Abstract
Late infantile neuronal ceroid lipofuscinosis (LINCL) is a fatal inherited neurodegenerative disease caused by loss of lysosomal protease tripeptidyl peptidase 1 (TPP1). We have investigated the effects of chronic intrathecal (IT) administration using enzyme replacement therapy (ERT) to the brain of an LINCL mouse model, in which locomotor function declines dramatically prior to early death. Median lifespan was significantly extended from 126 days to >259 days when chronic IT treatment was initiated before the onset of disease. While treated animals lived longer and showed little sign of locomotor dysfunction as measured by stride length, some or all (depending on regimen) still died prematurely. One explanation is that cerebrospinal fluid (CSF)-mediated delivery may not deliver TPP1 to all brain regions. Morphological studies support this, showing delivery of TPP1 to ventral, but not deeper and dorsal regions. When IT treatment is initiated in severely affected LINCL mice, lifespan was extended modestly in most but dramatically extended in approximately one-third of the cohort. Treatment improved locomotor function in these severely compromised animals after it had declined to the point at which animals normally die. This indicates that some pathology in LINCL is reversible and does not simply reflect neuronal death.
Keywords: neuronal ceroid lipofuscinosis, tripeptidyl peptidase 1, enzyme replacement therapy, chronic, intrathecal
Introduction
Late infantile neuronal ceroid lipofuscinosis (LINCL) is an inherited neurodegenerative disease caused by mutations in the gene encoding the lysosomal protease tripeptidyl peptidase 1 (TPP1).1, 2 Typically, LINCL affects children and results in death in the first or second decade of life. Currently, there is no approved treatment for LINCL.
Enzyme replacement therapy (ERT), where a recombinant form of the protein that is missing or defective is administered to patients, is currently the most effective and widely used therapy for non-neurological forms of lysosomal diseases (reviewed in Hollak and Wijburg3). Restoration of TPP1 to the brain of LINCL patients and individuals with related lysosomal diseases is needed to cure the disease. However, the blood-brain barrier (BBB) represents a major obstacle to enzyme delivery. Several clinical trials have taken different approaches to this problem in LINCL, including the use of stem cells (https://ClinicalTrials.gov: NCT00337636), gene therapy to the parenchyma of the brain (https://ClinicalTrials.gov: NCT00151216), and cerebrospinal fluid (CSF)-mediated delivery of enzyme via intracerebroventricular (ICV) injection (https://ClinicalTrials.gov: NCT01907087). Preliminary data appear promising in the latter.4, 5
A TPP1-deficient mouse model of LINCL6 has been used extensively to explore different strategies for treatment, including gene therapy and ERT.7, 8, 9, 10, 11, 12, 13, 14 The LINCL mouse recapitulates many aspects of the human disease, including progressive locomotor decline (compare Movies S1, S2, and S3 [untreated LINCL mice] with Movies S4 and S5 [unaffected wild-type controls]), and it has a greatly shortened lifespan (∼4–5 months), which allows ready evaluation of the effects of treatment on survival.6 We have explored several different ERT approaches to delivery of recombinant TPP1 to the brain of the LINCL mouse, potentially to provide rationale for clinical studies. We conducted CSF-mediated administration of TPP1 to the brain by lumbar intrathecal (IT) injection of recombinant protein.12 This allowed delivery of supraphysiological levels of TPP1 to the brain, and an acute treatment significantly extended the lifespan of the LINCL mice. This treatment also ameliorated cellular pathology and slowed progression of ataxia. In addition, while there is negligible delivery of TPP1 alone to the brain when it is administered intravenously, when co-administered with K16ApoE, a 36-residue peptide containing polylysine and apolipoprotein E sequences, there is robust delivery of TPP1 throughout the brain.14 As with acute IT administration, intravenous (i.v.) administration of K16ApoE and TPP1 stems neurological symptoms, and acute treatment at 4 weeks of age extends the lifespan of the LINCL mice.
In this study, we have investigated chronic IT ERT in the LINCL mouse, focusing on the effect of timing of treatment initiation on survival and locomotor function. The conclusions drawn from this study may have important translational implications for clinical studies of ERT in LINCL and related disorders.
Results
Chronic IT ERT in Presymptomatic LINCL Mice
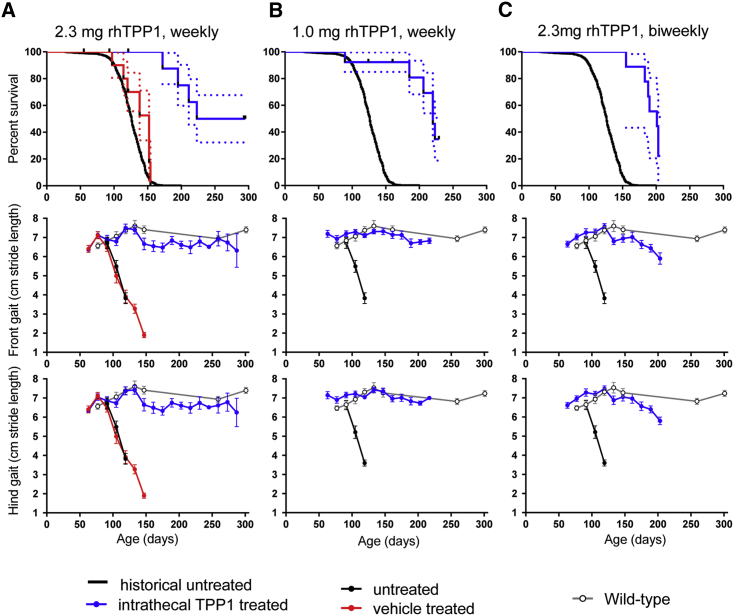
Acute administration of recombinant human TPP1 (rhTPP1) by IT injection to LINCL mice at 4 weeks of age significantly extended the lifespan of the animals from 16 weeks (vehicle control) to 23 weeks.12 In this study, one goal was to determine whether chronic administration of rhTPP1 by IT injection could further extend survival. Our experimental plan was to establish cohorts of 13 animals, most of which were allowed to die naturally but sacrificing two at an early 119-day time point (17 weeks) and either the last two to three survivors or all at a predetermined study endpoint of 294 days (42 weeks) for biochemical and histopathological analyses. LINCL mice were dosed with rhTPP1 starting at 6 weeks of age using different treatment regimens and monitored for survival and locomotor function. Median survival of vehicle-treated animals (152 days) was not significantly different from untreated LINCL mice (126 days, Figure 1A). While premature death was observed in some of the treated animals, a weekly administration of 2.3 mg rhTPP1 (Figure 1A) significantly (log-rank test p < 0.0001 and p < 0.0001 compared to vehicle-treated or untreated, respectively) extended the survival of half of the cohort to more than 300 days (median survival > 259 days), at which point the study was terminated. It is important to consider the survival data obtained by IT ERT in context with previous studies and these are summarized in Table 1. There is no significant difference between IT ERT survival data and those obtained from the most effective gene therapy studies to date in the LINCL mouse model (log-rank test p = 0.1001 when compared with treatment at 4 weeks8 and p = 0.0758 compared with neonatal treatment15).
