Abstract
Immunotherapy using chimeric antigen receptor-modified T cells has demonstrated high response rates in patients with B cell malignancies, and chimeric antigen receptor T cell therapy is now being investigated in several hematologic and solid tumor types. Chimeric antigen receptor T cells are generated by removing T cells from a patient’s blood and engineering the cells to express the chimeric antigen receptor, which reprograms the T cells to target tumor cells. As chimeric antigen receptor T cell therapy moves into later-phase clinical trials and becomes an option for more patients, compliance of the chimeric antigen receptor T cell manufacturing process with global regulatory requirements becomes a topic for extensive discussion. Additionally, the challenges of taking a chimeric antigen receptor T cell manufacturing process from a single institution to a large-scale multi-site manufacturing center must be addressed. We have anticipated such concerns in our experience with the CD19 chimeric antigen receptor T cell therapy CTL019. In this review, we discuss steps involved in the cell processing of the technology, including the use of an optimal vector for consistent cell processing, along with addressing the challenges of expanding chimeric antigen receptor T cell therapy to a global patient population.
Keywords: chimeric antigen receptor, T lymphocytes, manufacturing, lentiviral vector, global regulatory environment
Main Text
Chimeric antigen receptor (CAR) T cell therapy is a cellular therapy that redirects a patient’s T cells to specifically target and destroy tumor cells. CARs are genetically engineered fusion proteins composed of (1) an antigen recognition domain derived from a monoclonal antibody and (2) intracellular T cell signaling and costimulatory domains.1, 2, 3, 4, 5 Use of CAR T cells as a treatment for cancer has been most extensively investigated in patients with B cell malignancies, and early results have been encouraging. For example, CAR T cell therapy has demonstrated complete response rates of 69%–90% in pediatric patients with relapsed or refractory acute lymphoblastic leukemia (ALL) in phase 1 trials.6, 7, 8, 9, 10 The development of CAR T cell therapy has now expanded beyond phase 1 trials and moved into phase 2 multi-site trials (NCT02435849 and NCT02228096), and a major consideration for academic institutions and industry is how to scale out the production of CAR T cells in an efficient, effective manner. Here we describe the process of manufacturing CAR T cells, and we discuss regulatory concerns that must be addressed to successfully produce CAR T cells for larger numbers of patients.
Production of CAR T Cells
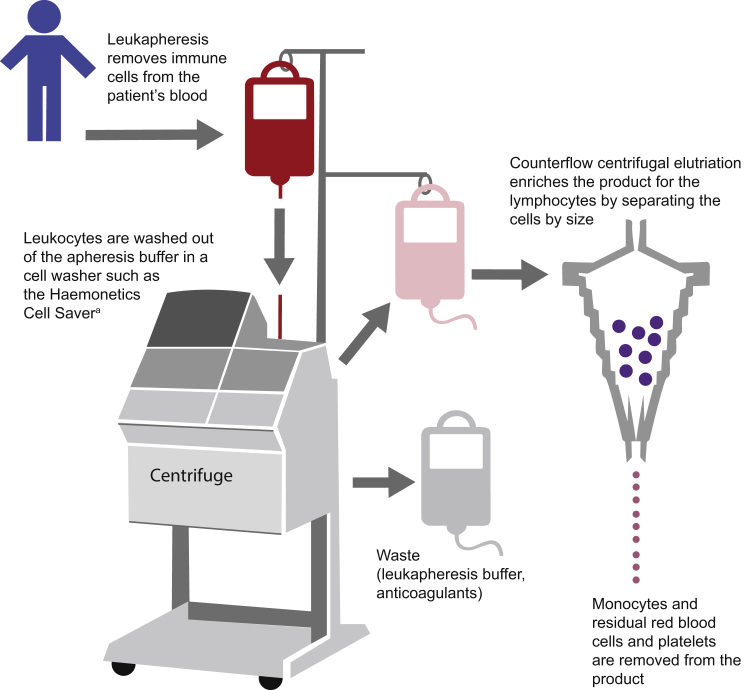
The production of CAR T cells requires several carefully performed steps, and quality control testing is performed throughout the entire protocol.11 First, the process involves using leukapheresis to remove blood from the patient’s body, separate the leukocytes, and return the remainder of the blood to the circulation.12 After a sufficient number of leukocytes have been harvested, the leukapheresis product is enriched for T cells (Figure 1). This process involves washing the cells out of the leukapheresis buffer, which contains anticoagulants.13 Enrichment of lymphocytes can be accomplished subsequently through counterflow centrifugal elutriation, which separates cells by size and density and maintains cell viability.14 Separation of T cell subsets at the level of CD4/CD8 composition using specific antibody bead conjugates or markers is an additional step that may be performed.15
Figure 1.
Leukapheresis and T Cell Isolation
After a sufficient number of leukocytes have been harvested from the patient’s blood via leukapheresis, the anticoagulants in the leukapheresis buffer are washed out of the product and the cells are concentrated by counterflow centrifugal elutriation, which separates cells by size and density. aHaemonetics Corporation.
