Abstract
Oxycodone DETERx® (Collegium Pharmaceutical Inc, Canton, Massachusetts) is an extended‐release, microsphere‐in‐capsule, abuse‐deterrent formulation designed to retain its extended‐release properties after tampering (eg, chewing/crushing). This randomized, double‐blind, placebo‐controlled, triple‐dummy study evaluated the oral abuse potential of intact and chewed oxycodone DETERx capsules compared with crushed immediate‐release oxycodone. Subjects with a history of recreational opioid use who were nondependent/nontolerant to opioids were enrolled. Treatments included intact oxycodone DETERx (high‐fat, high‐calorie meal and fasted), chewed oxycodone DETERx (high‐fat, high‐calorie meal and fasted), crushed immediate‐release oxycodone (fasted), and placebo (high‐fat, high‐calorie meal). Plasma samples were collected to determine pharmacokinetic parameters. The primary endpoint was drug liking at the moment; other endpoints included drug effects questionnaire scores, Addiction Research Center Inventory/Morphine Benzedrine Group score, pupillometry measurements, and safety. Thirty‐eight subjects completed the study. Chewed and intact oxycodone DETERx were bioequivalent, unlike crushed immediate‐release oxycodone, which yielded higher peak oxycodone plasma concentrations compared with all methods of oxycodone DETERx administration. The mean maximum (peak) effect (Emax) for drug liking was significantly lower for chewed and intact oxycodone DETERx than for crushed immediate‐release oxycodone (P < .01). The time to Emax was significantly longer for chewed and intact oxycodone DETERx than for crushed immediate‐release oxycodone (P < .0001). Scores for feeling high and Addiction Research Center Inventory/Morphine Benzedrine Group scores demonstrated lower abuse potential for chewed and intact oxycodone DETERx compared with crushed immediate‐release oxycodone. Study treatments were well tolerated; no subjects experienced serious adverse events. These results demonstrate the lower oral abuse potential of chewed and intact oxycodone DETERx than crushed immediate‐release oxycodone.
Keywords: abuse‐deterrent formulations, opioids, oxycodone DETERx, human abuse potential
When used as prescribed, extended‐release (ER) opioid formulations offer numerous advantages to patients with chronic cancer or noncancer pain severe enough to require daily, around‐the‐clock, long‐term opioid treatment and for whom alternative treatment options are inadequate. Less frequent dosing, more consistent analgesia, and less nighttime awakening due to pain1, 2 are advantages of ER opioids that may result in improved compliance (ie, medication adherence) in those patients on long‐term therapy, particularly elderly patients.1, 3 Because ER opioids are made with higher amounts of active drug than immediate‐release (IR) formulations, tampering with conventional ER opioid formulations (eg, by grinding, crushing, or chewing) results in a more rapid release of most, if not all, of the active drug. This rapid release of the full drug load in an ER formulation is particularly attractive to individuals who engage in recreational opioid use or those with psychological dependence.4, 5, 6 In addition to increased abuse liability, immediate exposure to a larger amount of opioid also presents a significant safety risk, including potential for overdose and death.4, 5, 6
Abuse‐deterrent formulations are designed to discourage abuse via specific routes of administration while still preserving analgesic benefits for patients.7, 8, 9, 10, 11 Postmarketing reports of a reformulated opioid have indicated a lower incidence of abuse, abuse‐related fatalities, and doctor shopping.12 Most abuse‐deterrent formulations impose physicochemical barriers that make mechanical tampering with the solid oral dosage forms difficult. To date, the prevailing marketed abuse‐deterrent formulation technology is in the form of hard‐to‐crush tablets. This formulation is problematic for patients with chronic pain who also have difficulty swallowing tablets (ie, dysphagia) and must resort to crushing or breaking the tablet to ingest their medication or have it administered via enteral tube. It is estimated that as many as 11 million patients cannot take their prescribed, solid, monolithic, oral opioid medications because they are unable to swallow these formulations intact.13 Therefore, there is a need for continued innovation pertaining to opioid abuse‐deterrent formulations that also meet the needs of those patients who have difficulty swallowing.
Oxycodone DETERx® (Xtampza® ER, Collegium Pharmaceutical Inc, Canton, Massachusetts) is an abuse‐deterrent formulation of ER oxycodone with the active drug (oxycodone base) formulated within a novel, abuse‐deterrent drug delivery technology platform (called DETERx), which is comprised of hydrophobic, waxy microspheres. Unlike currently marketed ER opioid formulations, which lose a portion if not all of their ER properties if successfully crushed,14 the small particle size and physiochemical properties of the oxycodone DETERx microspheres protect the ER mechanism from tampering and allow for administration by sprinkling onto soft food or via gastrostomy or nasogastric tube. This is an important feature because only 1 other ER opioid formulation15 can be administered via gastrostomy tube; there are currently no other ER opioid formulations that can be administered via nasogastric tube.
Previous studies revealed that manipulation of oxycodone DETERx by crushing did not significantly change the oxycodone pharmacokinetic profile when compared with intact oxycodone DETERx; crushed and intact oxycodone DETERx were bioequivalent with lower peak exposure and longer times to peak exposure than IR oxycodone.16, 17 The aim of the current study was to investigate the impact of manipulation by chewing on the pharmacokinetic profile and abuse potential of oxycodone DETERx relative to IR oxycodone in recreational drug users with a history of moderate opioid use. This study examined the oral human abuse potential of oxycodone DETERx when administered with a high‐fat, high‐calorie meal and fasted in comparison with IR oxycodone and placebo.
Methods
This was a randomized, double‐blind, triple‐dummy, active‐ and placebo‐controlled, single‐dose, 6‐way crossover, hypothesis‐driven study (Figure 1). The study was conducted at a single center in the United States (PRA Health Sciences, Salt Lake City, Utah) in accordance with the International Conference on Harmonization guideline for Good Clinical Practice, the Food and Drug Administration regulations governing clinical study conduct, and the Declaration of Helsinki (and its amendments). Study materials were reviewed by an independent ethics review committee (New England Institutional Review Board, Newton, Massachusetts) as required by local regulations. All subjects provided written informed consent after a complete explanation of the study and before any study‐related procedures or assessments were performed. Subjects were informed that they could discontinue the study at any time.
Figure 1.
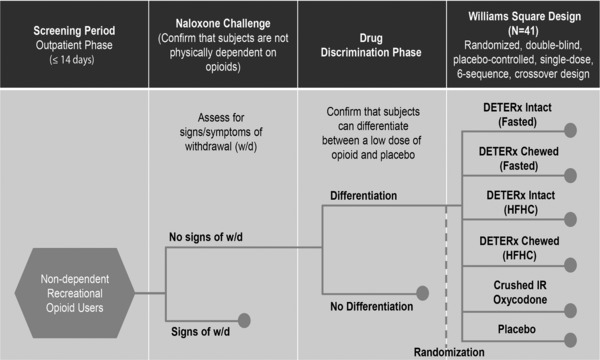
Oral human abuse potential study design. DETERx, oxycodone DETERx; HFHC, high‐fat high‐calorie meal; IR, immediate‐release.
Participants
During a standard medical screening visit, potential subjects were evaluated for study eligibility. Subjects were healthy male or female nondependent recreational opioid users aged 18 to 55 years. Recreational drug use was defined as opioid use for nontherapeutic purposes (ie, for psychoactive effects) on at least 10 occasions within the previous year and at least once in the 12 weeks before the screening phase. Subjects were also required to have reported previously taking and tolerating a 40‐mg dose of oxycodone hydrochloride. Enrolled subjects must not have had clinically significant abnormalities on medical history, vital sign measurements, physical examination results, 12‐lead electrocardiograms, or clinical laboratory tests. Subjects with a lifetime history of drug or alcohol dependence were excluded, as were heavy users of tobacco products (eg, subjects who smoked more than 20 cigarettes per day and were unable to abstain from smoking for at least 5 hours during the day). Subjects with a known allergy to any of the test products or subjects with a condition for which an opioid is contraindicated were excluded.
