Abstract
Outcomes for patients with refractory diffuse large B cell lymphoma (DLBCL) are poor. In the multicenter ZUMA-1 phase 1 study, we evaluated KTE-C19, an autologous CD3ζ/CD28-based chimeric antigen receptor (CAR) T cell therapy, in patients with refractory DLBCL. Patients received low-dose conditioning chemotherapy with concurrent cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days followed by KTE-C19 at a target dose of 2 × 106 CAR T cells/kg. The incidence of dose-limiting toxicity (DLT) was the primary endpoint. Seven patients were treated with KTE-C19 and one patient experienced a DLT of grade 4 cytokine release syndrome (CRS) and neurotoxicity. Grade ≥3 CRS and neurotoxicity were observed in 14% (n = 1/7) and 57% (n = 4/7) of patients, respectively. All other KTE-C19-related grade ≥3 events resolved within 1 month. The overall response rate was 71% (n = 5/7) and complete response (CR) rate was 57% (n = 4/7). Three patients have ongoing CR (all at 12+ months). CAR T cells demonstrated peak expansion within 2 weeks and continued to be detectable at 12+ months in patients with ongoing CR. This regimen of KTE-C19 was safe for further study in phase 2 and induced durable remissions in patients with refractory DLBCL.
Keywords: CAR T, CD19, diffuse large B cell lymphoma, refractory NHL, KTE-C19
In a multicenter phase 1 study, Locke, Neelapu, et al. report tolerability and safety of KTE-C19, a CD19 chimeric antigen receptor technology, in patients with chemorefractory DLBCL. More importantly, KTE-C19 could provide durable clinical benefit in this difficult-to-treat patient population, demonstrating broad clinical applicability of KTE-C19.
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) in the United States, accounting for approximately 30%–40% of all cases of NHL.1, 2, 3 Studies examining outcomes in patients with relapsed/refractory DLBCL show that the response rates to subsequent therapy varies from 14% to 63%.4, 5, 6, 7, 8 However, relapsed/refractory DLBCL is broadly defined and consists of a heterogeneous patient population. Outcomes are particularly poor in those patients with truly refractory DLBCL, defined as no response to last line of chemotherapy or relapse within 1 year of autologous stem cell transplant (ASCT).6, 7, 8, 9 A large patient-level meta-analysis of patients with refractory DLBCL (Retrospective Non-Hodgkin Lymphoma Research, SCHOLAR-1) found that outcomes in this homogeneous population are significantly worse, with a complete response (CR) rate of 8%, a partial response (PR) rate of 18%, and median overall survival (OS) of 6.6 months,10 indicating a major unmet need for effective therapies for these patients.
Adoptive cell therapy with T cells genetically engineered to express chimeric antigen receptor (CAR) targeting CD19 is a promising approach for treatment of B cell malignancies. A recent single-institution study conducted at the National Cancer Institute (NCI) demonstrated high response rates with an overall response rate of 73% and a CR rate of 55% with anti-CD19 CAR T cells containing CD3ζ/CD28 signaling domains administered in conjunction with low-dose cyclophosphamide conditioning regimen in patients with relapsed/refractory B cell lymphomas.11 KTE-C19 is an autologous CD3ζ/CD28-based anti-CD19 CAR T cell product that uses the same CAR construct as in the NCI study but is manufactured in a centralized, closed, and streamlined process of approximately 8 days. ZUMA-1 is the first multicenter study evaluating the safety and efficacy of anti-CD19 CAR T cells in patients with refractory NHL (NCT02348216). We report here the safety, efficacy, and correlative studies of apheresis product, KTE-C19, and in vivo effects from the phase 1 portion of ZUMA-1.
Results
Demographics and Baseline Characteristics
As of August 24, 2016, the median follow-up time was 9 months. Nine patients were enrolled in the study. Two patients experienced adverse events (AEs) due to disease progression, discontinued the study, and never received KTE-C19 (one prior to leukapheresis and one prior to conditioning chemotherapy). KTE-C19 manufacturing was successful for all eight leukapheresed patients (Figure S1). Seven patients received conditioning chemotherapy and KTE-C19. One additional patient was added to have the pre-specified dose-limiting toxicity (DLT) set of six patients treated at a target of 2 × 106 CAR+ T cells/kg (see Materials and Methods). Patients ranged from 29 to 69 years of age and had received two to four prior lines of therapy. Three were refractory to second-line or later lines of therapy, and four patients had relapsed post-ASCT within 1 year (Table 1). Two of the four that relapsed post-ASCT received another regimen before enrollment: patient 4 had stable disease after three cycles of R-GEMOX and patient 6 had progressive disease after two cycles of ipilimumab and lenalidomide therapy, meeting the definition of refractoriness as defined in the protocol. Median absolute lymphocyte count at time of enrollment was 900 lymphocytes/μL (range, 100–1,400 lymphocytes/μL). Four patients were non-germinal center B cell (GCB) subtype, and three were GCB as per the Hans algorithm.12
Table 1.
Baseline Characteristics
| Characteristic | Patient |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Age (years) | 59 | 68 | 69 | 67 | 34 | 40 | 29 |
| Sex | male | male | male | male | female | male | female |
| ECOG PS | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Prior therapies | (1) R-CHOP | (1) R-CHOP | (1) R-CHOP | (1) R-CHOP | (1) R-CHOP | (1) R-EPOCH | (1) R-CHOP |
| (2) R-ICE | (2) R-ICE | (2) R-CEOP | (2) R-ESHAP | (2) R-ICE | (2) R-BEAM-ASCT | (2) R-GDP | |
| (3) R-BEAM-ASCT | (3) R-ICE | (3) R-BEAM-ASCT | (3) R-AZA/ SAHA/GEMBUM-ASCT | (3) Ipilimumab+Lenalidomide | (3) ICE | ||
| (4) R-GEMOX + lenalidomide | (4) R-GEMOX | ||||||
| Prior lines of therapy | 3 | 2 | 4 | 4 | 3 | 3 | 3 |
| Relapsed/refractory status | relapsed post-ASCT within 12 months | refractory second or higher line of therapy | refractory second or higher line of therapy | relapsed post-ASCT within 12 months | relapsed post-ASCT within 12 months | relapsed post-ASCT within 12 months | refractory second or higher line of therapy |
| Primary diagnosis/sub-type | DLBCL/non-GCB | DLBCL/GCB | DLBCL/non-GCB | DLBCL/GCB | DLBCL/non-GCB | DLBCL/GCB | DLBCL/non-GCB |
| Disease stage | IV | II | III | I | IV | I | III |
ASCT, autologous stem cell transplant; AZA, azacitidine; BEAM, carmustine, etoposide, cytarabine, melphalan; CEOP, cyclophosphamide, etoposide, vincristine, prednisone; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; DLBCL, diffuse large B cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, cisplatin; GCB, germinal center B cell; GDP, gemcitabine, dexamethasone, cisplatin; GEMBUM, gemcitabine, busulfan, melphalan; GEMOX, gemcitabine, oxaliplatin; ICE, ifosfamide, carboplatin, etoposide; R, rituximab; SAHA, vorinostat.
