Abstract
Aims
To estimate the risk of stroke in people with Type 2 diabetes with different blood pressure levels compared with the risk in the general population in Sweden.
Methods
This prospective case–control study included 408 076 people with Type 2 diabetes, aged ≥ 18 years, and free of prior stroke, registered in the Swedish National Diabetes Register 1998–2011. Age‐ and sex‐matched control subjects (n = 1 913 507) without stroke from the general population were included. Stroke diagnoses were retrieved using International Classification of Disease codes from the Swedish patient and death registers. Cox hazard ratios and 95% confidence intervals (CIs) were estimated at six different blood pressure levels.
Results
During a median follow‐up of 4 years, 19 548 (4.8%) people with Type 2 diabetes and 61 690 (3.2%) without diabetes were diagnosed with stroke, corresponding to an adjusted hazard ratio of 1.43 (95% CI 1.41–1.46) for people with Type 2 diabetes as a group. Compared with people without diabetes, the risk of stroke for people with Type 2 diabetes with different blood pressure levels was significantly higher, starting at blood pressure levels > 130/80 mmHg. Hazard ratios for stroke were 1.20 (95% CI 1.16–1.24), 1.47 (95% CI 1.43–1.50), and 1.97 (95% CI 1.90–2.03) for blood pressure categories of 130–139/80–89 mmHg, 140–159/90–99 mmHg and ≥ 160/≥ 100 mmHg, respectively, after adjustment for age, sex, diabetes duration, being born in Sweden, maximum education level and baseline comorbidities.
Conclusions
People with Type 2 diabetes and blood pressure < 130/80 mmHg had a risk of stroke similar to that of the general population.
What's new?
Type 2 diabetes and blood pressure level are well known predictors of stroke, but the risk of stroke for people with Type 2 diabetes at different blood pressure levels compared with the risk for the general population is not known.
We found that people with Type 2 diabetes and blood pressure < 130/80 mmHg had a risk of any stroke similar to that of the general population.
People with Type 2 diabetes and blood pressure in the range of 120–139/70–89 mmHg had a significantly lower risk of haemorrhagic stroke compared with the general population.
What's new?
Type 2 diabetes and blood pressure level are well known predictors of stroke, but the risk of stroke for people with Type 2 diabetes at different blood pressure levels compared with the risk for the general population is not known.
We found that people with Type 2 diabetes and blood pressure < 130/80 mmHg had a risk of any stroke similar to that of the general population.
People with Type 2 diabetes and blood pressure in the range of 120–139/70–89 mmHg had a significantly lower risk of haemorrhagic stroke compared with the general population.
Introduction
Stroke is the second most common cause of death and a common cause of disability worldwide 1, 2. Knowledge regarding risk factors and their importance in different groups is essential for preventive actions. People with Type 2 diabetes have a 1.5–3 times higher risk of stroke than people without diabetes 3, and often have several other conventional risk factors for stroke, which further enhances the risk. Hypertension is the most important risk factor for stroke 4, with risk gradually increasing from blood pressure levels below the limits for hypertension 5. Numerous studies exclusively in people with Type 2 diabetes have shown that reducing blood pressure lowers the risk of stroke 6, 7.
Even though it is well documented that the risk of stroke is elevated for people with Type 2 diabetes as a group, this may not apply equally to all individuals with Type 2 diabetes. Blood pressure and Type 2 diabetes may interact synergistically and greatly increase the stroke risk for people above a certain blood pressure level, which could result in an increased risk for people with Type 2 diabetes as a group. Large studies evaluating the risk of stroke in people with Type 2 diabetes at different blood pressure levels compared with the general population are sparse. Using the Swedish National Diabetes Register (NDR), we conducted a large observational case–control study in which we investigated the risk of stroke at different blood pressure levels in people with Type 2 diabetes and compared their risk with that of the general Swedish population.
Patients and methods
People with and without diabetes
The NDR was initiated in 1996 as a nationwide instrument for quality improvement in diabetes care, covering patients aged ≥ 18 years. Annually, information on risk factors, diabetes complications and medications is reported to the register from the local care provider. The number of patients in the register has increased since 1996 8. The NDR annual report of 2013 estimated that 88% of all adults with diabetes in Sweden were included in the register. In the NDR, Type 2 diabetes is defined as diabetes treated with diet only, oral hypoglycaemic agents only or insulin only or in combination with oral hypoglycaemic agents if onset of diabetes was at age ≥ 40 years 9. We identified a cohort of 435 660 people with Type 2 diabetes registered at least once during 1998–2011. For each individual with Type 2 diabetes, we randomly selected five age‐, sex‐ and county‐matched controls from the Swedish Total Population Register, a method used in previous studies 10, 11, altogether 2 144 567 people. We excluded control subjects registered in the NDR (394), people with Type 2 diabetes, those who died before the start of the study (26 981), and people with Type 2 diabetes and control subjects with a diagnosis of stroke registered before the start of the study (231 269). Finally, a total of 408 076 people with and 1 913 507 without Type 2 diabetes remained for analysis. Patients with Type 2 diabetes and their matched controls entered the study on the day of the first registration in the NDR, and were followed until first hospitalization for stroke, until death, or until 31st of December 2011.