Purifying autologous antigen-presenting cells (APCs) from the patient to use for T cell activation would require several additional steps, making it labor intensive and difficult to obtain a potent CAR T cell product.11 For this reason, an approach was developed to activate T cells in a more standardized, efficient manner using, for example, beads coated with anti-CD3/anti-CD28 monoclonal antibodies (Life Technologies). While the use of anti-CD3 antibodies alone or in combination with feeder cells and growth factors, such as IL-2, has been the practice for many years, in comparison to beads coated with anti-CD3/anti-CD28 monoclonal antibodies or cell-based artificial APCs (aAPCs), the activation and ex vivo expansion are suboptimal.16, 17 The beads, or aAPCs, can be easily removed from the culture through magnetic separation.18 In the presence of interleukin-2 and aAPCs, T cells can grow logarithmically in a perfusion bioreactor for several weeks.11, 16, 18, 19, 20 The use of aAPCs derived from the chronic myelogenous leukemia cell line K562, which can be engineered to express the required costimulatory ligands, also has been investigated as a method of expanding T cells ex vivo.17, 21 Culture conditions may be further refined to polarize T cells to a specific phenotype (i.e., Th2 or Th17) during expansion. Indeed, CAR T cells that were polarized to a Th17 phenotype demonstrated efficacy in a preclinical model, suggesting that T cell polarization is a strategy that may enter the clinic in the future.22
During the activation process, the T cells are incubated with the viral vector encoding the CAR, and, after several days, the vector is washed out of the culture by dilution and/or medium exchange. The viral vector uses viral machinery to attach to the patient cells, and, upon entry into the cells, the vector introduces genetic material in the form of RNA.23 In the case of CAR T cell therapy, this genetic material encodes the CAR. The RNA is reverse-transcribed into DNA and permanently integrates into the genome of the patient cells; therefore, CAR expression is maintained as the cells divide and are grown to large numbers in the bioreactor. The CAR is then transcribed and translated by the patient cells, and the CAR is expressed on the cell surface. Lentiviral vectors, which have a safer integration site profile than gammaretroviral vectors,24, 25 are commonly used in clinical trials of CAR T cell therapies, including CTL019. Other methods of gene transfer, including the Sleeping Beauty transposon system or mRNA transfection, have been investigated as alternative approaches to express a CAR in T cells.26, 27 CAR T cells generated using transient mRNA transfection have been used in the clinic; however, this approach requires several rounds of CAR T cell infusion.28 Furthermore, while the Sleeping Beauty transposon system is considered to be inexpensive and has been tested in early-phase clinical trials, there are still several concerns, including efficiency relative to lentiviral vectors, the unknown potential of insertional mutagenesis, and remobilization of transposons.29
Bioreactor culture systems are designed to provide the optimal gas exchange requirements and culture mixing necessary to grow large numbers of cells for clinical use (Figure 2). The WAVE Bioreactor (now known as the Xuri; GE Healthcare Life Sciences), which utilizes a rocking platform, has been used to expand the CD19-targeted CAR T cell therapy CTL019.11, 20, 30 Another culture system that can be used is the G-Rex (Wilson Wolf), which has the ability to expand cells from low seeding densities.31, 32 The G-Rex uses gas-permeable membranes, allowing the flask to be placed directly in a cell culture incubator. One drawback of this system, however, is that the flask must be opened during cell inoculation. The CliniMACS Prodigy (Miltenyi Biotec) is a single device that accomplishes cell preparation, enrichment, activation, transduction, expansion, final formulation, and sampling.33 This contrasts with other methods, which use separate machines for the cell culture, cell washing, and other steps in the preparation. It was shown recently that the CliniMACs Prodigy is feasible for generating CAR T cells, and this device is expected to be used soon to prepare CAR T cells for clinical trials.34
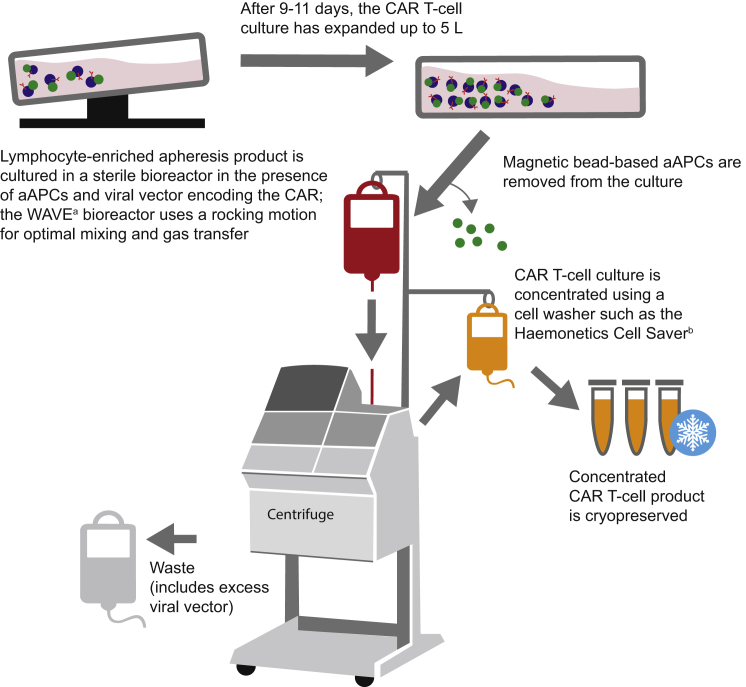
Figure 2.
T Cell Culture and Transduction
The T cells are activated using aAPCs, transduced with the CAR-encoding viral vector, and expanded to large numbers in a bioreactor. After expansion, the cells are washed, concentrated, and cryopreserved. aAPC, artificial antigen-presenting cell; CAR, chimeric antigen receptor. aGE Healthcare Life Sciences. bHaemonetics Corporation.
When the cell expansion process is finished, the cell culture, which may reach a volume of up to ≈5 L, must be concentrated to a volume that can be infused into the patient.11, 18 The washed and concentrated cells are cryopreserved in infusible medium, and, following product release, the frozen cells are transported to and thawed at the center where the patient will be treated. While aspects of cell washing, isolation, and culture are semi-automated, improving the throughput of currently manual parts of processing will be critical for developing CAR T cell therapies that can be used for a wider range of indications and larger populations.