To minimize the risk of drug interaction, subjects were restricted from using other prescription or nonprescription drugs (with the exception of acceptable forms of birth control and acetaminophen), herbal remedies, or nutritional supplements during the study. Subjects were also told to avoid caffeine and alcohol for 24 hours before admission to the drug discrimination phase and each treatment period. Subjects were required to have a negative urine drug screen and alcohol breath test before dosing in the drug discrimination phase and in each treatment period. Due to the long half‐life of cannabinoids, positive urine drug screen results for tetrahydrocannabinol were permitted but were required to be stable for the duration of the study (ie, tetrahydrocannabinol results were to remain negative or positive from the start of the study through to end of the study). Subjects who were positive for tetrahydrocannabinol had to pass a targeted neurological examination (assessing lightheadedness, sedation, dizziness, and confusion) administered by the investigator, or designee, showing that subjects were not cognitively impaired. Additionally, subjects were to abstain from food or drinks containing Seville oranges, grapefruit, pomegranate, pomelo, star fruit, and poppy seeds from 1 week before the start of the drug discrimination phase until the end of the study to avoid the risk of cytochrome P450 3A4 inhibition and the ensuing effects on the pharmacokinetic analysis (ie, metabolism of oxycodone DETERx) in the study. Female subjects of childbearing potential could not be pregnant or breastfeeding, had to use acceptable methods of contraception during the study, and were required to have a negative urine pregnancy test before dosing in each treatment period.
Study Design and Treatment
Drug Discrimination Phase
After a standard medical screening visit, subjects underwent a 3‐day drug discrimination phase that included a naloxone challenge test, which was administered first to exclude subjects who were physically dependent on opioids, and a pharmacologic qualification test. Naloxone (0.2‐mg bolus followed by 0.6 mg) was given on day –1, dosing occurred on days 1 and 2, and then discharge was on the morning of day 3 (Figure 1). During the pharmacologic qualification test, subjects received oral IR oxycodone 20 mg (fasted) or placebo (fasted) in a double‐blind, crossover fashion with an approximate 24‐hour washout period between placebo and IR oxycodone treatments. To identify subjects who were more sensitive to the effects of oxycodone (and therefore able to discriminate subtle differences between formulations), the Food and Drug Administration draft guidance recommendations18 suggest using a lower dose of the active comparator. Thus, a 20‐mg dose of IR oxycodone was administered in the drug discrimination phase. Subjects completed a series of subjective questionnaires before dosing and up to 6 hours postdose. To qualify for enrollment into the double‐blind treatment phase, subjects were required to successfully discriminate between placebo and IR oxycodone based on responses to the bipolar drug‐liking visual analog scale (measure from 0 to 100, where 50 is neutral). Subjects had to meet 3 requirements: show a minimum peak score of 65 points on the bipolar drug‐liking visual analog scale, have a greater than or equal to 15‐point difference between active drug and placebo at 1 or more time points during the first 2 hours after dosing, and have a placebo response that fell between 40 and 60 points on the bipolar drug‐liking visual analog scale within the first 2 hours postdose. In addition, subjects were eligible to proceed to the double‐blind treatment phase if they were able to tolerate 20 mg of IR oxycodone hydrochloride and were judged by the clinical staff to be capable of successfully completing the study.
Double‐Blind Treatment Phase
This phase consisted of 6 treatment periods. Enrolled, non‐naltrexone‐blocked subjects received the following 6 oral single doses of study drug in a randomized, crossover, triple‐dummy fashion: intact oxycodone DETERx in a fasted state, intact oxycodone DETERx after a high‐fat, high‐calorie meal, chewed oxycodone DETERx in a fasted state, chewed oxycodone DETERx after a high‐fat, high‐calorie meal, crushed IR oxycodone in solution in a fasted state (IR oxycodone), and placebo after a high‐fat, high‐calorie meal. All doses of oxycodone DETERx contained the equivalent of 40 mg oxycodone hydrochloride (36 mg oxycodone base). IR oxycodone contained 40 mg oxycodone hydrochloride.
Subjects were admitted to the research unit the day before dosing and remained housed at the clinical site approximately 36 hours after dosing or until subjects were deemed safe to be discharged. Each treatment was separated by a minimum of 5 days. Subjects were assigned to 1 of 6 treatment sequences according to a 6 × 6 Williams square randomization design and received 1 dose of each of the assigned treatments. For the fed treatments, subjects fasted overnight for at least 10 hours, then started a standardized high‐fat, high‐calorie breakfast (approximately 150, 250, and 500 to 600 calories from protein, carbohydrate, and fat, respectively, as per Food and Drug Administration guidance19) 30 minutes before the scheduled dosing time. Subjects were required to consume the entire meal within 20 minutes. Subjects who had emesis after the high‐fat, high‐calorie meal before dosing or within 6 hours relative to dosing were discontinued from the study. For the fasted treatments, study drug dosing took place after an overnight fast of at least 10 hours. All subjects were required to fast for at least 4 hours after dosing. Subjects were allowed to consume water ad libitum other than the 1 hour before and 1 hour after drug administration.
In all treatment periods, pharmacodynamic measurements were collected predose through 24 hours postdose, serial blood samples for pharmacokinetic evaluation were collected predose through 36 hours postdose, and standard safety measures were collected from admission to the research unit through discharge from the research unit. Subjects were to be deemed medically stable by the investigator before discharge.
Study Drugs
To maintain blinding, treatment was administered in a triple‐dummy fashion, so that during each treatment period, a subject received an intact capsule, capsule contents to chew, and solution. Intact study drug (oxycodone DETERx or DETERx placebo) was always administered first with 50 mL of solution (IR oxycodone or placebo) followed by chewed study drug.
For each study drug, subjects swallowed intact capsules directly from the dosing container. Intact doses of oxycodone DETERx were administered with 50 mL of placebo solution (microcrystalline cellulose mixed with room temperature, noncarbonated water with denatonium benzoate to create a 1‐ppm solution added for blinding purposes). Intact doses of DETERx placebo were administered with either 50 mL of IR oxycodone solution (room temperature, noncarbonated water with denatonium benzoate added to create a 1‐ppm solution for blinding purposes, then mixed with 40 mg of crushed IR oxycodone) or 50 mL of placebo solution. After intact dosing with solution, 2 50‐mL rinses of room‐temperature, noncarbonated water were administered from the dosing container. Subjects were then provided with study drug (oxycodone DETERx or DETERx placebo) to chew. For the chewed study drug dose, staff opened the capsule and poured the capsule contents (microspheres) into a small dosing cup immediately before dosing. Subjects were instructed to pour the capsule contents from the dosing cup onto their tongue and to chew the capsule contents for 2 minutes without swallowing or talking. After the chewed dose, 2 additional 50‐mL rinses of room‐temperature, noncarbonated water were administered from the dosing container. Study staff conducted a visual oral cavity check to ensure that all study drug had been consumed.