Safety
All AEs occurring within 30 days of KTE-C19 infusion were graded and reported for the seven (100%) treated patients, with maximum grade 3, 4, and 5 events reported in three (43%), three (43%), and one (14%) patient(s), respectively (Table 2). All patients experienced KTE-C19-related AEs of any grade, with grade 3 and 4 events reported in four (57%) and one (14%) patient(s), respectively. No patient had a grade 5 KTE-C19-related event. B cell aplasia and hypogammaglobulinemia was observed in patients 4, 5, and 6 (data not shown).
Table 2.
Grade 3 or Higher Treatment-Emergent Adverse Events
| Event | Any, n (%) | Worst Grade 3, n (%) | Worst Grade 4, n (%) | Worst Grade 5, n (%) |
|---|---|---|---|---|
| Any grade 3 or higher AE within 30 days of KTE-C19 infusion | 7 (100) | 3 (43) | 3 (43) | 1 (14) |
| Febrile neutropenia | 4 (57) | 3 (43) | 1 (14) | 0 |
| Encephalopathy | 3 (43) | 2 (29) | 1 (14) | 0 |
| Neutropenia | 3 (43) | 0 | 3 (43) | 0 |
| Anemia | 2 (29) | 2 (29) | 0 | 0 |
| Hypoxia | 2 (29) | 2 (29) | 0 | 0 |
| Somnolence | 2 (29) | 2 (29) | 0 | 0 |
| Thrombocytopenia | 2 (29) | 0 | 2 (29) | 0 |
| Acute kidney injury | 1 (14) | 0 | 1 (14) | 0 |
| Agitation | 1 (14) | 1 (14) | 0 | 0 |
| Ascites | 1 (14) | 1 (14) | 0 | 0 |
| Aspartate aminotransferase increased | 1 (14) | 1 (14) | 0 | 0 |
| Cardiac failure | 1 (14) | 1 (14) | 0 | 0 |
| Delirium | 1 (14) | 1 (14) | 0 | 0 |
| Fatigue | 1 (14) | 1 (14) | 0 | 0 |
| Hemorrhage intracranial | 1 (14) | 0 | 0 | 1 (14) |
| Hypocalcemia | 1 (14) | 1 (14) | 0 | 0 |
| Hyponatremia | 1 (14) | 1 (14) | 0 | 0 |
| Hypophosphatemia | 1 (14) | 1 (14) | 0 | 0 |
| Hypotension | 1 (14) | 1 (14) | 0 | 0 |
| Metabolic acidosis | 1 (14) | 1 (14) | 0 | 0 |
| Oral herpes | 1 (14) | 1 (14) | 0 | 0 |
| Pseudomonal sepsis | 1 (14) | 0 | 1 (14) | 0 |
| Pyrexia | 1 (14) | 1 (14) | 0 | 0 |
| Restlessness | 1 (14) | 1 (14) | 0 | 0 |
| Tremor | 1 (14) | 1 (14) | 0 | 0 |
| Urinary tract infection | 1 (14) | 1 (14) | 0 | 0 |
One patient (14%) experienced a DLT. Patient 7 was a 29-year-old female with DLBCL with an Eastern Cooperative Oncology Group (ECOG) performance status of 1 and refractory to three lines of combination immuno-chemotherapy (Table 1). She experienced grade 3 hypotension, grade 3 metabolic acidosis, and grade 4 encephalopathy on day 0 and required intubation on day 1; grade 3 acute systolic heart failure on day 2; and grade 4 cytokine release syndrome (CRS) comprising grade 4 acute kidney injury on day 6. The grade 4 KTE-C19-related events in patient 7 initially improved with tocilizumab, corticosteroids, and supportive care including dialysis. However, the patient’s condition subsequently worsened with grade 4 pseudomonal sepsis, grade 4 thrombocytopenia, grade 4 neutropenia, and subsequent grade 5 intracranial hemorrhage. Death was deemed unrelated to KTE-C19 per the investigator, and on the day of death, the patient was on ongoing heparin for deep vein thrombosis (DVT) prophylaxis in the setting of grade 4 thrombocytopenia and pseudomonas sepsis. Retrospective biomarker analysis revealed that immediately prior to initiation of fludarabine and cyclophosphamide, the patient had a C-reactive protein (CRP) of 655 mg/L (normal range, 0–10 mg/L), which was approximately 100-fold higher than the baseline values of the other patients (median, 7 mg/L; range 4–34 mg/L), indicating an elevated baseline inflammatory state. With the exception of the patient experiencing a DLT, all grade ≥3 KTE-C19-related toxicities resolved within 1 month.
Cytokine release syndrome was reported in six (86%) patients treated with KTE-C19. Five (71%) patients experienced grade ≤2 CRS and one (14%) patient experienced grade 4 CRS (occurring in the patient with a DLT; Table 3). The most common CRS-related symptoms were pyrexia (71%), hypotension (43%), and tachycardia (29%). All grade 3 and 4 CRS-related events, except for one grade 3 pyrexia and one grade 3 hypoxia, occurred in the same patient experiencing the DLT. Six of seven (86%) patients (patients 1, 2, 3, 4, 5, and 7) received tocilizumab and four of seven (57%; patients 1, 3, 4, and 7) received corticosteroids for management of CRS and/or neurotoxicity symptoms as described by Lee et al.;13 four patients (patients 1, 3, 4, and 7) received both tocilizumab and corticosteroids. All evaluable patients experienced at least one neurotoxicity event of any grade (Table 3), with three (43%) having maximum grade 3, and one (14%) having a maximum grade 4 event (occurring in the patient with the DLT). The median time to development of CRS and neurotoxicity were 1 day (range: 0–3 days; n = 6) and 4 days (1–4 days; n = 6), respectively, with a median duration of 7 days (range: 3–17 days; n = 6) and 8 days (range: 2–20 days; n = 6), respectively. Except for the patient who experienced a DLT, CRS and neurotoxicity were found to be self-limiting and reversible. Four of the seven patients have died. With the exception of the patient who experienced the DLT, all deaths were due to progressive disease. Based on the safety profile of the six evaluable DLT patients in phase 1, this regimen was deemed safe for study in phase 2.
Table 3.
