Abstract
Pompe disease results from acid α-glucosidase (GAA) deficiency, and enzyme replacement therapy (ERT) with recombinant human (rh) GAA has clinical benefits, although its limitations include the short half-life of GAA and the formation of antibody responses. The present study compared the efficacy of ERT against gene transfer with an adeno-associated viral (AAV) vector containing a liver-specific promoter. GAA knockout (KO) mice were administered either a weekly injection of rhGAA (20 mg/kg) or a single injection of AAV2/8-LSPhGAA (8 × 1011 vector genomes [vg]/kg). Both treatments significantly reduced glycogen content of the heart and diaphragm. Although ERT triggered anti-GAA antibody formation, there was no detectable antibody response following AAV vector administration. The efficacy of three lower dosages of AAV2/8-LSPhGAA was evaluated in GAA-KO mice, either alone or in combination with ERT. The minimum effective dose (MED) identified was 8 × 1010 vg/kg to reduce glycogen content in the heart and diaphragm of GAA-KO mice. A 3-fold higher dose was required to suppress antibody responses to ERT. Efficacy from liver gene therapy was slightly greater in male mice than in female mice. Vector dose correlated inversely with anti-GAA antibody formation, whereas higher vector doses suppressed previously formed anti-GAA antibodies as late as 25 weeks after the start of ERT and achieved biochemical correction of glycogen accumulation. In conclusion, we identified the MED for effective AAV2/8-LSPhGAA-mediated tolerogenic gene therapy in Pompe disease mice.
Keywords: gene therapy, adeno-associated virus, acid α-glucosidase, acid maltase, glycogen storage disease type II, immune tolerance induction
Introduction
Pompe disease is an inherited rare disorder caused by mutations in the gene for the enzyme acid α-glucosidase (GAA; >1 in 40,000 births) that affects the heart and skeletal muscles, and is often fatal.1 Enzyme replacement therapy (ERT) with recombinant human GAA (rhGAA) has been shown to decrease heart size; maintain normal heart function; improve muscle function, tone, and strength; and reduce glycogen accumulation.
Although ERT has prolonged survival in the majority of patients with infantile Pompe disease, many patients have died or remained very weak despite compliance with ERT. Among the poor responders to ERT were many cross-reacting immune material-negative (CRIM-negative) patients, who lack any residual GAA protein and who formed high, sustained anti-rhGAA IgG antibody titers (HSATs). Patients with HSATs demonstrated greatly increased mortality, in comparison with patients who formed no or low titer antibodies.2 Furthermore, suppressing anti-rhGAA antibody formation with immunosuppression significantly prolonged the survival of CRIM-negative infants, although immunosuppression has associated risks.3, 4 A small minority of adult patients with late-onset Pompe disease (LOPD) also formed HSATs during ERT, which in some cases were associated with reduced efficacy.5, 6
Multiple preclinical experiments have demonstrated the ability of gene therapy to prevent antibody formation in mice with Pompe disease.7, 8, 9, 10 Preventing HSATs also reduced mortality from hypersensitivity that had occurred during ERT in GAA-knockout (KO) mice, whereas ERT was efficacious only in the setting of immune tolerance to GAA following AAV vector administration.8 We and others demonstrated that AAV-vector-mediated gene transfer consistently induced immune tolerance to GAA by expressing GAA exclusively in the liver and by activating regulatory T cells in preclinical experiments.7, 8, 9, 10, 11
The efficacy from ERT in Pompe disease is limited by the short half-life of GAA and the formation of antibody responses that interfere with the uptake of GAA. We hypothesized that liver-specific expression of GAA with a recombinant (r) AAV8 vector expressing human GAA under the transcriptional control of a liver-specific promoter (AAV2/8-LSPhGAA12) would suppress the antibody response, continually secrete GAA in the blood, and improve efficacy in comparison with ERT. Previous studies suggested that the efficacy of this rAAV8 vector at a low dose12 (2 × 1010 vector genomes [vg], equivalent to 8 × 1011 vg/kg body weight) was comparable with long-term ERT13, 14 with regard to biochemical correction. Importantly, a higher vector dose (4 × 1012 vg/kg) reduced glycogen in skeletal muscle by 70% more than intensive ERT in GAA knockout (KO) mice.7, 14 The current study directly compares ERT with AAV2/8-LSPhGAA, and supported a successful investigational new drug (IND) application to the Food and Drug Administration in anticipation of a clinical trial of liver depot gene therapy in Pompe disease.
Results
AAV8-GAA Liver Gene Transfer Is as Effective as ERT and Prevents Anti-GAA Antibody Formation
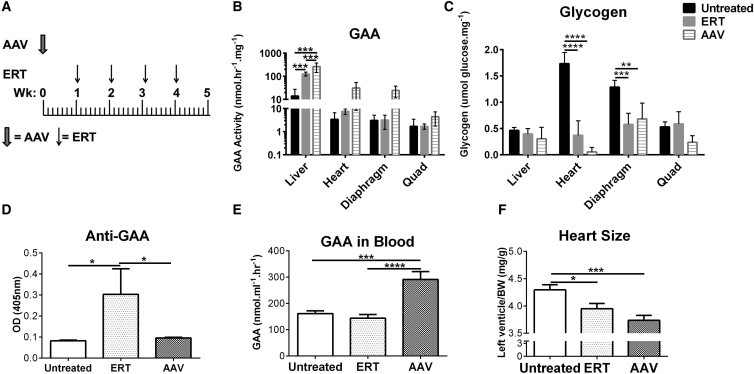
We hypothesized that liver depot gene therapy for Pompe disease could potentially improve clinical outcomes (Figure 1). Therefore, we directly compared the efficacy of intensive ERT with AAV2/8-LSPhGAA gene transfer at the established12 low dose. GAA-KO mice were assigned (both male and female; Figure 1A; Table 1) to receive either a weekly injection of rhGAA for intensive ERT15 (20 mg/kg/week; n = 10) or a single injection of AAV2/8-LSPhGAA for low-dose gene therapy (8 × 1011 vg/kg; n = 10). The primary endpoints included GAA activity and glycogen content in the tissues and anti-GAA antibody formation. In both ERT- and gene-therapy-treated animals, GAA activity was significantly increased in liver following both treatments, whereas GAA activity was higher without reaching statistical significance in the heart and muscles following gene therapy (Figure 1B). Glycogen content was reduced by both treatments in the heart and diaphragm, demonstrating that glycogen content is a more sensitive measure of biochemical correction than GAA activity (Figure 1C) as previously observed.12 Although ERT provoked anti-GAA antibody formation, there was no detectable antibody response following AAV vector administration (Figure 1D). GAA activity was continuously elevated in the blood of mice following a single injection of vector and was not detectable 7 days following ERT (Figure 1E). The left ventricle mass was reduced significantly following either ERT or vector injection, demonstrating the reversal of cardiac hypertrophy (Figure 1F). As expected, AAV2/8-LSPhGAA demonstrated slightly greater efficacy in male mice than in female mice, including higher liver GAA activity and lower diaphragm glycogen (Figure S1). Additionally, female mice had higher anti-GAA IgG1 following ERT, in comparison with male mice (Figure S1). Thus, gene therapy with AAV2/8-LSPhGAA at a dose of 8 × 1011 vg/kg was confirmed to have similar efficacy to ERT in both sexes, with the added benefit of avoiding anti-GAA immune responses.
