for grading the severity of adult and pediatric adverse events. National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS; Rockville, MD: 2004. [Google Scholar]
- 21.Conen A, Wang Q, Glass TR, Fux CA, Thurnheer MC, Orasch C, et al. Association of alcohol consumption and HIV surrogate markers in participants of the swiss HIV cohort study. J Acquir Immune Defic Syndr. 2013;64(5):472–8. doi: 10.1097/QAI.0b013e3182a61ea9. [DOI] [PubMed] [Google Scholar]
- 22.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 23.Stabinski L, Reynolds SJ, Ocama P, Laeyendecker O, Ndyanabo A, Kiggundu V, et al. High prevalence of liver fibrosis associated with HIV infection: a study in rural Rakai, Uganda. Antivir Ther. 2011;16(3):405–11. doi: 10.3851/IMP1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stockdale AJ, Phillips RO, Geretti AM, Group HS. The gamma-glutamyl transpeptidase to platelet ratio (GPR) shows poor correlation with transient elastography measurements of liver fibrosis in HIV-positive patients with chronic hepatitis B in West Africa. Response to: 'The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa' by Lemoine et al. Gut. 2016;65(5):882–4. doi: 10.1136/gutjnl-2015-311133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 revision. World Health Organization; Geneva: 2013. Available at http://www.who.int/hiv/pub/guidelines/arv2013/en/ [PubMed] [Google Scholar]
- 26.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 28.El-Serag HB, Kramer J, Duan Z, Kanwal F. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. The American journal of gastroenterology. 2014;109(9):1427–35. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 29.Farrell G. Insulin resistance, obesity, and liver cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):117–9. doi: 10.1016/j.cgh.2013.07.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.