Abstract
The 13th Banff Conference on Allograft Pathology was held in Vancouver, British Columbia, Canada from October 5 to 10, 2015. The cardiac session was devoted to current diagnostic issues in heart transplantation with a focus on antibody‐mediated rejection (AMR) and small vessel arteriopathy. Specific topics included the strengths and limitations of the current rejection grading system, the central role of microvascular injury in AMR and approaches to semiquantitative assessment of histopathologic and immunophenotypic indicators, the role of AMR in the development of cardiac allograft vasculopathy, the important role of serologic antibody detection in the management of transplant recipients, and the potential application of new molecular approaches to the elucidation of the pathophysiology of AMR and potential for improving the current diagnostic system. Herein we summarize the key points from the presentations, the comprehensive, open and wide‐ranging multidisciplinary discussion that was generated, and considerations for future endeavors.
Keywords: clinical research/practice, heart transplantation/cardiology, rejection, rejection: antibody‐mediated (ABMR), rejection: subclinical, translational research/science
Short abstract
This article summarizes the Banff conference on heart transplantation with a focus on antibody‐mediated rejection, strengths and limitations of the current rejection grading system, the important role of serologic antibody detection and the potential application of new molecular approaches to the elucidation of the pathophysiology of antibody‐mediated rejection, and the potential for improving the current diagnostic system. See the companion report on page 28.
Abbreviations
- ACR
acute cellular rejection
- AECVP
European Association for Cardiovascular Pathology
- AMR
antibody‐mediated rejection
- CAV
cardiac allograft vasculopathy
- DSA
donor‐specific antibodies
- EMB
endomyocardial biopsy
- IAMC
intravascular activated mononuclear cells
- ISHLT
International Society for Heart & Lung Transplantation
Introduction
The XIIIth Banff meeting was held October 5–10, 2015 in Vancouver, British Columbia, Canada in conjunction with the Annual Scientific Meeting of the Canadian Society of Transplantation. A total of 451 delegates from 28 countries attended the conference, including pathologists, immunologists, immunogeneticists, and transplant physicians and surgeons. Heart transplant diagnostics was covered as part of a dedicated session during the Banff conference. The main goal was to explore and enhance the common issues facing the different solid organ transplant groups, to identify new challenges in thoracic transplant diagnostics, and to foster a collaborative effort among transplant teams to address these unmet needs. The commonalities and challenges between kidney and heart transplant rejection was stressed during the meeting introduction by the program chairs G. Berry, MD and A. Angelini, MD. This provided a great opportunity to explore and for building an integrative network among the different specialties and solid organ transplant groups. The present report summarizes some of the outstanding issues in heart transplant diagnostics identified by the panel and members of the audience together with the main results presented by experts from centers from different parts of the world and summary from live discussions. Lastly, this report addresses proposals for future investigations to elucidate specific issues in heart transplantation (Table 1).
Table 1.
Key questions to address in the setting of heart transplant diagnostics identified by the panel
| Microcirculation inflammation |
|
| Chronic antibody‐associated allograft damage |
|
| Antibody detection in cardiac AMR |
|
| Molecular approaches in heart TX |
|
Ab, antibody; ACR, acute cellular rejection; AMR, antibody‐mediated rejection; CAV, cardiac allograft vasculopathy; ISHLT, The International Society for Heart & Lung Transplantation; MI, microvascular injury; pAMR, pathologic antibody‐mediated rejection; TX, transplant.
The Current Diagnosis System for Antibody‐Mediated Rejection: Certainties and Uncertainties
Currently, the endomyocardial biopsy (EMB) serves as a primary diagnostic tool for the diagnosis of antibody‐mediated rejection (AMR). The EMB permits the identification of AMR‐induced tissue damage and the myocardial response to injury. The histopathological changes in AMR have been formally addressed in the working formulation for the pathologic diagnosis, grading, and reporting of cardiac AMR 1 under the auspices of the International Society for Heart & Lung Transplantation (ISHLT). Although the authors of this working formulation recognized that unresolved pathologic questions remain, the current grading paradigm represents a standardization of nomenclature, diagnostic criteria, and a reporting scheme to facilitate communication between pathologists and clinicians to promote future multicenter studies and serves as a foundation for pathologic and other research investigations.
Certainties
pAMR working formulation is a purely pathology‐based approach relying on histopathology and immunohistochemistry
The main histopathologic feature of cardiac AMR is microvascular injury with accumulation of intravascular macrophages representing microvascular inflammation (Figure 1). As swollen endothelial cells and T‐lymphocytes 2 are part of the spectrum of cells that can be present in the lumens of interstitial capillaries and venules in AMR and other processes, the descriptive term “activated mononuclear cells” is applied to indicate the difficulty in distinguishing the cellular components by routine staining alone. The immunophenotypic component requires multifocal/diffuse (>50%) C4d capillary endothelial deposition or ≥10% CD68‐positive intravascular macrophages within capillaries or venules. The grading of AMR is based on the combination of morphologic and immunopathologic findings as follows: pAMR 0 negative for pathologic AMR when both histology and immunohistochemistry are negative; pAMR 1 (H+) when histopathologic findings are present and immunohistochemistry negative; pAMR 1 (I+) when histopathologic findings are negative and immunohistochemistry C4d and /or CD68 is positive; pAMR 2 or pathologic AMR when both histopathologic and immunopathologic findings are present; pAMR 3 or severe pathologic AMR: immunopathologic findings and myocyte necrosis, microvascular thrombosis, interstitial hemorrhage and/or polymorphic inflammation are present.
Figure 1.