Figure 1.
Comparison of Liver Depot Gene Therapy with ERT
In a 5-week study, GAA-KO mice were treated with (A) either a weekly injection of rhGAA (ERT; 20 mg/kg; n = 10) or a single injection of AAV2/8-LSPhGAApA (AAV; 8 × 1011 vg/kg; n = 10). (B–F) The endpoints included: (B) GAA activity and (C) glycogen content in the tissues, (D) antibody formation, (E) blood GAA, and (F) heart size. GAA activity was increased and glycogen content was reduced in the heart and skeletal muscles. Mean ± SD is shown. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 from ANOVA.
Table 1.
Experimental Group Designation
| Experiment | Group | No. of Animals | Study Day 1 | Additional Dosing Days |
Experimental Goal | Duration | |
|---|---|---|---|---|---|---|---|
| Vectora Dose (vg/kg) | rhGAAb (Study Day) | ||||||
| 1 | 1 | 10 (5 M/5 F) | none | 0 | 7, 14, 21, 28c | evaluate intensive ERT | 5 weeks |
| 2 | 10 (5 M/5 F) | AAV | 8 × 1011 | none | evaluate low-dose AAV | ||
| 3 | 8 (4 M/4 F) | none | 0 | none | negative control | ||
| 2 | 1 | 8 (5 M/3 F) | none | 6 × 109 | none | evaluate dose reduction to determine minimum effective dose for biochemical correction | 8 weeks |
| 2 | 8 (5 M/3 F) | none | 2 × 1010 | none | |||
| 3 | 9 (4 M/5 F) | none | 8 × 1010 | none | |||
| 4 | 9 (5 M/4 F) | none | 2 × 1011 | none | |||
| 5 | 10 (5 M/5 F) | rhGAA | 0 | 14, 28, 42 | ERT-only control | ||
| 6 | 10 (5 M/5 F) | rhGAA | 6 × 109 | 14, 28, 42 | evaluate dose reduction to determine minimum effective dose for immune tolerance induction | ||
| 7 | 9 (5 M/4 F) | rhGAA | 2 × 1010 | 14, 28, 42 | |||
| 8 | 9 (5 M/4 F) | rhGAA | 8 × 1010 | 14, 28, 42 | |||
| 9 | 9 (5 M/4 F) | rhGAA | 2 × 1011 | 14, 28, 42 | |||
| 10 | 8 (5 M/3 F) | none | 0 | none | untreated control | ||
| 3 | 1 | 11 (6 M/5 F) | rhGAA | 0 | 15, 36, 220d | ERT to provoke antibody responses in comparison with Grp 2 | 36 weeks |
| 2 | 9 (5 M/4 F) | rhGAA | 2 × 1012 (week 5) | 15, 120, 134, 220 | ERT followed by AAV at week 5 to evaluate induction and duration of immune tolerance | ||
| 3 | 11 (7 M/4 F) | rhGAA | 2 × 1011 (week 25) | 15, 120, 134, 220e | ERT to provoke antibody responses in comparison with Grp 2, followed by AAV at week 31 to evaluate suppression of antibody responses | ||
| 4 | 4 (3 M/1 F) | none | 0 | none | untreated control | ||
M, male; F, female.
AAV2/8-LSPhGAA administered intravenously on study day 1, or at a later date as indicated.
ERT consisting of injecting rhGAA intravenously (20 mg/kg). The second and subsequent ERT injections were preceded by 15 min with diphenhydramine through intraperitoneal injection (20 mg/kg) to prevent hypersensitivity.
Two mice died: one male died following ERT at day 28, and one female died following ERT at day 7.
One mouse died: one male died after day 162, unrelated to ERT.
One mouse died: one male died following ERT at day 134.
Definition of the Minimum Effective Dose of Liver Depot Gene Therapy for Pompe Disease
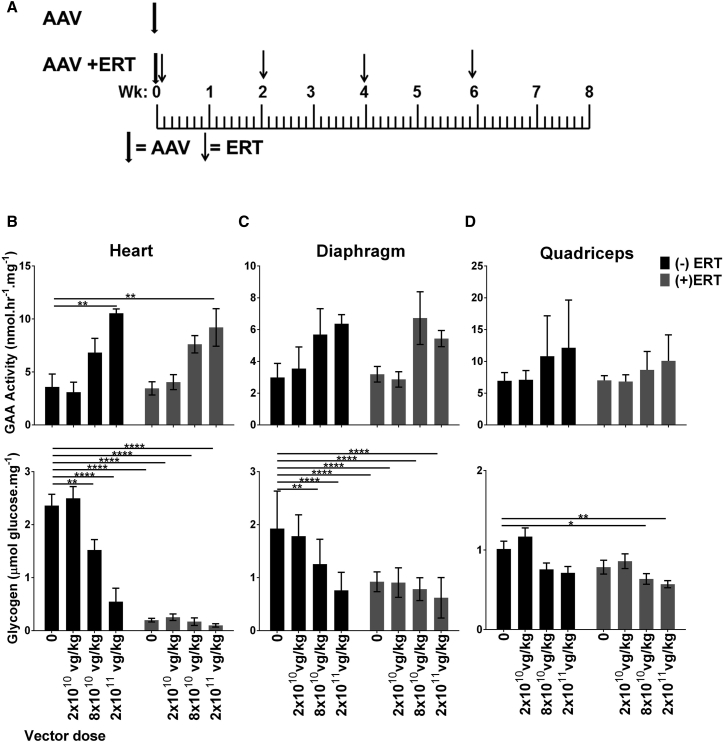
Next, we evaluated the biochemical efficacy of four lower dosages of AAV2/8-LSPhGAA in GAA-KO mice, either alone or in combination with ERT (Figure 2A; Table 1). No biochemical correction was observed following administration of 6 × 109 vg/kg (data not shown). The highest dose (2 × 1011 vg/kg) significantly increased GAA activity in the heart, either with or without ERT (Figure 2B). Administration of the highest vector dose reduced glycogen content of the heart to a greater extent in male mice than in female mice (Figure S2). Lower vector dosages were associated with lower GAA activity in the heart and skeletal muscles that did not achieve statistical significance, in comparison with no vector administration (Figure 2B). However, AAV2/8-LSPhGAA significantly reduced glycogen content in the heart and diaphragm of GAA-KO mice at a dose of 8 × 1010 vg/kg (p < 0.01; Figure 2C), which demonstrated that the glycogen storage in muscle associated with Pompe disease was substantially cross-corrected by GAA secretion from liver. Furthermore, administering ERT by itself had no significant effect on the glycogen content of quadriceps, but ERT following administration of the second highest dose (8 × 1010 vg/kg) of AAV2/8-LSPhGAA reduced glycogen content of quadriceps by 38% (p < 0.05; Figure 2D), indicating that gene therapy with AAV2/8-LSPhGAA could possibly have a synergistic effect with ERT. Therefore, the minimum effective dose (MED) with regard to biochemical correction was established as 8 × 1010 vg/kg.