Figure 1.
Chronic IT Enzyme Replacement Therapy of Asymptomatic Animals
Treatment was initiated at 42 days of age. Dashed lines in survival curves are SE; gait error bars are SEM. Treatment groups were compared with untreated animals using log-rank (Mantel-Cox) tests. (A) Survival of animals treated weekly with 2.3 mg rhTPP1 (n = 13 animals) was significantly increased (p < 0.0001) from vehicle (aCSF) alone (n = 10) or untreated animals. (B) Survival of animals treated with a weekly administration of 1 mg rhTPP1 (n = 12) was significantly (p < 0.0001) increased. (C) Survival of animals treated with a biweekly administration of 2.3 mg rhTPP1 (n = 12) was significantly (p < 0.0001) increased.
Table 1.
Summary of Survival Data from Gene Therapy and Enzyme Replacement Studies Conducted in LINCL Mice
| Study | Treatment Method | Strain | Treatment Age (days) | Median Survival (days) | Maximum Survival (days) |
|---|---|---|---|---|---|
| Sleat et al.6 | none | mixed C57BL/6:129Sv | 138 | ∼170 | |
| none | 129Sv | 164 | ∼185 | ||
| Cabrera-Salazar et al.8 | none | NR | 151 | ∼175 | |
| gene therapy | NR | 28 | >330 | >330 | |
| gene therapy | NR | 77 | 216 | ∼230 | |
| Sondhi et al.15 | none | C57BL/6 | 121 | 133 | |
| gene therapy | C57BL/6 | 2 | 376 | 400 | |
| gene therapy | C57BL/6 | 21 | 277 | 370 | |
| gene therapy | C57BL/6 | 49 | 168 | 350 | |
| Xu et al.12 | none | C57BL/6 | 125 | 170 | |
| 1.2 mg rhTPP1, intrathecal, acute, replicate 1 | C57BL/6 | 28 | 163 | 190 | |
| 1.2 mg rhTPP1, intrathecal, acute, replicate 2 | C57BL/6 | 28 | 164 | 180 | |
| 1.2 mg rhTPP1, intrathecal, acute, replicate 3 | C57BL/6 | 28 | 155 | 168 | |
| 0.038 mg rhTPP1, intrathecal, acute | C57BL/6 | 28 | 135 | 160 | |
| 0.12 mg rhTPP1, intrathecal, acute | C57BL/6 | 28 | 130 | 145 | |
| 0.38 mg rhTPP1, intrathecal, acute | C57BL/6 | 28 | 145 | 145 | |
| Meng et al.14 | acute i.v. administration of rhTPP1 with peptide mediator | C57BL/6 | 42 | 156 | 190 |
| Current study | none | C57BL/6 | 126 | 175 | |
| 2.3 mg rhTPP1, intrathecal, chronic, weekly | C57BL/6 | initiated at 42 | >259 | >300 | |
| 1.0 mg rhTPP1, intrathecal, chronic, weekly | C57BL/6 | initiated at 42 | 220 | 223 | |
| 2.3 mg rhTPP1, intrathecal, chronic, biweekly | C57BL/6 | initiated at 42 | 201 | 203 | |
| 2.3 mg rhTPP1, intrathecal, chronic, weekly | C57BL/6 | initiated at 105 | 167 | >300 |
NR, not reported.
Other doses/dosing regimens extended lifespan of the LINCL animals, but they did not extend survival more than ∼225 days. When LINCL mice were treated with 1 mg rhTPP1 at a weekly dosing interval from 6 weeks of age, survival was significantly (log-rank p < 0.0001 compared to untreated) extended to a median 220 days (Figure 1B). Similarly, a biweekly dose of 2.3 mg IT-administered rhTPP1 significantly (log-rank p < 0.0001 compared to untreated) increased survival of the LINCL mouse (Figure 1C, median survival 201 days).
Locomotor deficits are a prominent manifestation of LINCL in patients and are present in the LINCL mice6 (compare Movies S1, S2, and S3 [untreated LINCL mice] with Movies S4 and S5 [unaffected wild-type controls]). Gait analysis16 is a sensitive measure of locomotor dysfunction in the LINCL mouse14 that can detect deficits earlier than Rotarod;6 therefore, we used this approach to evaluate efficacy of chronic IT treatment. In untreated or vehicle-treated animals, stride length started to decline at ∼90 days and decreased precipitously from ∼7 to ∼2 cm until death between 100 and 150 days (Figure 1A). When mice were treated with IT rhTPP1 in the three survival studies, there was no significant decline in motor function, and, prior to their natural death, animals appeared relatively healthy compared to untreated end-stage LINCL mice (Figures 1A–1C; compare Movies S6 and S7 [229-day LINCL mice treated with 1 mg TPP1 weekly] and Movies S8 and S9 [206-day LINCL mice treated with 2.3 mg TPP1 biweekly] with Movie S3 [untreated LINCL mouse at 146 days]). This indicates that IT TPP1 treatment can have differential effects on disease, as some animals that appeared outwardly healthy still died prematurely. At the conclusion of the study (42 weeks), surviving animals treated with 2.3 mg weekly appeared to be healthy. There was no visible tremor; animals were hydrated, feeding, and nesting normally; and they lacked the hunched appearance characteristic of symptomatic LINCL mice. Consistent with these observations, histopathological analysis of brain, kidney, liver, and adrenals from 42-week-old treated animals revealed no notable changes, with the exception of occasional intracellular eosinophilic inclusions in neurons in the brainstem and/or deep cortical regions (data not shown).