Challenges of Bringing CAR T Cell Therapy to a Global Patient Population
Although protocols for manufacturing clinical-grade CAR T cells have now been established, CAR T cell therapies have been used to treat only a few hundred patients to date. When scaling out this complex manufacturing process to treat more patients in larger trials at an increased number of clinical centers, the process should be carefully evaluated to ensure production efficiency without compromising the integrity and potency of the final product. Because CAR T cells can be used to target several types of cancer, the scale of production for the vector and the CAR T cells also will depend on the incidence of each indication. Additional considerations include generating consistently high-quality vector for predictable genetic modification of cells, understanding the long-term safety of gene therapy, and anticipating global regulatory concerns.
Toward Consistent Cell Processing: Use of the Optimal Vector
In the United States, the viral vector used to transduce the CAR into T cells is considered a key raw material of the CAR T cell manufacturing process, and the modified T cell is considered the investigational final product, also known as the medicinal product in the European Union. In contrast to the final CAR T cell product, which must be individually generated for each patient, the viral vector encoding the CAR can be made in large quantities and stored at −80°C for 4 years in our experience. Other reports suggest that frozen viral vector stocks are stable for up to 9 years at this temperature.35, 36 As with the CAR T cell manufacturing process, generation of the vector stocks must take place in Good Manufacturing Practice (GMP) facilities. The sterility of the vector is crucial because the final CAR T cell product cannot be sterilized by filtration; manufacture of the vector under controlled, clean room conditions with minimal open processing and sterile filtration during the final aseptic stages of production, all supported and verified by an array of safety testing, ensures sterility and the absence of packaging cells from the final vector product. In addition, use of a third-generation minimal lentiviral vector, incorporating key safety features, enhances safety.37, 38
In our experience, the batch manufacture of viral vector for cellular therapies takes a minimum of 2 weeks. Most of this time is spent growing adequate numbers of cells, such as HEK293T cells, to produce large quantities of replication-defective viral vector.39 Starting from a cryopreserved aliquot of an appropriate working cell bank, the cells are expanded in culture for several days to the appropriate number for production, allowing considerable expansion from the original number of cells seeded. The cells are then transfected with plasmids that collectively result in the production of the minimal lentiviral vector. These plasmids are typically (1) a Gag/Pol packaging construct that encodes the viral structural proteins (Gag) and enzymes (Pol); (2) a construct encoding a suitable envelope glycoprotein from a heterologous source, resulting in vector particle pseudotyping (e.g., VSV-G); (3) a construct for the expression of viral accessory protein Rev; along with (4) a vector plasmid encoding the CAR construct as well as other sequences required for efficient reverse transcription, RNA packaging, and integration.40 The vector systems should employ a number of key safety features that collectively prevent the reacquisition of replication competence (e.g., codon-optimized Gag/Pol that minimizes homology between vector components to prevent recombination, self-inactivating long terminal repeat sequences, and removal of all unnecessary sequences and accessory genes).41, 42, 43 Within 48 hr of transfection, the production cells begin to release CAR-expressing lentiviral vector, which can be collected from the culture medium.39 Over the course of several days, medium exchange allows for the harvest of multiple batches of vector-containing medium, typically two harvests. After filtration to remove production cells and debris, the viral vector is purified through downstream processing to enrich for the viral vector while removing impurities and to formulate the vector into an appropriate storage buffer. In our experience, the vector may be frozen at this point to allow for the production of multiple sub-batches to make larger quantities of the final vector product for increased economic efficiency. Once the target quantity of vector is available, a further GMP process, involving sterilizing filtration and vialing under aseptic conditions, is performed. Once production is complete, the vector is cryopreserved until later use.
It is important to establish quality control testing for safety, sterility, purity, potency, identity, and titer so that manufacturing centers can be assured that each batch of vector meets defined standards before it is used to transduce T cells.44 These quality control tests are described in guidance documents written by the U.S. Food and Drug Administration (FDA) and are briefly summarized here. Safety testing, which can involve preclinical experimentation or toxicity studies in animals, aims to determine that the product is safe for humans when administered appropriately. The sterility of the product is tested to ensure that it is free of contaminating microorganisms; in the case of gammaretroviral vectors and lentiviral vectors, assays for replication-competent retroviruses/lentiviruses (RCRs/RCLs) and mycoplasma testing of the cells and media used in the process help determine that the final CAR T cell product is free from adventitious agents and safe for infusion into patients. Impurities in the vector product are broadly tested to (1) verify consistent purification afforded by the vector manufacturing process, and (2) consequently ensure that the quality of the vector is consistent prior to its use in the T cell manufacturing process. Impurity testing includes testing for process-related impurities, such as Benzonase (Merck KGaA; used to degrade and facilitate the removal of DNA) and bovine serum albumin (originating from fetal bovine serum), and it also includes characterization of both residual host cell DNA and residual plasmid DNA. In addition, the T cells are tested for bacterial and fungal contamination from the cell culture process. The potency of the vector product is tested to assess whether it functions as anticipated, and its identity is proven through relevant physical, chemical, or biological tests. For example, the titer of viral vector can be measured by analyzing the percentage of healthy donor cells transduced by predefined MOIs, and this can verify whether a particular batch of vector is expected to optimally transduce patient cells.39