Assessments
Pharmacokinetic Measures
During each treatment period, blood samples to determine plasma oxycodone concentrations were obtained for each subject at predose and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 12.0, 24.0, and 36.0 hours postdose. For each sample, approximately 3 mL of venous blood was collected. Pharmacokinetic variables were calculated from plasma concentration data using standard, noncompartmental methods by SAS® for Windows® version 9.3 (SAS Institute, Cary, North Carolina). Data included area under the plasma concentration‐time curve from time 0 to infinity (AUC0‐∞), area under the plasma concentration‐time curve from time 0 to last measurable plasma concentration (AUC0‐t), maximum observed plasma concentration (Cmax), time to reach maximum plasma concentration (Tmax), and abuse quotient (Cmax/Tmax). The abuse quotient is a measure of average rate of rise in plasma concentration between dosing and Tmax; the score is thought to be related to a product's abuse potential.20
Pharmacodynamic Measures
Pharmacodynamic measures in this study were consistent with the guidelines prepared for abuse potential studies18, 21, 22 and were selected to evaluate multiple subjective and objective effects associated with opioids. During each treatment period, subjects were required to rate their current perception of their subjective state and of the drug's effects. A drug effects questionnaire visual analog scale (including the primary endpoint of drug liking at the moment) and Addiction Research Center Inventory/Morphine Benzedrine Group questions were administered predose (except measures assessing specific drug effects) and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 12.0, and 24.0 hours postdose. Global measures (ie, overall drug liking visual analog scale and “take drug again” visual analog scale) were assessed at 8.0 and 24.0 hours postdose. Bipolar measurements (visual analog scale 100‐mm scales where 50 = neutral) included drug liking, overall drug liking, and the desire to take the drug again. Unipolar measurements of the drug effects questionnaire visual analog scale (100‐mm scales where 0 = neutral) included “feeling high,” “good effects,” “bad effects,” “feeling sick,” “nausea,” “sleepy,” “dizzy,” and “any effects.” The Addiction Research Center Inventory/Morphine Benzedrine Group assessment consisted of 16 true/false statements used to assess euphoria and positive mood. Pupil diameter was measured using a NeurOptic® VIP‐200 pupillometer (Neuroptics Inc, Irvine, California). Measurements were taken during each treatment period at predose and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, 6.0, and 8.0 hours postdose. Each measurement was made for a minimum of 1 minute after subject's acclimation to the dark. The same eye was used for all assessments.
Mean maximum (peak) effect scores (Emax) were calculated for pharmacodynamic measures, as appropriate, for each subject in each treatment period of the double‐blind treatment phase. Each subject was assessed for percentage reduction in drug‐liking visual analog scale between oxycodone DETERx and IR oxycodone as outlined in the recent Food and Drug Administration guidance.18 For the objective measure of pupillometry, maximum pupil constriction was measured.
Safety Monitoring
Safety and tolerability evaluations included assessment of treatment‐emergent adverse events, monitoring of vital signs, oxygen saturation, physical examinations, and results of clinical laboratory tests.
Statistical Analysis
Subjects who completed all 6 treatment periods were included in the primary pharmacodynamic analysis. Subjects who completed at least 2 active treatment periods, had sufficient quantifiable plasma concentration data to provide Cmax and AUC data, and who did not experience emesis within 12 hours of dosing were included in the pharmacokinetic analyses. Subjects who received at least 1 dose of study drug and for whom there was at least 1 posttreatment safety observation were included in the safety analyses.
The pharmacokinetic parameters for oxycodone were compared among treatments using an analysis of variance statistical model with sequence, treatment, and period as the fixed effects and subject within sequence as a random effect, using the natural logarithms of the data. Confidence intervals (90%) were constructed for the least‐squares geometric mean ratios of all 3 parameters (Cmax, AUC0‐t, and AUC0‐∞) for the treatments being compared using the natural log‐transformed data and the 2, 1‐sided t‐test procedure. The geometric mean ratios and associated 90% confidence intervals were transformed back to the original scale. Bioequivalence was concluded if the 90% confidence intervals of the geometric mean ratios for a specific comparison fell entirely within 80.0% to 125.00% (per Food and Drug Administration guidance19). Time to reach maximum plasma concentration was analyzed using a nonparametric analysis without transformation. The Wilcoxon signed‐rank test23 was used to compare Tmax among the different treatments. All pharmacokinetic calculations were prepared using SASۚ for Windowsۚ.
The primary outcome measure was drug liking at the moment; the primary endpoint was drug‐liking Emax during the 24 hours after dosing. Secondary outcome measures were feeling high, any drug effects, good effects, bad effects, feeling sick, nauseous, sleepy, and dizzy; overall drug liking; Addiction Research Center Inventory/Morphine Benzedrine Group scores; take drug again assessment; and pupillometry measurements. The primary analysis was based on the pairwise comparison between the control (ie, crushed IR oxycodone fasted) and the 2 chewed oxycodone DETERx treatments for drug‐liking Emax. The drug‐liking Emax comparison between crushed IR oxycodone fasted and placebo (high‐fat, high‐calorie meal) was used for validation of the appropriateness of the positive control. Secondary analyses included comparisons between intact oxycodone DETERx treatments and IR oxycodone, intact and chewed oxycodone DETERx fasted treatments, and intact and chewed oxycodone DETERx (high‐fat, high‐calorie meal) treatments.
Pharmacodynamic endpoints were analyzed using a mixed‐effects model with fixed effects for sequence, period, and treatment with subject nested within sequence as a random effect. Least‐squares means and 95% confidence intervals were calculated for each treatment. The least‐squares means differences and 95% confidence intervals were generated for each pairwise treatment comparison. Pairwise comparisons were not adjusted for multiplicity. This study was hypothesis‐driven; the null hypothesis vs alternate hypothesis is described by
where μC is the mean of the control treatment, crushed IR oxycodone in solution fasted, and μT is the mean of the test treatment, chewed oxycodone DETERx high‐fat, high‐calorie fed or chewed oxycodone DETERx fasted. For the pharmacodynamic statistical analyses, significance comparisons between control and test for the drug‐liking primary endpoint, Emax, were 1‐tailed using a nominal α = 0.025; all other comparisons were tested with a 2‐sided test using a nominal α = 0.05. Assumptions of normality of residuals were investigated for each response measurement. If the normality assumption was rejected at the 1% level with the Shapiro‐Wilk test,24 then an analysis using ranked values was performed. The median differences for the pairwise comparisons were derived using Hodges‐Lehmann25 estimates. The 95% confidence intervals were provided as estimated by Moses.26
The percentage reduction in drug‐liking Emax was used to analyze the data by means of a responder analysis for chewed oxycodone DETERx (high‐fat, high‐calorie meal) and chewed oxycodone DETERx (fasted). A responder was defined as a subject who had at least a prespecified level of reduction, where levels from 0% to 100% in 10% increments are presented in a sensitivity analysis. The number and percentage of subjects determined as responders and nonresponders are presented. The binominal test of proportions was utilized to test the null hypothesis that 50% or fewer subjects were responders.
Results
Subject Disposition and Demographics
Of the 111 male and female subjects who entered the study, 110 subjects passed the naloxone challenge test; of these, 107 patients were enrolled and participated in the pharmacologic qualification test. Sixty‐four subjects proceeded to the double‐blind treatment phase. One subject experienced emesis after the high‐fat, high‐calorie meal before receiving the first treatment dose and was subsequently withdrawn; therefore, 63 subjects received at least 1 dose of study drug. Thirty‐eight subjects received all 6 study treatments and were included in the pharmacodynamic analysis. Twenty‐five subjects were discontinued before completing the double‐blind treatment phase (9 subjects discontinued due to treatment‐emergent adverse events, 8 subjects discontinued due to protocol deviations, 7 subjects withdrew consent, and 1 subject was discontinued due to a drug administration error). For subjects included in the pharmacodynamic analysis, the mean (range) age was 26.2 (18–46) years. Most subjects were male (66%) and were either white (87%), black/African American (3%), Asian (5%), or other (5%). All subjects reported a history of recreational opioid use (hydrocodone, oxycodone, morphine, buprenorphine, codeine, oxymorphone, or heroin).
Pharmacokinetics
Mean oxycodone plasma concentration‐vs‐time profiles for the 5 active oral treatments are shown in Figure 2A. Oral administration of crushed IR oxycodone in solution under fasted conditions resulted in the highest mean (standard deviation) Cmax (77.7 [24.5] ng/mL) and shortest Tmax (1.08 hours) compared with all methods of administration of oxycodone DETERx (mean Cmax range 30.9 [9.9] ng/mL to 41.9 [12.4] ng/mL; median Tmax range 3.07 hours to 5.12 hours). The mean abuse quotient score for crushed IR oxycodone (108 ng/[mL·h]) was approximately 10‐fold higher than the scores observed for intact and chewed oxycodone DETERx (Table 1), indicating a relatively slower and more gradual rise in plasma concentration after administration of all DETERx treatments. None of the oxycodone DETERx treatments were equivalent to IR oxycodone on Cmax, but were equivalent on AUC based on the confidence intervals and point estimates falling within the 80% to 125% range (Table 2).