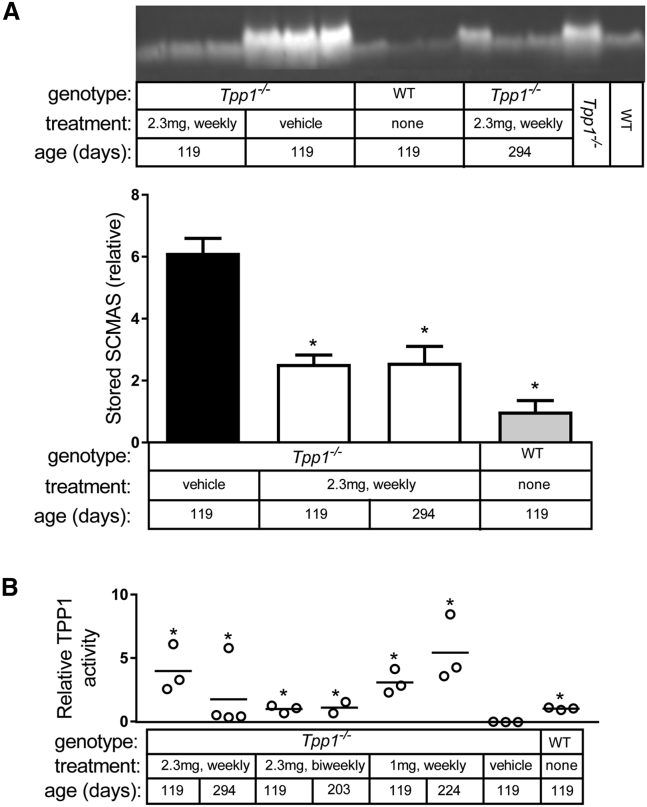
Levels of the major storage material in LINCL, subunit c of mitochondrial ATP synthase (SCMAS), were lower in chronic IT-treated animals compared to vehicle-treated controls (Figure 2A). At 17 weeks, there was a significant accumulation of SCMAS in vehicle-treated LINCL mice (6.1-fold higher than wild-type animals). Levels of SCMAS in treated animals were higher than in wild-type mice but significantly lower than in vehicle-treated animals. Animals were killed 24 hr after the last IT administration, which allowed us to evaluate the efficacy of rhTPP1 delivery to the brain by enzyme assay. While there was animal-to-animal variability as seen previously with IT administration,12 TPP1 activities in the groups of treated animals were higher than in vehicle-treated LINCL mice (Figure 2B).
Figure 2.
Storage Material and TPP1 Activity
Significance of changes in SCMAS and TPP1 activity relative to vehicle-treated LINCL mice was calculated by fitting data to a linear model, as described in the Materials and Methods. (A) Detection of SCMAS in isolated storage material by immunoblotting in animals treated with a weekly administration of 2.3 mg rhTPP1 and controls. Samples from 16-week-old wild-type and LINCL animals are shown as reference standards. Analysis of older wild-type animals indicated that there were no detectable age-related changes in SCMAS levels (data not shown). Relative SCMAS levels obtained from multiple loadings (4 and 8 μg protein equivalents of original homogenate) were normalized to average signal obtained from 17-week-old wild-type animals; error bars show SEM (n = 3 per treatment group). Significance of change compared to vehicle-treated LINCL mice, *p < 0.05/3 (i.e., 0.05 after Bonferroni correction for three individual comparisons). (B) TPP1 activities measured in brain, 24 hours after administration of the final dose. Data are expressed relative to average TPP1 activity in 17-week-old wild-type animals. Significance of change compared to vehicle treated animals, *p < 0.05/7 (i.e., 0.05 after Bonferroni correction for seven individual comparisons).
Chronic IT ERT at Late-Stage Disease
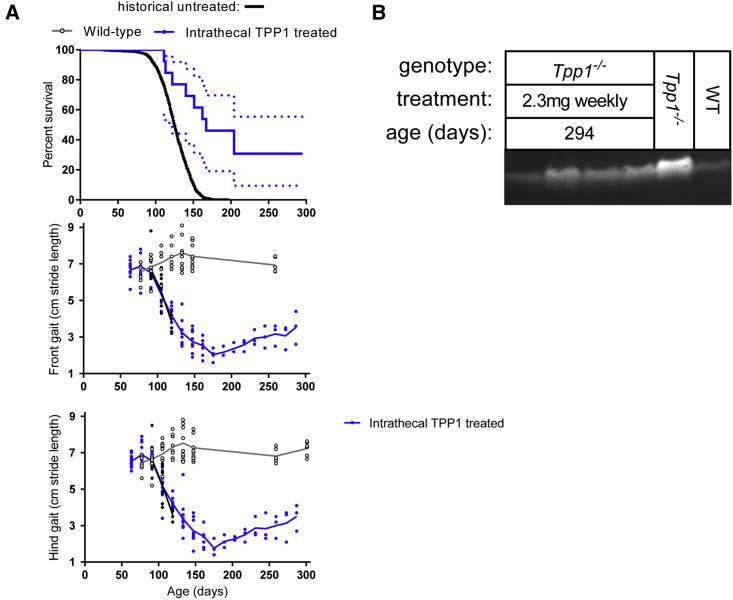
Earlier gene therapy studies in LINCL mice demonstrated that efficacy increases the earlier treatment is initiated.8, 15 However, given that stage of disease may not be the only variable (e.g., viral distribution and, thus, TPP1 expression may be more widespread when treating the smaller brains of younger mice), we have investigated chronic IT ERT in symptomatic animals. A previous gene therapy study in LINCL mice focused on treatment at the earliest stages of detectable disease, i.e., 77 days (11 weeks),8 but we have instead focused on late-stage disease. At 105 days of age (15 weeks), the cohort of LINCL mice was beginning to die, and there was constant tremor, while locomotor defects were evident from gait analysis (Figure 1A, stride length at 105 days was diminished from ∼7 to ∼5 cm).
The 15-week LINCL mice were treated with a weekly IT administration of 2.3 mg rhTPP1 (Figure 3). For most of the animals, IT ERT resulted in a modest increase in lifespan, with median survival increased from 126 to 167 days. However, a subgroup of approximately one-third of the treated late-stage animals was still alive at 42 weeks when the study was terminated.
Figure 3.
IT ERT in Late-Stage Disease
Weekly administration of 2.3 mg rhTPP1 was initiated at 105 days of age (n = 12 animals). (A) Survival and gait analysis data. Dashed lines in survival plot indicate SE. Survival of treated animals was significantly increased compared to untreated animals (p < 0.0001, log-rank [Mantel-Cox] test). Stride length is plotted for each individual animal to demonstrate variability. Linear regression of stride length data from 175 to 287 days indicates that the increase in stride length observed after 175 days is highly significant (hind gait, slope = 0.015 ± 0.002, p < 0.0001; front gait, slope = 0.013 ± 0.002, p < 0.0001). (B) SCMAS in LINCL mice at 42 weeks of age. The ∼16-week-old wild-type and LINCL animals are shown as reference standards.
Stride length of the treated animals continued to decline rapidly after initiating chronic IT ERT (Figure 3A), similarly to untreated animals (Figure 1A). This included the subgroup of animals that responded well to treatment in terms of survival. After continuing to decline, locomotor function in long-term survivors then started to improve significantly (see Figure 3A legend) after 10 weeks of treatment, and it was continuing to improve at the conclusion of the study. At 42 weeks, levels of SCMAS were somewhat elevated compared to control but were lower than younger (17-week) untreated LINCL mice (Figure 3B).
