Abstract
Aim
In Type 2 diabetes, there is no clear understanding of how people perceive their risk of experiencing diabetes‐related complications. To address this issue, we undertook an evidence‐based synthesis of how people with Type 2 diabetes perceive their risk of complications.
Methods
We performed a systematic search of nine electronic databases for peer‐reviewed articles published on or before 1 March 2016. Data from 18 studies reporting lay perceptions of risks for complications in Type 2 diabetes populations were included. Publication year ranged between 2002 and 2014.
Results
Methods used to assess risk perceptions were heterogeneous, ranging from questionnaires measuring the accuracy of perceived risks to semi‐structured and focus group interviews. We found evidence of low risk awareness in most dimensions of risk perceptions measured and the existence of optimistic bias.
Conclusions
Perceptions were generally biased and varied according to the dimension of risk measured, the subpopulation concerned and the type of complications considered. Future work is needed to identify the best practical ways of correcting for biased risk perceptions so as to encourage self‐care behaviours and treatment adherence.
What's new?
This systematic review is the first to provide an evidence‐based synthesis of risk perceptions for diabetes‐related complications in Type 2 diabetes populations.
The review highlights the large heterogeneity in study designs and methods used to assess risk perceptions.
Risk perceptions of people with Type 2 diabetes are generally biased, characterized by low risk awareness with most measurement methods and contaminated by optimistic bias.
The specific biases identified in this review will help design more effective risk communication interventions.
What's new?
This systematic review is the first to provide an evidence‐based synthesis of risk perceptions for diabetes‐related complications in Type 2 diabetes populations.
The review highlights the large heterogeneity in study designs and methods used to assess risk perceptions.
Risk perceptions of people with Type 2 diabetes are generally biased, characterized by low risk awareness with most measurement methods and contaminated by optimistic bias.
The specific biases identified in this review will help design more effective risk communication interventions.
Introduction
Self‐care behaviour is a necessary requirement for the management of Type 2 diabetes. Although many factors are known to influence health behaviours, risk perceptions are thought to play a key role in the behavioural process. People who underestimate their risks may be less likely to adopt recommended behaviours, while overestimating their risks may cause so much anxiety that it hampers people from taking precautionary measures 1. There is strong evidence supporting the causality of the association between perceived risks and precautionary behaviours; empirical data have shown a highly significant, albeit small‐to‐moderate, positive association 2, 3, 4. In addition, perceived risks are significant predictors of behavioural intentions 5, 6. Although behavioural processes are complex, wrong risk perceptions are a major impediment to the adoption of self‐care behaviours and, as a result, an additional risk for the occurrence of adverse outcomes.
In different disease areas, regardless of the dimension of perceived risk measured, there is strong evidence that people's risk perceptions ‘often go awry’ 7. For example, a substantial body of literature has examined risk perceptions of people concerned by genetic counselling. Most studies have reported erroneous perceptions of the risks of developing inherited disorders—in particular cancers—with a strong tendency of people to overestimate their risks 8. Although this may result from a lack of information, personal factors (e.g. numeracy, socio‐economic status, type of condition) and bounded rationality (e.g. the tendency of people to overestimate the occurrence of low‐probability events) are thought to bias the cognitive assessment process of risk estimates. In the area of Type 2 diabetes, however, there is still no clear understanding of how people perceive their risk of experiencing complications. In order to best inform structured education programmes and improve the design of tailored risk communication interventions, we report an evidence‐based synthesis of how people with Type 2 diabetes perceive their risks for complications. Identifying particular trends and/or systematic biases associated with their perceptions allows targeted and more effective risk communication interventions to be developed.
Methods
Search strategy and selection criteria
We conducted a systematic review to identify all studies that investigated lay risk perceptions for diabetes‐related complications in people with Type 2 diabetes. We performed the review in accordance with the PRISMA Statement methodology checklist 9.
We searched nine electronic databases (MEDLINE, Embase, Global Health, PsycINFO, EconLit, Scopus, Web of Science, International Bibliography of the Social Sciences and Cochrane Library) for peer‐reviewed articles published on or before 1 March 2016. The search strategy consisted of different combinations of keywords related to (a) risk perceptions (e.g. awareness, susceptibility, likelihood); and (b) diabetes (e.g. Type 2 diabetes, t2dm, non‐insulin dependent diabetes). Full details are given in the online Supporting Information.
Two inclusion criteria were applied, the study had to: (a) include people diagnosed with Type 2 diabetes and (b) examine lay risk perceptions for diabetes‐related complications (i.e. perceptions not influenced by a preliminary risk communication intervention). We excluded studies that: (a) were not peer‐reviewed articles; (b) included people with Type 1 diabetes or without diabetes; (c) reported perceptions measured after a risk communication intervention; and (d) reported perceptions of diabetes treatment benefits and risks, or general perceptions about Type 2 diabetes. Screening by title, abstract and full‐texts was undertaken independently by two reviewers (TR, SK). Results were compared and any disagreements regarding inclusion were resolved by consensus.
Data extraction and quality assessment
For each study that met the inclusion criteria, we extracted information on sample size, population characteristics, types of perceived risks measured, types of actual risks used as comparators (when appropriate) and outcomes. These elements were summarized in tabular form.
Two reviewers (TR, SK) independently assessed the quality of identified studies using two quality assessment tools. For qualitative studies without a quantitative component, we used the Critical Appraisal Skills Programme (CASP) Qualitative Checklist 10. Each of the 10 items and the overall quality were rated as good, fair or poor. A study received a good global rating if it had no poor item ratings or at most one fair item rating, a fair global rating if it had one poor item rating or at most three fair item ratings, and a poor global rating if it had two or more poor item ratings or four or more fair item ratings. Other studies were evaluated using a simplified version of the Quality Assessment Tool (QAT) for Observational Cohort and Cross‐Sectional Studies (those items not considered relevant to the design of included studies were removed) 11, which is an instrument designed to assess risk of bias and methodological quality of cohort and cross‐sectional studies. The score on each item of the tool allowed computation of an overall quality score in accordance with the QAT guidance. Similarly, the overall quality of a study was rated as good, fair, or poor, using the same rating system.
Data synthesis and analysis
We found significant heterogeneity in the methods and outcomes reported in the studies. Hence, we were unable to perform a quantitative synthesis with meta‐analyses. For this reason, we qualitatively synthesized results for each study within summary tables, in order to highlight similarities and differences among studies that addressed similar research questions.