Procedure
Information on stroke endpoints and comorbidities was linked to our study from the Swedish National Patient Register and Cause of Death Register via the personal identification number, unique for every citizen in Sweden. Likewise, information concerning place of birth and educational level were linked from the Longitudinal Integration Database for Health Insurance and Labour Market Studies register. For people with diabetes, data on risk factors, medications and lifestyle were retrieved from the NDR; no similar data were available for people without diabetes. After matching, all personal identifiers were replaced by codes. All patients in the NDR have agreed to be included in the register. The study was approved by the regional ethics review board at the University of Gothenburg.
Variables
All discharges from hospitals in Sweden are registered in the Swedish National Patient Register, with principal and contributory diagnoses recorded using the International Classification of Disease (ICD) codes The register has had nationwide coverage since 1987. All deaths are registered in the Cause of Death Register. The positive predictive value for discharge diagnoses in the hospital discharge register differs, but is generally 85–95% for major cardiovascular diagnosis categories 12. In 2004, the two registers showed a sensitivity for stroke diagnosis of 92% when combined 13.
The following diagnostic codes were used to identify previous stroke: haemorrhagic stroke: 431, 432X (ICD‐9), I61, I62.9 (ICD‐10); and ischaemic stroke: 433, 434, 436, 437X (ICD‐9), I63, I64, I67.9 (ICD‐10). To identify comorbidities at study entry, the following codes were used: coronary heart disease 410‐414 (ICD‐9) I20‐I25 (ICD‐10); atrial fibrillation 427D (ICD‐9) I48 (ICD‐10); and heart failure 428 (ICD‐9) I50 (ICD‐10).
To define stroke endpoints, we used the following ICD‐10 diagnoses: any stroke (I61, I62.9, I63, I64, I67.9); ischaemic stroke (I63, I64, I67.9); and haemorrhagic stroke (I61, I62.9).
Education was categorized as low (compulsory only), intermediate or high (university or similar), and country of birth as Sweden or other.
The standard for blood pressure measurement used in the NDR is the mean value (mmHg) of two readings in the supine position using a cuff of appropriate size and after at least 5 min of rest. People with Type 2 diabetes were categorized into one of the following six predefined blood pressure categories: < 110/< 65 mmHg; 110–119/65–69 mmHg; 120–129/70–79 mmHg; 130–139/80–89 mmHg; 140–159/90–99 mmHg; and ≥ 160/≥ 100 mmHg. Participants with discordant systolic (SBP) and diastolic blood pressure (DBP) were classified into the higher category. Laboratory analyses of HbA1c and microalbuminuria were performed at the local laboratory. All healthcare laboratory units in Sweden are associated with a quality assessment organization with regular validations. Healthcare units in Sweden used HbA1c methods, which were calibrated to the high‐performance liquid chromatography mono‐S method until September 2010, when there was a national change in favour of the calibration recommended by the International Federation of Clinical Chemistry and Laboratory Medicine and the National Glycohemoglobin Standardization Program (NGSP). HbA1c values are reported as mmol/mol and percentage, and were converted according to the NGSP 14. Microalbuminuria was defined as at least two positive tests out of three taken within 1 year. Renal impairment was categorized as normoalbuminuria, microalbuminuria [albumin/creatinine ratio of 3–30 mg/mmol (~ 30–300 mg/g) or U‐albumin of 20–200 μg/min (20–300 mg/l)], macroalbuminuria [albumin/creatinine ratio > 30 mg/mmol (> 300 mg/g) or U‐albumin > 200 μg/min (> 300 mg/l)] or stage 5 chronic renal disease (defined as the need for renal dialysis or renal transplantation or as an estimated GFR < 15 ml/min).
Statistical analysis
Unadjusted incidence rates for stroke endpoints were estimated by exact Poisson confidence limits and presented as events per 1000 person‐years of follow‐up with 95% CIs.
To study the relationship between people with Type 2 diabetes with different updated mean blood pressure and stroke endpoints, three Cox regression models were constructed with people without diabetes as a reference. Updated mean blood pressure was defined as the mean value of all preceding measures and this was updated for each new measurement (for example, when the third measurement from baseline was performed, the updated mean blood pressure was the mean of the three first measurements). The mean number of blood pressure registrations used was four. Only values recorded before the index stroke event or censoring were used. Time at risk was calculated from the date of entry of study to that of first admission to hospital with a principal diagnosis of stroke, to death, or to 31 December 2011. Model 1 was an unadjusted model stratified by match set (according to age and sex). Model 2 was adjusted for time‐updated age and sex, and stratified by diabetes duration. Model 3 was additionally adjusted for being born in Sweden or elsewhere, maximum education level and comorbidities (atrial fibrillation, coronary heart disease and heart failure) at baseline. In models 2 and 3 the matching by set was not kept because we also wanted to study the effect of age and sex on stroke events, and hence, they were included as covariates. In models 2 and 3 the risk of stroke for each group of people with diabetes in a certain blood pressure category was estimated against the risk of all controls grouped together. Subgroup analyses on model 3 were performed to analyse the effect of sex, age group and comorbid cardiovascular disease [diagnosis: I00‐99 (ICD‐10)].