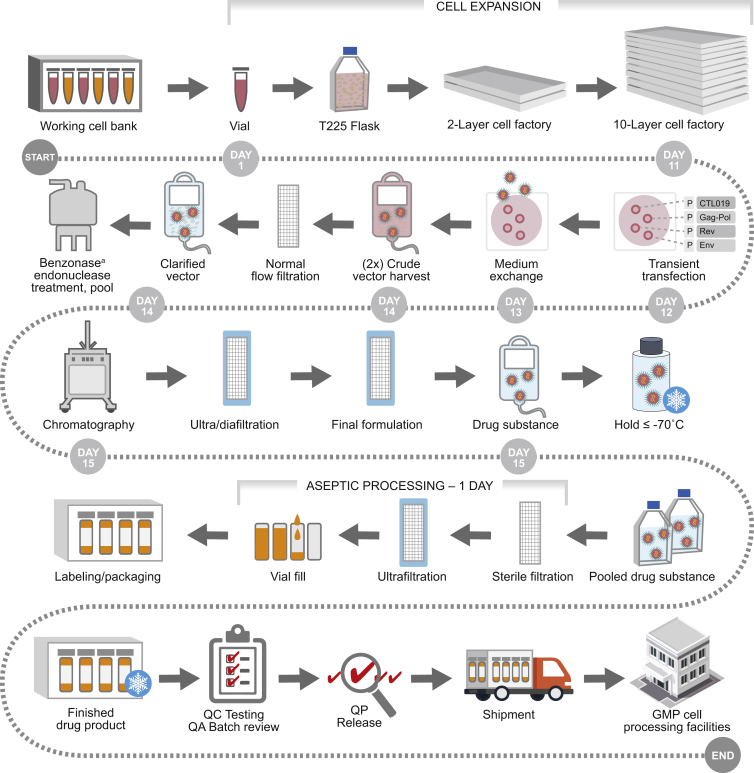
In early clinical trials performed at the University of Pennsylvania, multiple vector suppliers were used during the generation of the CD19-targeting therapy CTL019. Using vectors from different suppliers as starting material raises additional questions about the comparability of vector purity, stability, and function. Therefore, it is desirable that a single vector source is used for the generation of CTL019 cells in current and future clinical trials. Additionally, the vector supplier should be able to meet requirements, such as GMP compliance, as well as meet the large production demand. Therefore, our approach was to adopt a lentiviral vector manufacturing process that can meet global health authority requirements and that has the appropriate supporting process validation (Figure 3). In our experience with lentiviral vector manufactured by Oxford BioMedica (OXB), we found that consistent vector quality minimized site-to-site variation in the subsequent CAR T cell manufacturing process. To provide batches of sufficient size to support clinical and commercial requirements, the process has been designed and optimized to manufacture vector in a series of multiple sub-batches within a given manufacturing campaign spanning several weeks. This approach relies on a purification and formulation step prior to cryopreservation and a subsequent hold period. Once sub-batches have been demonstrated to meet a key subset of specifications for that manufacturing stage, specific sub-batches are selected for filling, and final processing is completed in a single day. The intermediate container closure system (and associated fill volume), GMP processing parameters (freeze/thaw conditions, further aseptic processing conditions, final container, etc.), and the formulation buffer have all been selected to ensure that any loss in titer is minimal and so that the resultant filled product meets target specifications and performs consistently and robustly in the T cell manufacturing process. In addition, the hold stage is supported by a stability testing program, which shows that vector titer is stable in storage for an extended period.
Figure 3.
The Lentiviral Vector Manufactured by Oxford BioMedica Ltd. Undergoes Several Rounds of Filtration and Testing to Ensure that a High-Quality Product with Minimal Variability Is Produced
The process has been designed and optimized to manufacture vector in a series of multiple sub-batches. This approach relies on a purification and formulation step prior to cryopreservation and a subsequent hold at ≤−70°C. Specific sub-batches are then selected for final aseptic processing, which is completed in a single day. QA, quality assurance; QC, quality control; QP, qualified person. aMerck KGaA.
The transduction efficiency of the OXB CTL019 vector was examined in a two-site study, and consistent, dose-dependent transduction efficiency was observed at both sites (unpublished data). This study also provided an understanding of the variability in transduction efficiency among healthy donors, which provides a meaningful baseline to which we can compare patient data. The data demonstrated that the OXB vector can be used with equivalent performance across donors and manufacturing sites. These qualities are both desirable and necessary when choosing a vector that can be produced and used at large scale, and they reduce the risk in process validation.
Optimizing the vector for CAR T cell transduction before beginning large-scale manufacturing reduces variability and maximizes efficiency. The scale of vector production ultimately needs to take into account the potential size of the patient population on an indication-by-indication basis. On this basis, our strategy is to focus current clinical supply, as well as initial commercial supply, on current and well-established production platforms, while investing in the development of next-generation production processes that will provide an equivalent quality of functional vector on a significantly larger scale. The most common vectors used in CAR T cell manufacturing are replication-defective vector systems based on two types of retroviruses: gammaretroviruses and lentiviruses. Both gammaretroviruses and lentiviruses deliver RNA that is reverse-transcribed into DNA in the target cell; this DNA, which encodes the CAR construct, then integrates into the host genome via a process catalyzed by the vector integrase enzyme and several key sequences within the vector construct.23 An additional advantage of lentiviral-derived vectors is that they retain the ability of lentiviruses to infect non-dividing cells, thus increasing their ability to transduce a wide variety of cells, including quiescent and difficult-to-transduce cells.45 However, as described previously, T cell activation is required for increased transduction efficiency, and the lentiviral vector is introduced during cell activation.11