Figure 2.
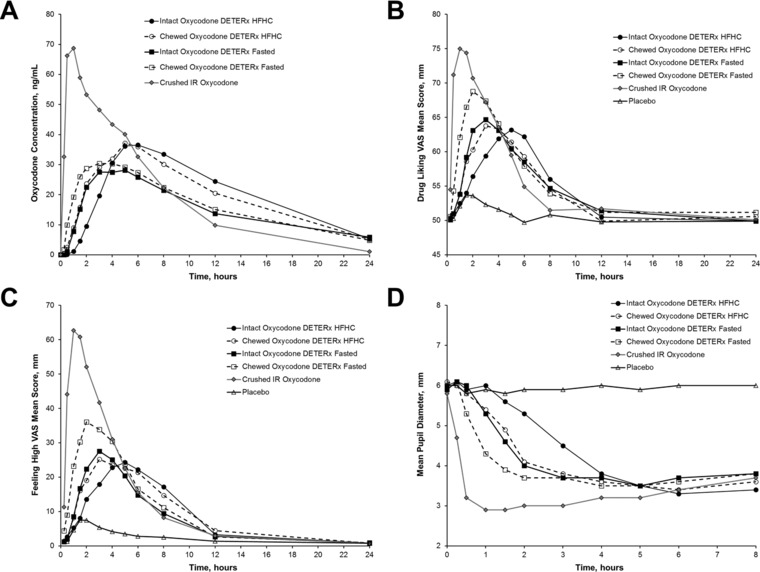
Oxycodone DETERx pharmacokinetic and pharmacodyamic measurements over time. Mean oxycodone plasma concentration over time (A), mean drug liking over time (B), mean feeling high over time (C), and mean pupil diameter over time (D). HFHC, high‐fat, high‐calorie meal; IR, immediate‐release; VAS, visual analog scale.
Table 1.
Summary of Pharmacokinetic Measures
| Parametera | Intact Oxycodone DETERx HFHC (n = 38) | Chewed Oxycodone DETERx HFHC (n = 38) | Intact Oxycodone DETERx Fasted (n = 38) | Chewed Oxycodone DETERx Fasted (n = 38) | Crushed IR Oxycodone (n = 38) |
|---|---|---|---|---|---|
| Cmax, ng/mL | 41.9 (12.4) | 40.3 (12.2) | 30.9 (9.9) | 35.5 (12.5) | 77.7 (24.5) |
| Tmax, hours | 5.1 (1.6‐12.1) | 5.1 (2.1‐12.1) | 4.1 (1.6‐8.1) | 3.1 (1.1‐6.2) | 1.1 (0.2‐5.1) |
| AUC0‐t, ng · h/mL | 511 (155) | 498 (123) | 408 (113) | 433 (123) | 468 (106) |
| AUC0‐∞, ng · h/mL | 553 (131) | 515 (122) | 469 (107) | 469 (126) | 476 (106) |
| t1/2, hours | 5.2 (0.9) | 5.3 (0.7) | 8.8 (2.6) | 7.4 (2.3) | 3.6 (0.5) |
| AQ, ng/(mL·h) | 7.8 (4.9) | 8.1 (4.3) | 8.2 (4.0) | 13.0 (8.2) | 108.0 (84.1) |
AQ, abuse quotient; AUC0‐∞, area under the plasma concentration‐time curve extrapolated to infinity; AUC0‐t, area under the plasma concentration‐time curve from 0 hours to the time of the last measurable plasma concentration; Cmax, maximum observed plasma concentration; HFHC, high‐fat, high‐calorie meal; IR, immediate‐release; t1/2, terminal elimination half‐life; Tmax = time to reach maximum plasma concentration.
All parameters are the mean (standard deviation) except for Tmax, which is the median (range).
Table 2.
Bioequivalence Comparison of Oxycodone Cmax, AUC0‐t, and AUC0‐∞ a
| Treatment | LS Geometric Mean Ratio | 90% Confidence Interval |
|---|---|---|
| Intact oxycodone DETERx HFHC vs chewed oxycodone DETERx HFHC | ||
| Cmax | 96.0 | 88.3–104.4 |
| AUC0‐t | 101.7 | 90.5–114.3 |
| AUC0‐∞ | 96.9 | 91.5–102.7 |
| Intact oxycodone DETERx fasted vs chewed oxycodone DETERx fasted | ||
| Cmax | 113.2 | 104.1–123.1 |
| AUC0‐t | 105.9 | 94.2–119.0 |
| AUC0‐∞ | 99.5 | 94.0–105.4 |
| Intact oxycodone DETERx HFHC vs crushed IR oxycodone | ||
| Cmax | 54.1 | 49.8–58.8 |
| AUC0‐t | 110.4 | 98.2–124.0 |
| AUC0‐∞ | 109.0 | 103.1–115.3 |
| Intact oxycodone DETERx fasted vs crushed IR oxycodone | ||
| Cmax | 40.4 | 37.2–43.9 |
| AUC0‐t | 94.4 | 84.0–106.0 |
| AUC0‐∞ | 96.5 | 91.4–101.9 |
| Chewed oxycodone DETERx HFHC vs crushed IR oxycodone | ||
| Cmax | 51.9 | 47.9–56.4 |
| AUC0‐t | 112.3 | 100.1–125.9 |
| AUC0‐∞ | 105.7 | 100.3–111.4 |
| Chewed oxycodone DETERx fasted vs crushed IR oxycodone | ||
| Cmax | 45.8 | 42.2–49.6 |
| AUC0‐t | 99.9 | 89.3–111.7 |
| AUC0‐∞ | 96.1 | 91.3–101.1 |
AUC0‐t, area under the plasma concentration‐time curve from 0 hours to the time of the last measurable plasma concentration; AUC0‐∞, area under the plasma concentration‐time curve extrapolated to infinity; Cmax, maximum observed plasma concentration; HFHC, high‐fat, high‐calorie meal; IR, immediate‐release, LS, least‐squares.
Comparisons were considered bioequivalent (highlighted in bold) if the 90% confidence intervals of the geometric mean ratios fell entirely within the interval between 80% and 125%.
Overall, mean plasma oxycodone concentrations were comparable after administration of chewed or intact oxycodone DETERx with or without a high‐fat, high‐calorie meal (Figure 2A). Chewed oxycodone DETERx (high‐fat, high‐calorie meal) was bioequivalent to intact oxycodone DETERx (high‐fat, high‐calorie meal) treatment on Cmax, AUC0‐t, and AUC0‐∞; similarly, chewed oxycodone DETERx fasted was bioequivalent to intact oxycodone DETERx fasted on Cmax, AUC0‐t, and AUC0‐∞ (Table 2).
Pharmacodynamic Measures
For the primary outcome measure of drug liking at the moment, IR oxycodone (Table 3) showed significantly higher mean visual analog scale Emax values compared with both chewed oxycodone DETERx treatments (P < .01) and both intact oxycodone DETERx treatments (P < .0001). Chewed oxycodone DETERx fasted showed significantly higher mean drug‐liking visual analog scale scores compared with fasted intact oxycodone DETERx treatment (P = .0457). No significant differences were observed between chewed oxycodone DETERx (high‐fat, high‐calorie meal) and intact oxycodone DETERx (high‐fat, high‐calorie meal). Oral administration of all active treatments resulted in significantly higher mean drug‐liking visual analog scale scores compared with placebo (P < .0001).
Table 3.