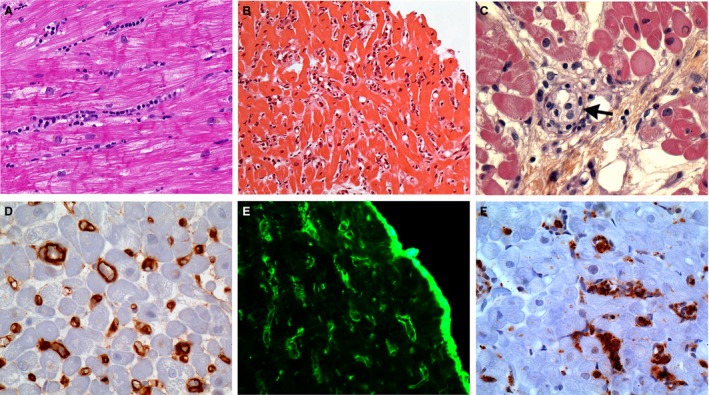
Main histopathologic and immunophenotypic features of cardiac AMR on endomyocardial biopsies. (A) Intravascular accumulation of intravascular mononuclear cells within the myocardial capillaries. H&E stain; original ×20. (B) Diffuse hypercellularity within the myocardium resulting in a “busy pattern” at low magnification. H&E stain; original ×10. (C) Histopathology cannot clearly differentiate the cell types accumulating in intravascular location (arrow). They are referred to as intravascular activated mononuclear cells. H&E stain; original ×40. (D) Diffuse labeling of capillaries with C4d antibody by immunohistochemistry. Formalin‐fixed paraffin‐embedded biopsy; original ×40. (E) Diffuse labeling of capillaries with C4d antibody by immunofluorescence. Frozen tissue; original ×40. (F) Many intravascular CD68‐positive macrophages. Formalin‐fixed paraffin‐embedded biopsy; original ×40. AMR, antibody‐mediated rejection.
It is a pathologic grading scheme independent of clinical and serologic data
One of the main conclusions of the 2010 ISHLT consensus meeting on AMR 3 was that pAMR would be a purely pathologic diagnosis akin to the diagnosis of acute cellular rejection (ACR), without the requirements of donor‐specific antibodies (DSA) and allograft dysfunction.
Currently, it is a diagnostic working formulation rather than a predictive or prognostic scheme
Except for pAMR3 or severe AMR that is usually associated with marked cardiac dysfunction, the other AMR grades (pAMR0 to pAMR2) have not been correlated with DSA levels, immediate prognosis, or clinical AMR in prospective studies and importantly, have not yet established treatment thresholds 4.
It is a consensus working formulation
pAMR working formulation resulted from the cumulative work of four consensus meetings: Allograft Pathology Conference in Banff 2009 and ISHLT meetings in Chicago, IL in 2010, San Diego, CA 2011, and Prague, Czech Republic 2012. Among its designated purposes will be a standardized format for reporting AMR in institutional programs and for multicenter studies 1, 4.
It is quite easy to use and learn
It has been validated by a reproducibility study on digitalized slides hosted on a web site testing 24 AMR‐positive EMBs among a panel of 13 pathologists 1. A tutorial for pAMR learning is now available at http://scvp.net/amr/index.html.
pAMR working formulation is supported by scientific evidences
pAMR grades are correlated with DSA and mTOR pathway activation in endothelial cells 5 and a limited number of retrospective clinical studies 6.
Uncertainties
Although the pAMR working formulation is now widely used as a diagnostic tool for cardiac AMR, there are limitations that will need to be addressed in future studies. These could include refinement of diagnostic thresholds, morphologic criteria, and importantly, clinicopathologic correlations to determine indications for therapeutic intervention.
The morphologic basis of pAMR, intravascular activated mononuclear cells (IAMC) and positive C4d and/or CD68 immunohistochemistry will require quantitative assessment
The threshold for which IAMC are considered significant for the possibility of AMR in hematoxylin and eosin (H&E)–stained sections has not been formally established. In the majority of cases the morphologic changes are diffusely present throughout the biopsy pieces 1. The clinical significance of focal or patchy histopathologic changes, while uncommon, is not understood. The establishment of a numeric threshold will require a formal study. The immunopathologic assessment of the thresholds for C4d and CD68 at 50% and 10%, respectively, were established by agreement of the participants in the working formulation. These parameters will need validation to better characterize the morphological diagnosis of AMR and establish a clinically relevant grading system.
The application of pAMR working formulation can present technical challenges
A major component of pAMR is the presence of mononuclear cells within the lumens of interstitial capillaries. The intravascular location of cells can be difficult to recognize by routine histology or by immunohistochemistry for a variety of technical and interpretative reasons as discussed in the working formulation 1. Double labeling with an endothelial marker (CD31 or CD34) has limited availability in many practices. An expanded repertoire of antibodies including PU.1 (BD Bioscience, San Jose, CA) may be helpful to distinguish intravascular from extravascular locales. The true nature of IAMC may not be apparent by routine H&E staining as the possible cell types include endothelial cells, lymphocytes, and macrophages among others. Phenotyping of the IAMC by immunohistochemistry is mandatory since only macrophages are assessed in the evaluation of AMR 2. The presence of intravascular lymphocytes and occasional macrophages not uncommonly accompany the perivascular and interstitial infiltrates of ACR. For this reason the evaluation of IAMC on EMB is assessed away from foci of ACR to avoid the misinterpretation of intravascular cells. Lastly, there are very few studies examining the pathologic differences between early‐onset and late AMR 7.
Entities that can complicate the interpretation of pAMR
In addition to the interpretative issues that ACR raises in assessing AMR, the issue of mixed ACR and AMR adds additional complexity. Depending on the patient population, the incidence of mixed ACR‐AMR is significant and the prognostic implications have been reported by a number of groups 8. One of the immunophenotypic criteria for AMR is deposition of C4d on the capillary endothelium. The issue of C4d‐negative AMR was recently raised by Tible and colleagues 5. The incidence and significance of this pattern are currently unknown but draw some parallels with the renal experience and warrant further investigation.
The correlation between pAMR and its pathophysiology and clinical phenotypes remains poorly understood
The correlation of the pAMR grades with treatment regimens, molecular characteristics, long‐term allograft outcomes, and patient survival is currently limited to only a few retrospective studies. These will require detailed prospective studies and ideally, multicenter projects. In addition, complementary approaches using IHC immunohistochemistry‐based biomarkers 5, 9, 10 in pAMR as well as tissue‐based transcriptomics are under active investigation to determine whether the pAMR grades correlate with distinct allograft injury phenotypes, commonalities/overlaps/and specificity 4.