Distribution of rhTPP1 after IT Administration
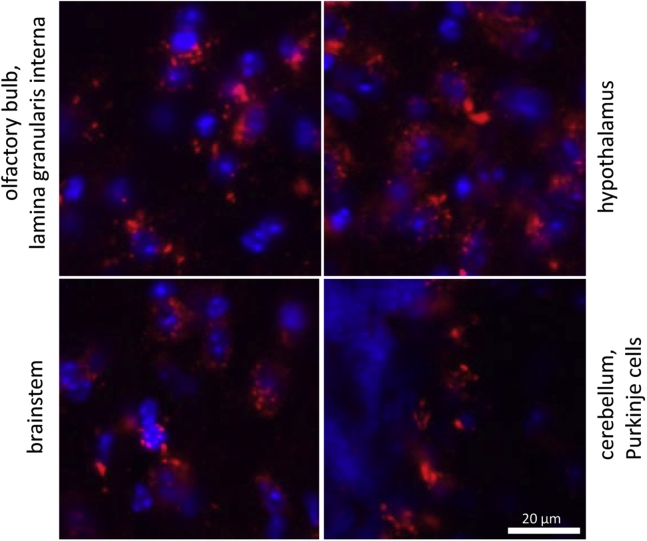
IT treatment successfully corrected locomotor deficits in LINCL mice, but outwardly healthy animals still died prematurely (Figure 1). One possible explanation is that IT administration may not effectively deliver rhTPP1 to all regions of the mouse brain. As an example, the cerebellum, which is associated with locomotor function, might be effectively treated because it is readily accessible to the CSF while deeper regions of the brain may not, and subsequent pathology in such untreated regions could result in death. This hypothesis was tested by evaluating the brain distribution of a fluorescent TPP1 derivative delivered via IT administration. In cells where TPP1 was delivered successfully (Figure 4), its distribution was punctate and cytoplasmic, which is consistent with delivery to the lysosome.
Figure 4.
Intracellular Distribution of Fluorescent TPP1
Wild-type mice were treated with 1 mg Alexa-647-labeled rhTPP1 via IT administration. Then 18 hr later, animals were killed, and brains were analyzed by confocal microscopy to measure the distribution of fluorescently labeled TPP1. Images were captured using a 40× objective. Blue, DAPI; red, Alexa-647 rhTPP1. Note that images were chosen for each brain region to illustrate intracellular distribution and are not meant to be representative of each respective brain region as a whole.
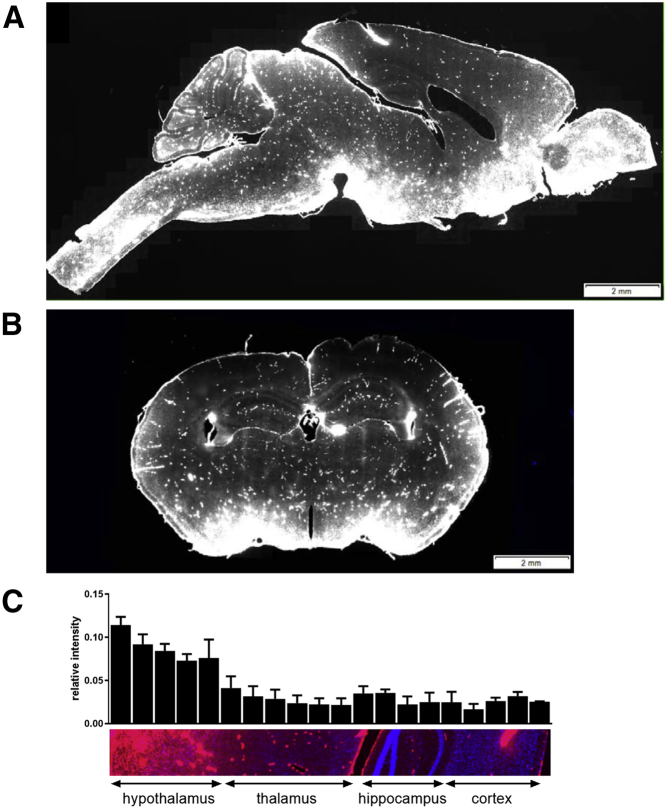
Note that in our previous studies of acute IT administration, we used double-label immunofluoresence microscopy to demonstrate that TPP1 delivered to the brain colocalized with lysosomes containing storage material.12 Use of chemically labeled fluorescent TPP1 allowed us to clearly visualize the fate of administered TPP1 with minimal non-specific interferences. When the whole brain was imaged in sagittal (Figure 5A) or coronal section (Figure 5B), distribution was variable at the anatomic level. We detected strong staining for TPP1 on the surface of the brain in regions proximal to the CSF, including the olfactory bulb and the brainstem, in the ventral regions of anterior olfactory nucleus, ventral striatum, basal forebrain, and hypothalamus. However, there was a gradient of intensity that diminished toward the dorsal surface (Figure 5C). There was strong staining in what appeared to be blood vessel endothelial cells throughout the brain, which is consistent with endocytosis of some of the administered IT dose that escapes the brain and reaches the plasma.12 Alternatively, this staining may reflect TPP1 retained within the CSF-accessible perivascular space surrounding blood vessels.
Figure 5.
Distribution of Fluorescent TPP1
(A–C) Wild-type mice (n = 3) were treated with Alexa-647-labeled rhTPP1 via IT administration. Then 18 hr later, animals were killed, and brains were analyzed by confocal microscopy to measure the distribution of fluorescently labeled rhTPP1. (A) Sagittal section, (B) coronal section, and (C) quantitation of relative fluorescent rhTPP1 signal through a dorsal-ventral section are shown (n = 3 animals, error bars depict SEM). Images were captured using a 20× objective. Blue, DAPI; red, Alexa-647 rhTPP1.
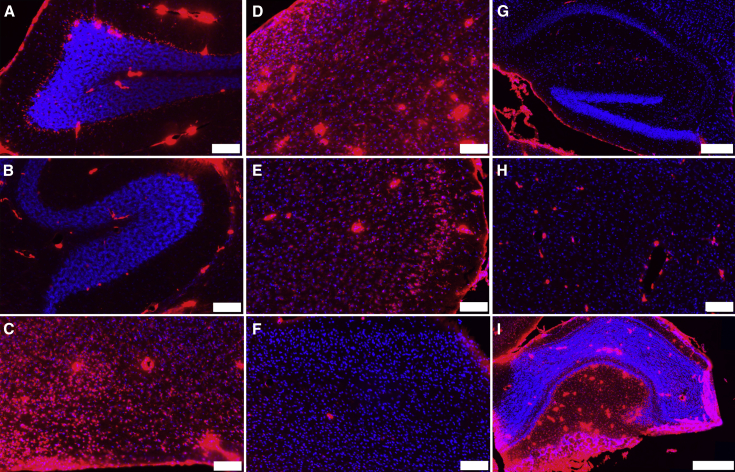
Regional dependence in delivery is illustrated by close examination of individual structures of the brain (Figure 6). In the cerebellum, there was significant rhTPP1 staining at the outer surface with IT delivery and rhTPP1 delivery to the Purkinje cells of lobes at regions proximal to the surface, e.g., lobe VIII (Figure 6A). However, Purkinje cells in deeper regions of the cerebellum, e.g., in lobe I, appeared to have undetectable levels of rhTPP1 (Figure 6B). Delivery to the ventral hypothalamus (Figure 6C), brainstem (Figure 6D), anterior cerebral cortex (Figure 6E), and olfactory bulb (Figure 6I) was very efficient. However, there was considerably less delivery to the posterior cerebral cortex (Figure 6F) and deeper regions of the brain, including the hippocampus (Figure 6G) and thalamus (Figure 6H). Thus, while chronic treatment might potentially deliver more TPP1 to some of the regions that received little or none in this acute study, TPP1 distribution, when delivered via IT administration, is not uniform.