Cytokine Release Syndrome and Neurotoxicity
| Event | Any, n (%) | Worst Grade 3, n (%) | Worst Grade 4, n (%) |
|---|---|---|---|
| CRS, anya | 6 (86) | 0 | 1 (14) |
| CRS, Specific Symptomsb | |||
| Pyrexia | 5 (71) | 1 (14) | 0 |
| Hypotension | 3 (43) | 1 (14)c | 0 |
| Tachycardia | 2 (29) | 0 | 0 |
| Acute kidney injury | 1 (14) | 0 | 1 (14)c |
| Cardiac failure | 1 (14) | 1 (14)c | 0 |
| Headache | 1 (14) | 0 | 0 |
| Hypoxia | 1 (14) | 1 (14) | 0 |
| Metabolic acidosis | 1 (14) | 1 (14)c | 0 |
| Neurotoxicity, any | 6 (86) | 3 (43) | 1 (14) |
| Neurotoxicity, Specific Symptoms | |||
| Encephalopathy | 5 (71) | 2 (29) | 1 (14)c |
| Tremor | 4 (57) | 1 (14) | 0 |
| Somnolence | 2 (29) | 1 (14) | 0 |
| Agitation | 1 (14) | 1 (14) | 0 |
| Aphasia | 1 (14) | 0 | 0 |
| Delirium | 1 (14) | 1 (14) | 0 |
| Dizziness | 1 (14) | 0 | 0 |
| Dyskinesia | 1 (14) | 0 | 0 |
| Hallucination | 1 (14) | 0 | 0 |
| Restlessness | 1 (14) | 1 (14) | 0 |
CRS was graded per a modified grading system proposed by Lee et al.13
Individual symptoms of CRS are graded per CTCAE, version 4.03.
Events occurred in patient 7.
Efficacy
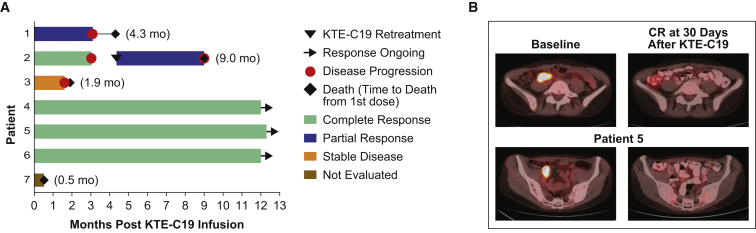
Five of seven (71%) patients achieved an objective response within 1 month of KTE-C19 infusion, with four of seven (57%) achieving a CR. Three patients are in ongoing CR at 12 months post-KTE-C19 infusion. All three patients with ongoing CR had previously relapsed within 5.8 months of ASCT (Table S1; Figure 1A). Of the three patients with ongoing CR at 12 months, one received tocilizumab, corticosteroids, and supportive care for the management of both CRS and neurotoxicity, one received only tocilizumab and supportive care for the treatment of both CRS and neurotoxicity during the first week post-treatment, and one received supportive care only. Figure 1B shows a representative positron emission tomography-computed tomography (PET-CT) with induction of CR 30 days after KTE-C19 infusion in patient 5. The response is ongoing at 12+ months. Patient 5 was previously treated with R-CHOP and R-ICE followed by ASCT conditioned with rituximab-gemcitabine-busulfan-melphalan+azacitidine-vorinostat and relapsed with DLBCL in the terminal ileum, ileocolic, and retroperitoneal lymph nodes 3.2 months after ASCT (Table 1).
Figure 1.
Clinical Efficacy after KTE-C19 Infusion
(A) Duration of response and survival post-infusion with KTE-C19. (B) CR at 30 days post KTE-C19 infusion in patient 5. Representative PET-CT scans at baseline and 30 days post KTE-C19 infusion in a patient with DLBCL relapsing after prior therapy with R-CHOP, R-ICE, and ASCT with Rituximab-gemcitabine-busulfan-melphalan+azacitidine-vorinostat.
Patient 2 achieved CR at 1 month after KTE-C19; however, progressive disease was noted at 3-month restaging and was subsequently proven as CD19+ relapse by biopsy. Per protocol, patient 2 was re-treated with exactly the same low-dose conditioning chemotherapy followed by the same target dose of KTE-C19. After the second infusion, KTE-C19 expansion was detected and the patient achieved a PR at 1 month. This patient progressed 3 months after the second infusion of KTE-C19 and died at 9 months following the initial KTE-C19 infusion due to rapidly progressive bulky lymphoma (Figure 1A). After the second KTE-C19 treatment, the patient developed myelodysplastic syndrome (MDS) with a complex karyotype and nonsynonymous DNMT3A and TP53 (double) mutations. Retrospective analysis demonstrated that these mutations were present in peripheral blood prior to study enrollment, indicating a pre-existing smoldering MDS.
Immunophenotyping and Biomarker Analysis
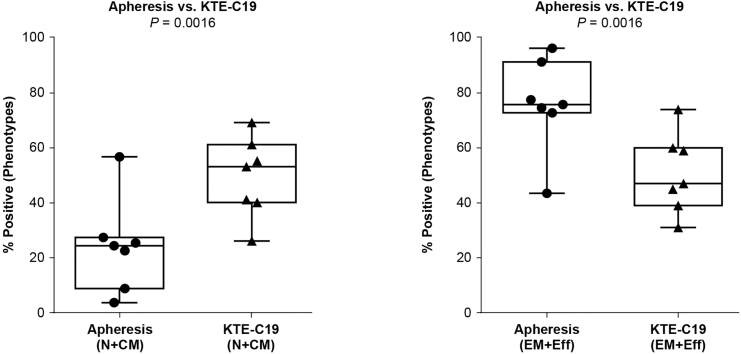
Detailed phenotypic characterization of the patients’ apheresis material, KTE-C19, and subsequent biomarker samples were conducted as outlined in Materials and Methods. The initial apheresis material (Table S2) and subsequent KTE-C19 product had similar intrapatient CD4/CD8 ratios (data not shown). Unfractionated CD4 and CD8 T cells were effectively transduced and showed ex vivo reactivity against CD19+ target cells (Table 4). T cells within the apheresis product typically showed more differentiated phenotypes with higher proportions of cells with effector memory (Tem [CCR7−, CD45RA−]) and effector (Teff [CCR7−, CD45RA+]) phenotypic profiles (Figure 2). The post-manufacture KTE-C19 CAR T cell product showed a less differentiated phenotype (Figure 2) based on CCR7 and CD45RA expression, with lower proportions of cells with Tem and Teff phenotypes compared to apheresis T cells and higher proportions of T cells with central memory (Tcm [CCR7+, CD45RA−]) and naive (TN [CCR7+, CD45RA+]) phenotypes (Figure S2).
Table 4.