Risk perception is a complex construct that covers different dimensions. Perceived risks may be absolute, comparative or conditional, depending on how they are measured. In the literature, it has been shown that there is limited overlap across the three dimensions 12; it is thus important to distinguish them and analyse each dimension separately. We classified the selected studies according to their ‘outcomes’, i.e. to the specific dimension(s) of perceived risks they assessed. In total, 23 outcomes were identified; we allocated them into five categories. First, ‘absolute accuracy’ refers to the difference between perceived and actual absolute risks. An absolute risk refers to the likelihood of experiencing an outcome over a specific period (e.g. ‘I think that my risk of developing a complication within the next 10 years is 10%’). Second, studies measuring comparative risk were labelled as studies of ‘optimistic bias’. A comparative risk refers to how likely a person is to experience a hazard compared with another person or compared with the average person 7. It may be direct, in which case an individual is asked to evaluate their own risk relative to an average person (e.g. ‘I think that my risk of developing a complication is above average’), or indirect, which requires separate judgements of one's own absolute risk and others’ absolute risk (e.g. ‘I think that my risk of developing a complication is 10%, but that the average risk is only 5%’). An indirect comparative risk is then computed as a difference score (own absolute risk – others’ absolute risk) 13. The term ‘optimistic bias’ refers to the tendency that people have to underestimate their risks of developing health problems, compared with their peers 14. Third, some studies investigated multiple aspects of risk perceptions, either using questionnaires that measure several dimensions of perceived risks (and then generate an overall risk perception score) or through qualitative studies. Last, the ‘complementary evidence’ category includes studies that focused on a very specific aspect of risk perceptions—for example, the measurement of perceived conditional risks (e.g. ‘I think that my risk of developing a complication within the next 10 years is 5%, providing that I start exercising’)—or studies providing valuable information that cannot be classified into one of the four previous categories.
Results
Search results
A total of 5482 references were obtained from the search strategy. After eliminating duplicate records (n = 3373), 2109 references remained. These records were all screened and 2087 irrelevant studies were excluded. The remaining 22 full‐text articles were assessed for eligibility. Of these, eight studies did not meet the inclusion criteria. In addition, the cited references of the 14 selected articles were searched for further studies of interest. Four new studies met the inclusion criteria, bringing the total to 18 studies included in the review 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, providing information for 23 different outcomes (see Fig. 1). Extracted data are summarized in Table 1.
Figure 1.
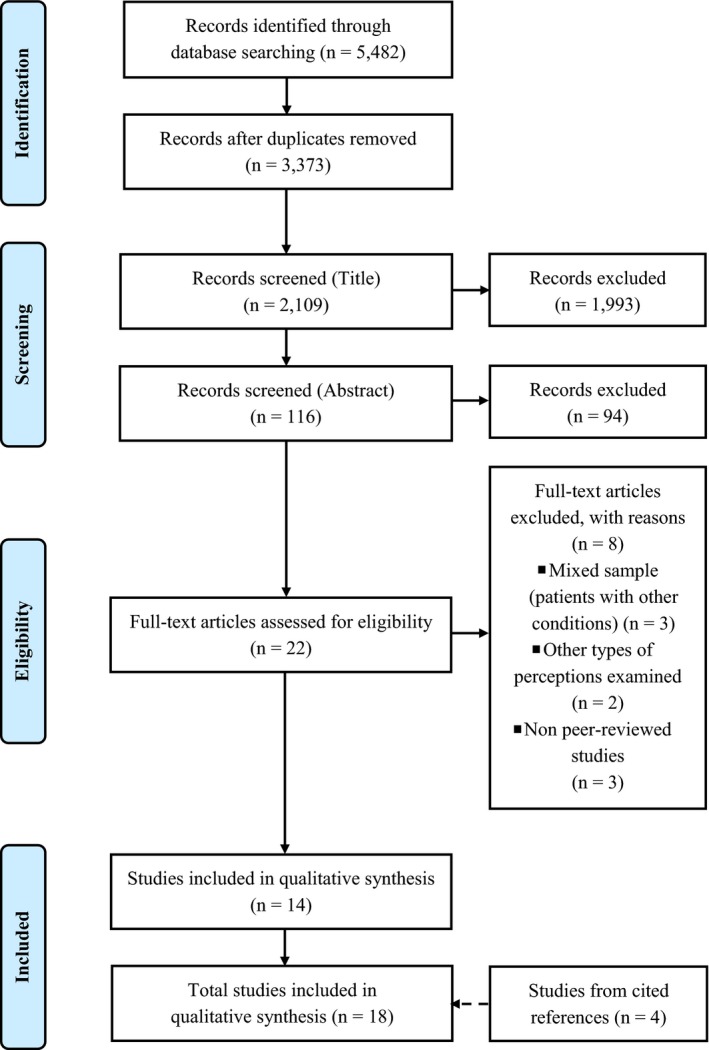
PRISMA statement flow diagram: summary of systematic search and review process.
Table 1.