Summary of Pharmacodynamic Measures
| Parameter | Intact Oxycodone DETERx HFHC (n = 38) | Chewed Oxycodone DETERx HFHC (n = 38) | Intact Oxycodone DETERx Fasted (n = 38) | Chewed Oxycodone DETERx Fasted (n = 38) | Crushed IR Oxycodone (n = 38) | Placebo (n = 38) |
|---|---|---|---|---|---|---|
| Drug‐Liking VAS Emax, mma | ||||||
| Mean (SD) | 68.6 (13.1)b | 70.8 (11.5)b | 68.8 (13.0)b | 73.4 (13.9)c | 81.8 (11.5) | 54.9 (8.4)b |
| Median | 70.0 | 70.0 | 72.0 | 76.0 | 82.5 | 51.0 |
| Good‐Effects VAS Emax, mm | ||||||
| Mean (SD) | 36.4 (31.3)b | 39.9 (23.8)b | 35.9 (29.0)b | 44.5 (32.5)b | 69.0 (26.4) | 9.2 (18.2)b |
| Median | 31.0 | 37.5 | 36.0 | 50.0 | 74.0 | 2.0 |
| Feeling‐High VAS Emax, mma | ||||||
| Mean (SD) | 36.0 (26.9)b | 37.4 (24.4)b | 33.6 (26.2)b | 44.7 (29.0)b | 68.9 (25.0) | 10.3 (19.2)b |
| Median | 34.5 | 33.0 | 34.5 | 51.5 | 72.0 | 2.0 |
| Bad‐Effects Emax, mmd | ||||||
| Mean (SD) | 8.1 (12.4) | 8.5 (14.1)e | 8.0 (12.3)e | 10.5 (16.4) | 19.1 (25.4) | 2.6 (3.1)b |
| Median | 4.0 | 4.0 | 4.0 | 3.5 | 5.5 | 2.0 |
| Sick VAS Emax, mm | ||||||
| Mean (SD) | 5.4 (13.0)e | 6.2 (12.7)e | 4.2 (6.9)e | 6.3 (10.6) | 14.5 (20.2) | 1.7 (4.3) |
| Median | 1.0 | 1.0 | 1.0 | 1.0 | 2.5 | 1.0 |
| Nausea VAS Emax, mm | ||||||
| Mean (SD) | 9.1 (19.4) | 6.3 (13.5) | 3.9 (6.4) | 6.0 (11.0) | 14.0 (20.5) | 1.7 (5.8) |
| Median | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 |
| Sleepy VAS Emax, mm | ||||||
| Mean (SD) | 30.3 (27.0)c | 35.4 (29.6)c | 28.3 (28.7)b | 34.7 (29.0)c | 48.1 (34.0) | 7.3 (11.7)b |
| Median | 23.5 | 26.5 | 19.0 | 32.0 | 45.0 | 2.0 |
| Any‐Effects VAS Emax, mm | ||||||
| Mean (SD) | 36.0 (28.8)b | 37.2 (24.5)b | 34.7 (26.9)b | 44.7 (29.4)b | 69.4 (25.5) | 9.9 (18.6)b |
| Median | 31.5 | 35.0 | 37.0 | 50.0 | 73.5 | 2.0 |
| ARCI/MBG score | ||||||
| Mean (SD) | 4.1 (4.8)c | 4.0 (4.3)c | 4.3 (5.0)c | 5.3 (5.0)e | 7.1 (5.6) | 1.4 (2.7)b |
| Median | 2.0 | 2.5 | 3.0 | 4.0 | 7.0 | 0.0 |
| Overall Drug‐Liking VASd Emax, mm | ||||||
| Mean (SD) | 68.5 (16.5)c | 69.8 (17.4)e | 69.4 (15.3)e | 74.2 (14.4) | 76.2 (16.4) | 54.4 (10.1)b |
| Median | 72.0 | 70.0 | 69.5 | 75.5 | 77.5 | 50.0 |
| Take‐Drug‐Again VASd Emax, mm | ||||||
| Mean (SD) | 70.6 (18.1) | 69.3 (18.9) | 70.2 (16.0) | 73.7 (14.9) | 75.4 (16.8) | 52.7 (13.4) |
| Median | 74.0 | 69.0 | 68.5 | 74.0 | 75.5 | 50.0 |
ARCI/MBG, Addiction Research Center Inventory/Morphine Benzedrine Group; Emax, maximum (peak) effect; HFHC, high‐fat, high‐calorie meal; IR, immediate‐release; SD, standard deviation; VAS, visual analog scale.
Data analyzed using a mixed‐effects model.
Significantly lower scores vs IR oxycodone (P < .0001).
Significantly lower scores vs IR oxycodone (P < .01).
Data analyzed nonparametrically using the ranks as the dependent variable.
Significantly lower scores vs IR oxycodone (P < 0.05).
The drug‐liking visual analog scale scores over time are shown in Figure 2B. Immediate‐release oxycodone had mean drug‐liking visual analog scale scores that were higher than those of all other treatments at early time points. Scores were in the “liking” range of the scale (drug‐liking score greater than 50) between 0.5 and 8 hours postdose, after which they returned to just above neutral. Mean peak effects were delayed with all oxycodone DETERx treatments relative to IR oxycodone, particularly for oxycodone DETERx treatments administered with a high‐fat, high‐calorie meal compared with their fasted comparators. Drug‐liking visual analog scale scores after placebo administration were lower than all active treatments and showed little change over time, remaining around the neutral mark (drug‐liking score of 50) throughout the time course.
Mean scores on the feeling‐high visual analog scale for crushed IR oxycodone quickly increased postdose, peaking at 1 hour (Figure 2C). Feeling‐high visual analog scale scores for all forms of oxycodone DETERx were much lower, with intact oxycodone DETERx peak feeling‐high visual analog scale scores being approximately half that of IR oxycodone. Trends for pupillometry measurements were similar to drug‐liking and feeling‐high results; maximum pupil constriction was greatest for IR oxycodone (Figure 2D). Intact and chewed oxycodone DETERx exhibited more gradual pupil constriction over time, unlike the rapid pupil constriction that occurred with crushed IR oxycodone administration.
For secondary measures (Table 3), both intact and chewed oxycodone DETERx (fasted and high‐fat, high‐calorie meal) had significantly lower mean Emax scores than did IR oxycodone on positive‐effects measures including feeling high (P < .0001), good effects (P < .0001), and Addiction Research Center Inventory/Morphine Benzedrine Group scores (P < .05). Fewer differences between treatments were observed on measures of negative effects, with IR oxycodone showing significantly greater scores on the bad‐effects visual analog scale Emax compared with intact oxycodone DETERx fasted and chewed oxycodone DETERx (high‐fat, high‐calorie meal) (P < .05 for both). Similar patterns were seen for sick visual analog scale, nausea visual analog scale, and sleepy visual analog scale, with most significant differences observed between oxycodone DETERx treatments and IR oxycodone. The any‐effect visual analog scale Emax scores also showed significantly lower scores for oxycodone DETERx treatments compared with IR oxycodone (P < .0001 for all). For global measures, mean overall drug‐liking visual analog scale Emax scores were significantly higher for IR oxycodone compared with intact oxycodone DETERx treatments (fasted and high‐fat, high‐calorie meal) and chewed oxycodone DETERx after a high‐fat, high‐calorie meal (P < .01 for intact oxycodone DETERx after a high‐fat, high‐calorie meal; P < .05 for intact fasted oxycodone DETERx and chewed oxycodone DETERx after a high‐fat, high‐calorie meal). No significant differences were observed between any of the active treatments on the take‐drug‐again visual analog scale.
The least‐squares means of the drug‐liking area under the drug effect curve (AUE) and feeling‐high AUE over the first hour support the primary outcome result demonstrating lower abuse potential of oxycodone DETERx when compared with IR oxycodone (Figure 3). IR oxycodone exhibited increased drug‐liking AUE when compared with oxycodone DETERx after only 1 hour postdose, an effect that was more pronounced in feeling‐high AUE measurements.