Figure 2.
AAV2/8-LSPhGAApA Reduced the Glycogen Content of Muscle and Increased the Benefit from Simultaneous ERT
(A) GAA-KO mice were treated with the AAV2/8-LSPhGAA vector (AAV), either with or without simultaneous ERT for four doses (rhGAA, 20 mg/kg every 2 weeks). Number of mice per group was as follows: (−)ERT: 0 (n = 8), 2 × 1010 (n = 8), 8 × 1010 (n = 9), 2 × 1011 (n = 8); (+)ERT: 0 (n = 10), 2 × 1010 (n = 9), 8 × 1010 (n = 9), 2 × 1011 (n = 9). Biochemical correction in GAA-KO mice was evaluated 8 weeks following gene therapy, either with (shaded bars) or without (black bars). GAA activity and glycogen content shown for (B) heart, (C) diaphragm, and (D) quadriceps. Mean ± SD is shown. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 from two-way ANOVA.
Administration of Low-Dose AAV2/8-LSPhGAA Reduces Humoral Responses to GAA following an Immune Challenge with ERT
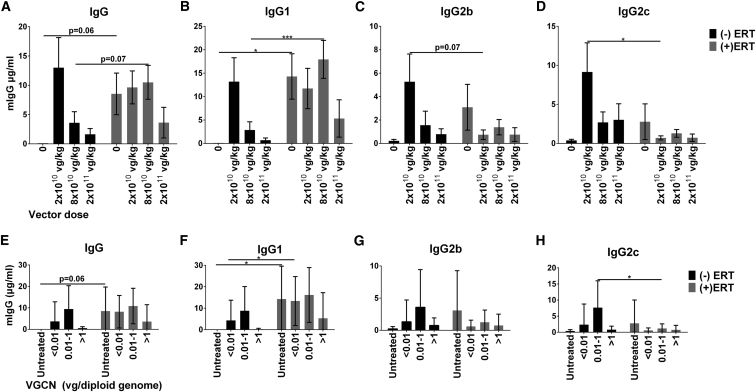
Total IgG and IgG subclasses were quantified in the serum of mice obtained 1 week following the last dose of ERT. In this experiment IgG2a and IgG3 were not detected (data not shown). As expected,16 we observed an increased level of total IgG specific for human GAA following four doses of ERT without AAV, in comparison with mice that were untreated (Figure 3A, vector dose “0”). Similarly, significant increases in anti-GAA IgG1 were observed following ERT alone, or administration of a vector dose of 8 × 1010 vg/kg (Figure 3B). Interestingly, at a dose of 2 × 1011 vg/kg, vector administration prevented significantly increased total IgG or IgG1 in response to ERT (Figures 3A and 3B). Surprisingly, the administration of vector alone at the dose of 2 × 1010 vg/kg induced a humoral immune response in four out of eight mice (Figures 3A and 3B). However, the administration of vector alone at the dose of 8 × 1010 vg/kg induced a humoral response in only two of nine mice, and the dose of 2 × 1011 vg/kg did not induce a humoral response. When ERT was administered following a vector dose of 8 × 1010 vg/kg, a humoral response was induced in seven of nine mice; however, following a vector dose of 2 × 1011 vg/kg, a humoral response was induced in only two of nine mice. Furthermore, in no group was total IgG or IgG1 increased to a greater extent following vector administration, in comparison with ERT alone (Figures 3A and 3B). Taken together, these data indicated that the highest dose of vector delivered to the liver prevented the induction of humoral immune responses to GAA. Thus, the MED with regard to immune tolerance induction was established at 2 × 1011 vg/kg.
Figure 3.
Liver Gene Transfer Reduces IgG Levels in a Dose-Dependent Manner
Number of mice per group was as follows: (−)ERT: 0 (n = 8), 2 × 1010 (n = 8), 8 × 1010 (n = 9), 2 × 1011 (n = 8); (+)ERT: 0 (n = 10), 2 × 1010 (n = 9), 8 × 1010 (n = 9), 2 × 1011 (n = 9). (A–D) The histograms indicate the levels of total IgG (A), IgG1 (B), IgG2b (C), and IgG2c (D), measured in plasma 9 weeks after vector injection and 1 week following the fourth dose of ERT. The quantification of antibody isotypes has been performed using purified mouse IgG isotypes as standard. Statistical analysis has been performed by comparison of untreated and ERT-treated mice at the same vector dose using multiple t tests [p < 0.05, (+)ERT versus (−)ERT]. Nine weeks after vector injection mice were sacrificed and the VGCN was measured in the liver. IgG isotype titers were divided in three groups depending on the genome copy number measured in the liver, less than 0.1 (<0.01), between 0.01 and 1 (0.01–1), and more than 1 (>1) copy of vector genome per diploid genome. (E–H) The histograms indicate the levels of total IgG (E), IgG1 (F), IgG2b (G), and IgG2c (H) for each level of VGCN. Statistical analysis has been performed by comparison of untreated and ERT-treated mice at the same vector dose using multiple t tests [p < 0.05, (+)ERT versus (−)ERT]. Mean ± SD is shown. *p < 0.05, ***p < 0.001 from multiple t tests.