We also constructed Cox regression models to study the relationship between blood pressure and stroke within the group of people with Type 2 diabetes. The updated mean blood pressure category of 120–129/70–79 mmHg was used as a reference. Separate models for SBP and DBP categories were also constructed. Hazard ratios (HRs) and associated 95% CIs for stroke were estimated in all Cox regression models. All P values were two‐sided and values < 0.05 were taken to indicate statistical significance. The assumption of the proportional hazard was tested for all Cox regression analyses and was found to hold. All analyses were performed using SAS software version 9.3.
Results
Baseline characteristics
People with Type 2 diabetes were less educated and less often born in Sweden than people without diabetes (Table 1). Blood pressure had a linear relationship with age in people with Type 2 diabetes, 64% of whom received antihypertensive treatment (Table S1). Baseline comorbidities (coronary heart disease, atrial fibrillation and heart failure) were more common in people with Type 2 diabetes compared with those without diabetes. The number of comorbid conditions (coronary heart disease, heart failure and atrial fibrillation) decreased with increasing blood pressure.
Table 1.
Characteristics of people with Type 2 diabetes at first inclusion in the National Diabetes Register, 1998–2011, and of controls, all free of previous stroke
| Controls N = 1 913 507 | All type 2 diabetes N = 408 076 | |
|---|---|---|
| Women | 869 045 (45.4%) | 182 486 (44.7%) |
| Age, years (sd) | 64.6 (12.5) | 65.3 (12.6) |
| Born in Sweden | 1 673 823 (87.5%) | 337 235 (82.6%) |
| Education category | ||
| Low | 682 929 (36.3%) | 175 217 (43.9%) |
| Mid | 744 627 (39.6%) | 160 153 (40.1%) |
| High | 453 805 (24.1%) | 63 730 (16.0%) |
| Registrations in the National Patient Register prior to baseline | ||
| Coronary heart disease (I20‐I25) | 137 957 (7.2%) | 62 037 (15.2%) |
| Atrial fibrillation (I48) | 89 814 (4.7%) | 32 166 (7.9%) |
| Heart failure (I50) | 53 104 (2.8%) | 25 986 (6.4%) |
| Variables in the National Diabetes Register only | ||
| Systolic blood pressure, mmHg (sd) | 140.3 (18.3) n = 351 847 | |
| Diastolic blood pressure, mmHg (sd) | 78.6 (9.8) n = 351 847 | |
| HbA1c, mmol/mol (sd), NGSP % (sd) | 54.3 (14.6), 7.1 (1.3) n = 364 237 | |
| Diabetes duration, y (sd) | 5.53 (6.96) n = 363 024 | |
| Antihypertensive treatment | 245 495 (64.0%) | |
| Smoking, n (%) | 51 153 (15.5%) | |
Incidence of stroke
The median follow‐up in the study was 4.0 years and 4.1 years for people with and without Type 2 diabetes, respectively. Out of 408 076 individuals with Type 2 diabetes, 19 548 (4.8%) received a diagnosis of stroke compared with 61 690 out of 1 913 507 (3.2%) individuals without diabetes; 13.8% were fatal within 28 days. The unadjusted incidences of stroke according to blood pressure category are shown in Figure 1. For people with Type 2 diabetes as a group, the incidence rate per 1000 person‐years was 10.63 for any stroke, 9.62 for ischaemic stroke and 1.01 for haemorrhagic stroke. Corresponding rates among people without diabetes were 6.80, 5.92 and 0.88 cases per 1000 person‐years, respectively.
Figure 1.
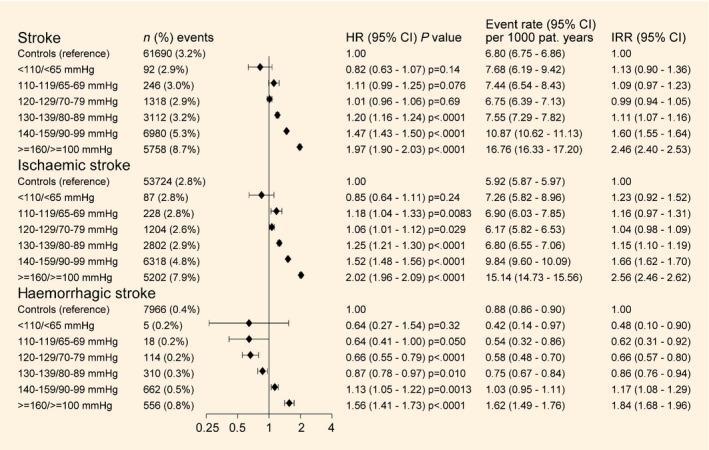
Crude incidence of stroke, incidence rate ratios and adjusted hazard ratios (HR) of stroke and stroke subtypes by blood pressure category. Blood pressure categories are baseline blood pressure category for incidence and incidence rate ratio (IRR) and time‐updated mean blood pressure category for HRs. Forest plot shows HRs and CIs. HRs and 95% CIs were estimated by Cox regression, adjusted for time‐updated age, sex, stratified by diabetes duration, being born in Sweden, maximum education level and baseline comorbidities (atrial fibrillation, coronary heart disease and heart failure).