Figure 6.
Distribution of Fluorescent rhTPP1 in Defined Brain Regions in Sagittal Section
See Figure 5 for details. Color ranges in all panels are identical. (A) Cerebellum, lobe VIII; (B) cerebellum, lobe I; (C) hypothalamus; (D) brainstem; (E) anterior cerebral cortex; (F) posterior cerebral cortex; (G) hippocampus; (H) thalamus; and (I) olfactory bulb are shown. Scale bars represent 100 μm except in (G) (200 μm) and (I) (500 μm). Blue, DAPI; red, Alexa-647-labeled rhTPP1.
Discussion
ERT is conceptually a straightforward approach to neurodegenerative lysosomal storage diseases (LSDs), but delivery of proteins to the brain remains problematic. Thus, in proof-of-principle studies of chronic ERT in mouse models, success has been modest in terms of extending survival and improving phenotypes of LSDs. For example, in a recent study,17 GM2 gangliosidosis mice were treated with chronic ICV administration of a modified β-hexosaminidase A, and this increased median survival by 22% and slowed, but did not prevent, locomotor decline.
In this study, we conducted chronic IT administration, terminating each experiment either at a predetermined endpoint of animal age 42 weeks or when there were only two to three surviving animals in each cohort. While technically challenging, we could conduct up to ∼35 IT injections repeated weekly, but continued administration using this route became difficult due to scarring. We chose IT rather than ICV delivery as previous ERT experiments in LINCL mice10 demonstrated that implantation of catheters resulted in glial scarring and premature death at 100–110 days. Despite these challenges, we have shown that chronic IT ERT to the brain can be an effective treatment in the LINCL mouse model, extending a median lifespan of 125 days for untreated animals to more than 259 days, with half of the animals surviving at 42 weeks when the study was concluded (Figure 1A). This represents an increase in lifespan of at least 100%. IT ERT did not prevent premature death in all LINCL mice when initiated at a presymptomatic age. While animals are predosed with cyproheptadine to prevent acute anaphylactic response to rhTPP1 administration, it is possible that there may be differences in immune response in the treated symptomatic animals, with those that die earlier mounting stronger and possibly neutralizing responses. Regardless, chronic IT ERT is similar in efficacy to the most successful gene therapy approaches reported for the LINCL mouse (Table 1).8, 15 To our knowledge, this is the most successful demonstration of chronic ERT to the brain of a lysosomal disease mouse model to date.
There are currently no data to indicate whether treatment of late-stage LINCL may or may not provide benefits. In previous studies of ERT in LINCL animal models, treatment was initiated before the onset of disease.12, 13, 18 In gene therapy studies where LINCL mice were treated at different ages, including at the earliest stage of detectable disease,8, 15 better results were obtained with earlier treatment. A number of studies evaluated the effects of ERT and gene therapy to symptomatic animal models of other lysosomal diseases.19, 20, 21, 22, 23 Again, the overall consensus from these studies is that treatment of symptomatic LSD animal models is less effective than that of presymptomatic animals. Treatment of symptomatic animals may correct some biochemical and histopathological features, but, in terms of survival, it is either ineffective or it extends lifespan to a lesser degree than when administered at a presymptomatic age. In terms of locomotor function, late-stage treatment either has no effect or it slows, but does not stop, decline. Thus, a major goal of this study was to determine whether chronic treatment might benefit symptomatic, late-stage LINCL mice.
We found that in about two-thirds of late-stage mice (15 weeks old), IT ERT extended survival only modestly. However, an unexpected finding was that, in the remaining third, lifespan was extended to more than 42 weeks, at which point the study was concluded (Figure 3). This extension in survival was equivalent to that observed in mice treated before disease onset. It is not clear why only a subset of the symptomatic animals responded well to treatment, but, as discussed earlier, this could reflect differences in immune response. However, there is a window in which the LINCL mice die (100–150 days). It is possible that the ones that responded well to treatment are the animals that would have naturally died toward the end of this window. If so, this may indicate that there is a committed point in the progression of the disease at which treatment, no matter how effective in terms of TPP1 amounts and distribution, will have a modest or no effect on survival.
In late-stage animals that responded well to treatment, locomotor function measured by gait analysis continued to decrease after treatment was initiated, to or below the level of untreated LINCL mice shortly before death. However, as they survived beyond this point, locomotor function then began to show a steady and significant improvement. While previous studies have shown that treatment can prevent or slow decline in locomotor function when administered to presymptomatic or symptomatic lysosomal disease mouse models,8, 15, 19, 20, 21, 22, 23 our data indicate that successful treatment has the potential to actually rescue locomotor function in animals that are already compromised. This establishes an important precedent that at least one phenotype of a neurological lysosomal disease can potentially be reversed with effective treatment. The underlying basis for phenotypic rescue remains unclear. It is possible that effective delivery of rhTPP1 corrects neurons that are alive but compromised and not functioning correctly. Alternatively, this may reflect plasticity of the brain, with compensatory changes resulting in improved locomotor function as further neurodegeneration is halted. Regardless, the fact that some of the highly symptomatic animals survived with treatment and that they showed a partial rescue of a complex phenotype of disease potentially has implications for the treatment of LINCL patients.