Anti-GAA IgG1 was the isotype most prevalent in mice that received ERT combined with a low vector dose. Interestingly, we also observed increased levels of IgG2b and IgG2c following the administration of vector alone at the 2 × 1010 vg/kg dose, in comparison with mice that received vector and ERT (Figures 3C and 3D). The vector dose of 2 × 1010 vg/kg induced a humoral response characterized by high titers of IgG1, IgG2b, and IgG2c (greater in male than in female mice; Figure S3), whereas a higher vector dose generally prevented IgG formation. This unique isotype profile was not observed in the other groups treated with vector or ERT alone or in combination. This unique profile was most pronounced in male mice, in comparison with female mice (Figure S3). Notably, an isotype switch from IgG1 to IgG2b and IgG2c isotypes has been associated with the induction of peripheral tolerance mediated by the liver.17
Induction of Immunological Tolerance to GAA following Gene Transfer Correlates with Vector Genome Copy Number in Liver
The induction of liver-mediated transgene tolerance is highly dependent upon a robust liver transduction.10, 12 Vector genome copy number (VGCN) in liver represents a surrogate for liver transduction. To correlate antibody formation in mice treated with AAV8-LSPhGAA with the VGCN, we arbitrarily divided mice into three groups: (1) those with less than 0.01 copy, considered as the limit of detection of VGCN measurement; (2) those with between 0.01 and 1 copy; and (3) those with more than 1 copy per diploid genome. Total IgG and IgG subclasses were quantified in the three groups and compared with untreated mice and with mice treated with ERT without liver gene transfer. As previously shown, ERT treatment in the absence of the vector induced IgG (Figure 3E) and IgG1 (Figure 3F) against rhGAA, which could not be prevented by liver transduction corresponding to less than 0.01 copy of vector per diploid genome. Conversely, the treatment with AAV8-LSPhGAA doses that resulted in more than one copy per diploid genome (>1) prevented the induction of a significant humoral immune response against the transgene. Furthermore, intermediate transduction of 0.01–1 copy allowed significant formation of IgG2c (Figure 3H). The formation of IgG2c was significantly increased in the presence of intermediate vector transduction without ERT, in comparison with intermediate transduction with ERT (Figure 3H).
Eradication of Anti-GAA Humoral Immunity and Long-Term Efficacy of AAV2/8-LSPhGAA Gene Transfer
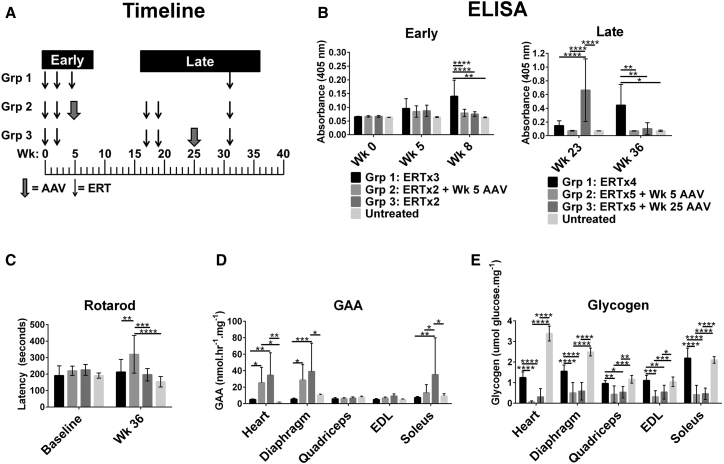
The long-term effect of AAV2/8-LSPhGAA in combination with ERT was evaluated with regard to immune tolerance induction (Figure 4; Table 1), given the experience that vector administration following the start of ERT might fail to prevent anti-GAA formation.16 The ability of AAV2/8-LSPhGAA to suppress anti-GAA antibody formation following exposure to ERT was evaluated by administering the vector either 5 or 25 weeks following the initiation of ERT (Figure 4A). Initially the mice were treated with two doses of ERT according to standard recommendations (20 mg/kg every other week). At week 5 mice were treated with an additional dose of ERT (group [Grp] 1) or with AAV2/8-LSPhGAA at a highly effective dose to evaluate the antibody response to each agent. Early monitoring revealed that ERT (Grp 1; Figure 4B) provoked significantly higher anti-GAA formation in comparison with AAV2/8-LSPhGAA (Grp 2; Figure 4B), whereas the administration of only two doses of ERT did not provoke anti-GAA formation (Grp 3; Figure 4B). Long-term monitoring revealed that the administration of AAV2/8-LSPhGAA at week 5 suppressed anti-GAA formation in response to two subsequent doses of ERT at weeks 17 and 19 (Grp 2; Figure 4B). However, administration of ERT at weeks 17 and 19 provoked significantly elevated anti-GAA by 23 weeks in mice that had received only ERT (Grp 3; Figure 4B). Thus, administration of AAV2/8-LSPhGAA as late as week 5 following the initiation of ERT was sufficient to induce long-term immune tolerance to rhGAA.
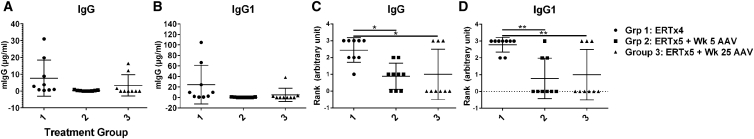
Figure 4.
Long-Term Clearance of Glycogen from the Heart, Diaphragm, and Skeletal Muscle and Preservation of Neuromuscular Function from Higher Dosage rAAV8
(A) Experimental design. GAA-KO mice were monitored for 36 weeks following administration of ERT with or without a single dose of AAV2/8-LSPhGAA (2 × 1012 vg/kg) at the indicated times. (B) Histograms for anti-GAA ELISA (1:200) detecting IgG1 at the early and late time points. All three groups were challenged with rhGAA at week (Wk) 31. The week 25 administration of rAAV8 suppressed anti-GAA from very elevated at Wk 23 to background levels by week 36. However, the ERT-only group formed higher antibodies following the immune challenge. AAV at week 5 maintained suppression of anti-GAA. The background signal for untreated mice was <0.01 in the ELISA. (C) Rotarod at week 0 (baseline) and week 36. Mice were euthanized at week 36. (D and E) Muscle GAA (D) and muscle glycogen (E). Mean ± SD is shown. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, per two-way ANOVA.