Insertional mutagenesis caused by the integration of vector DNA into host cells near an oncogene is a potential concern with all integrating vectors.46, 47 However, lentiviral integration patterns favor sites away from cellular promoters, while gammaretroviral integration more frequently occurs near transcriptional start sites, suggesting that lentiviral vectors may carry a lower risk of mutagenesis.45 Risk for insertional mutagenesis also appears to be dependent on factors other than the vector system, such as the transgene that is encoded, the promoter, and the cell type or disease that is targeted. CAR T cell viral vector-mediated oncogenicity has not been observed in patients treated with CAR T cells in our experience nor in a decade-long study.48 Oncogenic potential may be increased if RCRs/RCLs are present in the vector products; however, current vector designs make the generation of RCRs/RCLs highly unlikely.49 The FDA 2006 guidance considers lentiviral and retroviral vectors to be potentially oncogenic; therefore, vectors used in the clinic for CAR T cell therapy are carefully tested for RCRs/RCLs. In addition to testing for RCRs at multiple stages throughout the vector manufacturing process and the vector-modified cell product, the FDA also recommends that patients be followed for RCRs/RCLs for up to 15 years to monitor any potential delayed adverse event related to these vectors.50
Investigating the Long-Term Safety of Viral Vectors Requires Patient Follow-up
Evaluating the long-term safety of using viral vectors for cell and gene therapies will require extensive follow-up. As mentioned above, health authority guidelines also require long-term follow-up of studies using viral vectors; however, requirements may differ depending on the individual countries involved. For example, a United States (USA) trial has been developed to monitor patients who received the CD19 CAR T cell therapy CTL019 for 15 years after treatment (NCT02445222). This study will include patients of any age who have been treated with CTL019 for any B cell malignancy. The primary objective of this follow-up study is to describe delayed adverse events suspected to be related to CD19 CAR T cell therapy, such as the development of new malignancies, incidence or exacerbation of a preexisting neurologic or autoimmune disorder, or new incidence of a hematologic disorder. The secondary objectives of this study are monitoring the persistence of the CTL019 cells in peripheral blood and the long-term efficacy of the therapy. The persistence of CTL019 cells will be examined by using qPCR to detect the CD19 CAR transgene at specified time points. Additionally, the proportion of patients who relapse or experience disease progression will be monitored, as well as the incidence of patient death from any cause. Because the effects of persistent CAR T cells on pregnancy are unknown, reproductive health and pregnancy outcomes also will be followed in female patients who were treated with CAR T cells. Thus, the careful design and manufacture of viral vectors for CAR T cell therapy to maximize safety, along with quality and safety testing and long-term patient follow-up, ensure that patient safety is the top priority.
Ensuring Quality of Product in Moving from a Single Institution to a Multi-site, Large-Scale Manufacturing Process
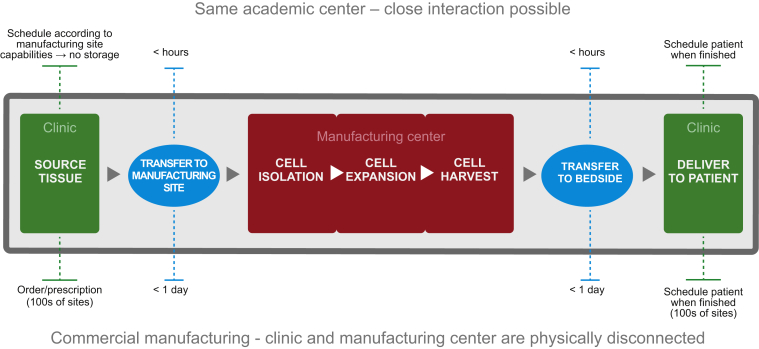
A major challenge with scaling out the production of CAR T cell therapies is the transition from a flexible process at a single academic institution to a highly controlled process that can be implemented across many collection, manufacturing, and treatment sites (Figure 4). Therefore, effective coordination among the collection, manufacturing, and treatment sites involved is crucial to ensure that the material is handled correctly and patients are appropriately scheduled throughout the therapeutic process. Success in developing a global manufacturing process of CAR T cells will be driven by a robust understanding of both the product and the process in order to establish the target product profile and critical quality attributes. For CTL019, the target product profile includes those qualities already discussed: target-specific, highly potent T cells that are capable of robust expansion and long-term persistence in vivo. Using this product profile, critical quality attributes were explored. Cell number, transduction efficiency, growth rate, cellular phenotype, and functional analysis are all critical quality attributes of CTL019 that are well understood and controlled. Cellular phenotype includes measures such as T cell subset distribution (helper or cytotoxic, effector or memory, etc.) and functionality (cell killing, cytokine release, proliferative capacity, exhaustion, apoptosis, etc.). These critical quality attributes will be coordinated with process understanding to develop a consistent manufacturing process and control strategy that will ensure a uniform product. Examples include the percentage of CD3+ T cells in the product and measurement of potency. However, it is to be noted that values for CAR positivity, viability, phenotype, and potency will vary from product to product. As more experience is gained and these data begin to be made public, fair comparisons on the utility and appropriate ranges may be assessed.51, 52
Figure 4.
Transition to Commercial Manufacturing
As CAR T cell therapies transition from flexible processes at single academic institutions to highly controlled processes that can be implemented across many collection, manufacturing, and treatment sites, the coordination among these sites will be crucial to ensure that the material is handled correctly and patients are scheduled appropriately.
Vendor agreements, such as our agreement with OXB described previously, are critical for maintaining a controlled manufacturing process. In the case of CAR T cell therapy, product comparability has the additional challenge that variability between patient apheresis starting materials is likely to contribute much greater differences than factors related to the manufacturing site. Therefore, we have controlled our processes for CTL019 manufacturing and vector production so that we minimize variability related to the manufacturing process.