Implementing a Microcirculation Inflammation Grading System in EMB (Figure 2)
Figure 2.
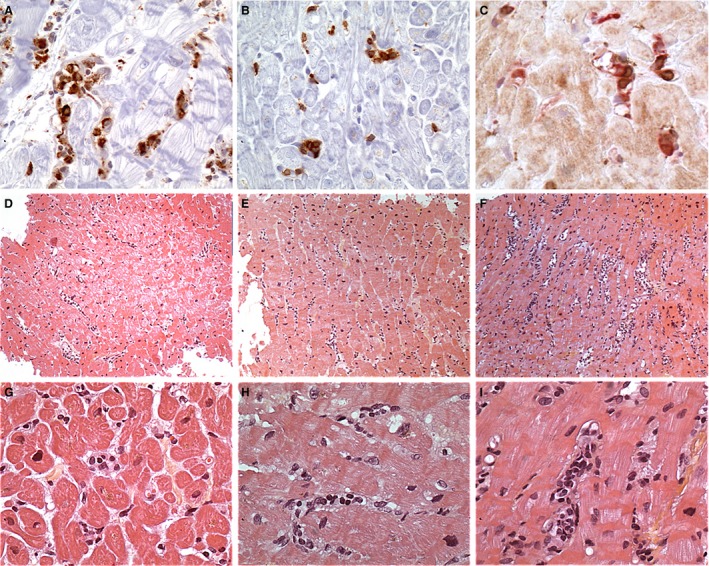
Microcirculation inflammation in cardiac AMR . (A) CD68‐positive intravascular mononuclear cell in several capillaries reflecting their monocyte–macrophage lineage. In this field, most of the labeled cells are clearly intravascular. CD68 immunohistochemistry; original ×40. (B) CD3‐positive intravascular mononuclear cell in capillaries reflecting that T‐lymphocytes are a significant cell component of the intravascular activated mononuclear cells in AMR. CD3 immunohistochemistry; original ×40. (C) Double labeling using an endothelial marker (CD31) in red and a T‐lymphocyte marker (CD3) in brown demonstrating the intravascular location of the CD3‐positive cells. This pattern is different from the T‐lymphocyte riming around capillaries and venules in cellular rejection. Double CD31‐CD3 immunohistochemistry; original ×40. (D–F) Low‐magnification views showing different intensities of the extension of the microcirculation inflammation in biopsies: D– minimal; E–focal; F–diffuse. Hematoxylin and eosin (H&E) stain; original ×10. (G–I) Higher magnification views showing different patterns of accumulation of the intravascular activated mononuclear cells (IAMC) in biopsies: G–rare IAMC; H–some IAMC; I–many IAMC forming intravascular plug. H&E stain; original ×20. AMR, antibody‐mediated rejection.
Microvascular inflammation (MI) is recognized as a key indicator of AMR in kidney and heart allografts. As discussed above, the primary histopathologic marker in the assessment of cardiac microvascular injury (MI) is the IAMC, which include both intravascular macrophages and swollen endothelial cells.
This consensus reflected the current state of knowledge and experience in the morphology of AMR at that time, but the group was aware of the many questions left open such as (i) evaluating IAMC is at present purely binary (presence or absence); (ii) in many cases, distinguishing between intravascular macrophages and swollen endothelial cells or other cell types, i.e. lymphocytes, is difficult if the phenotype of intracapillary cells is not routinely assessed by immunohistochemistry; (iii) recognizing the predominant pattern of intravascular versus extravascular localization of inflammatory cells may be challenging. This distinction is important as it is utilized to differentiate ACR from AMR.
A recent pilot study 2 from the European Association for Cardiovascular Pathology (AECVP) focused on the inflammatory burden (IB) in 35 cases of pAMR1 or pAMR2 cases without significant ACR (ISHLT 0 or ≤ 1R), using an antibody panel consisting of the complement C4d, pan‐T‐cell CD3‐T, pan‐B‐cell CD20, macrophage CD68, and a plasma cell marker, CD138. The primary findings in this study included the following: (i) the number of intracapillary inflammatory cells vary greatly between AMR cases (even in cases classified in the same categories); (ii) macrophages are not the sole cell type present in AMR, as a variable proportion of T‐cells can be present and fewer B‐cells and plasma cells; (iii) extravascular pericapillary inflammatory cells consisting of both lymphocytes and macrophages are frequently observed in the presence of intracapillary hypercellularity. The authors promoted the need for further studies to address these issues in large collaborative multicenter studies. Some interesting points addressed by the study warrant highlighting: (i) the heterogeneity of IB in cardiac pAMR in general, and in early and late or very late pAMR; (ii) the presence of inflammatory cell gradients and heterogeneous cell profiles in pAMR grades; (iii) the complex interplay between cellular‐ and antibody‐mediated immunological processes, with the reciprocal influence of ACR and AMR, and consequently the issue of mixed ACR‐AMR rejection. The ISHLT working group proposed to address the issue of mixed rejection in subsequent multicenter projects. At the 2016 Washington ISHLT Meeting, the Pathology Council proposed that the IB study be extended to a wider multicenter study to address these points and to focus on mixed rejection in more detail.
Another issue discussed at Banff 2015 was the assessment of MI using a semiquantitative histologic grading system. In kidney transplantation, a semiquantitative histologic evaluation of MI that combines glomerulitis and peritubular capillaritis scores is now part of the Banff schema for the diagnosis of renal AMR. High‐grade MI has been associated with worse renal allograft outcome in some studies 11. The French transplant group reported its preliminary data applying a semiquantitative grading scheme to assess MI severity. The score incorporates both the extent (percentage of the specimen involved) and pattern (maximum number of inflammatory cells in the most affected capillaries or venules) of IAMC. The preliminary results showed an association between the MI grade and AMR disease activity measured by molecular analysis. During the group discussion it was recognized that the MI grading scheme should be further evaluated in a multicenter study to address its reproducibility and correlation with allograft outcomes. At the subsequent 2016 ISHLT meeting in Washington, DC, the Pathology Council proposed that the project on histologic MI grading be included in a multicenter study, with particular attention to the issues of sensitivity and specificity of histologic aspects in MI, inter‐, and intraobserver reproducibility, and correlation with current AMR histologic criteria, clinical, and immunologic findings and ultimately with outcome data.