We further evaluated the ability of AAV2/8-LSPhGAA to suppress previously formed antibody responses against ERT. AAV2/8-LSPhGAA was administered at week 25 to suppress previously formed anti-GAA, reducing the anti-GAA IgG1 to background levels by week 36 (Grp 3; Figure 4B). Initially, ELISA confirmed that 10 of 10 mice were positive for anti-GAA at week 23, whereas only one of nine mice were positive at week 36 following AAV2/8-LSPhGAA administration (Grp 3; Figure 4B). When all three groups were challenged with ERT at week 31, GAA-KO mice treated with ERT only formed significantly increased anti-GAA at week 36 (Grp 1; Figure 4B). GAA-KO mice that were treated with AAV2/8-LSPhGAA at week 5 developed increased Rotarod latency in comparison with all other groups (Grp 2; Figure 4C), which demonstrated the importance of early vector treatment to improve muscle function. Both vector-treated groups had improved biochemical correction after 36 weeks, in comparison with mice treated with ERT alone (Figures 4D and 4E). GAA activity was significantly increased in the heart, diaphragm, and soleus of GAA-KO mice following administration of AAV2/8-LSPhGAA, in comparison with ERT-treated or untreated mice (Figure 4D). Vector administration significantly reduced glycogen content in multiple skeletal muscles of vector-treated mice (Figure 4E), and glycogen content was again a more sensitive measure of biochemical correction than GAA activity (Figure 4D) as previously demonstrated.12 The soleus demonstrated a greater elevation of GAA (Figure 4D) and reduction in glycogen content (Figure 4E) following vector administration that was observed for other skeletal muscles. This observation was consistent with improved response of type I muscles to GAA replacement,14 in comparison with other muscles examined that were comprised mainly of type II myofibers. As expected,16 AAV2/8-LSPhGAA-treated mice demonstrated slightly greater efficacy in males than in females, achieving higher GAA activity and lower glycogen content (Figure S4). In general the degree of biochemical correction achieved by early and late vector injection was equivalent with regard to reducing glycogen content, confirming the efficacy of suppression of anti-GAA with AAV2/8-LSPhGAA at either timepoint (Figure 4E).
Circulating IgG isotypes in the three groups were quantified at week 36 and ranked according to the degree of elevation (Figure 5; Table S1). As expected, ERT-only mice formed the highest total IgG with nine out of nine mice generating elevated total IgG (Grp 1; Figure 5A) and IgG1 (Figure 5B). Administering AAV2/8-LSPhGAA at week 5 reduced total IgG and IgG isotypes, with six out of nine mice showing detectable IgG1 levels less than 0.25 μg/ml (Grp 2; Figure 5B; Table S1). Vector-treated mice had significantly lower IgG (Grp 2 and 3; Figure 5C) and IgG1 (Grp 2 and 3; Figure 5D) responses, in comparison with the ERT-only Grp 1. Finally, AAV2/8-LSPhGAA at week 25 resulted in elevated total IgG (Grp 3; Figure 5C) or IgG1 (Grp 3; Figure 5D) in only three out of nine mice according to the ranking scale, thus indicating an efficient eradication of the immune response 11 weeks following vector administration. Interestingly, following AAV2/8-LSPhGAA at week 25, only very low titers of IgG1 were observed, along with IgG2b and IgG2c (Grp 3; Figure S5). IgG1 was elevated to >10 μg/ml in only one of nine Grp 3 mice (Figure 5B), which would correspond to an ELISA titer >1:1,600 (Figure S6) that would be expected to interfere with efficacy.8 Together, these data suggest that AAV-vector-mediated liver gene transfer for GAA can eradicate established immune responses to the enzyme and enhance efficacy of GAA replacement and, consequently, clinical outcome in Pompe disease.
Figure 5.
Liver Gene Transfer Eradicates Humoral Immune Response Triggered by Multiple rhGAA Infusions
GAA-KO mice from the three groups were injected with 20 mg/kg rhGAA and 8 × 1011 vg/kg AAV8-LSPhGAA as indicated in the experimental plan (Figure 4A). Scatterplots show total IgG (A), IgG1 (B), and ranked IgG (C) and IgG1 (D), which was measured in plasma 36 weeks after vector injection and ranked by the degree of antibody production. Antibodies were ranked as follows: <0.25 = 0, 0.25–0.5 = 1, 0.51–0.75 = 2, 0.76–1 = 3, and >1 = 4 (Table S1). The quantification of antibody isotypes has been performed using purified mouse IgG isotypes as standard. Mean ± SD is shown. *p < 0.05, **p < 0.01 from ANOVA.
Discussion
This study modeled the clinical translation of a gene therapy approach aimed at preventing IgG antibody formation in patients at risk for HSATs and addressed important questions related to the successful filing of an IND. This strategy induced immune tolerance to ERT through liver-specific expression of GAA. Furthermore, liver expression of GAA treats Pompe disease by the continuous secretion of GAA from the liver accompanied by receptor-mediated uptake in the heart and skeletal muscles. Three experiments investigated the effectiveness of liver depot gene therapy and its interactions with ERT. A single dose of AAV2/8-LSPhGAA was as effective as intensive, weekly ERT (Figure 1). This direct comparison confirmed previous experiments that suggested that gene therapy with a very low amount of AAV2/8-LSPhGAApA, equivalent to 8 × 1011 vg/kg body weight, was as effective as ERT,13, 14 and more precisely quantitated the reduction in glycogen content following GAA replacement than a previous comparison between ERT and gene therapy.18 We established the MED for biochemical correction with AAV2/8-LSPhGAA (8 × 1010 vg/kg), which is approximately 10-fold lower than previous data suggested (Figure 2).8, 9 The MED for immune tolerance is 2.5-fold higher (2 × 1011 vg/kg). As previously observed, glycogen content was a more sensitive indicator of biochemical correction than GAA activity, because glycogen content was significantly reduced in muscles where GAA activity was not significantly increased (Figures 1, 2, and 3).12, 19 Finally, the ability of AAV2/8-LSPhGAA to suppress or eradicate antibody following the start of ERT was demonstrated, as well as the long-term efficacy of liver depot therapy (Figure 4). These experiments modeled the effects of AAV2/8-LSPh-GAA administration in: (1) the naive Pompe subject, prior to ERT; (2) in conjunction with ERT, either at the MED or slightly higher dose; and (3) 4 months following ERT initiation, in a Pompe subject immunized against rhGAA.
The eradication of long-term memory antibody responses to rhGAA is perhaps the most novel aspect of this study, never achieved previously with gene therapy in Pompe disease. Previously the administration of an AAV vector to express coagulation factor VIII in dogs with hemophilia A was shown to suppress inhibitory antibodies and to establish immune tolerance.20 Subsequently, two groups showed that expression of coagulation factor IX could similarly induce immune tolerance following inhibitory antibody formation in mice with hemophilia.21, 22 Similar effects have been demonstrated following AAV vector administration in mice and dogs with hemophilia B using a high activity factor IX.23 In Pompe disease rAAV8 vectors have been administered 2–3 weeks following the start of ERT to suppress anti-GAA antibody formation in GAA-KO mice.9, 11 In the current study immune tolerance induction was possible as late as week 25. As shown in Figure 4, when ERT was administered three times by week 5 and again at week 31, GAA-KO mice formed antibodies by week 8 that were boosted to a robust HSAT following the fourth injection (Grp 1; Figure 4B). Mice that received two ERT treatments before the intravenous injection of AAV8-LSPhGAA in week 5 formed no significant humoral response against GAA, even after five ERT treatments over 31 weeks (Grp 2; Figure 4B). In contrast, mice that received four ERT treatments in the first 20 weeks developed a strong humoral response against the transgene (Grp 3; Figure 4B). Subsequently, treatment with AAV8-LSPhGAA at week 25 dramatically decreased the humoral response to rhGAA (Grp 3; Figure 4C). Furthermore, an immune challenge with rhGAA at week 31 did not induce a humoral response following treatment with AAV8-LSPhGAA at week 25, reflecting a robust peripheral tolerance induced by liver gene transfer. Therefore, we anticipate that AAV2/8-LSPhGAA could be administered to patients with Pompe disease in order to prevent or suppress anti-GAA antibodies and achieve efficacious GAA replacement.