Risk of stroke
The estimates from the Cox regression models are shown in Fig. 1 and Table S2. In the final model with adjustment for updated age, sex, stratified by diabetes duration, being born in Sweden, maximum education level and baseline comorbidities, the HR of any stroke for people with Type 2 diabetes as a group was 1.43 (95% CI 1.41–1.46) compared with people without diabetes. When the multiple‐adjusted risk was estimated for people with Type 2 diabetes at different updated mean blood pressure levels, the risk was significantly elevated in the three highest blood pressure categories. The HRs of any stroke were 1.20 (95% CI 1.16–1.24), 1.47 (95% CI 1.43–1.50) and 1.97 (95% CI 1.90–2.03) for blood pressure categories of 130–139/80–89 mmHg, 140–159/90–99 mmHg and ≥ 160/≥ 100 mmHg, respectively.
The multiple‐adjusted HR for ischaemic stroke for people with Type 2 diabetes as a group was 1.48 (95% CI 1.46–1.51) compared with people without diabetes. The multiple‐adjusted risk of ischaemic stroke was only very slightly elevated for people with Type 2 diabetes compared with those without diabetes at blood pressure levels < 130/80 mmHg. For blood pressure levels > 130/80 mmHg, the HR for ischaemic stroke increased gradually to a maximum HR of 2.02 (95% CI 1.96–2.09) in the blood pressure category of ≥ 160/≥ 100 mmHg.
For haemorrhagic stroke, the multiple‐adjusted HR for people with Type 2 diabetes as a group was 1.08 (95% CI 1.02–1.14) compared with people without diabetes, with a significantly lower multiple‐adjusted risk for people with Type 2 diabetes compared with people without diabetes in the blood pressure categories of 120–129/70–79 mmHg and 130–139/80–89 mmHg, with corresponding HRs of 0.66 (95% CI 0.55–0.79) and 0.87 (95% CI 0.78–0.97), respectively; however, people with Type 2 diabetes in the two highest blood pressure categories had a significantly higher risk of haemorrhagic stroke with a multiple‐adjusted HR of 1.56 (95% CI 1.41–1.73) in the highest blood pressure category. Figure 2 and Figs S1 and S2 show the results from the analyses by sex, age group and circulatory comorbidities, with essentially similar findings.
Figure 2.
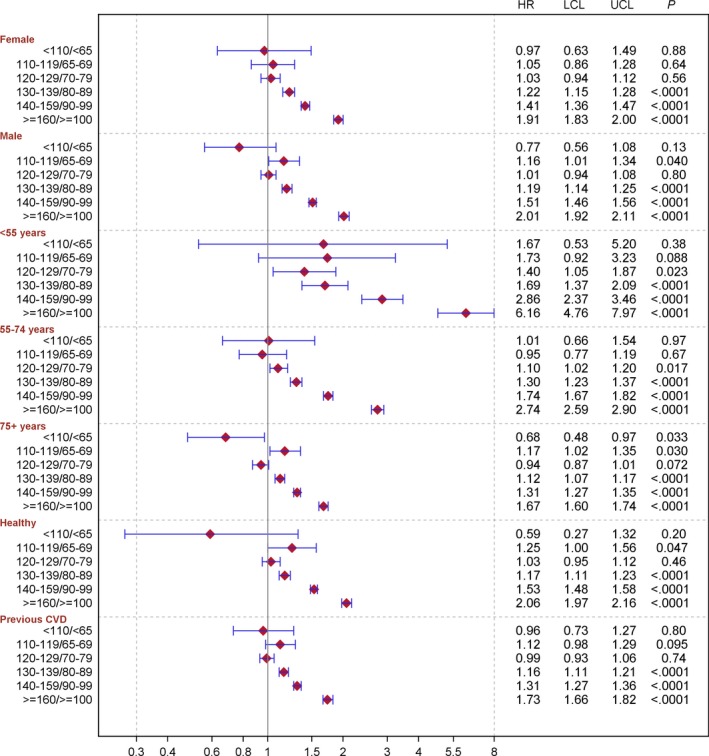
Adjusted hazard ratios (HRs) for stroke in people with Type 2 diabetes compared with people without diabetes. HRs and 95% CIs were estimated by means of Cox regression. Subgroup analyses by sex were adjusted for time‐updated age, stratified by diabetes duration, being born in Sweden, maximum education level and baseline comorbidities (atrial fibrillation, coronary heart disease and heart failure). Subgroup analyses by age group were adjusted for sex, stratified by diabetes duration, being born in Sweden, maximum education level and baseline comorbidities (atrial fibrillation, coronary heart disease and heart failure). Subgroup analyses by previous cardiovascular disease (CVD; ICD‐10 I00‐99) were adjusted for sex, time‐updated age, stratified by diabetes duration, being born in Sweden, and maximum education level. LCL, lower confidence limit; UCL, upper confidence limit.