Assigning Specific Phenotypes of Alloimmune‐Mediated Vascular Injury (Figure 3)
Figure 3.
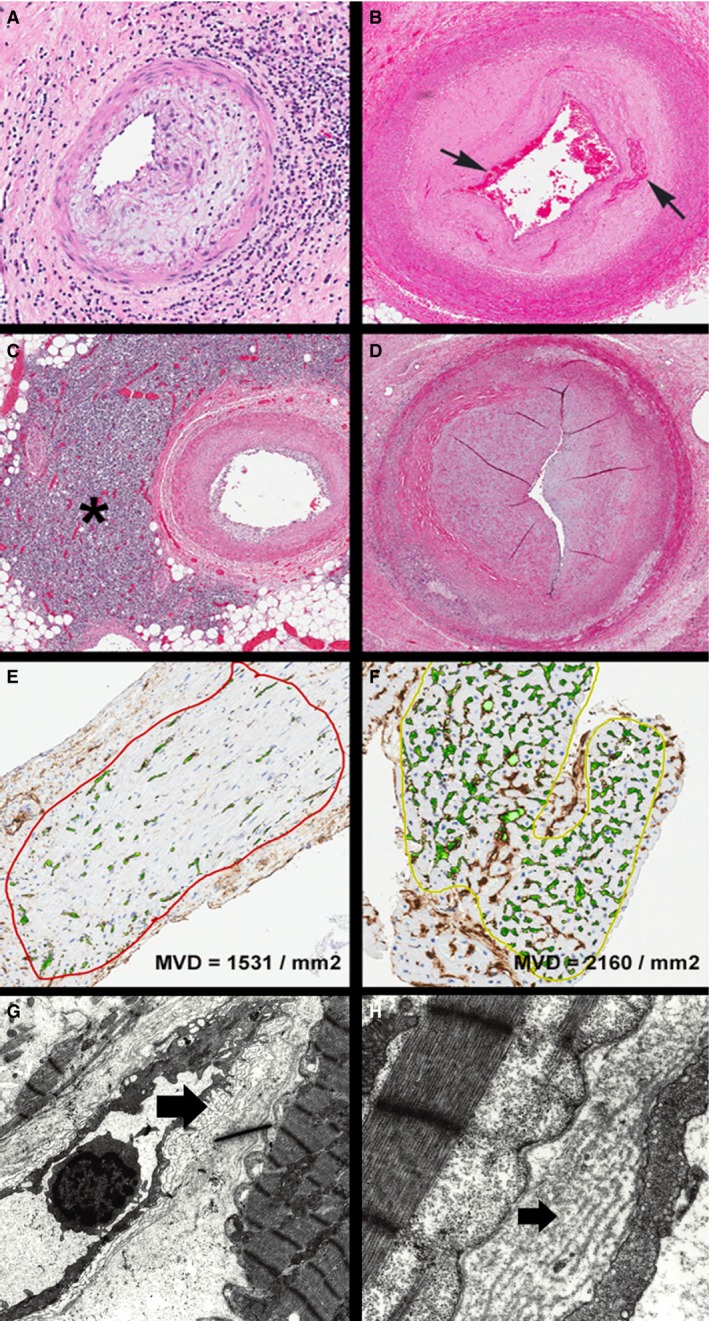
Spectrum of cardiac allograft vasculopathy (from epicardial arteries to myocardial capillaries). (A) Allograft epicardial coronary artery showing intimal and adventitial inflammation (hematoxylin and eosin [H&E], ×100). (B) Allograft epicardial coronary artery showing intimal fibrosis with shallow fibrin thrombus at the luminal aspect and some entrapped fibrin deeper in the intimal wall (arrows) (H&E, ×20). (C) Allograft epicardial coronary artery with less intimal thickening but dramatic adventitial lymphoid aggregate (asterisk) (H&E, ×20). (D) Allograft epicardial coronary artery showing advanced narrowing with a slit‐like lumen; there is very little outward remodeling of the vessel wall (H&E, ×40). (E) Allograft endomyocardial biopsy photomicrograph after computer‐assisted image analysis for capillary density. This case showed reduced capillaries (CD34 stain, ×200) (MVD, microvascular density). (F) Allograft endomyocardial biopsy photomicrograph after computer‐assisted image analysis for capillary density. This case showed preserved capillary density (CD34 stain, ×200). (G) and (H) Electron photomicrographs of allograft myocardium showing an interstitial capillary with basement membrane multilayering (arrows) (original ×4000 and ×10 000).
The recognition of AMR in cardiac allografts has evolved over the last quarter century 12. Originally it was recognized as a cause of early allograft dysfunction but late‐onset AMR is now encountered. Furthermore, asymptomatic AMR has been reported, although the clinical significance remains controversial 13, 14, 15. The natural history of AMR and specifically the cumulative effects of repetitive AMR episodes on allograft function and survival have been addressed in a limited number of studies 16.
AMR affects the endothelium throughout the heart and the morphologic manifestations vary according to vessel caliber. The epicardial coronary and penetrating arteries show alterations termed “cardiac allograft vasculopathy (CAV)”. The small vessels and microcirculation are also affected, initially manifesting as MI with IAMC 1 with progression to loss of capillaries per unit area (microvascular density) 17 and structural changes (thickening) 18.
Morphologic manifestations of CAV in epicardial and myocardial muscular arteries are both inflammatory and proliferative in nature. Inflammation may be limited to the intimal layer (endothelialitis or intimitis) or extend transmurally. Shallow nonocclusive thrombus accompanies the inflammatory lesion in some cases. The proliferative lesions are confined to the intimal layer, but differ from native atherosclerosis by their concentric distribution, limited lipid deposition, and absence of outward remodeling of the media and adventitia 19, 20.