A pharmacology and toxicology study with AAV2/8-LSPhGAApA was completed under good laboratory practice (GLP), which was designed to detect early or late toxicity.16 In brief, intravenous administration of the AAV2/8LSPhGAA vector at 1.6 × 1013 vp/kg (8-fold higher than the proposed higher dose in the proposed clinical trial described in the IND) did not cause significant short- or long-term toxicity. The vector genome was sustained in all tissues through 16 weeks post-dosing, except for in blood, with a similar tissue tropism between males and females.16 Administration of the vector alone, or combined with the ERT, was effective in producing significantly increased GAA activity and consequently decreased glycogen accumulation in multiple tissues, in comparison with administration of vehicle. The only complication demonstrated was formation of low-titer anti-GAA IgG in some female mice. Anti-hGAA antibody formation has been associated with anaphylactic responses to ERT following the third dose in GAA-KO mice,13 although anaphylaxis is rare in humans undergoing ERT for Pompe disease.5 The only mortality in the current study occurred in mice treated solely with ERT (Table 1), consistent with the suppression of anti-GAA observed following administration of AAV2/8-LSPhGAApA.
Limitations of the current study include the lack of GAA-KO mice treated with long-term ERT, either alone or following AAV2/8-LSPhGAApA administration. Long-term ERT was not attempted because of the risk for hypersensitivity and mortality,8, 9, 13 and because of the limited supply of rhGAA available to us. Because of this limitation we did not demonstrate long-term immune tolerance induction to GAA following AAV2/8-LSPhGAApA at week 25. However, immune tolerance induction from AAV2/8-LSPhGAApA has been persistent in this study following vector administration at week 5 and in prior studies.7, 8, 9
We have confirmed that the sex-dependent lower efficacy of AAV vector in female mice also applies to Pompe disease,16, 24 as suggested by other published studies in murine models.25, 26, 27 Our pharmacology and toxicology study of AAV2/8-LSPhGAA in GAA-KO mice further supported the hypothesis that AAV vectors transduced tissues more efficiently in male mice.16 The biodistribution analysis revealed a significantly higher number of vector genomes in the liver and heart of vector-treated male GAA-KO mice, in comparison with female mice.16 Furthermore, biochemical correction was greater in male mice, in comparison of female mice, demonstrated by significantly lower glycogen content in the quadriceps and diaphragm of male mice, in comparison with female mice.16 The current study showed greater biochemical efficacy and reduced immune responses in male mice with Pompe disease. Intriguingly, we demonstrated slightly higher glycogen content in the muscle of untreated male GAA-KO mice, in comparison with female mice, which correlated with lower background GAA activity in male GAA-KO mice (Figure S2). These data indicated that, in GAA-KO female mice, previously observed GAA-KO16 and, in other mouse models,25 AAV-mediated gene transfer appeared to be less efficacious, However, in non-human primates, this difference was not observed28; thus, sex-related differences reported in this study are less likely to be relevant in the clinic. To this end, in a recently published clinical trial of AAV-mediated gene transfer, no evidence of differential transduction between male and female liver was observed.29 Thus, available data suggest that efficacy of liver transduction in male and female Pompe patients will be equivalent.
Importantly, our data indicate that a minimum level of liver transduction is required to control transgene immunity in Pompe disease models. In translating these results to the clinic, it therefore will be important to ensure sufficient liver transduction. In our proposed clinical study we plan to administer a first dose that will be 2-fold higher than the MED defined by immune tolerance induction in the current study (i.e., 4 × 1011 vg/kg). The second cohort will receive a highly effective dose of AAV2/8-LSPhGAA, shown to suppress previously formed antibodies (Figure 4), which is identical to the higher dose of an rAAV8 vector (2 × 1012 vg/kg) administered in the hemophilia B clinical trials.30 This higher dose was sufficient to decrease the glycogen content of skeletal muscle by >50%, demonstrating a high degree of biochemical efficacy (Figure 4E). The selection of vector dosages that were effective in prior clinical trials further justifies the design of our proposed clinical trial. Moving forward, additional improvements to the platform could be implemented, such as the use of serotypes with reported high tropism for human hepatocytes,31, 32 which seem to outperform rAAV8 in non-human primates and, possibly, in humans. However, rAAV8 vectors have performed very well in humanized mouse models, suggesting that proceeding with the clinical translation of AAV2/8-LSPhGAA-mediated gene therapy for Pompe disease should be considered.33, 34
In conclusion, these experiments define a range of doses, starting from the MED, able to prevent humoral immune responses to GAA, to a therapeutic dose of AAV2/8-LSPhGAA, highly effective in eradicating a previously formed anti-GAA IgG response. In combination with the assessment of pharmacology and toxicology of AAV2/8-LSPhGAA gene transfer for Pompe disease,16 these data support the clinical translation of liver depot gene therapy in Pompe disease. The strategy of liver-targeted gene therapy is an innovative emerging therapy with no parallel among currently approved therapies. Eventual marketing approval would represent a systemic, non-invasive gene therapy in Pompe disease. Successful clinical development would provide immunomodulatory gene therapy in Pompe disease, which would induce immune tolerance to GAA and prevent HSAT formation. This strategy could be useful in other lysosomal storage disorders and in hemophilia, where antibody responses to therapeutic proteins frequently complicate replacement therapy.2, 35, 36, 37, 38, 39
Materials and Methods
In Vivo Evaluation of AAV-Vector-Mediated Efficacy
The AAV vector was prepared as described previously and administered intravenously to GAA-KO mice with a C57BL/6 background.24, 40 ERT was administered at the standard dose (20 mg/kg), injected intravenously either weekly or every other week, and diphenhydramine was injected intraperitoneally 15 min prior to the second and subsequent doses of ERT to prevent anaphylaxis. Age- and sex-matched GAA-KO mice were housed in groups of three to five, and mice from different groups were co-housed when possible. Rotarod testing was performed as described previously.24 GAA activity and glycogen content were analyzed as described previously.24 All animal procedures were done in accordance with Duke University Institutional Animal Care and Use Committee-approved guidelines.