IT ERT can effectively ameliorate locomotor deficits and maintain SCMAS at levels observed in wild-type animals without preventing premature death of the LINCL mice. One possibility is that death may result from peripheral defects, but, with extensive delivery of IT-administered rhTPP1 to visceral organs in addition to the brain,12 this appears unlikely. Another possibility is that effective delivery of rhTPP1 to the spinal cord via intrathecal delivery, as has been observed in a previous acute IT ERT study,24 may provide locomotor benefits without preventing death. However, perhaps the likeliest scenario, which is supported by our acute distribution experiments, is that death results from pathology in deeper regions of the brain where rhTPP1 appears to be less efficiently delivered by IT administration. This explanation is consistent with several previous studies. rhTPP1 was administered to an LINCL dog model and wild-type controls,25 and, in the former, extremely high rhTPP1 activity was detected in the hypothalamus and cerebellum (∼12-fold higher than wild-type), while low or undetectable levels of rhTPP1 were delivered to deep regions, including the thalamus (0.4-fold of wild-type). Cynomolgus monkeys were treated with IT administration of rhTPP1,26 and, again, levels in cerebellum were high (∼12-fold higher than wild-type) and low in the thalamus (∼0.5-fold of wild-type). This uneven distribution may reflect the fact that the CSF-brain interface is anatomically limited, requiring significant diffusion of a therapeutic for complete coverage.27 In addition, efficient delivery of TPP1 to the ventral areas by IT administration in the mouse may reflect the flow pattern of CSF throughout the brain, with outflow from the ventricles essentially pushing the IT dose under or around the brain. ICV administration, which is the approach used in the recent clinical trial of rhTPP1 for LINCL (ClinicalTrials.gov: NCT01907087), provides a better distribution of rhTPP1 throughout the monkey brain,26 but rhTPP1 is still delivered with variable efficiency to different regions. It is worth noting that TPP1 deficiency is associated with cerebellar atrophy in both spinocerebellar ataxia 728 and LINCL (reviewed in Anderson et al.29), and loss of cerebellar neurons occurs in the mouse model.6 The fact that rhTPP1 is delivered by IT ERT to the cerebellum, at least to outer layers (Figure 6), may explain why locomotor function is preserved with treatment.
Two major conclusions from this study may have implications for the treatment of LINCL and possibly related LSDs. First, IT ERT (and presumably other methods that restore TPP1 to the brain) at symptomatic late-stage disease has the potential to significantly extend lifespan and at least partially correct existing locomotor deficits. Second, while IT ERT prevents locomotor decline when initiated presymptomatically, it does not necessarily prevent premature death. While ataxia is the only locomotor parameter that we evaluated in the mouse model, this is a precedent that raises specific concerns when individual phenotypes of disease are used as part of a measure of disease progression.30, 31 It is possible that other measurable parameters, e.g., vision and speech, also respond to treatment in ways that are not necessarily indicative of survival. This should be borne in mind when using tests for discrete neurological functions to monitor overall disease progression when evaluating the efficacy of ERT to the brain.
Materials and Methods
Materials
The rhTPP1 proenzyme in artificial CSF (aCSF) at a concentration of 29 mg/mL was generously provided by Biomarin, and it was used directly for IT injection. Note that our earlier prediction12 for a solubility limit for TPP1 of ∼20 mg/mL was an underestimate. Fluorescently labeled rhTPP1 was generated by reaction with Alexa Fluor 647 NHS Ester (Thermo Fisher Scientific), and salts and unreacted dye were removed by buffer exchange using PD10 columns. Labeling efficiency was 0.7 mol dye/mol TPP1 based on absorbance using molar extinction coefficients of ε280 = 82,195 for TPP1 and ε280 = 7,170 and ε467 = 239,000 for Alexa Fluor 647.
Animals
Animals were maintained and used following protocols approved by the Rutgers University and Robert Wood Johnson Medical School Institutional Animal Care and Use Committee (“Preclinical evaluation of therapy in an animal model for LINCL,” protocol I09-0274-4). Male and female LINCL mice were in a C57BL/6 background and genotyped as described previously.6 The rhTPP1 was administered by IT injection essentially as described,12 except animals were pretreated by intraperitoneal administration of 5 mg/kg cyproheptadine from the second or third dose onward to prevent anaphylactic response. In brief, animals were anesthetized with isoflurane using an anesthesia inhalation system (VetEquip), and 80 μL TPP1 solution was administered at a rate of 10 μL/min using a syringe pump (New Era Pump Systems). Mice were housed singly from 6 weeks old (asymptomatic animals) or 9 weeks old (symptomatic animals) and gently handled to avoid fatal startle seizures.6 Locomotor function was measured by biweekly gait analysis.32 For analysis of TPP1 uptake, animals were IT-injected with 1 mg fluorescent TPP1, killed, perfused with saline, and brains were dissected sagitally or coronally into two segments. One half was frozen for enzyme activity and other biochemical analyses, and the other, along with liver, kidney, and adrenals, was fixed for histopathology as described.14
Microscopy
For the determination of fluorescent TPP1 distribution, animals were perfused with saline and then perfusion-fixed with 4% paraformaldehyde in PBS (PFA/PBS). Brains were excised, fixed for 48 hr in 4% PFA/PBS, and then transferred to 30% sucrose/PBS at 4°C until they sunk. After embedding in Richard Allen Scientific NEG-50 (Thermo Fisher Scientific), 20-μm sections were cut using a Leica CM1900 cryostat (Leica Biosystems) and mounted using Fluoro-Gel II with DAPI (Electron Microscopy Sciences). Whole-slide images were acquired using an Olympus VS120 whole-slide scanner equipped with an Olympus XM10 cooled monochrome 14-bit camera.
Experimental Design for Survival and Gait Studies
Survival data from various treatments were compared with historical data from 1,560 untreated C57BL/6 LINCL mice that were acquired over many years of maintaining this model. In the first survival study (2.3 mg TPP1 administered weekly from 6 weeks of age), cohorts were treated either with rhTPP1 or with vehicle alone. Given that survival of vehicle-treated LINCL mice was equivalent to that of historical control untreated animals, this group was not included for subsequent survival studies of LINCL mice treated with other regimens.
Gait analyses were initially conducted on cohorts of wild-type and LINCL mice and subsequently on the cohort administered 2.3 mg TPP1 weekly from 6 weeks of age and accompanying vehicle controls. Given that gait measurements of the vehicle-treated LINCL mice were indistinguishable from the previous cohort of untreated LINCL mice, we excluded this control group from subsequent treatment groups. In plotting survival curves, data from the initial wild-type and untreated LINCL cohorts are plotted with all treatment groups for reference.
SCMAS Immunoblotting
Mouse brain samples were homogenized in 1:50 volumes (w/v) of 0.15 M NaCl/0.1% Triton X-100 using a Polytron tissue homogenizer (Kinematica). One hundred microliters of each homogenate (∼1.5 mg protein/mL) were centrifuged at 14,000 × g for 30 min to separate the insoluble storage material containing SCMAS from normal mitochondrial SCMAS, as described previously.14 Pellets were resuspended in 100 μL homogenization buffer and recentrifuged. The pellet was dissolved in reducing lithium dodecyl sulfate PAGE buffer and analyzed by immunoblotting as described.12
Statistical Analyses
Statistical analyses were conducted using Prism 5.03 (GraphPad) and R-3.3.33 For comparison of SCMAS levels in vehicle-treated LINCL mice with other treatment groups (Figure 2A), measurements were log-transformed according to y = log(x) and a linear model was fitted, with the transformed variables as the response variable and the groups as predictors. The vehicle group was used as the reference, and all other parameter estimates represent differences between the respective groups and the reference group. Standard analysis of the residuals was performed to verify that the statistical assumptions underlying the linear model were met.34 For comparison of TPP1 activities in vehicle-treated LINCL mice with other treatment groups (Figure 2B), the same procedure was used except that measurements were log-transformed according to y = log(x + 0.15).