CAV has been associated with AMR in published studies for more than two decades now 21, but the stringency of that association as well as the apparent correlation between CAV and other non‐AMR factors suggests a more complex interplay 21, 22, 23, 24. At the capillary level, the classic features of AMR are endothelial swelling and accumulation of intravascular macrophages. Complement activation, characterized by C3d and C4d deposition in tissue sections, is a key component of AMR, but may not be demonstrable in every case and is then captured in the AMR grading scheme as pAMR 1(H+) 1. This may be explained, in part, by the decrement in myocardial capillary density and morphologic alteration of capillaries manifesting as multilayering of their supporting basement membranes in cases of repetitive microvascular injury 25.
Much work remains to be performed to unravel the immunobiology, morphology, and pathophysiology of AMR's influence on the cardiac vasculature from the epicardial arteries to the interstitial capillaries. While many studies have highlighted a correlation, other studies have not shown a link between these processes 26. Additional clinical and pathologic studies along with more robust animal models will be needed to clarify the morphologic, immunopathologic, serologic, and molecular components of AMR. The current diagnostic criteria and grading scheme serve as an initial platform for this work. As emphasized by the working formulation of the ISHLT, there will be a need to reconvene in the future to reassess the concepts and approaches to the diagnosis of AMR. Lastly, there is now a standardization of nomenclature for the angiographic diagnosis and classification of CAV, but there remains a need for uniform pathologic terminology. The renal transplant community has established morphologic criteria for “acute” and “chronic” AMR 27. In the cardiac literature the terms are often applied according to temporal presentation. The term “chronic rejection” has been used inconsistently in the literature, often to indicate late onset (or recognition) of AMR rather than AMR persisting over time. Likewise, the term “rejection” has been questioned since there may be no demonstrable impairment of graft function and nonimmune mechanisms may play an important mechanistic role in these changes. “Chronic antibody‐associated allograft damage” was proposed as a term appropriately encompassing the changes described herein. More precise terminology and definitions will be needed to establish databases and design studies examining “early‐onset acute,” “late‐onset acute,” “recurrent,” “persistent,” and “chronic” AMR.
The Place of Antibody Detection in AMR Assessment in Heart Transplantation
The current ISHLT grading system for cardiac AMR is based exclusively on pathologic evidence, without requiring the additional features of clinical graft dysfunction and/or presence of donor‐specific HLA or non‐HLA antibodies 1. That said, the vital role and importance of clinical information and serologic data in the overall assessment of the patient is heavily underscored. Currently, most patients with suspected AMR will undergo evaluation to identify the causative agent, namely, HLA or non‐HLA antibodies. Numerous studies have shown that cardiac AMR diagnosis is associated with donor‐specific HLA antibodies (DSA) and cardiac transplant survival is lower in recipients with DSA at the time of AMR 3, 16, 28, 29. Furthermore, DSA are associated with progression to cardiac allograft vasculopathy and reduced long‐term cardiac allograft survival 23, 30. DSA testing shows outstanding sensitivity and negative predictive value for biopsy‐diagnosed AMR in both adult and pediatric cardiac transplant recipients 31. In posttransplant care, quantitative DSA should be an essential component in the surveillance for AMR. Moreover, the presence of mixed AMR and ACR concurrently 8 may be explained by the fact that antibodies mediate leukocyte recruitment to the allograft via IgG‐Fc receptor‐mediated effector functions 32. Table 2 summarizes outstanding questions identified and potential recommendations made by the Ab expert panel. As the immunologic testing and interpretation of test results become more standardized across transplant centers, some investigators have raised the issue of reintroducing HLA DSA and non‐HLA antibody testing information for use in the diagnosis of AMR and for risk assessment of persistent AMR and CAV 3. Whether there is currently sufficient standardization and consensus among immunologists and clinicians is controversial and awaits future investigation, consensus analysis, and debate.
Table 2.
Prospects for implementing HLA Ab detection into the AMR classification in cardiac transplantation: outstanding question identified and potential recommendations made by the Ab expert panel
| Questions | Recommendations | Definitions |
|---|---|---|
| What is the optimum timing of DSA testing posttransplantation? | Stratify the patients based on risk for AMR and monitor:
|
|
| When DSA should be treated? |
|
|
| Should DSA testing be performed with diagnosis of pAMR ? |
|
Ab, antibody; AMR, antibody‐mediated rejection; DSA, donor‐specific antibodies; MFI, mean fluorescence intensity.
New advances in antibody testing by introduction of multiplex‐bead array assays have revolutionized the field and significantly improved the sensitivity and precision of circulating DSA detection. The benefits and limitations of the solid‐phase assay using single antigen beads have been captured in many reviews 33, 34, 35, 36, 37, 38, 39. In Table 3 the American Society for Histocompatibilty and Immunogenetics HLA panel experts describe problems that may impact test interpretation and provide potential solutions to avoid false positive or incomplete results that may influence patient management. The continuous dialogue between the clinical laboratory and clinicians is the key to provide the most accurate information. The clinical impact of non‐HLA‐specific antibodies either alone or together with DSA recently has been an intense area of research in solid organ transplantation 40, 41. Newly developed enzyme‐linked immunosorbent assay solid‐phase assays have allowed a reliable means of detecting antibodies to the G protein–coupled receptors, angiotensin‐II type 1 receptor (AT1R) and endothelin type A receptor (ETAR). These reagents together with the availability of proficiency testing programs have allowed their implementation in testing for clinical transplantation. Elevated levels of AT1R and ETAR have been associated with early onset of microvasculopathy as well as with AMR and ACR 42. Furthermore, freedom from AMR and/or ACR was observed to be significantly decreased when both de novo DSA and increased AT1R antibodies levels were considered 43. A growing body of evidence supports the role of alloimmune and autoimmune mechanisms involving antibodies directed against non‐HLA antigens in transplant allograft damage 44, 45, 46.
Table 3.