Characteristics of KTE-C19 Products
| Patient No. |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| CAR T cells/kg × 106 | 2.0 | 2.0 | 2.0 | 1.1 | 2.0 | 1.9 | 1.2 |
| CD4 T cells (%) | 18 | 73 | 30 | 34 | 51 | 30 | 68 |
| CD8 T cells (%) | 82 | 27 | 70 | 66 | 49 | 70 | 32 |
| CD8/CD4 T cell ratio | 4.6 | 0.4 | 2.3 | 1.9 | 1 | 2.3 | 0.5 |
| IFN-γ production in co-culture (pg/mL)a | 20,930 | 8,589 | 3,356 | 7,598 | 6,948 | 2,278 | 816 |
| Manufacturing time (days) | 8 | 8 | 8 | 8 | 8 | 9 | 8 |
Co-culture experiments were performed using Toledo cells mixed in a 1:1 ratio with KTE-C19 product cells. IFN-γ was measured in cell culture media 24 hr post-incubation using a qualified ELISA.
Figure 2.
Apheresis and Product Phenotype as Determined by Flow Cytometry Using CD45RA and CCR7 Cell Surface Markers
N, naive; CM, central memory; EM, effector memory; Eff, effector. The bars and boxes show the minimum, maximum, median, and interquartile range.
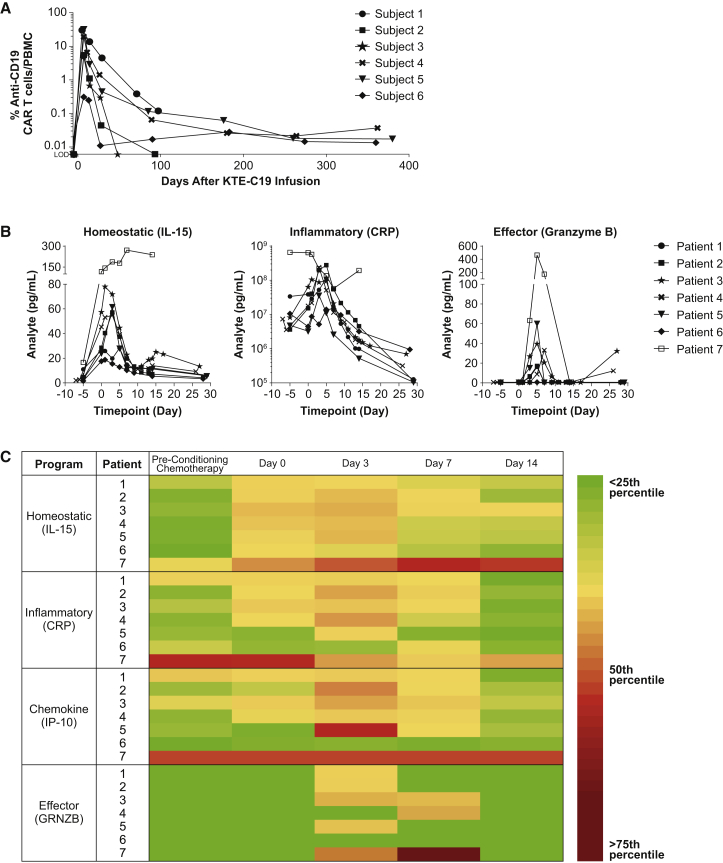
Peak expansion of CAR T cells occurred within the first 7–14 days post-infusion (Figure 3A) and were detectable at low levels by qPCR analysis for up to 12 months in the peripheral blood of all three patients with ongoing CRs. Expansion of KTE-C19 was mirrored by sequential induction, elevation, and general clearance of a range of homeostatic (IL-15), inflammatory and immune modulating cytokines, chemokines (such as IP-10), and T cell effector proteins (Figures 3B, 3C, and S3). Some of these cytokines and markers, notably IL-15 (median fold change from baseline, 9.9; range, 7.6–17.8), were initially elevated by the cyclophosphamide and fludarabine conditioning chemotherapy, paralleled by reduction of perforin and endogenous lymphocyte numbers. No antibodies for the scFv portion of KTE-C19 were detected in any of the patients during the course of the study (data not shown).
Figure 3.
Kinetics of Peripheral Blood CAR T Cells and Serum Biomarkers
(A) PCR data demonstrates exponential expansion and persistence of CD19 CAR T cells in blood. Expansion occurs rapidly with peak levels achieved within the first 7–14 days post-KTE-C19 infusion (note: patient 7 was not tested). Persisting CD19 CAR T cells were detectable in six of six (100%) patients at week 4 and in four of five (80%) patients with samples available for testing at month 3. Three patients with ongoing CR had detectable CAR T cells at 12 months. Limit of detection of the qPCR assay is 0.001% or 1 × 10−5. (B) Analysis of patient serum reveals a biomarker profile composed of specific cytokines, chemokines, and effector proteins associated with KTE-C19 treatment. The expansion of CD19 CAR T cells (Figure 3A) was mirrored by induction and elevation of a range of cytokines that regulate proliferation, activation, and effector function. Induction of IL-15 occurs during conditioning chemotherapy and levels continue to rise post-infusion, promoting anti-CD19 CAR T cell expansion. CRP levels parallel CRS and generally resolve within the first 28 days. Granzyme B levels peak 3–7 days post-infusion, during peak anti-CD19 CAR T cell expansion, and provide evidence of effector function and tumor killing. (C) Heat map of serum biomarkers demonstrates sequential induction and gradual resolution within the first 2 weeks after KTE-C19 infusion of key cytokines, chemokines, and effector proteins. Patients 1–6 demonstrated similar baseline levels and post-infusion kinetics for induction of IL-15 (T cell proliferation), CRP (marker of inflammation), granzyme B (evidence of effector function), and IP-10 (chemokine that promotes CAR T cell homing). Early induction of IL-15, CRP, and IP-10 was observed 1–3 days post-infusion and effector function occurred around days 3–7 for these patients. In contrast, patient 7 demonstrated a dysregulated profile relative to the other six patients both at baseline (IL-15, CRP, and IP-10) and after KTE-C19 administration (all markers), indicative of an inflammatory state prior to KTE-C19.
Discussion
To our knowledge, this is the first multicenter study of anti-CD19 CAR T cell therapy in patients with NHL. Our results demonstrated that (1) the conditioning regimen of cyclophosphamide at 500 mg/m2 (days −5, −4, and −3) and fludarabine at 30 mg/m2 (days −5, −4, and −3) followed by KTE-C19 (day 0) at a dose of 1–2 × 106 CAR T cells/kg is safe for further study and the toxicity is manageable; (2) a centralized, ∼8-day, closed manufacturing process of CAR T cells is feasible for a multicenter trial; and (3) therapy with cryopreserved KTE-C19 product is associated with robust CAR T cell expansion and durable clinical responses that are similar to those observed with fresh anti-CD19 CAR T cell therapy in studies conducted at single institutions.14 The results of this phase 1 study led to the initiation of the pivotal ZUMA-1 phase 2 registration trial.