Analysis of people with Type 2 diabetes only
Table 2 and Tables S3 and S4 show the results from Cox regression analysis with different adjustment models on people with Type 2 diabetes only. The blood pressure category of 120–129/70–79 mmHg was used as a reference. For any stroke and ischaemic stroke, we found a J‐shaped association over the blood pressure categories, with a slightly higher risk in the second lowest category, but paradoxically, not the lowest, compared with the third blood pressure category (reference). For haemorrhagic stroke, there was a significant gradually increasing risk by each higher blood pressure category above the reference. For any stroke and for both ischaemic and haemorrhagic stroke, any blood pressure of 130/80 mmHg or above was associated with significantly increased risk in all models. Table S3 Model E (additionally adjusted for antihypertensive treatment, smoking and time‐updated mean SBP/DBP) shows the increased risk for ischaemic stroke by 1 sd mmHg which was for SBP 1.17 (95% CI 1.15–1.19) and for 1 sd mmHg DBP 1.11 (95% CI 1.09–1.13). Corresponding estimates for haemorrhagic stroke were 1.16 (95% CI 1.09–1.22) for 1 sd mm Hg SBP and 1.29 (95% CI 1.21–1.36) for 1 sd mmHg DBP (Table S4 Model E).
Table 2.
Adjusted hazard ratios for risk of stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes
| HR (95% CI) P value | |||||||
|---|---|---|---|---|---|---|---|
| Model A | Model B | Model C | Model D | Model E | Model F | Model G | |
|
Stroke n events/n individuals |
18 598/393 425 | 17 245/353 653 | 16 778/346 672 | 16 604/342 984 | 16 024/331 852 | 7554/236 160 | 10 131/270 768 |
| < 110/< 65 mmHg |
1.12 (0.88–01.42) 0.34 |
1.05 (0.81–1.36) 0.71 |
0.90 (0.69–1.17) 0.43 |
0.91 (0.70–1.18) 0.48 |
0.90 (0.68–1.18) 0.44 |
0.93 (0.63–1.39) 0.74 |
0.92 (0.64–1.32) 0.65 |
| 110–119/65–69 mmHg |
1.25 (1.11–1.41) 0.0002 |
1.27 (1.12–1.44) 0.0002 |
1.19 (1.05–1.35) 0.0065 |
1.20 (1.06–1.36) 0.0036 |
1.17 (1.03–1.33) 0.015 |
1.10 (0.92–1.32) 0.29 |
1.28 (1.10–1.49) 0.0013 |
| 120–129/70–79 mmHg | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 130–139/80–89 mmHg |
1.15 (1.08–1.21) < .0001 |
1.14 (1.08–1.22) < .0001 |
1.18 (1.12–1.26) < .0001 |
1.18 (1.11–1.26) < .0001 |
1.19 (1.11–1.26) < .0001 |
1.19 (1.10–1.30) < .0001 |
1.18 (1.10–1.28) < .0001 |
| 140–159/90–99 mmHg |
1.43 (1.36–1.51) < .0001 |
1.42 (1.34–1.50) < .0001 |
1.50 (1.42–1.59) < .0001 |
1.48 (1.40–1.57) < .0001 |
1.50 (1.42–1.59) < .0001 |
1.43 (1.32–1.55) < .0001 |
1.48 (1.38–1.58) < .0001 |
| ≥ 160/≥ 100 mmHg |
2.06 (1.95–2.18) < .0001 |
2.00 (1.88–2.12) < .0001 |
2.14 (2.02–2.28) < .0001 |
2.08 (1.96–2.22) < .0001 |
2.15 (2.01–2.29) < .0001 |
1.77 (1.62–1.95) < .0001 |
1.95 (1.80–2.11) < .0001 |
| Increased by 1 sd mmHg SBP |
1.22 (1.20–1.23) < .0001 |
1.21 (1.19–1.22) < .0001 |
1.23 (1.22–1.25) < .0001 |
1.22 (1.20–1.24) < .0001 |
1.17 (1.15–1.19) < .0001 |
1.18 (1.16–1.21) < .0001 |
1.21 (1.19–1.24) < .0001 |
| SBP < 110 mmHg |
1.17 (0.99–1.39) 0.064 |
1.17 (0.98–1.40) 0.086 |
1.01 (0.84–1.21) 0.94 |
1.01 (0.84–1.22) 0.89 |
1.14 (0.94–1.38) 0.19 |
0.93 (0.69–1.25) 0.63 |
1.06 (0.83–1.35) 0.66 |
| SBP 110–119 mmHg |
1.12 (1.01–1.23) 0.024 |
1.16 (1.05–1.28) 0.0038 |
1.10 (0.99–1.21) 0.070 |