Prospects for implementing HLA‐Ab detection into the AMR classification in cardiac transplantation: limitations and potential solutions
| Problem | Interpretation | Resolution |
|---|---|---|
| HLA‐Ab to denatured antigens | False positive results: HLA‐Ab to cryptic epitopes, clinically irrelevant | Repeat testing after acid treatment of SAB; surrogate crossmatch |
| Intrinsic and extrinsic factors inhibiting the SAB assay | False low MFI or negative results: due to inhibition of SAB assay | Dilution of sera pretesting, adsorption, inhibition of C1q, addition of EDTA, heat treatment to remove and uncover the real reactivity |
| Low MFI on SAB resulting in higher reactivity using cellular targets | False low MFI: DSA to a shared target present on multiple beads | Adequate analysis of specific DSA epitope |
| Using MFI to evaluate level and strength of DSA for risk stratification | Low or high MFI level of DSA may not correlate with risk of AMR, or response to treatment following antibody removal therapies | Modified SAB assay to distinguish between complement and noncomplement binding DSA and determining titer of DSA (serial dilutions of patient sera) |
Ab, antibody; AMR, antibody‐mediated rejection; DSA, donor‐specific antibodies; EDTA, ethylenediamine tetraacetic acid; MFI, mean fluorescence intensity; SAB, single‐antigen bead.
Need for Complementary Tissue Molecular Approaches
The ISHLT working formulation has taken important steps to improve the pathological diagnosis and uniform reporting of AMR. The panel and live discussion at the Banff conference discussed some of the issues that remain unresolved such as regarding the pathophysiology of heart rejection and how activity, injury degree, and stage could be improved. As discussed in an earlier section, the emerging role of molecular diagnostics is a potential avenue to further our mechanistic understanding of ACR and AMR, to help refine our current diagnostic categories and elucidate thresholds for therapeutic intervention. Molecular diagnostics has been utilized in renal transplantation to identify the subset of C4d‐negative patients with AMR. There is currently limited but evolving data in the cardiac AMR arena. Preliminary data from the Paris‐Bologna‐Edmonton collaboration were presented showing the potential of gene expression in EMB to map the molecular architecture of AMR and its correlation with disease activity. The commonalities between cardiac and kidney transplant rejection suggests the molecular microscope as an important approach that should be actively investigated by transplant research groups. The panel cautioned about the need for a comprehensive clinical and pathologic detail including state‐of‐the‐art DSA assessment using sensitive assays and accepted thresholds such as mean fluorescence intensity before a specific set of genes could be correlated to specific allograft injury phenotypes. In this setting, it was also suggested that as there is a morphologic and immunophenotypic spectrum for AMR, it is unlikely that a single gene will be specific and that this very complex undertaking will require transcriptomics data based on methodical approaches such as classifiers, machine learning, etc. Finally, the panel supported and encouraged collaborations within centers and promoted multicenter studies.
Summary and Future Directions
The diagnostic, therapeutic, and mechanistic landscapes of allograft rejection have evolved and changed dramatically over the last 25 years. The incidence of clinically significant ACR has diminished in most transplant centers, with 5% to 15% of EMB being positive for T cell–mediated rejection of the total of EMB performed in the first year posttransplant. Cardiac allograft vasculopathy remains the persistent impediment to long‐term allograft and patient survival. While the angiographic findings and corresponding histopathologic features have been well known for many decades, the immunobiology continues to evolve through clinical and animal studies. The role of the EMB has emerged as a useful investigative tool. It was once thought that the myocardial changes were static and merely reflected effects of larger epicardial disease; however, the focus has now shifted to the microvascular changes in the capillaries, venules, and arterioles and their role in the clinical and pathophysiologic consequences of CAV. There is a need for more precise terminology, definitions, and classifications of the changes at the microvascular level and uniformity in approaches, morphometrics, and immunohistochemical analysis.
The role of AMR in the initiation of allograft dysfunction and the development of CAV has also matured. The working formulation for the diagnosis and reporting of AMR has now been utilized for less than 5 years and the results from single‐center studies are limited. Its original aim to provide a framework for clinical and investigative endeavors should remain, with the intended goal to reevaluate its functionality in the future based on published data. That said, the rapidly expanding influence of proteomics, transcriptomics, and other molecular diagnostic approaches will likely illuminate and clarify our current concepts.
The overlap of clinical, histopathologic, and immunophenotypic features of AMR in the different solid organ transplant groups encourages the multidisciplinary and transdisciplinary endeavors of the Banff Conference. Much can be learned from each group. In acute renal AMR, like its cardiac counterpart, microvascular injury is centered on the microvasculature such as the peritubular capillary injury and the glomerulitis resulting in endothelial alterations, interstitial edema, and in severe cases, vascular thrombosis and fibrinoid necrosis 27. The cardiac morphologic spectrum has been described in detail in earlier sections of this report. The acute manifestations of AMR in the liver allograft are evolving but include endothelial swelling in the portal veins, venulitis, and capillaritis with hepatocyte ballooning and cholestasis 47. Chronic AMR has been defined and criteria have been enumerated in the renal allograft 27. They include glomerular double contours, peritubular capillary multilayering of basement membranes, interstitial fibrosis, tubular atrophy, and intimal proliferation of arteries. In the liver interface hepatitis, lobular inflammation, periportal and sinusoidal fibrosis, and vasculopathy have been described 47. The cardiac allograft currently does not have a formal “chronic AMR” designation, but there is an overlap of findings with the kidney and liver. These similarities provide an impetus and directions for further investigations.
This rich array between the different solid organ groups provides a multitude of directions and opportunities for transdisciplinary collaborations. The molecular discoveries in kidney AMR, including the category of C4d‐negative AMR 48, are currently being evaluated in some cardiac groups. Together with future clinical, pathologic, serologic, and other discoveries, these molecular data may help to clarify clinical, therapeutic, predictive, and prognostic information. Ultimately, the goal of overcoming transplant vasculopathy and graft loss in the different transplant groups may yet be realized. To this end, collaborations among transplant groups and societies will expedite these goals.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Acknowledgments
We would like to acknowledge the instrumental support from the Roche Organ Transplantation Research Foundation (ROTRF) Grant 608390948 awarded to Dr. Kim Solez, which allowed establishment of the Banff Foundation for Allograft Pathology. The joint 2015 Banff and Canadian Transplant Society meeting acknowledges the receipt of sponsorship from Astellas, Alexion, Novartis, One Lambda, Renal Pathology Society, American Society of Transplantation, Wiley, Qiagen, Canadian Institute for Health Research, Immucor, Bridge to Life, Organ Recovery Systems, Transplant Connect, Glycorex Transplantation, Transpath Inc., and the University of Alberta.