The conditioning chemotherapy regimen doses utilized in ZUMA-1 were selected based on the results of the NCI clinical trial demonstrating that lower doses of chemotherapy afforded clinical efficacy with an attenuated toxicity profile versus higher doses of conditioning chemotherapy.15 Although the fludarabine dose was comparable, the dose of cyclophosphamide utilized here is significantly lower than that used in other trials of CAR T cell therapy.16, 17, 18 The lower doses of the conditioning chemotherapy with KTE-C19 were also associated with an acceptable toxicity profile. CRS and neurotoxicity were the two primary categories of KTE-C19-related AEs. Grade ≥3 CRS and neurotoxicity were observed in 14% (n = 1/7) and 57% (n = 4/7) of patients, respectively. One of seven patients (14%) experienced a DLT of grade 4 CRS and neurotoxicity, and none of the patient deaths was attributed to KTE-C19.
Close examination of the patient who experienced a DLT was revealing. The patient experienced cyclical fevers prior to cell infusion on day 0 attributed to rapidly progressive bulky disease with B symptoms. Although blood cultures remained negative, the patient had mucositis with underlying active HSV-1 that was being treated through KTE-C19 infusion. Biomarker analysis of this patient revealed a baseline CRP of 655 mg/L (normal range, 0–10 mg/L) in conjunction with the elevation of other inflammatory markers (IL-6, TNF-α). This timing and the nature of the cytokine profile were in stark contrast to the other patients in this trial (Figures 3B and 3C), indicating an extremely high level baseline inflammatory state. Following this DLT, the protocol was amended for safety so that patients having an active infection needing treatment or worsening of their end organ function at the time of planned initiation of conditioning chemotherapy would be ineligible to proceed with conditioning chemotherapy or infusion of KTE-C19. Except for the patient who experienced a DLT, all CRS and neurotoxicity AEs were self-limiting, and resolved within 1 month at a similar incidence with those observed on single institutional anti-CD19 CAR T cell trials for B cell malignancies.19, 20 Consistent with the “on-target, off-tumor” effect of KTE-C19, B cell aplasia and hypogammaglobulinemia were observed in subjects with ongoing complete response and persistent CAR T cells at 12 months post-infusion.
Despite the small numbers in this study, the overall and CR rates were high and durable relative to historical controls. Durable efficacy of the KTE-C19 regimen was observed in patients with rigorously defined chemotherapy refractory disease who had no viable treatment options. Rapid CRs were demonstrated after only 1 month of follow-up in only those four (57%) patients who relapsed after prior ASCT, and responses are ongoing at 12+ months in three of seven (43%) patients. In these three patients, the duration of response with KTE-C19 markedly exceeded the time to relapse after their prior ASCT. This is remarkable, as the expected CR rate in this chemotherapy refractory patient population is 8%, and median OS is 6.6 months with conventional therapies.10 Notably, responses in ZUMA-1 were observed without the use of any bridging chemotherapy: all patients proceeded directly from apheresis to conditioning chemotherapy and KTE-C19 infusion.
Comprehensive characterization of the apheresis product, KTE-C19, and blood/serum samples post-infusion provided insights into the possible mechanisms of efficacy and toxicity observed in these patients. The closed, approximately 8-day, centralized manufacture of a cryopreserved product consistently yielded active CAR T cells in all patients, across a broad range irrespective of lymphocyte counts at time of enrollment (median, 900 lymphocytes/μL; range, 100–1,400/μL). The product T cells showed a less differentiated phenotype based on CCR7 and CD45RA expression, considered desirable for adoptive T cell therapies (Figure S2) as naive and central memory T cells have increased proliferative capacity.21, 22, 23 Adequate CD4+ and CD8+ CAR T cells in the final product were obtained without pre-selection of T cell subsets.
The low-dose cyclophosphamide and fludarabine conditioning chemotherapy alone was capable of enhancing several homeostatic cytokines and chemokines, most notably IL-15, a critical T cell proliferative cytokine.24 Conditioning chemotherapy followed by KTE-C19 infusion resulted in a rapid and sequential induction, elevation, and general clearance of an array of cytokines, chemokines, and immune effector proteins (Figure 3C). More specifically, markers, including CRP, IL-6, TNF-α, IFN-γ, and IL-15, increased with peak values occurring 2–3 days post-infusion, and typically resolved to baseline within the first 28 days (Figure S3). Levels of Granzyme B, a key effector of T cell-mediated cytotoxicity, peaked later, 3–7 days post-infusion, providing evidence of when immune-mediated tumor cell killing may occur. This sequence of events paralleled CAR T cell expansion within 1–2 weeks after T cell infusion (Figure 3).
The timing of the elevations and general clearance of these systemic cytokines, chemokines, and immune effectors are similar in timing to the onset and resolution of CRS and neurotoxicity. Although the etiology of CRS is better understood, the pathophysiology of neurotoxicity that manifests as toxic encephalopathy remains unclear. The neurotoxicity reported here has been observed across other anti-CD19 CAR T cell studies and with blinatumomab, a bispecific T cell engager to CD19.18, 19, 25, 26, 27 Given that there is no direct evidence of CD19 expression in the CNS, two hypotheses that may not be mutually exclusive arise as the cause of neurotoxicity: either passive diffusion into the CNS of systemically generated cytokines; or activated CAR T cells migrating through the blood-brain barrier with subsequent local production of cytokines within the CNS.28, 29 Regardless, the neurotoxicity is generally reversible resolving in a similar temporal fashion to the abatement of peak CAR T cell and cytokines levels.
The agents effectively used for management of CRS and neurotoxicity, tocilizumab and/or systemic corticosteroids, did not appear to ablate CAR T cell expansion nor alter the CAR T cell-related elevation of cytokines, chemokines, and immune effector molecules. Importantly, durable responses were observed in patients who received tocilizumab and/or corticosteroids for toxicity management as well as those who did not. Overall, this analysis suggests the CAR T infusion in concert with chemotherapy conditioning creates an environment that promotes CAR T cell expansion, trafficking, anti-tumor activity, and persistence for an interval of time sufficient to mediate clinical activity.
In conclusion, the results of this phase 1 portion of ZUMA-1, the first multicenter study of anti-CD19 CAR T cell therapy in aggressive NHL, demonstrated that the KTE-C19 regimen was tolerable and safe for further study. It also validated that centralized manufacturing is feasible and established the logistics for transportation of patient-specific product door to door within approximately 2 weeks. More importantly, it showed that a single infusion of cryopreserved KTE-C19 cells could provide durable clinical responses in refractory DLBCL patients including those whose disease has failed to respond to ASCT. Together, these results fulfilled a prerequisite to broaden clinical applicability of this patient-specific adoptive T cell therapy and have led to the initiation of the pivotal multicenter phase 2 portion of ZUMA-1 for patients with refractory aggressive B cell NHL with an unmet clinical need.