Author Contributions
P.L., Y.M., J.A.W., and D.E.S. conceived and designed this study. Y.M., J.A.W., and Y.N. conducted chronic enzyme replacement experiments in mice; J.A.W., D.E.S., P.G.M., P.L., and J.H.M. conducted and interpreted morphological studies; D.F.M., D.E.S., and P.L. conducted statistical analyses. The initial draft of the manuscript was written by D.E.S. and P.L., while all authors contributed toward subsequent revisions prior to submission.
Conflicts of Interest
P.L. and D.E.S. have received royalty payments as Inventors on Patent 8029781 “Methods of treating a deficiency of functional tripeptidyl peptidase I (CLN2) protein,” which is licensed to BioMarin Pharmaceutical Inc. Other authors have declared that no conflicts of interest exist.
Acknowledgments
We would like to thank Dr. Wenjin Chen (Rutgers Cancer Institute of New Jersey) for her help with microscopy, Dr. Kenneth Reuhl (Environmental and Occupational Health Sciences Institute, Rutgers University) for histopathological analysis, and Dr. Derek Kennedy and colleagues at Biomarin for providing recombinant rhTPP1. This project was supported by NIH grant NS37918 (P.L.) and P30CA072720 (Rutgers Cancer Institute of New Jersey: D.F.M. and the Imaging Shared Resource). Y.M. was supported by fellowships from the Batten Disease Support and Research Association and Cures Within Reach funded by Noah’s Hope, Hope 4 Bridget, and Jasper Against Batten.
Footnotes
Supplemental Information includes nine movies and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.01.004.
Contributor Information
David E. Sleat, Email: sleat@cabm.rutgers.edu.
Peter Lobel, Email: lobel@cabm.rutgers.edu.
Supplemental Information
Genotype: LINCL; treatment: none; age: 116 days.
Genotype: LINCL; treatment: none; age: 133 days.
Genotype: LINCL; treatment: none; age: 146 days.
Genotype: WT; treatment: none; age: 104 days.
Genotype: WT; treatment: none; age: 225 days.
Genotype: LINCL; treatment: 1 mg rhTPP1 weekly; age: 229 days.
Genotype: LINCL; treatment: 1 mg rhTPP1 weekly; age: 229 days.
Genotype: LINCL; treatment: 2.3 mg rhTPP1 biweekly; age: 206 days.
Genotype: LINCL; treatment: 2.3 mg rhTPP1 biweekly; age: 206 days.
References
- 1.Sleat D.E., Donnelly R.J., Lackland H., Liu C.G., Sohar I., Pullarkat R.K., Lobel P. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science. 1997;277:1802–1805. doi: 10.1126/science.277.5333.1802. [DOI] [PubMed] [Google Scholar]
- 2.Mole S.E., Williams R.E., Goebel H.H. Oxford University Press; Oxford: 2011. The Neuronal Ceroid Lipofuscinoses (Batten Disease) [Google Scholar]
- 3.Hollak C.E., Wijburg F.A. Treatment of lysosomal storage disorders: successes and challenges. J. Inherit. Metab. Dis. 2014;37:587–598. doi: 10.1007/s10545-014-9718-3. [DOI] [PubMed] [Google Scholar]
- 4.Nickel M., Jacoby D., Lezius S., Down M., Simonati A., Genter F., Wittes J., Kohlschütter A., Schulz A. Natural history of CLN2 disease: quantitative assessment of disease characteristics and rate of progression. Neuropediatrics. 2016;47:FV04-03. [Google Scholar]
- 5.Schulz A., Specchio N., Gissen P., de los Reyes E., Williams R., Cahan H., Genter F., Jacoby D. Intracerebroventricular Cerliponase Alfa (BMN 190) in children with CLN2 disease: interim results from a phase 1/2, open-label, dose-escalation study. Neuropediatrics. 2016;47:FV02-06. [Google Scholar]
- 6.Sleat D.E., Wiseman J.A., El-Banna M., Kim K.H., Mao Q., Price S., Macauley S.L., Sidman R.L., Shen M.M., Zhao Q. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J. Neurosci. 2004;24:9117–9126. doi: 10.1523/JNEUROSCI.2729-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passini M.A., Dodge J.C., Bu J., Yang W., Zhao Q., Sondhi D., Hackett N.R., Kaminsky S.M., Mao Q., Shihabuddin L.S. Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J. Neurosci. 2006;26:1334–1342. doi: 10.1523/JNEUROSCI.2676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera-Salazar M.A., Roskelley E.M., Bu J., Hodges B.L., Yew N., Dodge J.C., Shihabuddin L.S., Sohar I., Sleat D.E., Scheule R.K. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease. Mol. Ther. 2007;15:1782–1788. doi: 10.1038/sj.mt.6300249. [DOI] [PubMed] [Google Scholar]
- 9.Sondhi D., Hackett N.R., Peterson D.A., Stratton J., Baad M., Travis K.M., Wilson J.M., Crystal R.G. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- 10.Chang M., Cooper J.D., Sleat D.E., Cheng S.H., Dodge J.C., Passini M.A., Lobel P., Davidson B.L. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 11.Kim K.H., Sleat D.E., Bernard O., Lobel P. Genetic modulation of apoptotic pathways fails to alter disease course in tripeptidyl-peptidase 1 deficient mice. Neurosci. Lett. 2009;453:27–30. doi: 10.1016/j.neulet.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S., Wang L., El-Banna M., Sohar I., Sleat D.E., Lobel P. Large-volume intrathecal enzyme delivery increases survival of a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 2011;19:1842–1848. doi: 10.1038/mt.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng Y., Sohar I., Wang L., Sleat D.E., Lobel P. Systemic administration of tripeptidyl peptidase I in a mouse model of late infantile neuronal ceroid lipofuscinosis: effect of glycan modification. PLoS ONE. 2012;7:e40509. doi: 10.1371/journal.pone.0040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng Y., Sohar I., Sleat D.E., Richardson J.R., Reuhl K.R., Jenkins R.B., Sarkar G., Lobel P. Effective intravenous therapy for neurodegenerative disease with a therapeutic enzyme and a peptide that mediates delivery to the brain. Mol. Ther. 2014;22:547–553. doi: 10.1038/mt.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sondhi D., Peterson D.A., Edelstein A.M., del Fierro K., Hackett N.R., Crystal R.G. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp. Neurol. 2008;213:18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernagut P.O., Diguet E., Labattu B., Tison F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J. Neurosci. Methods. 2002;113:123–130. doi: 10.1016/s0165-0270(01)00485-x. [DOI] [PubMed] [Google Scholar]