Studies included in the review
| Authors | Population | Perceived risks | Comparator | Outcomes |
|---|---|---|---|---|
| A. Studies investigating absolute accuracy | ||||
| Allen et al. (2010) 15 | (a) n = 143. (b) Urban, low SES, middle‐aged Black women. (c) No CVD. (d) At least one additional CV risk factor | Own absolute lifetime risk of CVD (numerical) | Based on physiological measures | Overestimation |
| Asimakopoulou et al. (2008) 16 | (a) n = 95. (b) No CVD | Own absolute lifetime risk of CHD (numerical) | UKPDS‐OM risk estimate | Overestimation (factor 3.5) |
| Own absolute lifetime risk of stroke (numerical) | UKPDS‐OM risk estimate | Overestimation (factor 5.5) | ||
| Frijling et al. (2004) 20 | (a) n = 450. (b) No atherosclerotic disease | Own absolute 10‐year risk of MI (numerical) | Framingham risk score | Overestimation (factor 4) |
| Own absolute 10‐year risk of stroke (numerical) | Framingham risk score | Overestimation (factor 4.7) | ||
| Hoffmann and Del Mar (2012) 21 | n = 91 | Average absolute 15‐year risk of diabetes‐related eye complication (numerical) | Klein et al. 33 | Underestimation (factor 2.3) |
| Martell‐Claros et al. (2011) 25 | (a) n = 511. (b) Diagnosed for < 1 year. (c) No history of CV event or disease. (d) No insulin therapy | Own absolute lifetime risk of CVD (verbal) | Based on physiological measures (ESH‐ESC 2007 guidelines) | No agreement between perceived and calculated risks |
| Portnoy et al. (2014) 28 | (a) n = 83. (b) Diagnosed for at least 1 year. (c) No history of CV event or CHD | Own absolute 10‐year/lifetime risk of developing/dying from CHD (numerical) | UKPDS‐OM risk estimate | No agreement between perceived and calculated risks |
| Welschen et al. (2012) 32 | (a) n = 204. (b) No history of CVA or TIA | Own absolute 10‐year risk of CVD, after being told estimates for average diabetic men and women (numerical) | UKPDS‐OM risk estimate | No agreement between perceived and calculated risks |
| B. Studies investigating optimistic bias | ||||
| Choi et al. (2008) 19 | (a) n = 143. (b) Diagnosed for at least 1 year. (c) Korean immigrants | Own risk perception score for CHD (optimistic bias section of the B&L index, verbal) | Perceived risk attributed to people of similar age and sex in the general population | Underestimation (optimistic bias) |
| Homko et al. (2010) 22 | (a) n = 211. (b) No history of CVD. (c) At least 10% 10‐year risk of CVD (Framingham risk score). (d) No ESRD or dialysis. (e) No participants from nursing or boarding home | Own comparative 10‐year risk of CHD, high BP and stroke (S&R risk score, verbal) | Perceived risk attributed to peers without diabetes | Higher risk perception among women. No agreement between perceived risks and actual risks (Framingham risk score) |
| Portnoy et al. (2014) 28 | (a) n = 83. (b) Diagnosed for at least 1 year. (c) No history of CV event or CHD | Own comparative 10‐year/lifetime risk of developing/dying from CHD (numerical) | Perceived risk attributed to the average person | Slight overestimation (pessimism) |
| Walker et al. (2007) 31 | (a) n = 250. (b) Socially deprived. (c) No dilated eye examination undergone over the past year | Own comparative risk perception (optimistic bias section of the RPS‐DM, verbal) | Perceived risk attributed to diabetic peers | Higher optimistic bias in people born abroad and with lower education |
| C. Studies assessing a risk perception score | ||||
| Calvin et al. (2011) 17 | (a) n = 143. (b) Diagnosed for < 5 years. (c) Urban African Americans. (d) No known complication | Own risk perception score for complications (RPS‐DM) | Based on physiological measures | Low perception of risks |
| Choi et al. (2008) 19 | (a) n = 143. (b) Diagnosed for at least 1 year. (c) Korean immigrants | Own risk perception score for CHD (B&L index) | Based on physiological measures | Low perception of risks |
| D. Qualitative studies investigating risk perceptions | ||||
| Carroll et al. (2003) 18 | (a) n = 20. (b) Half of participants with CVD | Own lifetime risk of CVD (verbal, semi‐structured interview) | – | Low perception of the link between diabetes and CVD risk |
| Macaden and Clarke (2006) 24 | (a) n = 20. (b) South Asian older people living in northeast England | Perception of risks in relation to sociocultural factors (verbal, focus group interview) | – | Bias induced by sociocultural norms |
| McKenzie and Skelly (2010) 26 | (a) n = 6. (b) Diagnosed for at least 1 year. (c) Southern African American women. (d) No self‐reported CHD | Lifetime risk of CVD (verbal, semi‐structured interview) | – | Low perception of risks |
| Price et al. (2009) 29 | n = 16 | Risks of CHD (verbal, focus group interview) | – | Low perception of the link between diabetes‐related risk factors and CHD |
| E. Studies providing complementary evidence | ||||
| Kausar et al. (2013) 23 | (a) n = 100. (b) No history of psychiatric condition, cognitive impairment, any other chronic condition, terminal illness, speech or hearing problems, comorbidities such as hypertension and obesity | Own risk perception for complications (RPS‐DM) | – | Higher risk perceptions were associated with higher emotional distress; men had better knowledge of diabetes complications |
| Merz et al. (2002) 27 | n = 2008 | Own risks for CV complications (telephone survey) | – | Low perception of risks, especially in people aged > 65 years; higher risk perception for microvascular complications |
| Portnoy et al. (2014) 28 | (a) n = 83. (b) Diagnosed for at least 1 year. (c) No history of CV event or CHD | Own conditional 10‐year/lifetime risk of developing/dying from CHD (numerical, conditional on physical activity level) | UKPDS‐OM risk estimate | No agreement between perceived and calculated risks |
| Saver et al. (2014) 30 | (a) n = 202. (b) At least one additional CV risk factor | Ranking of six complications (including fatal events) by likelihood of occurrence | – | Underestimation of the likelihood of fatal events |
B&L index, Becker & Levine index 34; CHD, coronary heart disease; CV, cardiovascular; CVA, cardiovascular accident; CVD, cardiovascular disease; ESH–ESC, European Society of Hypertension–European Society of Cardiology 35; ESRD, end‐stage renal disease; MI, myocardial infarction; RPS‐DM, Risk Perception Survey – Diabetes Mellitus 31; SES, socio‐economic status; S&R risk score, Schwarzer & Renner's risk score 36; TIA, transient ischaemic accident, UKPDS‐OM, UK Prospective Diabetes Study – Outcomes Model (UKPDS 56).
Study characteristics
Publication year ranged from 2002 to 2014, emphasizing the recent nature of the research. Half of the studies were conducted in the USA 15, 17, 19, 22, 26, 27, 28, 30, 31, followed by the UK 16, 18, 24, 29, The Netherlands 20, 32, Australia, Pakistan and Spain 21, 23, 25. Sample sizes varied widely, from 6 to 2008 individuals (median 143). Some studies reported in‐depth semi‐structured interviews and thus focused on a restricted number of persons, whereas others were large‐scale studies with self‐reported questionnaires.
Study populations
The study populations consisted of people with Type 2 diabetes, by decreasing order of frequency: (a) with no cardiovascular disease (CVD)/coronary heart disease (CHD) or no history of CVD/CHD; (b) recently diagnosed with Type 2 diabetes; (c) socially deprived or from minority ethnic populations; and (d) with at least one additional cardiovascular risk factor, e.g. hypertension, hyperlipidaemia or self‐reported smoking. Two studies did not specify whether people included in the study population had Type 1 or Type 2 diabetes (or if the study population was composed of both types) 20, 27. Despite a potential violation of our inclusion criteria, we decided not to exclude them because of the high probability that their study populations be composed of people with Type 2 diabetes and their substantial sample sizes.