Materials and Methods
Patient Population
The ZUMA-1 study is a phase 1/2, single-arm, open-label study evaluating the safety and efficacy of anti-CD19 CAR T cells (KTE-C19) in patients with refractory aggressive NHL. Eligible patients must have had all of the following: (1) histologically confirmed B cell NHL, including DLBCL not otherwise specified, primary mediastinal large B cell lymphoma, or transformed follicular lymphoma (TFL) as defined by the World Health Organization 2008 criteria; (2) chemotherapy refractory disease (as defined in the SCHOLAR-1 study:10 progressive disease or stable disease lasting ≤6 months, as best response to most recent chemotherapy regimen; or disease progression or recurrence ≤12 months after prior ASCT); (3) prior therapy must have included an anti-CD20 monoclonal antibody-containing regimen and an anthracycline-containing chemotherapy regimen; for patients with TFL, prior chemotherapy for follicular lymphoma and subsequent refractory disease after transformation to DLBCL; (4) at least one measurable lesion according to revised International Working Group (IWG) Response criteria;30 (5) no evidence of CNS lymphoma by magnetic resonance imaging; and (6) ≥2 weeks since prior radiation therapy or systemic therapy at the time of leukapheresis. Eligible patients were also aged ≥18 years, with ECOG performance status of 0 or 1, absolute neutrophil count of ≥1,000/μL, and platelet count of ≥50,000/μL. Patients must have had adequate renal, hepatic, and cardiac function defined as serum creatinine of ≤1.5 mg/dL, serum alanine aminotransferase/aspartate aminotransferase of ≤2.5 times the upper limit of normal, total bilirubin of ≤1.5 mg/dL (except in patients with Gilbert’s syndrome), cardiac ejection fraction of ≥50%, and no evidence of pericardial effusion, as determined by an echocardiogram. Key exclusion criteria included history of malignancy other than non-melanoma skin carcinoma in situ, ASCT within 6 weeks of informed consent, history of allogeneic hematopoietic stem cell transplant, prior CD19-targeted therapy, and prior CAR T cell therapy (except KTE-C19). All patients provided written, informed consent. The Institutional Review Board/Independent Ethics Committee of each study site approved the protocol. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Phase 1 Study Design and Toxicity Evaluation
In the phase 1 portion of ZUMA-1, the primary endpoint was incidence of DLTs. The study began with cohort A1. Progression to phase 2 would occur if the patient incidence of DLT was less than or equal to one of six DLT-evaluable patients in cohort A1. Dose-limiting toxicities were defined as KTE-C19-related events with onset within 30 days of infusion and included grade 4 neutropenia or thrombocytopenia lasting longer than 21 or 35 days, respectively; any KTE-C19-related AE requiring intubation; all other grade 3 toxicities lasting more than 3 days; and any grade 4 toxicities. Exceptions to this definition, not counting as DLT, included the following: aphasia/dysphasia or confusion/cognitive disturbance resolving to grade ≤1 within 2 weeks and baseline within 4 weeks; grade 3 fever or myelosuppression; or grade 3 immediate hypersensitivity reaction within 2 hr reversible to grade ≤2 within 24 hr; and grade 3 or 4 hypogammaglobulinemia.
The investigators were responsible for monitoring and reporting of all AEs through 3 months post-infusion. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. For CRS, a revised grading system created by Lee et al.13 was used. After 3 months, investigators monitored and reported on a targeted subset of AEs, including neurological and hematological AEs, infections, autoimmune disorders, and secondary malignancies for 24 months (or until disease progression, whichever occurred first). Secondary endpoints included objective response rate per revised IWG Response Criteria for Malignant Lymphoma per Cheson et al.,30 duration of response, progression-free survival, OS, incidence of AEs and clinically significant changes in laboratory values, incidences of anti-KTE-C19 antibodies, and levels of anti-CD19 CAR+ T cells and cytokines in blood and serum. Exploratory endpoints included objective response rate (ORR) and duration of second response among patients retreated with KTE-C19, and biomarker development based on assessment of blood and tumor cells and KTE-C19.
Study Procedures and Treatment
Patients were enrolled following screening and confirmation of eligibility and underwent leukapheresis within 5 days of enrollment to obtain peripheral blood mononuclear cells (PBMCs) to manufacture KTE-C19. Approximately 5 to 10 × 109 mononuclear cells from 12 to 15 L of apheresis material were shipped overnight to the central cell processing facility and enriched for the T cell-containing PBMC fraction. T cells were then stimulated to expand with anti-CD3 and IL-2 and transduced with a retroviral vector containing the CAR gene construct.20 Following expansion, the final KTE-C19 product was washed, cryopreserved, and tested for identity, potency, sterility, and adventitious agents. After meeting acceptance criteria, the KTE-C19 product was shipped back to the clinical sites using a validated cryo-shipper within approximately 2 weeks.31
Before receiving CAR T cell infusion, per cohort A1 parameters, patients received a non-myeloablative low-dose conditioning chemotherapy regimen of fludarabine at 30 mg/m2/day and cyclophosphamide at 500 mg/m2/day on day −5, day −4, and day −3. On day 0, hospitalized patients received a single intravenous infusion of KTE-C19 at a target dose of 2 × 106 CAR+ T cells/kg (minimum of 1 × 106 CAR+ T cells/kg) and remained hospitalized to recovery through day 7 or until all KTE-C19-related non-hematological toxicities returned to grade ≤1 or baseline.
Patients were followed in the post-treatment assessment period and returned to the clinic at week 2, week 4 (±3 days), month 2 (±1 week), and month 3 (±1 week). All patients completing the month 3 visit were followed in the long-term follow-up period for survival and disease status every 3 months (±2 weeks) through month 18, every 6 months (±1 month) between months 24 and 60; beginning with year 6 (month 72 ± 3 months), patients will return to the clinic once annually for up to 15 years. Patients achieving a CR or PR could have received a second course of conditioning chemotherapy and KTE-C19 if their disease progressed (not due to CD19− malignant cells) as part of an exploratory analysis. Each patient was allowed a maximum of one retreatment course.
Biomarker Analysis
Multiparametric flow cytometry was performed from apheresis through manufacturing, and final product, with in vivo characterization until end of study. Biomarker analyses were performed on blood and tumor samples to evaluate predictive and pharmacodynamic markers for KTE-C19. The presence, expansion, and persistence of transduced anti-CD19 CAR+ T cells in the blood were monitored by PCR. Levels of 44 serum cytokines, chemokines, circulating angiogenic factors, immune effector molecules, and markers of macrophage activating syndrome (MAS) were also assessed. Archived tumor tissue was collected for central pathology review.