Meeting Global Regulatory Expectations for Successful Implementation of CAR T Cell Therapies
Early results of CAR T cell therapy suggest significant patient benefit, and, thus, this therapy has the potential for clinical success, which could lead to greater global regulatory collaboration and harmonization. The patient benefit offered by CAR T cell therapies provides a promising opportunity to create a common ground between different regulatory authorities and improve international cooperation. Ensuring regulatory compliance is a critical factor for the successful development of this novel therapeutic approach, and this will be an interesting challenge in a global environment. The FDA has been regulating cell and gene therapies for many years and has developed a number of guidance documents regarding these products. However, these guidance documents are not harmonized with other countries or regions. In addition, each major country has a slightly different focus as they review clinical trial applications. For example, European Union (EU) countries require cGMP compliance as determined by a qualified person, while the United States assesses cGMP compliance through a paper review. Therefore, the more countries that are involved in clinical trials, the more robust the manufacturing process will need to be to meet all of the questions and regulatory requirements. To bring harmonization in the emerging area of cell and gene therapies, on October 11, 2012, nine members of the global regulatory community, including Brazil ANVISA; European Medicines Agency (EMA); Health Canada; India National Institute of Biologicals (NIB); Japan Ministry of Health, Welfare and Labour/Pharmaceutical and Medical Devices Agency; South Korea Ministry of Food and Drug Safety (previously known as Food and Drug Administration [KFDA]); Singapore Health Sciences Authority (HSA); Swissmedic; and the U.S. Food and Drug Administration, convened to form an integrated group with a goal to discuss best practices in the regulation of cell/gene-based therapies and support harmonization (https://www.i-p-r-f.org/en/working-groups/gene-therapy-working-group/).
CAR T cell developers should have a thorough understanding of this diverse regulatory landscape. Until harmonized regulations are established and there is common experience among regions, we can expect a significant level of uncertainty in product development and a greater reliance on subjective judgment by the regulators. Exemplifying this challenge are the different requirements associated with manufacturing that exist among regions. For example, donor screening and testing, traceability and labeling, patient confidentiality, and apheresis requirements can vary widely among countries. This is particularly challenging if the donor starting material and final product are shipped across international borders. Another example is that the definitions of the materials used (i.e., the starting or raw material) and the requirements for quality control of these materials vary across regions. Balancing different country requirements with starting material quality requirements can be challenging, but it is crucial for multi-national trials. Therefore, the origin, traceability, composition, and certification of each reagent must be readily accessible. Lastly, as each region has unique documents and recommendations related to materials used in cell and gene therapy manufacturing, the requirements for the human or animal serum used for cell culture differ among global regions. Thus, to address some of these regulatory concerns, we are establishing protocols for CAR T cell manufacturing using reagents that comply with the quality requirements for major global regions (e.g., using the same international supplier across global regions).
Additional Areas of Active Investigation in the Development and Manufacturing of CAR T Cell Therapy
There are several preclinical areas of investigation designed to improve CAR T cell therapies and provide benefit to a larger patient population. One aspect under investigation is the subtype of T cells used for CAR T cell therapy. The starting cell population used for many CAR T cell therapies consists of CD4+ and CD8+ T cells at the ratio present in the peripheral blood of the patient. However, the use of a fixed 1:1 ratio of CD4+:CD8+ CAR T cells in patients with ALL and non-Hodgkin lymphoma has been investigated.53 If the ratio of T cell subsets becomes an important factor in CAR T cell therapy, manufacturing protocols may need to be altered to include the extra purification steps necessary to administer CAR T cells in a fixed subset ratio.
Other areas of consideration for the future of manufacturing a CAR T cell therapy include mitigating possible adverse events, both short and long term. For example, one adverse effect related to CAR T cell therapy is severe cytokine release syndrome (CRS), which occurs in a minority of patients. CRS is caused by the release of proinflammatory cytokines directly from the CAR T cells and dying tumor cells. Although most cases of CRS are manageable with established treatment algorithms,54 it has been speculated that mechanisms to inactivate CAR T cells in patients experiencing adverse effects could be useful to improve the safety of CAR T cell therapy. Using a suicide switch incorporated into the CAR construct is a strategy that is currently being investigated as a potential way to specifically deplete CAR T cells in a controlled manner, and it may become important as CAR T cell therapy is investigated in an ever-growing clinical population.55, 56 For example, the iCasp9 safety switch has been effectively used to reduce graft-versus-host disease (GVHD) in patients receiving allogeneic stem cell transplant.57 However, depletion of CAR T cells also may abrogate the potential benefit of long-term CAR T cell-mediated tumor surveillance.
Another method of potentially increasing the safety of CAR T cell therapy involves improving the specificity of the modified T cells. This approach may be especially important for CAR T cells targeting solid tumors, which often do not have antigens uniquely expressed on the tumor. A preclinical study showed that CAR T cells could be used to target tumors expressing two antigens, PSCA and PSMA; the CAR T cells did not kill tumor cells expressing only one of these antigens.58 Combinatorial antigen recognition strategies may, therefore, be important to consider when designing CARs for the treatment of solid tumors. In our experience, CRS has not yet been observed in clinical trials using CAR T cells to target solid tumors, perhaps because fewer tumor cells are rapidly killed at once due to the solid tumor mass.
An additional strategy under investigation is the use of allogeneic off-the-shelf CAR T cells. This platform uses genome editing to inactivate the endogenous TCRα gene, which limits the ability of the allogeneic cells to cause GVHD.59, 60 Off-the-shelf CAR T cells have shown efficacy in lymphoma xenograft models; but, although there is a report of compassionate use in one patient, the results of controlled clinical trial use have not yet been reported.59, 61
How the quality of the patient cells used for CAR transduction affects the efficacy of the final CAR T cell product also should be more thoroughly investigated. It has been shown recently that the reduction of an exhausted T cell phenotype is associated with improved CAR T cell efficacy.62 Therefore, the choice of CAR-signaling domains, selection of cell subsets, and adjustment of cell culture conditions to decrease the percentage of cells that become exhausted during the transduction and expansion processes may be critical to generate the highest-quality CAR T cell product.