Bruneval P, Angelini A, Miller D, Potena L, Loupy A, Zeevi A, Reed EF, Dragun D, Reinsmoen N, Smith RN, West L, Tebutt S, Thum T, Haas M, Mengel M, Revelo P, Fedrigo M, Duong Van Huyen JP & Berry GJ. The XIIIth Banff Conference on Allograft Pathology: The Banff 2015 Heart Meeting Report: Improving Antibody‐Mediated Rejection Diagnostics: Strengths, Unmet Needs, and Future Directions. Am J Transplant 2017; 17: 42–53
See also: Loupy et al.
References
- 1. Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody‐mediated rejection in heart transplantation. J Heart Lung Transplant 2013; 32: 1147–1162. [DOI] [PubMed] [Google Scholar]
- 2. Fedrigo M, Leone O, Burke MM, et al. Inflammatory cell burden and phenotype in endomyocardial biopsies with antibody‐mediated rejection (AMR): A multicenter pilot study from the AECVP. Am J Transplant 2015; 15: 526–534. [DOI] [PubMed] [Google Scholar]
- 3. Kobashigawa J, Crespo‐Leiro MG, Ensminger SM, et al. Report from a consensus conference on antibody‐mediated rejection in heart transplantation. J Heart Lung Transplant 2011; 30: 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colvin MM, Cook JL, Chang P, et al. Antibody‐mediated rejection in cardiac transplantation: Emerging knowledge in diagnosis and management: A scientific statement from the American Heart Association. Circulation 2015; 131: 1608–1639. [DOI] [PubMed] [Google Scholar]
- 5. Tible M, Loupy A, Vernerey D, et al. Pathologic classification of antibody‐mediated rejection correlates with donor‐specific antibodies and endothelial cell activation. J Heart Lung Transplant 2013; 32: 769–776. [DOI] [PubMed] [Google Scholar]
- 6. Hammond ME, Revelo MP, Miller DV, et al. ISHLT pathology antibody mediated rejection score correlates with increased risk of cardiovascular mortality: A retrospective validation analysis. J Heart Lung Transplant 2016; 35: 320–325. [DOI] [PubMed] [Google Scholar]
- 7. Fedrigo M, Feltrin G, Poli F, et al. Intravascular macrophages in cardiac allograft biopsies for diagnosis of early and late antibody‐mediated rejection. J Heart Lung Transplant 2013; 32: 404–409. [DOI] [PubMed] [Google Scholar]
- 8. Kfoury AG, Miller DV, Snow GL, et al. Mixed cellular and antibody‐mediated rejection in heart transplantation: In‐depth pathologic and clinical observations. J Heart Lung Transplant 2016; 35: 335–341. [DOI] [PubMed] [Google Scholar]
- 9. Lepin EJ, Zhang Q, Zhang X, et al. Phosphorylated S6 ribosomal protein: A novel biomarker of antibody‐mediated rejection in heart allografts. Am J Transplant 2006; 6: 1560–1571. [DOI] [PubMed] [Google Scholar]
- 10. Li F, Wei J, Valenzuela NM, et al. Phosphorylated S6 kinase and S6 ribosomal protein are diagnostic markers of antibody‐mediated rejection in heart allografts. J Heart Lung Transplant 2015; 34: 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sis B, Jhangri GS, Riopel J, et al. A new diagnostic algorithm for antibody‐mediated microcirculation inflammation in kidney transplants. Am J Transplant 2012; 12: 1168–1179. [DOI] [PubMed] [Google Scholar]
- 12. Berry GJ, Angelini A, Burke MM, et al. The ISHLT working formulation for pathologic diagnosis of antibody‐mediated rejection in heart transplantation: Evolution and current status (2005‐2011). J Heart Lung Transplant 2011; 30: 601–611. [DOI] [PubMed] [Google Scholar]
- 13. Berry GJ. Antibody‐mediated rejection of the cardiac allograft: Where do we stand in 2012? Curr Opin Organ Transplant 2012; 17: 303–308. [DOI] [PubMed] [Google Scholar]
- 14. Chih S, Tinckam KJ, Ross HJ. A survey of current practice for antibody‐mediated rejection in heart transplantation. Am J Transplant 2013; 13: 1069–1074. [DOI] [PubMed] [Google Scholar]
- 15. Kfoury AG, Snow GL, Budge D, et al. A longitudinal study of the course of asymptomatic antibody‐mediated rejection in heart transplantation. J Heart Lung Transplant 2012; 31: 46–51. [DOI] [PubMed] [Google Scholar]
- 16. Loupy A, Toquet C, Rouvier P, et al. Late failing heart allografts: Pathology of cardiac allograft vasculopathy and association with antibody‐mediated rejection. Am J Transplant 2016; 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 17. Revelo MP, Miller DV, Stehlik J, et al. Longitudinal evaluation of microvessel density in survivors vs. nonsurvivors of cardiac pathologic antibody‐mediated rejection. Cardiovasc Pathol. 2012; 21: 445–454. [DOI] [PubMed] [Google Scholar]
- 18. Hiemann NE, Wellnhofer E, Knosalla C, et al. Prognostic impact of microvasculopathy on survival after heart transplantation: Evidence from 9713 endomyocardial biopsies. Circulation 2007; 116: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 19. Lu WH, Palatnik K, Fishbein GA, et al. Diverse morphologic manifestations of cardiac allograft vasculopathy: A pathologic study of 64 allograft hearts. J Heart Lung Transplant 2011; 30: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 20. Rahmani M, Cruz RP, Granville DJ, et al. Allograft vasculopathy versus atherosclerosis. Circ Res 2006; 99: 801–815. [DOI] [PubMed] [Google Scholar]
- 21. Hammond EH, Yowell RL, Price GD, et al. Vascular rejection and its relationship to allograft coronary artery disease. J Heart Lung Transplant. 1992; 11 (Pt 2):S111–S119. [PubMed] [Google Scholar]