Anti-GAA Antibody Isotypes Determination
Maxisorp 96-well plates (Thermo Fisher Scientific) were coated with Myozime protein in carbonate buffer at 4°C overnight. A standard curve of IgG isotype (Sigma-Aldrich) was coated to the wells in seven 2-fold dilution starting from 1 μg/ml. After blocking, plasma samples diluted at 1:100 were added to plates and incubated for 1 hr at 37°C. Isotype-specific secondary antibodies coupled to HRP were used for detection (Southern Biotech). Then, 3,3′,5,5′-tetramethylbenzidine substrate (BD Biosciences) was added to the wells and color development was measured at 450 and 570 nm (for background subtraction) on an Enspire plate reader (Perkin Elmer) after blocking the reaction with H2SO4.
Viral Vector Genome Copy Number Analysis
Total DNA was extracted from approximately 100 mg of frozen liver tissue by using the MagNA Pure 96 DNA and viral NA small volume kit (Roche Diagnosis) according to manufacturer’s instructions. Viral vector genome copy number (VGCN) measured by qPCR was normalized by the copies of titin gene measured in each sample. qPCR was performed on a LightCycler 480 (Roche Diagnostics) using SybrGreen mix (Thermo Fisher Scientific) and the following specific primers and probes: GAA, forward 5′-AGATCCCCCAGACAGTGCTG-3′ and reverse 5′-TTCCTGCTGGCAGTGGTGCTGA-3′; titin, forward 5′-AAAACGAGCAGTGACGTGAGC-3′ and reverse 5′-TTCAGTCATGCTGCTAGCGC-3′.
RNA Extraction and qRT-PCR
Total RNA was extracted from approximately 100 mg of frozen liver tissue by using the MagNA Pure 96 RNA extraction kit (Roche Diagnosis) according to the manufacturer’s instructions. Total RNA was reverse-transcribed using random hexamers and the RevertAid H minus first strand cDNA synthesis kit (Thermo Fisher Scientific). qRT-PCR was performed using SybrGreen (Thermo Fisher Scientific) with primers specific for CD4: forward 5′-GGTTCGGCATGACACTCT-3′, reverse 5′-CTGACTCTCCCTCACTCTTATAG-3′; CD8: forward 5′-ATCACTCTCATCTGCTACC-3′, reverse 5′-GCCTTCCTGTCTGACTAG-3′; FoxP3: forward 5′-AGGACAGACCACACTTCAT-3′, reverse 5′-GACGCACTTGGAGCACAG-3′; CTLA4: forward 5′-TATGTCATTGATCCAGAAC-3′, reverse 5′-CTGTTGTAAGAGGACTTC-3′; GAPDH: forward 5′-CATGGCCTTCCGTGTTCCTA-3′, reverse 5′-GCGGCACGTCAGATCCA-3′. Expression levels were normalized for the level of expression of GAPDH (ΔCt) and then to the average level measured in control group (ΔΔCt). By using this method, a ΔΔCt of 1 corresponds to one cycle difference in the expression levels measured by RT-PCR, and this corresponds to a two-fold difference in the RNA expression.
Statistical Analyses
Multiple comparisons were assessed with two-way ANOVA and Dunnett’s multiple comparisons test or with multiple t tests using Prism software (GraphPad). A p value <0.05 was considered to be statistically significant.
Author Contributions
S.H., G.R., B.A., C.L., and S.L. performed research and analyzed data. F.M. analyzed data, contributed new reagents, and wrote the paper. D.K. analyzed data and wrote the paper.
Conflicts of Interest
D.K. has developed the technology that is being used in this study. If the technology is commercially successful in the future, the developers and Duke University may benefit financially. D.K. has received research and grant support from Sanofi Genzyme Corporation in the past, and rhGAA for these studies was supplied by Sanofi Genzyme.
Acknowledgments
This study was supported by NIH grant R01AR065873 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and was supported by Genethon.
Footnotes
Supplemental Information includes six figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2016.12.010.
Supplemental Information
References
- 1.Hirschhorn R., Reuser A.J.J. Chapter 135. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Basis for Inherited Disease. Eighth Edition. McGraw-Hill; 2001. pp. 3389–3419. [Google Scholar]
- 2.Banugaria S.G., Prater S.N., Ng Y.K., Kobori J.A., Finkel R.S., Ladda R.L., Chen Y.T., Rosenberg A.S., Kishnani P.S. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet. Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messinger Y.H., Mendelsohn N.J., Rhead W., Dimmock D., Hershkovitz E., Champion M., Jones S.A., Olson R., White A., Wells C. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet. Med. 2012;14:135–142. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banugaria S.G., Prater S.N., Patel T.T., Dearmey S.M., Milleson C., Sheets K.B., Bali D.S., Rehder C.W., Raiman J.A., Wang R.A. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: a step towards improving the efficacy of ERT. PLoS ONE. 2013;8:e67052. doi: 10.1371/journal.pone.0067052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J., Herson S., Kishnani P.S., Laforet P., Lake S.L. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N. Engl. J. Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 6.Patel T.T., Banugaria S.G., Case L.E., Wenninger S., Schoser B., Kishnani P.S. The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol. Genet. Metab. 2012;106:301–309. doi: 10.1016/j.ymgme.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Franco L.M., Sun B., Yang X., Bird A., Zhang H., Schneider A., Brown T., Young S.P., Clay T.M., Amalfitano A. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Sun B., Bird A., Young S.P., Kishnani P.S., Chen Y.T., Koeberl D.D. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am. J. Hum. Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun B., Kulis M.D., Young S.P., Hobeika A.C., Li S., Bird A., Zhang H., Li Y., Clay T.M., Burks W. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol. Ther. 2010;18:353–360. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler R.J., Bercury S.D., Fidler J., Zhao M.A., Foley J., Taksir T.V., Ryan S., Hodges B.L., Scheule R.K., Shihabuddin L.S., Cheng S.H. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum. Gene Ther. 2008;19:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- 11.Doerfler P.A., Todd A.G., Clément N., Falk D.J., Nayak S., Herzog R.W., Byrne B.J. Copackaged AAV9 vectors promote simultaneous immune tolerance and phenotypic correction of Pompe disease. Hum. Gene Ther. 2016;27:43–59. doi: 10.1089/hum.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P., Sun B., Osada T., Rodriguiz R., Yang X.Y., Luo X., Kemper A.R., Clay T.M., Koeberl D.D. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum. Gene Ther. 2012;23:460–472. doi: 10.1089/hum.2011.