Conclusions
Given the success of CAR T cells in treating patients in the United States with B cell malignancies, scaling out CAR T cell manufacturing capacity will allow examination of the safety and efficacy of CAR T cell therapies in larger cohorts of patients around the globe. However, a number of manufacturing and regulatory challenges need to be considered when attempting to bring a cellular therapy with a complex manufacturing process to a larger, international patient population. We are currently manufacturing the CD19 CAR T cell therapy CTL019 in a way that will streamline the process of using the therapy globally. To accomplish this, we have established robust collaborations with academic institutions and vendors to thoroughly investigate both the product and process to ensure that our materials are controlled to maintain high quality. Anticipating regulatory and manufacturing issues before they arise and proactively addressing these concerns help expedite the process of bringing this promising therapeutic approach to more patients.
Author Contributions
B.L.L. wrote, edited, reviewed, coordinated, and submitted the paper and J.M., K.W., and C.K. wrote, edited, and reviewed the paper.
Conflicts of Interest
B.L.L. has commercial research grants from Novartis Pharmaceuticals and holds intellectual property assigned to the University of Pennsylvania and licensed to Novartis Pharmaceuticals. J.M. is an employee of Oxford BioMedica Ltd. K.W. and C.K. are employees of Novartis Pharmaceuticals.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Dr. Judith Murphy (ArticulateScience) for assistance with this manuscript.
References
- 1.Kuwana Y., Asakura Y., Utsunomiya N., Nakanishi M., Arata Y., Itoh S., Nagase F., Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 2.Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finney H.M., Lawson A.D., Bebbington C.R., Weir A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 4.Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat. Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 5.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude S.L., Pulsipher M.A., Boyer M.W., Grupp S.A., Davies S.M., Phillips C.L., Verneris M.R., August K.J., Schlis K., Driscoll T.A. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis. Blood. 2016;128:2801. [Google Scholar]
- 10.Grupp S.A., Laetsch T.W., Buechner J., Bittencourt H., Maude S.L., Verneris M.R., Myers G.D., Boyer M.W., Rives S., De Moerloose B. Analysis of a global registration trial of the efficacy and safety of CTL019 in pediatric and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) Blood. 2016;128:221. [Google Scholar]
- 11.Levine B.L. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther. 2015;22:79–84. doi: 10.1038/cgt.2015.5. [DOI] [PubMed] [Google Scholar]
- 12.Smith J.W. Apheresis techniques and cellular immunomodulation. Ther. Apher. 1997;1:203–206. doi: 10.1111/j.1744-9987.1997.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee G., Arepally G.M. Anticoagulation techniques in apheresis: from heparin to citrate and beyond. J. Clin. Apher. 2012;27:117–125. doi: 10.1002/jca.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell D.J., Jr., Brennan A.L., Zheng Z., Huynh H., Cotte J., Levine B.L. Efficient clinical-scale enrichment of lymphocytes for use in adoptive immunotherapy using a modified counterflow centrifugal elutriation program. Cytotherapy. 2009;11:923–935. doi: 10.3109/14653240903188921. [DOI] [PubMed] [Google Scholar]
- 15.Riddell S.R., Sommermeyer D., Berger C., Liu L.S., Balakrishnan A., Salter A., Hudecek M., Maloney D.G., Turtle C.J. Adoptive therapy with chimeric antigen receptor-modified T cells of defined subset composition. Cancer J. 2014;20:141–144. doi: 10.1097/PPO.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine B.L., Bernstein W.B., Connors M., Craighead N., Lindsten T., Thompson C.B., June C.H. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 17.Suhoski M.M., Golovina T.N., Aqui N.A., Tai V.C., Varela-Rohena A., Milone M.C., Carroll R.G., Riley J.L., June C.H. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine B.L. Personalized cell-based medicine: activated and expanded T cells for adoptive immunotherapy. BioProcessing J. 2007;6:14–19. [Google Scholar]
- 19.Hami L.S., Green C., Leshinsky N., Markham E., Miller K., Craig S. GMP production and testing of Xcellerated T Cells for the treatment of patients with CLL. Cytotherapy. 2004;6:554–562. doi: 10.1080/14653240410005348. [DOI] [PubMed] [Google Scholar]
- 20.Somerville R.P., Dudley M.E. Bioreactors get personal. OncoImmunology. 2012;1:1435–1437. doi: 10.4161/onci.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maus M.V., Thomas A.K., Leonard D.G., Allman D., Addya K., Schlienger K., Riley J.L., June C.H. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 22.Guedan S., Chen X., Madar A., Carpenito C., McGettigan S.E., Frigault M.J., Lee J., Posey A.D., Jr., Scholler J., Scholler N. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coffin J., Hughes S., Varmus H. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. Retroviruses. [PubMed] [Google Scholar]
- 24.McGarrity G.J., Hoyah G., Winemiller A., Andre K., Stein D., Blick G., Greenberg R.N., Kinder C., Zolopa A., Binder-Scholl G. Patient monitoring and follow-up in lentiviral clinical trials. J. Gene Med. 2013;15:78–82. doi: 10.1002/jgm.2691. [DOI] [PubMed] [Google Scholar]