Figure 3.
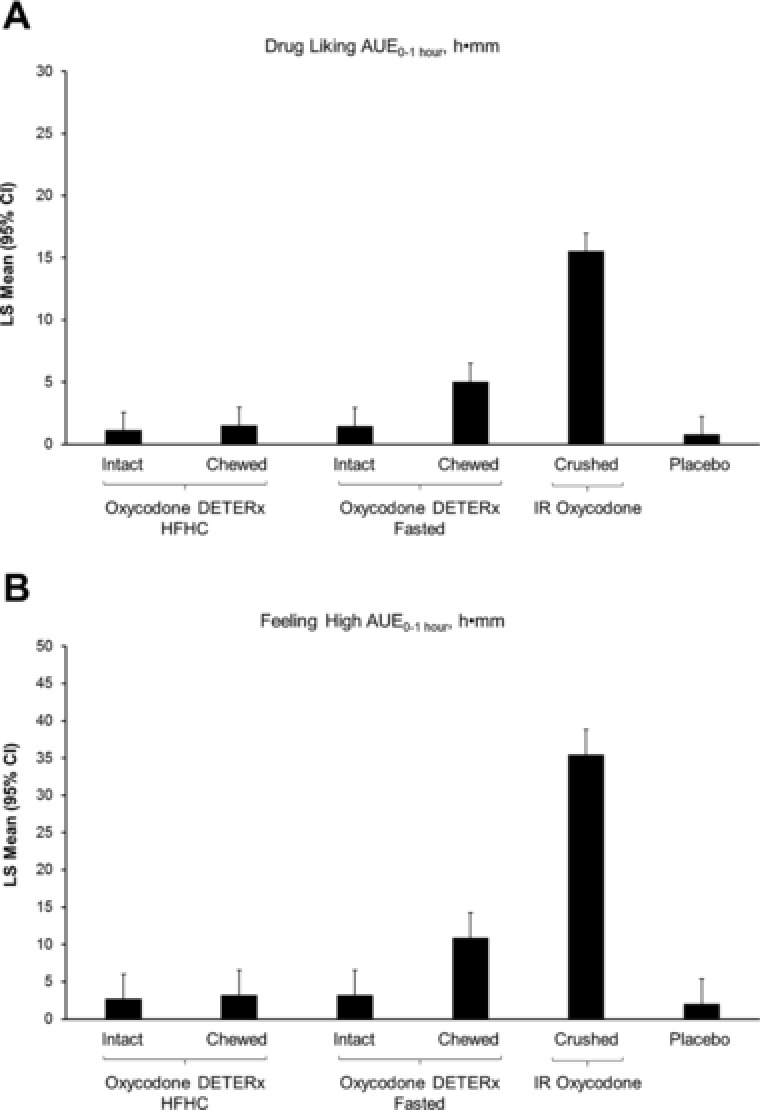
Least‐squares means (95% confidence interval) of the principal drug‐liking parameters. Drug‐liking AUE0‐1 hour (A) and feeling‐high AUE0‐1 hour (B). AUE, area under the drug effect curve; CI, confidence interval; HFHC, high‐fat, high‐calorie meal; IR, immediate‐release; LS, least‐squares.
Figure 4 presents the proportion of responders by percentage reduction deciles for drug‐liking visual analog scale Emax for the chewed and intact oxycodone DETERx treatments relative to IR oxycodone. After administration of chewed oxycodone DETERx, high‐fat, high‐calorie meal and fasted, 78.9% and 65.8% of subjects, respectively, showed some reduction (greater than 0) in Emax scores for the drug‐liking visual analog scale compared with IR oxycodone. These values were similar in magnitude to the 86.8% and 81.6% of subjects who showed some reduction (greater than 0) in drug‐liking visual analog scale scores compared with IR oxycodone after administration of intact oxycodone DETERx, high‐fat, high‐calorie meal and fasted, respectively. The same percentage of subjects showed greater than 30% reduction in Emax scores with intact and chewed oxycodone DETERx (high‐fat, high‐calorie meal) (47.4% for both) compared with IR oxycodone, demonstrating that particularly under high‐fat, high‐calorie meal conditions, reductions in drug liking were maintained for manipulated oxycodone DETERx compared with taking oxycodone DETERx intact.
Figure 4.
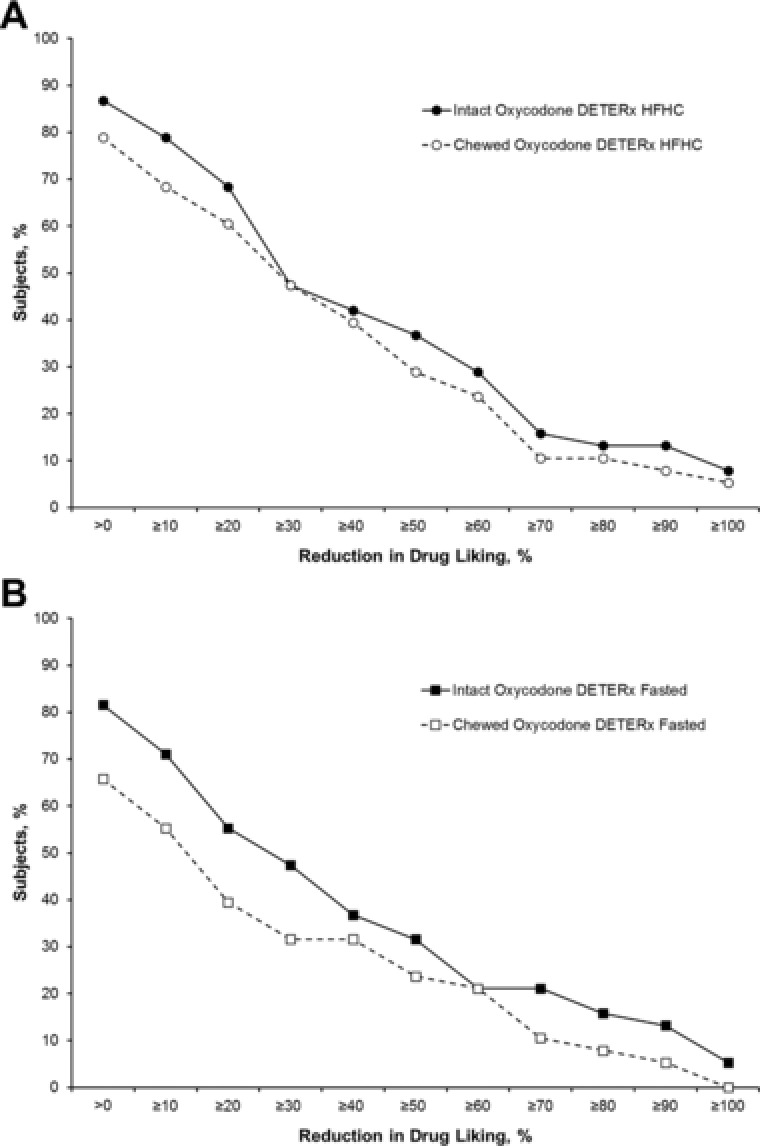
Proportion of responders for drug‐liking visual analog scale Emax for high‐fat, high‐calorie fed (A) and fasted subjects (B). Emax, maximum (peak) effect; HFHC, high‐fat, high‐calorie meal.
Safety
Single 36‐mg oral doses of intact and chewed oxycodone DETERx administered after a high‐fat, high‐calorie meal and fasted were well tolerated, as were equivalent doses of crushed IR oxycodone administered in solution. Most treatment‐emergent adverse events were assessed as mild or moderate in severity and were typical of known opioid pharmacological effects. Overall, the highest incidence of treatment‐emergent adverse events was observed after administration of crushed IR oxycodone (61%), followed by chewed oxycodone DETERx after a high‐fat, high‐calorie meal (52%), intact oxycodone DETERx after a high‐fat, high‐calorie meal (49%), chewed oxycodone DETERx fasted (38%), and intact oxycodone DETERx fasted (36%). The incidence of treatment‐emergent adverse events was lowest after administration of placebo (6%). Generalized pruritus was the most common treatment‐emergent adverse event (Table 4); the incidence was highest after administration of IR oxycodone (37%, 19 subjects). Other commonly reported treatment‐emergent adverse events (>5%) included vomiting, headache, nausea, somnolence, dizziness, and pruritus.