1.10 (1.00–1.22) 0.055 |
1.14 (1.03–1.27) 0.011 |
1.13 (0.98–1.30) 0.081 |
1.09 (0.97–1.24) 0.16 |
| SBP 120–129 mmHg | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| SBP 130–139 mmHg |
1.13 (1.07–1.20) < .0001 |
1.14 (1.07–1.21) < .0001 |
1.17 (1.10–1.24) < .0001 |
1.17 (1.10–1.24) < .0001 |
1.11 (1.05–1.19) 0.0006 |
1.21 (1.11–1.31) < .0001 |
1.19 (1.10–1.28) < .0001 |
| SBP 140–159 mmHg |
1.41 (1.34–1.49) < .0001 |
1.41 (1.33–1.49) < .0001 |
1.48 (1.40–1.56) < .0001 |
1.46 (1.38–1.54) < .0001 |
1.33 (1.26–1.41) < .0001 |
1.43 (1.32–1.55) < .0001 |
1.46 (1.36–1.56) < .0001 |
| SBP ≥ 160 mmHg |
2.02 (1.91–2.14) < .0001 |
1.97 (1.85–2.09) < .0001 |
2.09 (1.97–2.22) < .0001 |
2.04 (1.92–2.17) < .0001 |
1.75 (1.64–1.88) < .0001 |
1.77 (1.61–1.94) < .0001 |
1.91 (1.77–2.07) < .0001 |
| Increased by 1 sd mmHg diastolic blood pressure |
1.18 (1.17–1.20) < .0001 |
1.20 (1.18–1.21) < .0001 |
1.22 (1.20–1.24) < .0001 |
1.20 (1.19–1.22) < .0001 |
1.12 (1.10–1.14) < .0001 |
1.18 (1.15–1.20) < .0001 |
1.19 (1.16–1.21) < .0001 |
| DBP < 65 mmHg |
0.96 (0.90–1.02) 0.22 |
0.93 (0.87–0.99) 0.031 |
0.88 (0.82–0.94) 0.0002 |
0.89 (0.83–0.96) 0.0012 |
0.96 (0.90–1.03) 0.29 |
0.86 (0.78–0.95) 0.0034 |
0.86 (0.79–0.94) 0.0012 |
| DBP 65–69 mmHg |
0.95 (0.90–1.01) 0.082 |
0.93 (0.88–0.99) 0.017 |
0.90 (0.85–0.96) 0.0006 |
0.91 (0.86–0.96) 0.0011 |
0.96 (0.90–1.02) 0.16 |
0.91 (0.85–0.99) 0.024 |
0.93 (0.86–0.99) 0.030 |
| DBP 70–79 mmHg | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| DBP 80–89 mmHg |
1.26 (1.22–1.30) < .0001 |
1.28 (1.23–1.32) < .0001 |
1.30 (1.25–1.35) < .0001 |
1.28 (1.24–1.33) < .0001 |
1.20 (1.16–1.25) < .0001 |
1.22 (1.16–1.29) < .0001 |
1.23 (1.18–1.29) < .0001 |
| DBP 90–99 mmHg |
1.74 (1.65–1.84) < .0001 |
1.76 (1.66–1.86) < .0001 |
1.82 (1.72–1.92) < .0001 |
1.76 (1.66–1.86) < .0001 |
1.49 (1.40–1.59) < .0001 |
1.66 (1.50–1.85) < .0001 |
1.71 (1.58–1.86) < .0001 |
| DBP ≥ 100 mmHg |
2.46 (2.21–2.74) < .0001 |
2.42 (2.16–2.72) < .0001 |
2.51 (2.23–2.82) < .0001 |
2.34 (2.07–2.63) < .0001 |
1.75 (1.54–1.99) < .0001 |
1.93 (1.48–2.51) < .0001 |
2.38 (1.99–2.85) < .0001 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
HRs and CIs were estimated by means of Cox regression. The blood pressure category of 120–129/70–79 mmHg was used as a reference.
Model A (96.4% had no missing values): adjusted for time‐updated age and sex.
Model B (86.7% had no missing values): additionally stratified by diabetes duration.
Model C (85.0% had no missing values): additionally adjusted for being born in Sweden, maximum education level and baseline comorbidities (atrial fibrillation, coronary heart disease and heart failure).
Model D (84.0% had no missing values): Model C + adjusted for time‐updated type of diabetic treatment and time‐updated mean HbA1c.
Model E (81.3% had no missing values): Model C + adjusted for time‐updated antihypertensive treatment and smoking and time‐updated mean SBP/DBP
Model F (57.9% had no missing values): Model C + adjusted for time‐updated mean BMI, time‐updated physical activity, and renal impairment category.
Model G (66.4% had no missing values): Model C + adjusted for time‐updated mean LDL and HDL cholesterol and triglyceride levels, and time‐updated lipid‐lowering medication.