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raben N., Danon M., Gilbert A.L., Dwivedi S., Collins B., Thurberg B.L., Mattaliano R.J., Nagaraju K., Plotz P.H. Enzyme replacement therapy in the mouse model of Pompe disease. Mol. Genet. Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Raben N., Fukuda T., Gilbert A.L., de Jong D., Thurberg B.L., Mattaliano R.J., Meikle P., Hopwood J.J., Nagashima K., Nagaraju K., Plotz P.H. Replacing acid alpha-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol. Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Case L.E., Bjartmar C., Morgan C., Casey R., Charrow J., Clancy J.P., Dasouki M., DeArmey S., Nedd K., Nevins M. Safety and efficacy of alternative alglucosidase alfa regimens in Pompe disease. Neuromuscul. Disord. 2015;25:321–332. doi: 10.1016/j.nmd.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang G., Young S.P., Bali D., Hutt J., Li S., Benson J., Koeberl D.D. Assessment of toxicity and biodistribution of recombinant AAV8 vector-mediated immunomodulatory gene therapy in mice with Pompe disease. Mol. Ther. Methods Clin. Dev. 2014;1:14018–14027. doi: 10.1038/mtm.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mingozzi F., Liu Y.L., Dobrzynski E., Kaufhold A., Liu J.H., Wang Y., Arruda V.R., High K.A., Herzog R.W. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk D.J., Soustek M.S., Todd A.G., Mah C.S., Cloutier D.A., Kelley J.S., Clement N., Fuller D.D., Byrne B.J. Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice. Mol. Ther. Methods Clin. Dev. 2015;2:15007. doi: 10.1038/mtm.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S., Sun B., Nilsson M.I., Bird A., Tarnopolsky M.A., Thurberg B.L., Bali D., Koeberl D.D. Adjunctive β2-agonists reverse neuromuscular involvement in murine Pompe disease. FASEB J. 2013;27:34–44. doi: 10.1096/fj.12-207472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn J.D., Ozelo M.C., Sabatino D.E., Franck H.W., Merricks E.P., Crudele J.M., Zhou S., Kazazian H.H., Lillicrap D., Nichols T.C., Arruda V.R. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markusic D.M., Hoffman B.E., Perrin G.Q., Nayak S., Wang X., LoDuca P.A., High K.A., Herzog R.W. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol. Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annoni A., Cantore A., Della Valle P., Goudy K., Akbarpour M., Russo F., Bartolaccini S., D’Angelo A., Roncarolo M.G., Naldini L. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol. Med. 2013;5:1684–1697. doi: 10.1002/emmm.201302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crudele J.M., Finn J.D., Siner J.I., Martin N.B., Niemeyer G.P., Zhou S., Mingozzi F., Lothrop C.D., Jr., Arruda V.R. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125:1553–1561. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun B., Zhang H., Franco L.M., Young S.P., Schneider A., Bird A., Amalfitano A., Chen Y.T., Koeberl D.D. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol. Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Davidoff A.M., Ng C.Y., Zhou J., Spence Y., Nathwani A.C. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- 26.De B.P., Heguy A., Hackett N.R., Ferris B., Leopold P.L., Lee J., Pierre L., Gao G., Wilson J.M., Crystal R.G. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol. Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa K., Hirai Y., Ishizaki M., Takahashi H., Hanawa H., Fukunaga Y., Shimada T. Long-term inhibition of glycosphingolipid accumulation in Fabry model mice by a single systemic injection of AAV1 vector in the neonatal period. Mol. Genet. Metab. 2009;96:91–96. doi: 10.1016/j.ymgme.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Pañeda A., Lopez-Franco E., Kaeppel C., Unzu C., Gil-Royo A.G., D’Avola D., Beattie S.G., Olagüe C., Ferrero R., Sampedro A. Safety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman primates: a potential therapy for acute intermittent porphyria. Hum. Gene Ther. 2013;24:1007–1017. doi: 10.1089/hum.2013.166. [DOI] [PubMed] [Google Scholar]
- 29.D’Avola D., López-Franco E., Sangro B., Pañeda A., Grossios N., Gil-Farina I., Benito A., Twisk J., Paz M., Ruiz J. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 2016;65:776–783. doi: 10.1016/j.jhep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vercauteren K., Hoffman B.E., Zolotukhin I., Keeler G.D., Xiao J.W., Basner-Tschakarjan E., High K.A., Ertl H.C., Rice C.M., Srivastava A. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol. Ther. 2016;24:1042–1049. doi: 10.1038/mt.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisowski L., Dane A.P., Chu K., Zhang Y., Cunningham S.C., Wilson E.M., Nygaard S., Grompe M., Alexander I.E., Kay M.A. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Bell P., Somanathan S., Wang Q., He Z., Yu H., McMenamin D., Goode T., Calcedo R., Wilson J.M. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol. Ther. 2015;23:1877–1887. doi: 10.1038/mt.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S., Ling C., Zhong L., Li M., Su Q., He R., Tang Q., Greiner D.L., Shultz L.D., Brehm M.A. Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol. Ther. 2015;23:1867–1876. doi: 10.1038/mt.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponder K.P. Immune response hinders therapy for lysosomal storage diseases. J. Clin. Invest. 2008;118:2686–2689. doi: 10.1172/JCI36521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppola A., Di Capua M., Di Minno M.N., Di Palo M., Marrone E., Ieranò P., Arturo C., Tufano A., Cerbone A.M. Treatment of hemophilia: a review of current advances and ongoing issues. J. Blood Med. 2010;1:183–195. doi: 10.2147/JBM.S6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva E.M., Strufaldi M.W., Andriolo R.B., Silva L.A. Enzyme replacement therapy with idursulfase for mucopolysaccharidosis type II (Hunter syndrome) Cochrane Database Syst. Rev. 2011;11:CD008185. doi: 10.1002/14651858.CD008185.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox W.R., Linthorst G.E., Germain D.P., Feldt-Rasmussen U., Waldek S., Richards S.M., Beitner-Johnson D., Cizmarik M., Cole J.A., Kingma W., Warnock D.G. Anti-α-galactosidase A antibody response to agalsidase beta treatment: data from the Fabry Registry. Mol. Genet. Metab. 2012;105:443–449. doi: 10.1016/j.ymgme.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Jameson E., Jones S., Wraith J.E. Enzyme replacement therapy with laronidase (Aldurazyme(®)) for treating mucopolysaccharidosis type I. Cochrane Database Syst. Rev. 2013;11:CD009354. doi: 10.1002/14651858.CD009354.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.