Table 4.
Summary of Adverse Events Reported by ≥5% of Subjects for Any Treatment
| Subjects, n (%) | ||||||
|---|---|---|---|---|---|---|
| Preferred Term | Intact Oxycodone DETERx HFHC (n = 45) | Intact Oxycodone DETERx Fasted (n = 45) | Chewed Oxycodone DETERx HFHC (n = 48) | Chewed Oxycodone DETERx Fasted (n = 48) | Crushed IR Oxycodone (n = 51) | Placebo (n = 47) |
| Pruritus, generalized | 10 (22) | 5 (11) | 11 (23) | 6 (13) | 19 (37) | 0 |
| Vomiting | 9 (20) | 5 (11) | 9 (19) | 2 (4) | 11 (22) | 1 (2) |
| Headache | 7 (16) | 5 (11) | 9 (19) | 5 (10) | 6 (12) | 0 |
| Nausea | 4 (9) | 1 (2) | 6 (13) | 5 (10) | 5 (10) | 0 |
| Somnolence | 1 (2) | 3 (7) | 3 (6) | 1 (2) | 3 (6) | 0 |
| Dizziness | 1 (2) | 1 (2) | 4 (8) | 2 (4) | 3 (6) | 0 |
| Pruritus | 0 | 2 (4) | 0 | 2 (4) | 3 (6) | 0 |
HFHC, high‐fat, high‐calorie meal; IR, immediate‐release.
Although most of the treatment‐emergent adverse events reported were of mild to moderate severity, 2 subjects experienced severe treatment‐emergent adverse events (headache and pruritus). Nine subjects were withdrawn from the study due to treatment‐emergent adverse events, 7 of whom were withdrawn due to emesis, a per protocol exclusion criterion, which occurred within 6 hours after dosing. The 2 other subjects were withdrawn for irritability and toothache. None of the subjects experienced serious treatment‐emergent adverse events. There were no clinically significant treatment‐related changes in clinical laboratory results, vital sign measurements, blood oxygen saturation levels, or physical examination findings.
Discussion
This is the first human oral abuse potential study for oxycodone DETERx assessing the effect of chewing on abuse potential. The pharmacokinetic data show that chewing does not compromise the ER characteristics of the formulation. Moreover, the pharmacodynamic data show that there is a significantly lower abuse potential for both chewed and intact oxycodone DETERx compared with the control, IR oxycodone. Statistically significant differences in abuse potential in clinical studies have been associated with clinical importance.27, 28 However, the impact of oxycodone DETERx as an abuse‐deterrent formulation will not be fully established until it has been available on the market, allowing for confirmation that a lower abuse potential in controlled studies translates to lower rates of abuse in the community.
Comparisons of pharmacokinetic parameters for the oxycodone DETERx treatments demonstrate that intact and chewed oxycodone DETERx treatments were bioequivalent with respect to Cmax, AUC0‐t, and AUC0‐∞ and had comparable values for Tmax and abuse quotient. These results are consistent with those in a recent pharmacokinetic study conducted with oxycodone DETERx, which reported that manipulation of oxycodone DETERx by crushing did not significantly change the oxycodone pharmacokinetic profile when compared with intact oxycodone DETERx.17 Peak exposure to oxycodone was lower, and Tmax was delayed (5 hours and 3 hours for high‐fat, high‐calorie fed and fasted treatment groups, respectively, vs 1 hour postdose for IR oxycodone) after administration of chewed oxycodone DETERx in comparison to IR oxycodone. This delay in peak exposure suggests that the ER properties were not affected by manipulation (ie, there was no dose dumping).
Subjective measures, particularly the assessment of drug liking, are considered the most sensitive measures of abuse potential.18, 22 Results demonstrated that despite chewing, oxycodone DETERx administered fasted or after a high‐fat, high‐calorie meal showed significantly less drug liking compared with IR oxycodone. Secondary pharmacodynamic measures (eg, feeling high, good effects, and Addiction Research Center Inventory/Morphine Benzedrine Group scores) also demonstrated a lower abuse potential for intact and chewed oxycodone DETERx when compared with IR oxycodone. In addition, chewed oxycodone DETERx administered fasted was associated with significantly less pupil constriction, indicating less central exposure to oxycodone after chewing. When oxycodone DETERx was administered as intended (intact capsules), it also showed lower scores on positive subjective measures compared with IR oxycodone. Fewer statistically significant differences were observed on the “next day measures” of global effect (ie, overall drug‐liking visual analog scale and take‐drug‐again visual analog scale) when comparing oxycodone DETERx treatments with IR oxycodone, which was not unexpected. These scales, which were administered at the end of the sampling period, are thought to capture a subject's overall perception of the drug‐taking experience, taking into account the entire profile of the drug's effect (both positive and negative effects); this often results in lower and more variable scores (caused by recall bias) than scores typically observed on the drug‐liking visual analog scale, which may account for the lack of significance in some measures.
According to the Food and Drug Administration guidance on the assessment of abuse‐deterrent opioid products, an analysis of the percentage reduction in drug liking observed for each individual subject for the test product relative to the control product should be performed.18 This approach is intended to provide supportive data on the relative effect of the deterrent features of an abuse‐deterrent formulation and may provide more context to the broader claim that mean Emax scores were statistically significant. Comparisons among the different oxycodone DETERx administration conditions suggest that reductions in drug liking relative to IR oxycodone were minimally affected by manipulating oxycodone DETERx. For example, when drug was administered with a high‐fat, high‐calorie meal, the percentage of subjects who showed at least a 30% reduction (47%) in drug liking was identical across the chewed and intact conditions. Furthermore, administration of chewed or intact oxycodone DETERx under both high‐fat, high‐calorie fed and fasted conditions resulted in similar percentages of subjects who showed some reduction (greater than 0) in Emax scores for drug liking visual analog scale; however, for both the intact and chewed conditions, administration with a high‐fat, high‐calorie meal resulted in a greater reduction in drug‐liking scores. These findings are consistent with the results of the primary analysis.
The selected treatment groups evaluated the abuse potential of oxycodone DETERx via the oral route, both intact and with manipulation by chewing. The study design is relevant because oral administration is among the most widespread types of abuse, particularly for oxycodone, hydrocodone, and methadone products.29 Furthermore, because of the larger amounts of opioid in ER formulations, drug users will often chew or crush a product to increase the onset and magnitude of drug effects presumed to be reinforcing. Recent data30 suggest that despite the introduction of hard‐to‐crush tablets, manipulation (including chewing) is common before oral administration by abusers. Investigators found a prevalence rate of 41.5 of 100 oral abusers of crush‐resistant tablet products (which included reformulated oxycodone, tapentadol, and reformulated oxymorphone) reporting manipulation of the product before oral administration; crush‐resistant formulations were significantly more likely to be chewed than were all other non‐crush‐resistant formulation comparators examined.30 Therefore, in the current study, chewing represents a practical and common method of defeating the ER properties of an opioid product that might be employed by recreational drug users.
Limitations of the study include the fact that the pharmacokinetic and pharmacodynamic evaluations of oxycodone DETERx were assessed under various conditions after only 1 dose of each treatment. In addition, the sample size was relatively small, and although the study was designed to minimize bias, confounding, and intersubject variability, this study may have resulted in a cohort of subjects who are not entirely representative of all recreational opioid abusers. Nevertheless, the results of this study suggest that chewing has a minimal effect on the pharmacodynamic and pharmacokinetic profile of oxycodone DETERx.