Measurement of Serum Cytokines
Patient serum was harvested at the following time points: day −5 (prior to conditioning), day 0 (prior to KTE-C19 administration), every other day during hospitalization, day 14 and day 28. Serum samples were processed locally using standard serum separator tubes (BD Biosciences). Serum samples were held at −80°C for subsequent analysis by Luminex (EMD Millipore) or Meso Scale Discovery (MSD). Prior to processing, serum samples were briefly thawed on ice and aliquoted into 96-well U-bottom plates (BD Biosciences). Samples were analyzed using the following MSD kits: MSD V-PLEX Plus Angiogenesis Panel 1 (Human) Kit (bFGF, Flt-1/VEGFR-1, PlGF, Tie-2, VEGF-A, VEGF-C, VEGF-D), MSD V-PLEX Plus Chemokine Panel 1 (Human) Kit (Eotaxin, Eotaxin-3, IL-8 [HA], IP-10, MCP-1, MCP-4, MDC, MIP-1α, MIP-1β, TARC), MSD V-PLEX Plus Cytokine Panel 1 (Human) Kit (GM-CSF, IL-12/IL-23p40, IL-15, IL-16, IL-17A, IL-1α, IL-5, IL-7, TNF-β), MSD V-PLEX Plus Proinflammatory Panel 1 (Human) Kit (IFN-γ, IL-10, IL-12p70, IL-13, IL-1β, IL-2, IL-4, IL-6, IL-8, TNF-α), MSD V-PLEX Plus Vascular Injury Panel 2 (Human) Kit (CRP, ICAM-1, SAA, VCAM-1), and Luminex HCD8MAG-15K-04 (Granzyme A, Granzyme B, sFASL, and Perforin). All assays were carried out according to the manufacturer’s specifications. Quality and assay standard controls were included for independent runs per the manufacturer’s protocol.
All MSD assays were read using a MESO QuickPlex SQ 120 and analysis was performed using DISCOVERY WORKBENCH 4.0 (MSD). Luminex assays were read using a Luminex 200 system, and analysis was performed using Bio-Plex Data Pro software (Bio-Rad). Analyte values were reported as picograms per milliliter of serum.
Flow Cytometry Analysis of Product, Apheresis Material, and CAR T Cells after Co-culture with Target Cells
As part of standard release criteria, KTE-C19 products were evaluated by flow cytometry for anti-CD19 CAR surface expression, percentage of CD4 and CD8 T cells, and also memory phenotypes (Naive, Tcm, Tem, and Teff as defined by CCR7 and CD45RA). Testing was performed by Progenitor Cell Therapy, using validated protocols.
Cryopreserved apheresis samples were rapidly thawed in a 37°C water bath, washed twice with calcium-free/magnesium-free PBS (VWR Scientific) by gentle centrifugation, and resuspended in FACS Stain Buffer (BD Biosciences). Sample viability and cell density were measured using a Vi-cell as described. Cell densities were adjusted to 1.0 × 106 viable cells/mL in FACS stain buffer. Cells were aliquoted into 5-mL FACS tubes (BD Biosciences) at a final density of 1.0 × 106 viable cells/test. Cells were stained for 30 min on wet ice with the following commercially available fluorochrome-conjugated antibodies from BioLegend, CD3 fluorescein isothiocyante (FITC), CCR7 Brilliant Violet 650 (BV650), CD45RA allophycocyanin (APC), and CD4 Alexa Fluor 700 (AF700).
Cells from co-cultures of CAR T cells and target cell lines CD19-K562 or NGFR-K562 were pooled for analysis by flow cytometry. Sample viability and cell density were measured using a Vi-cell as previously described. Cell densities were adjusted to 1.0 × 106 viable cells/mL. Cells were aliquoted into 5-mL polystyrene FACS tubes (BD Biosciences) at a density of 1.0 × 106 viable cells. Staining was performed for 30 min on wet ice with the following commercially available fluorochrome-conjugated antibodies: (1) from BD Biosciences, PD1 phycoerytherin cyanine 7 (PE-Cy7) and CD57 Brilliant Violet 421 (BV421); (2) from BioLegend, CD3 (FITC), CCR7 (BV650), CD45RA (APC), CD4 (AF700), CD69 (BV510), CD137 (BV421), and CD107a (AF700); (3) from the Surgery Branch of the NCI, anti-CD19 CAR PE. All antibodies used in this study were titrated before use.
Prior to flow-cytometric analysis, samples were stained with propidium iodide (BD Biosciences) to exclude dead and apoptotic cells. Flow cytometry was performed using a FACSCanto II (BD Biosciences). For phenotypic markers, data were reported as percent positive (% pos) relative to the appropriate fluorescence minus one (FMO) control. For activation markers, data were reported relative to the CAR T cell products not subjected to co-culture. The analysis employed a cell-gating strategy that selected viable CD3+ and anti-CD19 scFV+ cells and excluded dead/apoptotic cells. A notable exception to the gating strategy in co-cultured CAR T cell products was a shifting of the gating strategy to viable CD3+ cells (and not anti-CD19 scFV+ cells) due to downregulation of surface CAR on T cells following engagement of the target antigen. Where feasible, data from a minimum of 1 × 104 viable cells were acquired. Data analysis was performed using FlowJo software, version 10 (FLOWJO) using standardized gating and compensation strategies.
Measurement of Anti-CD19 CAR T Cell Presence, Expansion, and Persistence
A qPCR assay, previously described,14, 15, 32 was optimized and validated by the University of Rochester Medical Center Central Lab Services (URMC CLS) for monitoring of anti-CD19 CAR T cell expansion and persistence. Sensitivity of the optimized method is 0.001% or 1 × 10−5. Testing was performed by URMC CLS using cryopreserved PBMC at baseline, prior to conditioning chemotherapy and KTE-C19 administration, at days 7, 14, and 28, and months 3, 6, 9, and 12.
Measurement of Anti-KTE-C19 Antibodies
Presence of anti-KTE-C19 antibodies were monitored by Intertek Laboratories using a qualified bridge ELISA designed to specifically detect anti-FMC63 antibodies in patient serum. Testing was performed at baseline, prior to conditioning chemotherapy and KTE-C19 administration, and at months 1 and 3.
Confirmatory Diagnosis of DLBCL in Archival Tumor Samples
Archived tumor tissue was collected for central pathology review at NeoGenomics Laboratories.
Author Contributions
F.L.L. (Moffitt Cancer Center) and S.S.N. (MD Anderson Cancer Center) participated in the study as investigators, reviewed and interpreted the data, and approved all drafts of the manuscript. F.L.L., S.S.N., N.L.B., T.S., J.C.C., C.M.H., A.G., L.E.B., A.B., J.M.R., Y.J., A.X.X., M.E., J.A., J.W., and W.Y.G. had access to and reviewed the study data, and carefully reviewed and approved all drafts of the manuscript including the final version.