Discussion
In the present nationwide observational study, people with Type 2 diabetes as a group had a higher risk of stroke compared with an age‐ and sex‐matched general population; however, when we analysed the risk in different blood pressure categories, we found no increased risk of stroke in Type 2 diabetes if blood pressure was < 130/80 mmHg. Above this level, the risk of any stroke rose gradually to a maximum of an almost doubled risk in the highest category compared with the general population.
The risk of ischaemic stroke was higher for people with Type 2 diabetes compared with the general population in all blood pressure categories. By contrast, those with Type 2 diabetes had a lower risk of haemorrhagic stroke compared with the general population in some lower blood pressure categories. This lower risk of haemorrhagic stroke offset the increase in risk of ischaemic stroke and indicates that individuals with Type 2 diabetes with a blood pressure < 130/80 mmHg have a total stroke risk similar to that of the general population.
Previous studies have found an approximately twofold increased risk of stroke associated with Type 2 diabetes 15, 16. We have not found any previous cohort study that examined the excess risk of stroke in people with Type 2 diabetes at different blood pressure levels in comparison with the general population. In a meta‐analysis, Sarwar et al. 15 found an increased risk of ischaemic stroke in people with diabetes at all SBP levels compared with people without diabetes, consistent with our findings regarding ischaemic stroke. A synergistic effect between high glucose levels and blood pressure that exponentially increases the risk of cardiovascular disease has been previously observed 17, 18, 19.
Earlier studies have differed concerning the relative risk of haemorrhagic stroke in people with Type 2 diabetes, with some studies indicating an increased risk 15, 16, but not others 4, 20. Our finding of a lower risk of haemorrhagic stroke in people with Type 2 diabetes, except in those with high blood pressure, could contribute to explaining these divergent results. The most important risk factor for haemorrhagic stroke is blood pressure, where risk is increased already in the upper‐normal blood pressure range 4, 21. High total cholesterol and LDL cholesterol levels have been found to reduce the risk of haemorrhagic stroke 4, 22, and high LDL cholesterol levels is one feature that characterizes dyslipidaemia in people with Type 2 diabetes 23; however, studies with information on blood pressure levels as well as other data in the general population are needed to further elucidate the relationships between Type 2 diabetes, blood pressure and stroke risk compared with the general population.
The present study has several strengths, including a large number of diagnosed people with Type 2 diabetes and stroke events, and data representative of people with Type 2 diabetes on routine treatment in hospital or primary care centres nationwide. We have repeated measurements of blood pressure and other risk factors in the Type 2 diabetes group, as well as information on comorbidities and education for people with and without diabetes.
The study also has several limitations. First, and most important, we did not have blood pressure measurements in people without diabetes, and were unable to compare the risk of stroke in people with and without diabetes at specific blood pressure levels. However, the study design adds information about the excess risk for people with Type 2 diabetes at different blood pressure levels compared with the general population, which is relevant information not only to the patients but also for the clinicians. Second, individuals diagnosed with Type 2 diabetes but not included in the NDR could be among the control population. The prevalence of diabetes in Sweden is ~ 4% (NDR annual report 2013). The percentage of all people with diabetes in Sweden included in the NDR was estimated to 35–40% in 2006 and 88% in 2013 (NDR annual reports 2006 and 2013); therefore, even if some individuals with Type 2 diabetes were included among the controls, they will have been few and ought not to have altered the association between blood pressure and stroke in a decisive way. Third, there were missing data for some variables in the NDR with only 57.9% of the original cohort of people with Type 2 diabetes included in one of the models; however, estimates of risk were not affected in a manner that would alter our conclusions. Fourth, because of the observational nature of the study we cannot eliminate the possibility of residual confounding.
In conclusion, people with Type 2 diabetes as a group have a moderately elevated risk of stroke, which is higher for ischaemic than for haemorrhagic stroke. Even so, individuals with Type 2 diabetes and a blood pressure < 130/80 mmHg did not have a higher risk of any stroke compared with the general population. These findings further emphasize the importance of good blood pressure control in people with Type 2 diabetes and indicate that they can have a risk of any stroke that is similar to overall risk in the general population if blood pressure is well controlled.
Funding sources
The study was financed by grants from the Swedish state, under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (the ALF agreement), as well as grants from the Swedish Society of Physicians, the Health and Medical Care Committee of the Regional Executive Board, Region Västra Götaland, Sweden, the Swedish Heart and Lung Foundation, the Swedish Research Council (SIMSAM; grant numbers 2013‐5187 and 2013‐4236), and the Swedish Council for Working Life and Social Research (Epilife).
Competing interests
None declared.
Supporting information
Table S1. Characteristics of people with Type 2 diabetes patients, by blood pressure categories at first inclusion in the Swedish National Diabetes Register, 1998–2011, and of controls, all free of previous stroke.
Table S2. Hazard ratios for risk of stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes vs controls with different adjustment models.