This human abuse liability study was designed to assess the potential for abuse of oxycodone DETERx intact or after tampering—this study was not an assessment of the addiction potential of oxycodone DETERx in the population. Addiction potential can only be addressed after an abuse‐deterrent formulation has been introduced into the market and is assessed in epidemiological studies.
Another objective of the current study was to evaluate the safety of orally administered intact and chewed oxycodone DETERx. Overall, single doses of oxycodone DETERx were generally safe and well tolerated. Most treatment‐emergent adverse events were mild or moderate in severity and were typical of known opioid effects. Immediate‐release oxycodone was associated with the highest incidence of treatment‐emergent adverse events; minimal differences were seen in incidence of treatment‐emergent adverse events across oxycodone DETERx treatments. These findings suggest that manipulating oxycodone DETERx and administering the product with food or fasted does not affect the overall safety profile compared with taking the capsule whole.
Conclusions
This study showed that the human abuse potential of oxycodone DETERx was similar before and after physical manipulation (ie, chewing). Chewing oxycodone DETERx did not change the pharmacokinetic profile, which supports the lower drug liking of intact and chewed oxycodone DETERx when compared with IR oxycodone. These data indicate that chewing oxycodone DETERx does not compromise the ER nature of the DETERx formulation, thereby mitigating the potential for increased drug liking on tampering and protecting against dose dumping and an associated potential fatal release of a bolus dose of oxycodone.
Funding
This study was funded by Collegium Pharmaceutical Inc. Medical writing assistance was funded by Collegium Pharmaceutical Inc, and was provided by The JB Ashtin Group, Inc.
Declaration of Conflicting Interests
E.A.K., A.B.F., and M.O. are full‐time employees of Collegium Pharmaceutical Inc and hold stock and/or stock options. N.L.C. is an employee of Altreos Research Partners Inc. E.S. is a principal in DL Global Partners Inc.
Acknowledgments
This study was supported by Collegium Pharmaceutical Inc, Canton, Massachusetts. Medical writing assistance was provided by Kelly M. Cameron, PhD, of The JB Ashtin Group, Inc, who, on behalf of Collegium Pharmaceutical Inc, provided editorial assistance and implemented author revisions after the authors developed the first draft. All authors reviewed and approved the final version of the manuscript.
References
- 1. McCarberg BH, Barkin RL. Long‐acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther. 2001;8(3):181‐186. [DOI] [PubMed] [Google Scholar]
- 2. Nicholson B. Benefits of extended‐release opioid analgesic formulations in the treatment of chronic pain. Pain Pract. 2009;9(1):71‐81. [DOI] [PubMed] [Google Scholar]
- 3. Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287‐313. [DOI] [PubMed] [Google Scholar]
- 4. Marsch LA, Bickel WK, Badger GJ, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self‐reported measures in humans. J Pharmacol Exp Ther. 2001;299(3):1056‐1065. [PubMed] [Google Scholar]
- 5. de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl). 1992;107(2‐3):352‐358. [DOI] [PubMed] [Google Scholar]
- 6. Schoedel KA, McMorn S, Chakraborty B, Potts SL, Zerbe K, Sellers EM. Positive and negative subjective effects of extended‐release oxymorphone versus controlled‐release oxycodone in recreational opioid users. J Opioid Manag. 2011;7(3):179‐192. [DOI] [PubMed] [Google Scholar]
- 7. Romach MK, Schoedel KA, Sellers EM. Update on tamper‐resistant drug formulations. Drug Alcohol Depend. 2013;130(1‐3):13‐23. [DOI] [PubMed] [Google Scholar]
- 8. Schaeffer T. Abuse‐deterrent formulations, an evolving technology against the abuse and misuse of opioid analgesics. J Med Toxicol. 2012;8(4):400‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cicero TJ, Ellis MS, Surratt HL. Effect of abuse‐deterrent formulation of OxyContin [correspondence]. N Engl J Med. 2012;367(2):187‐189. [DOI] [PubMed] [Google Scholar]
- 10. Larochelle MR, Zhang F, Ross‐Degnan D, Wharam JF. Rates of opioid dispensing and overdose after introduction of abuse‐deterrent extended‐release oxycodone and withdrawal of propoxyphene. JAMA Intern Med. 2015;175(6):978‐987. [DOI] [PubMed] [Google Scholar]
- 11. Kunins HV. Abuse‐deterrent opioid formulations: part of a public health strategy to reverse the opioid epidemic. JAMA Intern Med. 2015;175(6):987‐988. [DOI] [PubMed] [Google Scholar]
- 12. Coplan PM, Chilcoat HD, Butler SF, et al. The effect of an abuse‐deterrent opioid formulation (OxyContin) on opioid abuse‐related outcomes in the postmarketing setting. Clin Pharmacol Ther. 2016;100(3):275‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pergolizzi JV Jr., Taylor R Jr., Nalamachu S, et al. Challenges of treating patients with chronic pain with dysphagia (CPD): physician and patient perspectives. Curr Med Res Opin. 2014;30(2):191‐202. [DOI] [PubMed] [Google Scholar]
- 14. Webster LR, Bath B, Medve RA, Marmon T, Stoddard GJ. Randomized, double‐blind, placebo‐controlled study of the abuse potential of different formulations of oral oxycodone. Pain Med. 2012;13(6):790‐801. [DOI] [PubMed] [Google Scholar]
- 15. Kadian (morphine sulfate extended‐relase capsules, for oral use, CII) . Morristown, NJ: Actavis Pharma Inc; 2014. http://www.allergan.com/assets/pdf/kadian_pi. Accessed March 2016.
- 16. Kopecky EA, Fleming AB, Noonan PK, et al. Impact of physical manipulation on in vitro and in vivo release profiles of oxycodone DETERx(R): an extended‐release, abuse‐deterrent formulation. J Opioid Manag. 2014;10(4):233‐246. [DOI] [PubMed] [Google Scholar]
- 17. Gudin J, Levy‐Cooperman N, Kopecky EA, Fleming AB. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended‐release oxycodone formulations with abuse‐deterrent properties. Pain Med. 2015;16(11):2142‐2151. [DOI] [PubMed] [Google Scholar]
- 18. Food and Drug Administration . CDER Guidance for Industry: Abuse‐deterrent opioids—evaluation and labeling. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm334743.pdf. Published 2015. Accessed March 2016.
- 19. Food and Drug Administration . CDER Guidance for Industry: Food‐effect bioavailability and fed bioequivalence studies. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM126833.pdf. Published 2002. Accessed March 2016.
- 20. Harris SC, Perrino PJ, Smith I, et al. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse‐deterrent controlled‐release tablets in recreational opioid users. J Clin Pharmacol. 2014;54(4):468‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Food and Drug Administration . CDER Guidance for Industry: Assessment of abuse potential of drugs (draft). http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM198650.pdf. Published 2010. Accessed March 2016.
- 22. Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(3 Suppl):S41‐54. [DOI] [PubMed] [Google Scholar]
- 23. Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1(6):80‐83. [Google Scholar]
- 24. Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52:591‐611. [Google Scholar]
- 25. Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Statist. 1963;34(2):598‐611. [Google Scholar]
- 26. Moses LE. Rank test of dispersions. Ann Math Statist. 1963;34(3):973‐983. [Google Scholar]
- 27. Comer SD, Zacny JP, Dworkin RH, et al. Core outcome measures for opioid abuse liability laboratory assessment studies in humans: IMMPACT recommendations. Pain. 2012;153(12):2315‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eaton TA, Comer SD, Revicki DA, et al. Determining the clinically important difference in visual analog scale scores in abuse liability studies evaluating novel opioid formulations. Qual Life Res. 2012;21(6):975‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler SF, Black RA, Fleming AB. Relative abuse of abuse deterrent formulations via alternative oral routes. Presented at: 35th Annual Scientific Meeting of the American Pain Society; May 11‐14, 2016; Austin, TX.