Methods used to evaluate risk perceptions
Of 18 studies, four were qualitative, i.e. studies that used a purely qualitative research design (semi‐structured or focus group interviews) 18, 24, 26, 29. We refer to the remaining 14 studies as ‘quantitative studies’. Of these, seven studies measured the absolute accuracy of risk perceptions, four studies investigated the existence of optimistic bias, two studies assessed a risk perception score and four studies provided complementary evidence. Among the studies that measured absolute accuracy and/or optimistic bias, numerical probability estimates (e.g. ‘I think that my risk of developing a complication within the next 10 years is 1%’) were used more frequently than verbal qualifiers (e.g. ‘I think that I am at moderate risk of developing a complication in the next 10 years’) (nine vs. four outcomes, respectively).
Types of actual risks used as comparator
Actual risks were used to measure absolute accuracy (nine outcomes), to interpret risk perception scores (two outcomes) and to compare with conditional risks (one outcome). Three types of actual risks were identified: personalized risk estimates calculated from simulation models (seven outcomes), risk estimates extracted from the literature (one outcome) and level of actual risk based on physiological measures (four outcomes). Of the four studies using calculated personalized risk estimates, two simulation models were identified: (a) the UK Prospective Diabetes Survey–Outcomes Model (UKPDS‐OM) 37, which provides risks for diabetes‐related complications based on a 20‐year follow‐up study (three studies 16, 28, 32); and (b) the Framingham Risk Score 38, which provides 10‐year risk estimates of developing cardiovascular diseases (one study 20). Of the four studies that estimated actual risks based on physiological measures, only one indicated clearly how participants were classified into different risk categories 25.
Types of complications
Perceived risks for developing macrovascular complications were more frequently examined than perceived risks for experiencing microvascular events. Twelve of the 18 studies focused exclusively on cardiovascular risks (e.g. risks of stroke or myocardial infarctions), one focused exclusively on microvascular risks (risks of eye complications) 21, and five studies focused on both 17, 23, 24, 30, 31.
Study quality
Of the four qualitative studies, two were rated as good, one was rated as fair and one was rated as poor. Results were also mixed regarding quantitative studies: 58% of these studies were found to be of poor quality, 21% of fair quality and 21% of good quality. In particular, most studies were rated poorly with regard to presentation of quantitative data (40% poor, 33% fair, 27% good) and data analysis (31% poor, 44% fair, 25% good). Full details are given in the online Supporting Information.
Outcomes
Absolute accuracy
Seven studies measured the absolute accuracy of risk perceptions 15, 16, 20, 21, 25, 28, 32, reporting either people's own (eight outcomes) or average (one outcome) perceived absolute risks. One study clearly reported quantitative estimates of the difference between actual and perceived risks 16, two studies provided enough information (either numerically 20 or graphically 21) for us to calculate it, and four studies did not report sufficient quantitative information for an estimate to be derived 15, 25, 28, 32. Results were mixed, but tended towards overestimation: overestimation (three studies), underestimation (one study) and absence of a significant correlation between actual and perceived risks (meaning that no inference can be made towards under‐ or overestimation; three studies) were reported. For example, Frijling et al. 20 showed that people overestimated their 10‐year risk of experiencing myocardial infarction (by a factor of 4) and stroke (by a factor of 4.7). Similar results were reported by Asimakopoulou et al. 16 and Allen et al. 15, in studies demonstrating that people were unrealistically pessimistic about their risks of developing both CHD and stroke (by factors of 3.5 and 5.5, respectively). Conversely, most people in Hoffmann and Del Mar 2012's study underestimated their risk of eye complications 21. In addition, three studies examining the association between perceived and actual risks found no evidence of correlation 25, 28, 32. Finally, some studies reported that several people were unable to provide any (numerical) estimates of perceived risks 15, 20.
Optimistic bias
Four studies assessed comparative perceived risks 19, 22, 28, 31, providing support to the existence of optimistic bias. Choi et al. 19, focusing on Korean immigrants in the USA, observed that people underestimated their risks for CHD compared with those of the average person. Walker et al. 31 showed that the optimistic bias tended to be higher in minority ethnic populations and in people with a lower level of education. In addition, Homko et al. 22 provided further evidence on gender‐based differences in risk factors and risk perceptions, finding that women perceived their risk for CVD to be significantly higher than did men. Finally, one study reported a slight overestimation of perceived comparative risks, when people were asked to compare themselves with the average person 28.
Risk perception scores
The two studies that used composite scores (combination of different items to compute a risk perception score) as a proxy for perceived risks focused specifically on urban, socially deprived or minority ethnic populations 17, 19. They reported low perception of risks for various diabetes‐related complications.
Qualitative studies
Despite differences in study design and small sample sizes, findings from qualitative studies tended to be consistent, highlighting low risk perceptions. First, a focus group interview showed that people with Type 2 diabetes often had a low perception of the link between diabetes‐related risk factors and CHD 29. Second, two semi‐structured interviews exploring lifetime risk perceptions of CVD in two different populations (African American women 26 and Caucasian, UK residents 18) pointed out that significant proportions of people did not even know that they were at risk. In addition, Macaden and Clarke 24, conducting a series of focus group and individual interviews, showed that sociocultural norms (e.g. beliefs, food and religion) had a significant influence on how certain populations underestimated risks.
Complementary evidence
Four studies provided complementary information on risk perceptions in people with Type 2 diabetes. In a large‐scale study (n = 2008) where the dimensions of perceived risks measured were not specified, Merz et al. 27 found that more than two thirds of the persons did not consider CVD to be a serious complication of diabetes, and that very few respondents were able to name an approach to reduce their risk. Perceived risks were slightly higher for microvascular complications, but again were largely underestimated overall, especially in minority ethnic populations. In a study led in Pakistan, Kausar et al. 23 reported that men had better knowledge of diabetes complications, and that higher perceived risks were associated with higher levels of emotional distress. In addition, Portnoy et al. 28 did not obtain more accurate results when comparing perceived conditional risks for CHD. Lastly, a study by Saver and colleagues 30, in which people were asked to rank six complications—including death—by likelihood of occurrence, showed that the probability of experiencing fatal events was largely underestimated.
Discussion
This systematic review provides an overview of risk perceptions for diabetes‐related complications in people with Type 2 diabetes. We found evidence that perceptions were generally biased, characterized by low risk awareness in most dimensions of perceived risks measured and contaminated by optimistic bias.