Figure S1. Adjusted hazard ratios for ischaemic stroke by sex, age group and previous cardiovascular dise nase for people with Type 2 diabetes vs people without diabetes.
Figure S2. Adjusted hazard ratios for haemorrhagic stroke by sex, age group and previous cardiovascular disease for people with Type 2 diabetes vs people without diabetes.
Table S3. Adjusted hazard ratios for risk of ischaemic stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes.
Table S4. Adjusted hazard ratios for risk of haemorrhagic stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes.
Acknowledgements
We thank all the clinicians involved in the care of patients with diabetes for collecting data and staff at the NDR.
Prior presentation. Parts of this study were presented in abstract form at the 51st European Association for the Study of Diabetes annual meeting in Stockholm, 14–18 September 2015.
Diabet. Med. 34, 522–530 (2017)
References
- 1. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C et al Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V et al Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sander D, Sander K, Poppert H. Review: Stroke in type 2 diabetes. Br J Diabetes Vasc Dis 2008; 8: 222–229. [Google Scholar]
- 4. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P et al Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet 2010; 376: 112–123. [DOI] [PubMed] [Google Scholar]
- 5. Lee M, Saver JL, Chang B, Chang KH, Hao Q, Ovbiagele B. Presence of baseline prehypertension and risk of incident stroke: a meta‐analysis. Neurology 2011; 77: 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta‐analysis in 73,913 patients. J Hypertens 2011; 29: 1253–1269. [DOI] [PubMed] [Google Scholar]
- 7. Group UPDS . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998: 703–713. [PMC free article] [PubMed] [Google Scholar]
- 8. Eliasson B, Gudbjornsdottir S. Diabetes care–improvement through measurement. Diabetes Res Clin Pract 2014; 106(Suppl. 2): S291–294. [DOI] [PubMed] [Google Scholar]
- 9. Cederholm J, Eeg‐Olofsson K, Eliasson B, Zethelius B, Nilsson PM, Gudbjornsdottir S. Risk prediction of cardiovascular disease in type 2 diabetes: a risk equation from the Swedish National Diabetes Register. Diabetes Care 2008; 31: 2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lind M, Svensson A‐M, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H et al Glycemic Control and Excess Mortality in Type 1 Diabetes. N Engl J Med 2014; 371: 1972–1982. [DOI] [PubMed] [Google Scholar]
- 11. Emilsson L, Smith JG, West J, Melander O, Ludvigsson JF. Increased risk of atrial fibrillation in patients with coeliac disease: a nationwide cohort study. Eur Heart J 2011; 32: 2430–2437. [DOI] [PubMed] [Google Scholar]
- 12. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C et al External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koster M, Asplund K, Johansson A, Stegmayr B. Refinement of Swedish administrative registers to monitor stroke events on the national level. Neuroepidemiology 2013; 40: 240–246. [DOI] [PubMed] [Google Scholar]
- 14. Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I et al IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method‐comparison study. Clin Chem 2004; 50: 166–174. [DOI] [PubMed] [Google Scholar]
- 15. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E et al Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades‐Rodriguez M, Gale CP et al Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1· 9 million people. Lancet Diabetes Endocrinol 2015; 3: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Air EL, Kissela BM. Diabetes, the metabolic syndrome, and ischemic stroke epidemiology and possible mechanisms. Diabetes Care 2007; 30: 3131–3140. [DOI] [PubMed] [Google Scholar]
- 18. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–444. [DOI] [PubMed] [Google Scholar]
- 19. Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K et al Epidemiology of Ischemic Stroke in Patients With Diabetes The Greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 2005; 28: 355–359. [DOI] [PubMed] [Google Scholar]
- 20. Sturgeon JD, Folsom AR, Longstreth WT Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007; 38: 2718–2725. [DOI] [PubMed] [Google Scholar]
- 21. Fukuhara M, Arima H, Ninomiya T, Hata J, Yonemoto K, Doi Y et al Impact of lower range of prehypertension on cardiovascular events in a general population: the Hisayama Study. J Hypertens 2012; 30: 893–900. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta‐analysis. Stroke 2013; 44: 1833–1839. [DOI] [PubMed] [Google Scholar]
- 23. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009; 5: 150–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of people with Type 2 diabetes patients, by blood pressure categories at first inclusion in the Swedish National Diabetes Register, 1998–2011, and of controls, all free of previous stroke.
Table S2. Hazard ratios for risk of stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes vs controls with different adjustment models.
Figure S1. Adjusted hazard ratios for ischaemic stroke by sex, age group and previous cardiovascular dise nase for people with Type 2 diabetes vs people without diabetes.
Figure S2. Adjusted hazard ratios for haemorrhagic stroke by sex, age group and previous cardiovascular disease for people with Type 2 diabetes vs people without diabetes.
Table S3. Adjusted hazard ratios for risk of ischaemic stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes.
Table S4. Adjusted hazard ratios for risk of haemorrhagic stroke according to time‐updated mean blood pressure categories among people with Type 2 diabetes.