- 25.Montini E., Cesana D., Schmidt M., Sanvito F., Bartholomae C.C., Ranzani M., Benedicenti F., Sergi L.S., Ambrosi A., Ponzoni M. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huls M.H., Figliola M.J., Dawson M.J., Olivares S., Kebriaei P., Shpall E.J., Champlin R.E., Singh H., Cooper L.J. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. J. Vis. Exp. 2013;(72):e50070. doi: 10.3791/50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh H., Figliola M.J., Dawson M.J., Olivares S., Zhang L., Yang G., Maiti S., Manuri P., Senyukov V., Jena B. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty G.L., Haas A.R., Maus M.V., Torigian D.A., Soulen M.C., Plesa G., Chew A., Zhao Y., Levine B.L., Albelda S.M. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronovich E.L., McIvor R.S., Hackett P.B. The Sleeping Beauty transposon system: a non-viral vector for gene therapy. Hum. Mol. Genet. 2011;20(R1):R14–R20. doi: 10.1093/hmg/ddr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somerville R.P.T., Devillier L., Parkhurst M.R., Rosenberg S.A., Dudley M.E. Clinical scale rapid expansion of lymphocytes for adoptive cell transfer therapy in the WAVE® bioreactor. J. Transl. Med. 2012;10:69. doi: 10.1186/1479-5876-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin J., Sabatino M., Somerville R., Wilson J.R., Dudley M.E., Stroncek D.F., Rosenberg S.A. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J. Immunother. 2012;35:283–292. doi: 10.1097/CJI.0b013e31824e801f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bajgain P., Mucharla R., Wilson J., Welch D., Anurathapan U., Liang B., Lu X., Ripple K., Centanni J.M., Hall C. Optimizing the production of suspension cells using the G-Rex “M” series. Mol. Ther. Methods Clin. Dev. 2014;1:14015. doi: 10.1038/mtm.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaiser A.D., Assenmacher M., Schröder B., Meyer M., Orentas R., Bethke U., Dropulic B. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 2015;22:72–78. doi: 10.1038/cgt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mock U., Nickolay L., Cheung G., Zhan H., Peggs K., Johnston I., Kaiser A., Pule M., Thrasher A., Qasim W. Automated lentiviral transduction of T cells with CARs using the CliniMACS Prodigy. Blood. 2015;126:2043. [Google Scholar]
- 35.Lamers C.H., van Elzakker P., Luider B.A., van Steenbergen S.C., Sleijfer S., Debets R., Gratama J.W. Retroviral vectors for clinical immunogene therapy are stable for up to 9 years. Cancer Gene Ther. 2008;15:268–274. doi: 10.1038/sj.cgt.7701114. [DOI] [PubMed] [Google Scholar]
- 36.Przybylowski M., Hakakha A., Stefanski J., Hodges J., Sadelain M., Rivière I. Production scale-up and validation of packaging cell clearance of clinical-grade retroviral vector stocks produced in cell factories. Gene Ther. 2006;13:95–100. doi: 10.1038/sj.gt.3302648. [DOI] [PubMed] [Google Scholar]
- 37.Kim V.N., Mitrophanous K., Kingsman S.M., Kingsman A.J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J. Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Olszewska M., Qu J., Wasielewska T., Bartido S., Hermetet G., Sadelain M., Rivière I. Large-scale clinical-grade retroviral vector production in a fixed-bed bioreactor. J. Immunother. 2015;38:127–135. doi: 10.1097/CJI.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schambach A., Zychlinski D., Ehrnstroem B., Baum C. Biosafety features of lentiviral vectors. Hum. Gene Ther. 2013;24:132–142. doi: 10.1089/hum.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu S.F., von Rüden T., Kantoff P.W., Garber C., Seiberg M., Rüther U., Anderson W.F., Wagner E.F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc. Natl. Acad. Sci. USA. 1986;83:3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotsopoulou E., Kim V.N., Kingsman A.J., Kingsman S.M., Mitrophanous K.A. A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 gag-pol gene. J. Virol. 2000;74:4839–4852. doi: 10.1128/jvi.74.10.4839-4852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zufferey R., Dull T., Mandel R.J., Bukovsky A., Quiroz D., Naldini L., Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross P.J., Levine B.L. Assays for the release of cellular gene therapy products. In: Dropulic B., Carter B.J., editors. Concepts in Genetic Medicine. John Wiley & Sons; 2006. pp. 307–318. [Google Scholar]
- 45.Vannucci L., Lai M., Chiuppesi F., Ceccherini-Nelli L., Pistello M. Viral vectors: a look back and ahead on gene transfer technology. New Microbiol. 2013;36:1–22. [PubMed] [Google Scholar]
- 46.Varmus H.E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981;25:23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- 47.Shen-Ong G.L., Potter M., Mushinski J.F., Lavu S., Reddy E.P. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984;226:1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- 48.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bear A.S., Morgan R.A., Cornetta K., June C.H., Binder-Scholl G., Dudley M.E., Feldman S.A., Rosenberg S.A., Shurtleff S.A., Rooney C.M. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol. Ther. 2012;20:246–249. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briefing document—testing for replication competent retrovirus (RCR)/lentivirus (RCL) in retroviral and lentiviral vector based gene therapy products—revisiting current FDA recommendations. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/CellularTissueandGeneTherapiesAdvisoryCommittee/UCM232592.pdf. Accessed 12 April 2016.
- 51.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turtle C.J., Berger C., Sommermeyer D., Budiarto T., Hanafi L., Melville K., Pender B., Steevens N., Chaney C., Heimfeld S. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. J. Clin. Oncol. 2015;33 [Google Scholar]
- 54.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budde L.E., Berger C., Lin Y., Wang J., Lin X., Frayo S.E., Brouns S.A., Spencer D.M., Till B.G., Jensen M.C. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS ONE. 2013;8:e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gouble A., Philip B., Poirot L., Schiffer-Mannioui C., Galetto R., Derniame S., Cheung G., Arnould S., Desseaux C., Pule M., Smith J. In vivo proof of concept of activity and safety of UCART19, an allogeneic “off-the-shelf” adoptive T-cell immunotherapy against CD19+ B-cell leukemias. Blood. 2014;124:4689. [Google Scholar]
- 60.Poirot L., Philip B., Schiffer-Mannioui C., Le Clerre D., Chion-Sotinel I., Derniame S., Potrel P., Bas C., Lemaire L., Galetto R. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 61.Qasim W., Jal Amrolia P., Samarasinghe S., Ghorashian S., Zhan H., Stafford S., Butler K., Ahsan G., Gilmour K., Adams S. First clinical application of TALEN engineered universal CAR19 T cells in B-ALL. Blood. 2015;126:2046. [Google Scholar]
- 62.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]