First, there was a clear lack of awareness concerning the risk of diabetes‐related complications. People, particularly those from ethnic minorities 17, 19, 26, were often unaware of their risks of experiencing complications, and especially for macrovascular events 18, 19, 26, 27, 29. In the largest study, nearly 70% of people with Type 2 diabetes did not realize that they were at increased risk for CVD 27. Two studies also showed that people had only a vague idea of (or ignored) the link between Type 2 diabetes and cardiovascular risk factors 20, 29. Such findings are consistent with research conducted in other populations 34, 39 and disease areas 40. Second, studies examining comparative risk perceptions revealed the existence of an optimistic bias 19, 22, 31. This is in line with evidence found for the general population 41, 42, 43, 44. However, some results also suggested that people tended to overestimate their risk of developing complication when the absolute dimension of perceived risks was measured. Three of seven studies measuring absolute perceived risks reported unrealistic pessimism 15, 16, 20. Although apparently inconsistent with previous findings, this trend has also been documented in the recent literature 45, 46 and should be interpreted with caution, as absolute perceived risks are likely to be strongly affected by numeracy levels. Hence, people with Type 2 diabetes had generally biased perceptions of their risks for complications, characterized by low risk awareness in most dimensions measured. The direction and magnitude of the biases were related to the dimension of perceived risks measured, the subpopulation concerned, and the type of complication considered.
A major finding of our work was the large heterogeneity in study designs and methods. Several dimensions of perceived risks (e.g. absolute, comparative, conditional) were measured using very different methods (e.g. self‐reported numerical probability estimate, risk perception score, focus group interview), and various comparators were used to estimate actual risks (e.g. personalized estimates drawn from simulation models, estimates found in the literature). The aims of the identified studies partially explain the heterogeneity in study design. Although all studies assessed lay risk perceptions, their primary objectives varied widely. For example, Homko et al. 22 focused on gender differences in cardiovascular risk factors and risk perceptions; whereas Hoffman and Del Mar 21 examined how people with Type 2 diabetes understand evidence‐based health information. Furthermore, the heterogeneity in measurement methods was even larger. Despite a substantial, still growing, body of literature on risk perceptions, there has been no consensus about best practices for measurement and operationalization so far 12. One complexity is that different dimensions of risk perceptions measured do not exactly overlap. In fact, recent evidence suggests that these dimensions are only moderately related and not interchangeable 13, 28, 47. Such heterogeneity made data extraction and assessment of study quality challenging.
However, despite the heterogeneity in study designs and measurement methods, inclusion criteria were consistent across selected studies. More than half of the studies examined perceived risks for macrovascular complications, emphasizing the importance of this issue for self‐care behaviours. Several studies also focused on socially deprived or minority ethnic populations with Type 2 diabetes. These populations are described as the most at risk of having uncontrolled diabetes 48, 49, 50. Finally, almost half of the studies included people who were recently diagnosed. This population, by being more likely to experience the asymptomatic phase of the condition, may have lower perceptions of their risk of experiencing complications. The rationale for including recently diagnosed people may be driven by the expectation of higher gains from providing a risk communication intervention. Indeed, such an intervention could be expected to have a higher impact on those populations than on populations who self‐manage their condition effectively, or on people who have already experienced a complication.
A major strength of this review is that we conducted a systematic search of nine databases and followed PRISMA Statement guidelines. The selected studies provided a comprehensive overview of risk perceptions in Type 2 diabetes populations; they were diverse in design, methods and populations (six countries were represented, including minority ethnic populations), but consistent in terms of inclusion criteria. In addition, we evaluated the quality of the studies by using two tailored assessment tools. Most studies presented a well‐defined research question, provided a good description of their inclusion criteria and explained precisely how they measured lay risk perceptions in people with Type 2 diabetes. Moreover, only two studies were funded 27 or involved researchers attached to pharmaceutical companies 25, which reduces the risk of publication bias. However, study limitations associated with other quality criteria should be noted. In studies that used actual risks as a comparator to perceived risks, we found that some did not describe how these actual risks were calculated or how they were attributed to participants 15, 17, 19. For these studies, we reported how the authors interpreted their data (e.g. ‘overestimation’) without being able to quantify the gap between actual and perceived risks. Also, we found that more than half of the studies did poorly in analysing and interpreting the data. For example, when analysing the absolute accuracy of risk perceptions, some studies only reported an ‘absence of association between perceived and actual risks’. This is mainly due to the heterogeneity in primary objectives across selected studies. Lay risk perceptions for complications were only a secondary outcome in some studies, and only partial analyses and interpretations were provided in that case. Another limitation of the review is that selected studies varied widely in terms of sample sizes (from 6 to 2008; median 143). Because of the heterogeneity in study designs and methods, we were unable to address quantitatively these variations in the analysis.
To our knowledge, we conducted the first systematic review of perceptions of risks for diabetes‐related complications in Type 2 diabetes populations. This review provides an evidence‐based synthesis of the trends and biases that characterize lay risk perceptions. We focused exclusively on lay risk perceptions because this allows identification of ‘genuine biases’, i.e. biases not contaminated by any type of communication intervention. Identifying such biases is crucial as a first step towards the development of new types of interventions specifically designed to address them. By reviewing this literature, we establish a reliable starting point for the development of new, tailored interventions based on what could be called ‘debiasedness strategies’.
Existing risk communication interventions have shown mixed results, with many participants barely understanding the explanations of health professionals about risks and having poor recall of risk information 30, 32, 51. To our knowledge, five studies have investigated the impact of risk communication interventions on risk perceptions in Type 2 diabetes populations. First, a high‐quality randomized controlled trial investigating the effects of CVD risk communication showed that the intervention (a thorough six‐step procedure) improved the appropriateness of risk perceptions after 2 weeks, but that its effect was gone after 12 weeks 32. Second, a study by Hoffman and Del Mar 21 reported that people kept underestimating their risk of eye complications after attending a one‐day diabetes educational expo. Third, Saver et al. 30, asking people to rank some complications by frequency of occurrence before and after they were provided with personalized risk estimates, reported that their intervention had no impact on risk perceptions. Conversely, two studies reported a significant impact on risk perceptions after the intervention. Using short time frames (1 and 5 years) to communicate risks of CHD and stroke, Asimakopoulou et al. 52 successfully improved the appropriateness of perceived risks 6 weeks after the intervention. A randomized controlled trial recently conducted in Egypt showed an improvement of the appropriateness of CVD risk perceptions after 12 weeks. However, it should be noted that participants in this trial were provided with repeated communications (at baseline, after 4 weeks and after 8 weeks) 53. In that regard, it seems reasonable to assert that existing interventions aiming at improving risk perceptions in populations with Type 2 diabetes are not fully satisfactory. In particular, no effect lasting longer than 6 weeks has been shown.