- 22. Irving CA, Carter V, Gennery AR, et al. Effect of persistent versus transient donor‐specific HLA antibodies on graft outcomes in pediatric cardiac transplantation. J Heart Lung Transplant 2015; 34: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 23. Loupy A, Cazes A, Guillemain R, et al. Very late heart transplant rejection is associated with microvascular injury, complement deposition and progression to cardiac allograft vasculopathy. Am J Transplant 2011; 11: 1478–1487. [DOI] [PubMed] [Google Scholar]
- 24. Wu GW, Kobashigawa JA, Fishbein MC, et al. Asymptomatic antibody‐mediated rejection after heart transplantation predicts poor outcomes. J Heart Lung Transplant 2009; 28: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammond EH, Yowell RL. Ultrastructural findings in cardiac transplant recipients. Ultrastruct Pathol. 1994; 18: 213–220. [DOI] [PubMed] [Google Scholar]
- 26. Abu‐Qaoud MS, Stoletniy LN, Chen D, et al. Lack of relationship between microvascular and macrovascular disease in heart transplant recipients. Transplantation 2012; 94: 965–970. [DOI] [PubMed] [Google Scholar]
- 27. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: Inclusion of c4d‐negative antibody‐mediated rejection and antibody‐associated arterial lesions. Am J Transplant 2014; 14: 272–283. [DOI] [PubMed] [Google Scholar]
- 28. Ho EK, Vlad G, Vasilescu ER, et al. Pre‐ and posttransplantation allosensitization in heart allograft recipients: Major impact of de novo alloantibody production on allograft survival. Hum Immunol 2011; 72: 5–10. [DOI] [PubMed] [Google Scholar]
- 29. Reed EF, Demetris AJ, Hammond E, et al. Acute antibody‐mediated rejection of cardiac transplants. J Heart Lung Transplant 2006; 25: 153–159. [DOI] [PubMed] [Google Scholar]
- 30. Tran A, Fixler D, Huang R, et al. Donor‐specific HLA alloantibodies: Impact on cardiac allograft vasculopathy, rejection, and survival after pediatric heart transplantation. J Heart Lung Transplant 2016; 35: 87–91. [DOI] [PubMed] [Google Scholar]
- 31. Ware AL, Malmberg E, Delgado JC, et al. The use of circulating donor specific antibody to predict biopsy diagnosis of antibody‐mediated rejection and to provide prognostic value after heart transplantation in children. J Heart Lung Transplant 2016; 35: 179–185. [DOI] [PubMed] [Google Scholar]
- 32. Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcgammaRs, and endothelium in transplant rejection. Trends Mol Med 2015; 21: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckels DD, Stehlik J, Kfoury AG. The detection and role of circulating antibodies in rejection. Curr Opin Organ Transplant 2013; 18: 589–594. [DOI] [PubMed] [Google Scholar]
- 34. Haarberg KM, Tambur AR. Detection of donor‐specific antibodies in kidney transplantation. Br Med Bull 2014; 110: 23–34. [DOI] [PubMed] [Google Scholar]
- 35. Konvalinka A, Tinckam K. Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol 2015; 26: 1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tait BD, Susal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non‐HLA antibodies in transplantation. Transplantation 2013; 95: 19–47. [DOI] [PubMed] [Google Scholar]
- 37. Tambur AR, Herrera ND, Haarberg KM, et al. Assessing antibody strength: Comparison of MFI, C1q, and titer information. Am J Transplant 2015; 15: 2421–2430. [DOI] [PubMed] [Google Scholar]
- 38. Tyan DB. New approaches for detecting complement‐fixing antibodies. Curr Opin Organ Transplant 2012; 17: 409–415. [DOI] [PubMed] [Google Scholar]
- 39. Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti‐HLA antibody at high titer is complement binding and associated with increased risk of antibody‐mediated rejection in heart transplant recipients. J Heart Lung Transplant 2013; 32: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1‐receptor activating antibodies in renal‐allograft rejection. N Engl J Med 2005; 352: 558–569. [DOI] [PubMed] [Google Scholar]
- 41. Reinsmoen NL, Lai CH, Heidecke H, et al. Anti‐angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation 2010; 90: 1473–1477. [DOI] [PubMed] [Google Scholar]
- 42. Hiemann NE, Meyer R, Wellnhofer E, et al. Non‐HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation 2012; 94: 919–924. [DOI] [PubMed] [Google Scholar]
- 43. Reinsmoen NL, Lai CH, Mirocha J, et al. Increased negative impact of donor HLA‐specific together with non‐HLA‐specific antibodies on graft outcome. Transplantation 2014; 97: 595–601. [DOI] [PubMed] [Google Scholar]
- 44. Dragun D. Humoral responses directed against non‐human leukocyte antigens in solid‐organ transplantation. Transplantation 2008; 86: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 45. Dragun D, Catar R, Kusch A, et al. Non‐HLA‐antibodies targeting Angiotensin type 1 receptor and antibody mediated rejection. Hum Immunol 2012; 73: 1282–1286. [DOI] [PubMed] [Google Scholar]
- 46. Dragun D, Catar R, Philippe A. Non‐HLA antibodies against endothelial targets bridging allo‐ and autoimmunity. Kidney Int 2016; 90: 280–288. [DOI] [PubMed] [Google Scholar]
- 47. Demetris AJ, Bellamy C, Hubscher SG, et al. 2016. Comprehensive update of the Banff working group on liver allograft pathology: Introduction of antibody‐mediated rejection. Am J Transplant. doi: 10.1111/ajt.13909 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48. Sis B, Jhangri GS, Bunnag S, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody‐mediated damage despite lack of C4d staining. Am J Transplant 2009; 9: 2312–2323. [DOI] [PubMed] [Google Scholar]