Conflicts of Interest
F.L.L. has served as a scientific advisory board attendee for Kite Pharma. S.S.N. has received research funding and honoraria from and served as a consultant and Scientific Board Member for Kite Pharma. N.L.B. served in a consultancy or advisory role for Gilead. T.S. has served on speakers’ bureau for Pharmacyclics and Seattle Genetics and has received travel and lodging support from Kite Pharma. J.C.C., C.M.H., A.G., and L.E.B. declare no conflicts of interest. A.B., J.M.R., Y.J., A.X.X., M.E., J.A., J.W., and W.Y.G. are employed by and have equity ownership in Kite Pharma.
Acknowledgments
We thank the patients who participated in the study and their families, friends, and caregivers; the study staff and health care providers at City of Hope, Moffitt Cancer Center, MD Anderson Cancer Center, and Washington University School of Medicine. Medical writing support was provided by Dustin Khiem (Kite Pharma) and Luana Atherly-Henderson (Nexus Global Group Science LLC) funded by Kite Pharma. This study was funded by Kite Pharma, which provided all the study materials, and in part by the Leukemia & Lymphoma Society Therapy Acceleration Program, Moffitt Cancer Center Support Grant P30 CA076292, and MD Anderson Cancer Center Support Grant P30 CA016672. Effort for F.L.L. was in part supported by National Cancer Institute Cancer Clinical Investigator Team Leadership Award P30 CA076292-18S4.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.10.020.
Supplemental Information
References
- 1.Chaganti S., Illidge T., Barrington S., Mckay P., Linton K., Cwynarski K., McMillan A., Davies A., Stern S., Peggs K., British Committee for Standards in Haematology Guidelines for the management of diffuse large B-cell lymphoma. Br. J. Haematol. 2016;174:43–56. doi: 10.1111/bjh.14136. [DOI] [PubMed] [Google Scholar]
- 2.Morton L.M., Wang S.S., Devesa S.S., Hartge P., Weisenburger D.D., Linet M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sehn L.H., Gascoyne R.D. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. doi: 10.1182/blood-2014-05-577189. [DOI] [PubMed] [Google Scholar]
- 4.Crump M., Kuruvilla J., Couban S., MacDonald D.A., Kukreti V., Kouroukis C.T., Rubinger M., Buckstein R., Imrie K.R., Federico M. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J. Clin. Oncol. 2014;32:3490–3496. doi: 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- 5.Matasar M.J., Czuczman M.S., Rodriguez M.A., Fennessy M., Shea T.C., Spitzer G., Lossos I.S., Kharfan-Dabaja M.A., Joyce R., Fayad L. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood. 2013;122:499–506. doi: 10.1182/blood-2012-12-472027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisselbrecht C., Glass B., Mounier N., Singh Gill D., Linch D.C., Trneny M., Bosly A., Ketterer N., Shpilberg O., Hagberg H. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagle S.J., Woo K., Schuster S.J., Nasta S.D., Stadtmauer E., Mick R., Svoboda J. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am. J. Hematol. 2013;88:890–894. doi: 10.1002/ajh.23524. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri T., Stakiw J., Pintilie M., Keating A., Crump M., Kuruvilla J. Utility of subsequent conventional dose chemotherapy in relapsed/refractory transplant-eligible patients with diffuse large B-cell lymphoma failing platinum-based salvage chemotherapy. Hematology. 2008;13:261–266. doi: 10.1179/102453308X343527. [DOI] [PubMed] [Google Scholar]
- 9.Telio D., Fernandes K., Ma C., Tsang R., Keating A., Crump M., Kuruvilla J. Salvage chemotherapy and autologous stem cell transplant in primary refractory diffuse large B-cell lymphoma: outcomes and prognostic factors. Leuk. Lymphoma. 2012;53:836–841. doi: 10.3109/10428194.2011.643404. [DOI] [PubMed] [Google Scholar]
- 10.Crump M., Neelapu S.S., Farooq U., Van Den Neste E., Kuruvilla J., Ahmed M.A., Link B.K., Hay A.E., Cerhan J.R., Zhu L. Outcomes in refractory aggressive diffuse large B-cell lymphoma (DLBCL): results from the international SCHOLAR-1 study. J. Clin. Oncol. 2016;34 doi: 10.1182/blood-2017-03-769620. abstr 7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer J., Somerville R., Lu T., Shi V., Yang J.C., Sherry R., Klebanoff C., Kammula U.S., Goff S.L., Bot A. Anti-CD19 chimeric antigen receptor T cells preceded by low-dose chemotherapy to induce remissions of advanced lymphoma. J. Clin. Oncol. 2016;34 abstr LBA3010. [Google Scholar]
- 12.Hans C.P., Weisenburger D.D., Greiner T.C., Gascoyne R.D., Delabie J., Ott G., Müller-Hermelink H.K., Campo E., Braziel R.M., Jaffe E.S. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 13.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klebanoff C.A., Gattinoni L., Torabi-Parizi P., Kerstann K., Cardones A.R., Finkelstein S.E., Palmer D.C., Antony P.A., Hwang S.T., Rosenberg S.A. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 23.Sommermeyer D., Hudecek M., Kosasih P.L., Gogishvili T., Maloney D.G., Turtle C.J., Riddell S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steel J.C., Waldmann T.A., Morris J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topp M.S., Gökbuget N., Stein A.S., Zugmaier G., O’Brien S., Bargou R.C., Dombret H., Fielding A.K., Heffner L., Larson R.A. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 27.Topp M.S., Gökbuget N., Zugmaier G., Klappers P., Stelljes M., Neumann S., Viardot A., Marks R., Diedrich H., Faul C. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 28.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namuduri M., Brentjens R.J. Medical management of side effects related to CAR T cell therapy in hematologic malignancies. Expert Rev. Hematol. 2016;9:511–513. doi: 10.1080/17474086.2016.1183479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheson B.D., Pfistner B., Juweid M.E., Gascoyne R.D., Specht L., Horning S.J., Coiffier B., Fisher R.I., Hagenbeek A., Zucca E., International Harmonization Project on Lymphoma Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 31.Better M., Pugach O., Lu L., Somerville R., Kassim S., Kochenderfer J., Rosenberg S.A., Marshall M.A., Bot A., Nolop K.B. Rapid cell expansion (RACE) technology for production of engineered autologous T cell therapy: path toward manageable multi-center clinical trials in aggressive NHL with anti-CD19 CAR. J. Clin. Oncol. 2014;32(Suppl) abstr 3079. [Google Scholar]
- 32.Kochenderfer J.N., Dudley M.E., Carpenter R.O., Kassim S.H., Rose J.J., Telford W.G., Hakim F.T., Halverson D.C., Fowler D.H., Hardy N.M. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.