Moreover, studies including broader populations tend to confirm the need for better risk communication interventions. A study by van den Weijden et al. 54, who measured perceptions after people had discussed CVD risks with their general practitioner, highlighted a mismatch between perceived and actual risks. In particular, the authors insisted that incorrect perceptions among people with diabetes were striking. A systematic review assessing the effect of providing CHD risk information for adults without a history of CHD only showed a modest improvement in the accuracy of risk perceptions 55. Powers et al. 56, investigating the effects of both a standard and a personalized risk communication intervention for people with high risk of vascular events, found that perceived risks of CHD (stroke) were not more appropriate after immediately following the intervention or after 3 months. Finally, the results of a study by Koelewijn‐van Loon et al. 57, as noted by Welschen et al. 32, were promising, but they assessed the impact of a larger lifestyle intervention, making it difficult to untangle the effects of risk communication only.
Hence, in this context, the identification of biases characterizing lay risk perceptions provides valuable information to design better interventions, tailored for people with Type 2 diabetes. We think that identifying and addressing specific biases, wherever possible, will enhance the impact of risk communication interventions and help reduce misperceptions. As a result, we expect an improvement on self‐management behaviours. Ensuring that people have accurate perceptions of their risks for complications is, indeed, a prerequisite for the effective management of the condition. Spurious perceptions negatively impact self‐care behaviours. People who underestimate their risks, by feeling less vulnerable to the condition than they actually are, are less likely to comply with medical recommendations and engage in preventive behaviours 31, 51; by contrast, people with a low‐risk profile who overestimate their risks may develop unnecessary worries, which in turn might lead to overconsumption of healthcare services or, conversely, demotivate them from engaging in self‐care behaviour 16, 21, 32.
The rationale behind debiasedness strategies is to identify all levers for action that may support the delivery of risk information. For example, presenting the information through testimonials made by people who have already experienced a complication and who are similar to the people receiving that information (testimonials can be tailored to the sex, age and ethnic background of each person) may allow the optimistic bias issue to be addressed 58, 59. However, beyond the biases identified in this review, each characteristic of the targeted population may also be used as a lever for action. For instance, information materials can be tailored, as far as possible, to the numeracy level of individuals. Rather than increasing the quantity of information to be communicated (‘less is often more’ 60), it is necessary to improve its quality. The recent literature has encouraged this type of approach. Waters et al. 7, in particular, recommend that preliminary data be systematically collected, prior to any communication effort. New interventions based on such strategies are considered to be very promising in the risk communication field 61, 62. We believe that they will help to correct erroneous risk perceptions, and, in turn, have a greater impact on self‐care behaviours. However, one should note that if risk perceptions act as a key component for predicting and changing health behaviour, they are not the only factor specific to Type 2 diabetes populations that must be considered in practice. The recent literature on risk communication (see e.g. Ahmed et al. 63), as well as a better knowledge of other specific factors, including illness perceptions and perceptions of treatment benefits, would also add valuable inputs into the design of tailored risk communication interventions. We encourage further research on this topic.
Finally, we think that further research on patient risk perceptions would benefit from the emergence of a standardized method to measure perceived risks. Encouraging a certain ‘standardization’ in the way researchers measure risk perceptions would provide a reliable way of comparing studies investigating the impact of risk communication interventions, and thus help identify the most effective ones. In practice, we would encourage the design of standardized questionnaires assessing multiple dimensions of risk perceptions but generating a single risk perception score. Comparing studies investigating the impact of risk communication interventions on risk perceptions through the modification of this risk perception score would add clarity and ease to the assessment of such interventions. We also particularly recommend the use of personalized risk estimates as proxies for actual risks informed by validated prognostic models. Finally, we note that no studies included in the review reported uncertainty ranges along with the elicited perceived risks. Eliciting uncertainty ranges around patient absolute risk perceptions would add valuable information to assess the gap between perceived and actual risks. For the clinician, this would provide additional levers for action when designing effective risk communication interventions.
Funding sources
The research was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care Oxford at Oxford Health NHS Foundation Trust. The funding source had no involvement in the study design, the collection, analysis, and interpretation of the data, the writing of the report, or the decision to submit the paper for publication. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests
None declared.
Supporting information
Table S1. Study quality: studies assessed using the CASP.
Table S2. Study quality: studies assessed using an adapted version of the QAT.
Table S3. MEDLINE (via OvidSP) 1946 to February week 4, 2016.
Table S4. Embase (via OvidSP) 1974 to February week 4, 2016.
Table S5. Global Health (via OvidSP) 1973 to February week 4, 2016.
Table S6. PsycINFO (via OvidSP) 1967 to February week 4, 2016.
Table S7. SCOPUS (all years to 2016).
Table S8. EconLit (all years to 2016).
Table S9. IBSS (all years to 2016).
Table S10. Web of Science (all years to 2016).
Diabet. Med. 34, 467–477 (2017)
References
- 1. Cameron L. Conceptualizing and assessing risk perceptions: a self‐regulatory perspective. National Cancer Institute workshop on conceptualizing and measuring risk perception. Washington, DC, 2003. Available at http://cancercontrol.cancer.gov/brp/research/theories_project/cameron.pdf Last accessed 1 March 2016. [Google Scholar]
- 2. Sheeran P, Harris P, Epton T. Does heightening risk appraisals change people's intentions and behavior? A meta‐analysis of experimental studies. Psychol Bull 2014; 140: 511. [DOI] [PubMed] [Google Scholar]
- 3. Harrison J, Mullen P, Green L. A meta‐analysis of studies of the health belief model with adults. Health Educ Res 1992; 7: 107–116. [DOI] [PubMed] [Google Scholar]
- 4. Janz N, Becker M. The health belief model: a decade later. Health Educ Behav 1984; 11: 1–47. [DOI] [PubMed] [Google Scholar]
- 5. Floyd D, Prentice‐Dunn S, Rogers R. A meta‐analysis of research on protection motivation theory. J App Soc Psychol 2000; 30: 407–429. [Google Scholar]
- 6. Milne S, Sheeran P, Orbell S. Prediction and intervention in health‐related behavior: a meta‐analytic review of protection motivation theory. J Appl Soc Psychol 2000; 30: 106–143. [Google Scholar]
- 7. Waters E, McQueen A, Cameron L. Perceived risk and its relationship to health‐related decisions and behavior In: DiMatteo R, Martin L. eds. Handbook of Health Communication, Behavior Change, and Treatment Adherence. Oxford: Oxford University Press, 2013: 193–213. [Google Scholar]
- 8. Sivell S, Elwyn G, Gaff C, Clarke A, Iredale R, Shaw C et alHow risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: systematic review. J Genet Couns 2008; 17: 30–63. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 10. Singh J. Critical appraisal skills programme. J Pharmacol Pharmacother 2013; 4: 76. [Google Scholar]
- 11. National Institute of Health . Quality assessment tool for observational cohort and cross‐sectional studies, 2014. Available at http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort Last accessed 1 March 2016.