- 17.Kitakaze K., Mizutani Y., Sugiyama E., Tasaki C., Tsuji D., Maita N., Hirokawa T., Asanuma D., Kamiya M., Sato K. Protease-resistant modified human β-hexosaminidase B ameliorates symptoms in GM2 gangliosidosis model. J. Clin. Invest. 2016;126:1691–1703. doi: 10.1172/JCI85300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz M.L., Coates J.R., Sibigtroth C.M., Taylor J.D., Carpentier M., Young W.M., Wininger F.A., Kennedy D., Vuillemenot B.R., O’Neill C.A. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease) J. Neurosci. Res. 2014;92:1591–1598. doi: 10.1002/jnr.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroobants S., Gerlach D., Matthes F., Hartmann D., Fogh J., Gieselmann V., D’Hooge R., Matzner U. Intracerebroventricular enzyme infusion corrects central nervous system pathology and dysfunction in a mouse model of metachromatic leukodystrophy. Hum. Mol. Genet. 2011;20:2760–2769. doi: 10.1093/hmg/ddr175. [DOI] [PubMed] [Google Scholar]
- 20.King B., Setford M.L., Hassiotis S., Trim P.J., Duplock S., Tucker J.N., Hattersley K., Snel M.F., Hopwood J.J., Hemsley K.M. Low-dose, continual enzyme delivery ameliorates some aspects of established brain disease in a mouse model of a childhood-onset neurodegenerative disorder. Exp. Neurol. 2016;278:11–21. doi: 10.1016/j.expneurol.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Matthes F., Stroobants S., Gerlach D., Wohlenberg C., Wessig C., Fogh J., Gieselmann V., Eckhardt M., D’Hooge R., Matzner U. Efficacy of enzyme replacement therapy in an aggravated mouse model of metachromatic leukodystrophy declines with age. Hum. Mol. Genet. 2012;21:2599–2609. doi: 10.1093/hmg/dds086. [DOI] [PubMed] [Google Scholar]
- 22.Dickson P.I., Hanson S., McEntee M.F., Vite C.H., Vogler C.A., Mlikotic A., Chen A.H., Ponder K.P., Haskins M.E., Tippin B.L. Early versus late treatment of spinal cord compression with long-term intrathecal enzyme replacement therapy in canine mucopolysaccharidosis type I. Mol. Genet. Metab. 2010;101:115–122. doi: 10.1016/j.ymgme.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevin C., Verot L., Benraiss A., Van Dam D., Bonnin D., Nagels G., Fouquet F., Gieselmann V., Vanier M.T., De Deyn P.P. Partial cure of established disease in an animal model of metachromatic leukodystrophy after intracerebral adeno-associated virus-mediated gene transfer. Gene Ther. 2007;14:405–414. doi: 10.1038/sj.gt.3302883. [DOI] [PubMed] [Google Scholar]
- 24.Lu J.Y., Nelvagal H.R., Wang L., Birnbaum S.G., Cooper J.D., Hofmann S.L. Intrathecal enzyme replacement therapy improves motor function and survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2015;116:98–105. doi: 10.1016/j.ymgme.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Vuillemenot B.R., Katz M.L., Coates J.R., Kennedy D., Tiger P., Kanazono S., Lobel P., Sohar I., Xu S., Cahayag R. Intrathecal tripeptidyl-peptidase 1 reduces lysosomal storage in a canine model of late infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2011;104:325–337. doi: 10.1016/j.ymgme.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Vuillemenot B.R., Kennedy D., Reed R.P., Boyd R.B., Butt M.T., Musson D.G., Keve S., Cahayag R., Tsuruda L.S., O’Neill C.A. Recombinant human tripeptidyl peptidase-1 infusion to the monkey CNS: safety, pharmacokinetics, and distribution. Toxicol. Appl. Pharmacol. 2014;277:49–57. doi: 10.1016/j.taap.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y., Almomani R., Breedveld G.J., Santen G.W., Aten E., Lefeber D.J., Hoff J.I., Brusse E., Verheijen F.W., Verdijk R.M. Autosomal recessive spinocerebellar ataxia 7 (SCAR7) is caused by variants in TPP1, the gene involved in classic late-infantile neuronal ceroid lipofuscinosis 2 disease (CLN2 disease) Hum. Mutat. 2013;34:706–713. doi: 10.1002/humu.22292. [DOI] [PubMed] [Google Scholar]
- 29.Anderson G.W., Goebel H.H., Simonati A. Human pathology in NCL. Biochim. Biophys. Acta. 2013;1832:1807–1826. doi: 10.1016/j.bbadis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Worgall S., Kekatpure M.V., Heier L., Ballon D., Dyke J.P., Shungu D., Mao X., Kosofsky B., Kaplitt M.G., Souweidane M.M. Neurological deterioration in late infantile neuronal ceroid lipofuscinosis. Neurology. 2007;69:521–535. doi: 10.1212/01.wnl.0000267885.47092.40. [DOI] [PubMed] [Google Scholar]
- 31.Steinfeld R., Heim P., von Gregory H., Meyer K., Ullrich K., Goebel H.H., Kohlschütter A. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am. J. Med. Genet. 2002;112:347–354. doi: 10.1002/ajmg.10660. [DOI] [PubMed] [Google Scholar]
- 32.Sleat D.E., El-Banna M., Sohar I., Kim K.H., Dobrenis K., Walkley S.U., Lobel P. Residual levels of tripeptidyl-peptidase I activity dramatically ameliorate disease in late-infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2008;94:222–233. doi: 10.1016/j.ymgme.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- 34.Weisberg S. Wiley; Hoboken, NJ: 2014. Applied Linear Regression. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genotype: LINCL; treatment: none; age: 116 days.
Genotype: LINCL; treatment: none; age: 133 days.
Genotype: LINCL; treatment: none; age: 146 days.
Genotype: WT; treatment: none; age: 104 days.
Genotype: WT; treatment: none; age: 225 days.
Genotype: LINCL; treatment: 1 mg rhTPP1 weekly; age: 229 days.
Genotype: LINCL; treatment: 1 mg rhTPP1 weekly; age: 229 days.
Genotype: LINCL; treatment: 2.3 mg rhTPP1 biweekly; age: 206 days.
Genotype: LINCL; treatment: 2.3 mg rhTPP1 biweekly; age: 206 days.