- 12. Portnoy D, Ferrer R, Bergman H, Klein W. Changing deliberative and affective responses to health risk: a meta‐analysis. Health Psychol Rev 2014; 8: 296–318. [DOI] [PubMed] [Google Scholar]
- 13. Ranby K, Aiken L, Gerend M, Erchull M. Perceived susceptibility measures are not interchangeable: absolute, direct comparative, and indirect comparative risk. Health Psychol 2010; 29: 20. [DOI] [PubMed] [Google Scholar]
- 14. Weinstein N. Unrealistic optimism about future life events. J Pers Soc Psychol 1980; 39: 806. [Google Scholar]
- 15. Allen J, Purcell K, Szanton S, Dennison C. Perceptions of cardiac risk among a low‐income urban diabetic population. J Health Care Poor Underserved 2010; 21: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asimakopoulou K, Skinner T, Spimpolo J, Marsh S, Fox C. Unrealistic pessimism about risk of coronary heart disease and stroke in patients with type 2 diabetes. Patient Educ Couns 2008; 71: 95–101. [DOI] [PubMed] [Google Scholar]
- 17. Calvin D, Quinn L, Dancy B, Park C, Fleming S, Smith E et al African Americans’ perception of risk for diabetes complications. Diabetes Educ 2011; 37: 689–698. [DOI] [PubMed] [Google Scholar]
- 18. Carroll C, Naylor E, Marsden P, Dornan T. How do people with Type 2 diabetes perceive and respond to cardiovascular risk? Diabet Med 2003; 20: 355–360. [DOI] [PubMed] [Google Scholar]
- 19. Choi S, Rankin S, Stewart A, Oka R. Perceptions of coronary heart disease risk in Korean immigrants with type 2 diabetes. Diabetes Educ 2008; 34: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frijling B, Lobo C, Keus I, Jenks K, Akkermans R, Hulscher M et al Perceptions of cardiovascular risk among patients with hypertension or diabetes. Patient Educ Couns 2004; 52: 47–53. [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann T, Del Mar C. Exploring the understanding of evidence‐based concepts in people with type 2 diabetes. Int J Gen Med 2012; 5: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Homko C, Zamora L, Santamore W, Kashem A, McConnell T, Bove A. Gender differences in cardiovascular risk factors and risk perception among individuals with diabetes. Diabetes Educ 2010; 36: 483–488. [DOI] [PubMed] [Google Scholar]
- 23. Kausar R, Awan B, Khan N. Gender differences in risk perception and emotional distress in patients with Type 2 diabetes. J Indian Acad Appl Psychol 2013; 39: 222. [Google Scholar]
- 24. Macaden L, Clarke C. Risk perception among older South Asian people in the UK with type 2 diabetes. Int J Older People Nurs 2006; 1: 177–181. [DOI] [PubMed] [Google Scholar]
- 25. Martell‐Claros N, Aranda P, Gonzalez‐Albarran O, Dalfo‐Baque A, Dominguez‐Sardina M, de la Cruz J et al Perception of health and understanding of cardiovascular risk among patients with recently diagnosed diabetes and/or metabolic syndrome. Eur J Prev Cardiol 2011; 20: 21–28. [DOI] [PubMed] [Google Scholar]
- 26. McKenzie C, Skelly A. Perceptions of coronary heart disease risk in African American women with type 2 diabetes: a qualitative study. Diabetes Educ 2010; 36: 766–773. [DOI] [PubMed] [Google Scholar]
- 27. Merz C, Buse J, Tuncer D, Twillman G. Physician attitudes and practices and patient awareness of the cardiovascular complications of diabetes. J Am Coll Cardiol 2002; 40: 1877–1881. [DOI] [PubMed] [Google Scholar]
- 28. Portnoy D, Kaufman A, Klein W, Doyle T, de Groot M. Cognitive and affective perceptions of vulnerability as predictors of exercise intentions among people with type 2 diabetes. J Risk Res 2014; 17: 177–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price H, Dudley C, Barrow B, Griffin S, Holman R. Perceptions of heart attack risk amongst individuals with diabetes. Primary Care Diabetes 2009; 3: 239–244. [DOI] [PubMed] [Google Scholar]
- 30. Saver B, Mazor K, Hargraves J, Hayes M. Inaccurate risk perceptions and individualized risk estimates by patients with type 2 diabetes. J Am Board Fam Med 2014; 27: 510–519. [DOI] [PubMed] [Google Scholar]
- 31. Walker E, Caban A, Schechter C, Basch C, Blanco E, DeWitt T, et al. Measuring comparative risk perceptions in an urban minority population: the risk perception survey for diabetes. Diabetes Educ 2007; 33: 103–110. [DOI] [PubMed] [Google Scholar]
- 32. Welschen L, Bot S, Kostense P, Dekker J, Timmermans D, van der Weijden T et al Effects of cardiovascular disease risk communication for patients with type 2 diabetes on risk perception in a randomized controlled trial: the @RISK study. Diabetes Care 2012; 35: 2485–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klein R, Klein B, Moss S, Davis M, DeMets D. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984; 102: 527–532. [DOI] [PubMed] [Google Scholar]
- 34. Becker D, Levine D. Risk perception, knowledge, and lifestyles in siblings of people with premature coronary disease. Am J Prev Med 1986; 3: 45–50. [PubMed] [Google Scholar]
- 35. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G et al 2007 ESH–ESC practice guidelines for the management of arterial hypertension: ESH–ESC task force on the management of arterial hypertension. J Hypertens 2007; 25: 1751–1762. [DOI] [PubMed] [Google Scholar]
- 36. Schwarzer R, Renner B. Social–cognitive predictors of health behavior: action self‐efficacy and coping self‐efficacy. Health Psychol 2000; 19: 487. [PubMed] [Google Scholar]
- 37. Clarke P, Gray A, Briggs A, Farmer A, Fenn P, Stevens R et al A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004; 47: 1747–1759. [DOI] [PubMed] [Google Scholar]
- 38. Anderson K, Odell P, Wilson P, Kannel W. Cardiovascular disease risk profiles. Am Heart J 1991; 121: 293–298. [DOI] [PubMed] [Google Scholar]
- 39. Graham G, Leath B, Payne K, Guendelman M, Reynolds G, Kim S et al Perceived versus actual risk for hypertension and diabetes in the African American community. Health Promot Pract 2006; 7: 34–46. [DOI] [PubMed] [Google Scholar]
- 40. Gold R, Aucote H. ‘I'm less at risk than most guys’: gay men's unrealistic optimism about becoming infected with HIV. Int J STD AIDS 2003; 14: 18. [DOI] [PubMed] [Google Scholar]
- 41. Green J, Grant M, Hill K, Brizzolara J, Belmont B. Heart disease risk perception in college men and women. J Am Coll Health 2003; 51: 207–211. [DOI] [PubMed] [Google Scholar]
- 42. Marteau T, Kinmonth A‐L, Pyke S, Thompson S. Readiness for lifestyle advice: self‐assessments of coronary risk prior to screening in the British family heart study. Br J Gen Pract 1995; 45: 5–8. [PMC free article] [PubMed] [Google Scholar]
- 43. Avis N, Smith K, McKinlay J. Accuracy of perceptions of heart attack risk: what influences perceptions and can they be changed? Am J Public Health 1989; 79: 1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinstein N. Why it won't happen to me: perceptions of risk factors and susceptibility. Health Psychol 1984; 3: 431. [DOI] [PubMed] [Google Scholar]
- 45. Hamner J, Wilder B. Knowledge and risk of cardiovascular disease in rural Alabama women. J Am Acad Nurse Pract 2008; 20: 333–338. [DOI] [PubMed] [Google Scholar]
- 46. Williams A, Lindsell C, Rue L, Blomkalns A. Emergency Department education improves patient knowledge of coronary artery disease risk factors but not the accuracy of their own risk perception. Prev Med 2007; 44: 520–525. [DOI] [PubMed] [Google Scholar]
- 47. Zajac L, Klein W, McCaul K. Absolute and comparative risk perceptions as predictors of cancer worry: moderating effects of gender and psychological distress. J Health Commun 2006; 11: 37–49. [DOI] [PubMed] [Google Scholar]
- 48. Heisler M, Faul J, Hayward R, Langa K, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle‐aged and older Americans in the health and retirement study. Arch Intern Med 2007; 167: 1853–1860. [DOI] [PubMed] [Google Scholar]
- 49. Kirk J, D'Agostino R, Bell R, Passmore L, Bonds D, Karter A, et al. Disparities in HbA1c levels between African‐American and non‐Hispanic White adults with diabetes: a meta‐analysis. Diabetes Care 2006; 29: 2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirk J, Bell R, Bertoni A, Arcury T, Quandt S, Goff D et al Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother 2005; 39: 1489–1501. [DOI] [PubMed] [Google Scholar]
- 51. Sultan S, Hartemann‐Heurtier A, Grimaldi A. Understanding patients to promote self‐regulation in Type 2 diabetes: how to live with an illness beginning before its onset? Diabetes Metab 2003; 29: S21–S30. [PubMed] [Google Scholar]
- 52. Asimakopoulou K, Fox C, Spimpolo J, Marsh S, Skinner T. The impact of different time frames of risk communication on type 2 diabetes patients’ understanding and memory for risk of coronary heart disease and stroke. Diabet Med 2008; 25: 811–817. [DOI] [PubMed] [Google Scholar]
- 53. Tawfik M, Mohamed R. The impact of communicating cardiovascular risk in type 2 diabetics on patient risk perception, diabetes self‐care, glycosylated hemoglobin, and cardiovascular risk. J Public Health 2016; 24: 153–164. [Google Scholar]
- 54. van der Weijden T, van Steenkiste B, Stoffers H, Timmermans D, Grol R. Primary prevention of cardiovascular diseases in general practice: mismatch between cardiovascular risk and patients’ risk perceptions. Med Decis Making 2007; 27: 754–761. [DOI] [PubMed] [Google Scholar]
- 55. Sheridan S, Viera A, Krantz M, Ice C, Steinman L, Peters K et al The effect of giving global coronary risk information to adults: a systematic review. Arch Intern Med 2010; 170: 230–239. [DOI] [PubMed] [Google Scholar]
- 56. Powers B, Danus S, Grubber J, Olsen M, Oddone E, Bosworth H. The effectiveness of personalized coronary heart disease and stroke risk communication. Am Heart J 2011; 161: 673–680. [DOI] [PubMed] [Google Scholar]
- 57. Koelewijn‐van Loon M, van der Weijden T, Ronda G, van Steenkiste B, Winkens B, Elwyn G et al Improving lifestyle and risk perception through patient involvement in nurse‐led cardiovascular risk management: a cluster‐randomized controlled trial in primary care. Prev Med 2010; 50: 35–44. [DOI] [PubMed] [Google Scholar]
- 58. Klein W, Stefanek M. Cancer risk elicitation and communication: lessons from the psychology of risk perception. CA Cancer J Clin 2007; 57: 147–167. [DOI] [PubMed] [Google Scholar]
- 59. Roach P, Marrero D. A critical dialogue: communicating with type 2 diabetes patients about cardiovascular risk. Vasc Health Risk Manag 2005; 1: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk‐benefit information. Health Aff 2007; 26: 741–748. [DOI] [PubMed] [Google Scholar]
- 61. Arvai J. Rethinking of risk communication: lessons from the decision sciences. Tree Genet Genomes 2007; 3: 173–185. [Google Scholar]
- 62. Roach P, Klindukhova O, Saha C, Hudson B, Cantrell M, Marrero D. Project RedCar: Cardiovascular disease risk communication for people with type 2 diabetes combining the power of electronic health records and computer‐based multimedia technology. Diabetes Spectr 2010; 23: 155–160. [Google Scholar]
- 63. Ahmed H, Naik G, Willoughby H, Edwards A. Communicating risk. BMJ 2012; 344: 40–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study quality: studies assessed using the CASP.
Table S2. Study quality: studies assessed using an adapted version of the QAT.
Table S3. MEDLINE (via OvidSP) 1946 to February week 4, 2016.
Table S4. Embase (via OvidSP) 1974 to February week 4, 2016.
Table S5. Global Health (via OvidSP) 1973 to February week 4, 2016.
Table S6. PsycINFO (via OvidSP) 1967 to February week 4, 2016.
Table S7. SCOPUS (all years to 2016).
Table S8. EconLit (all years to 2016).
Table S9. IBSS (all years to 2016).
Table S10. Web of Science (all years to 2016).


