Abstract
Current anti-epileptic drugs (AEDs) act on a limited set of neuronal targets, are ineffective in a third of patients with epilepsy, and do not show disease-modifying properties. MicroRNAs are small noncoding RNAs that regulate levels of proteins by post-transcriptional control of mRNA stability and translation. MicroRNA-134 is involved in controlling neuronal microstructure and brain excitability and previous studies showed that intracerebroventricular injections of locked nucleic acid (LNA), cholesterol-tagged antagomirs targeting microRNA-134 (Ant-134) reduced evoked and spontaneous seizures in mouse models of status epilepticus. Translation of these findings would benefit from evidence of efficacy in non-status epilepticus models and validation in another species. Here, we report that electrographic seizures and convulsive behavior are strongly reduced in adult mice pre-treated with Ant-134 in the pentylenetetrazol model. Pre-treatment with Ant-134 did not affect the severity of status epilepticus induced by perforant pathway stimulation in adult rats, a toxin-free model of acquired epilepsy. Nevertheless, Ant-134 post-treatment reduced the number of rats developing spontaneous seizures by 86% in the perforant pathway stimulation model and Ant-134 delayed epileptiform activity in a rat ex vivo hippocampal slice model. The potent anticonvulsant effects of Ant-134 in multiple models may encourage pre-clinical development of this approach to epilepsy therapy.
Keywords: noncoding RNA, hippocampal sclerosis, epileptogenesis, chemoconvulsant, status epilepticus, anti-epileptic drug, temporal lobe epilepsy
Introduction
Epilepsy is a common and serious chronic neurological disease that affects over 50 million people worldwide. Anti-epileptic drugs (AEDs) are effective at controlling seizures in many patients, acting through a range of neuronal targets, including ion channels, neurotransmitter release, and receptor mechanisms.1, 2 However, a third of patients with epilepsy fail to achieve seizure freedom, and current AEDs do not show disease-modifying properties. Future treatments for epilepsy need to differentiate from currently marketed drugs, for example, by having a novel mechanism(s) of action or providing disease-modifying effects.1, 2
Acquired epilepsy is thought to result from disturbances to the expression and function of neurotransmitters and their receptors, ion channels, and signaling components, as well as altered metabolism, neuronal and glial microstructure, neuroinflammation, and other mechanisms.3, 4, 5 It is likely that disease-modifying treatments will need to influence multiple genes within a given pathway or in various independent pathways. Potential gene network control points were recently identified and include master regulators of transcription and neuroinflammation,6 epigenetic factors,7 and noncoding RNAs.8
MicroRNAs (miRNAs) are a conserved family of endogenous small noncoding RNAs that regulate protein levels in cells by post-transcriptional control of mRNA stability and translation.9, 10 This is achieved by sequence-specific binding of the miRNA to complementary bases in the mRNA target.11 Since this generally requires only a 7- to 8-nucleotide match, individual miRNAs can potentially target large numbers of mRNAs.12 This provides the ability to regulate entire gene networks or multiple biological processes and has been demonstrated in the brain.13 MicroRNA-134 (miR-134) was identified as a brain-enriched, neuronally expressed miRNA that controls dendrite spine morphogenesis, via repression of LIM domain kinase 1 and other targets.14, 15 This is potentially important for disorders of hyperexcitability, since dendritic spines are contact points for excitatory communication and there is evidence that reorganization of dendrites is a feature of experimental and human epilepsy.16, 17 We identified miR-134 among upregulated miRNAs profiled in areas of hippocampal damage following status epilepticus induced by intra-amygdala kainic acid (KA) in mice.18 Expression of miR-134 is also upregulated after neuronal stimulation and seizures in various other in vitro and in vivo models,15, 19, 20, 21, 22 and miR-134 is overexpressed in the resected human neocortex from patients with intractable temporal lobe epilepsy (TLE).19 Silencing miR-134 by intracerebroventricular (i.c.v.) injection of locked nucleic acid (LNA), cholesterol-tagged antagomirs (Ant-134) reduced levels of miR-134 for at least a month and rendered mice refractory to the convulsant effects of both KA and pilocarpine.19, 22 Consistent with known miR-134 targets,14 LIM domain kinase 1 and levels of other miR-134 targets were higher in Ant-134-treated mice after status epilepticus and the dendritic spine volume was increased.19, 22 Notably, injection of Ant-134 after status epilepticus suppressed the later occurrence of spontaneous recurrent seizures in the intra-amygdala KA model in mice.19
There is significant interest in developing miRNA-based therapies.23, 24 There are also barriers to antisense-based approaches and there has been some disengagement of industry from pursuing such efforts.25 This is likely to be a problem for any new therapy for epilepsy, particularly one that is not based on traditional small molecule chemistry.1, 2 Pre-clinical development of an miR-134-based treatment for epilepsy might be more attractive if additional important questions are answered. Principally, work to date has only tested Ant-134 in mouse models of status epilepticus. Proof of seizure-suppressive effects of Ant-134 in a non-status epilepticus model or in another species would bridge the gap in evidence and facilitate pre-clinical development of Ant-134-based treatment for epilepsy.26, 27 Here, we evaluated miR-134 expression in the resected hippocampus from patients with pharmacoresistant TLE and we tested Ant-134 in three different models. We used the pentylenetetrazol (PTZ) model in mice, a popular method for screening putative anticonvulsive effects that triggers seizures via γ-amino butyric acid (GABA) blockade.26, 28 Second, the perforant pathway stimulation (PPS) model, a toxin-free model of acquired epilepsy based on nonconvulsive status epilepticus that avoids the confounding influence of exposing the brain to chemoconvulsants, was used in rats.29 Finally, we used a high-potassium model of epileptiform activity,30 using ex vivo brain slices from rats pre-treated with Ant-134. Our results establish that targeting miR-134 offers broad anticonvulsant and possibly disease-modifying effects in experimental epilepsy, which may encourage pre-clinical development of Ant-134 as a treatment for epilepsy.
Results
miR-134 Levels in the Hippocampus from Patients with Pharmacoresistant TLE
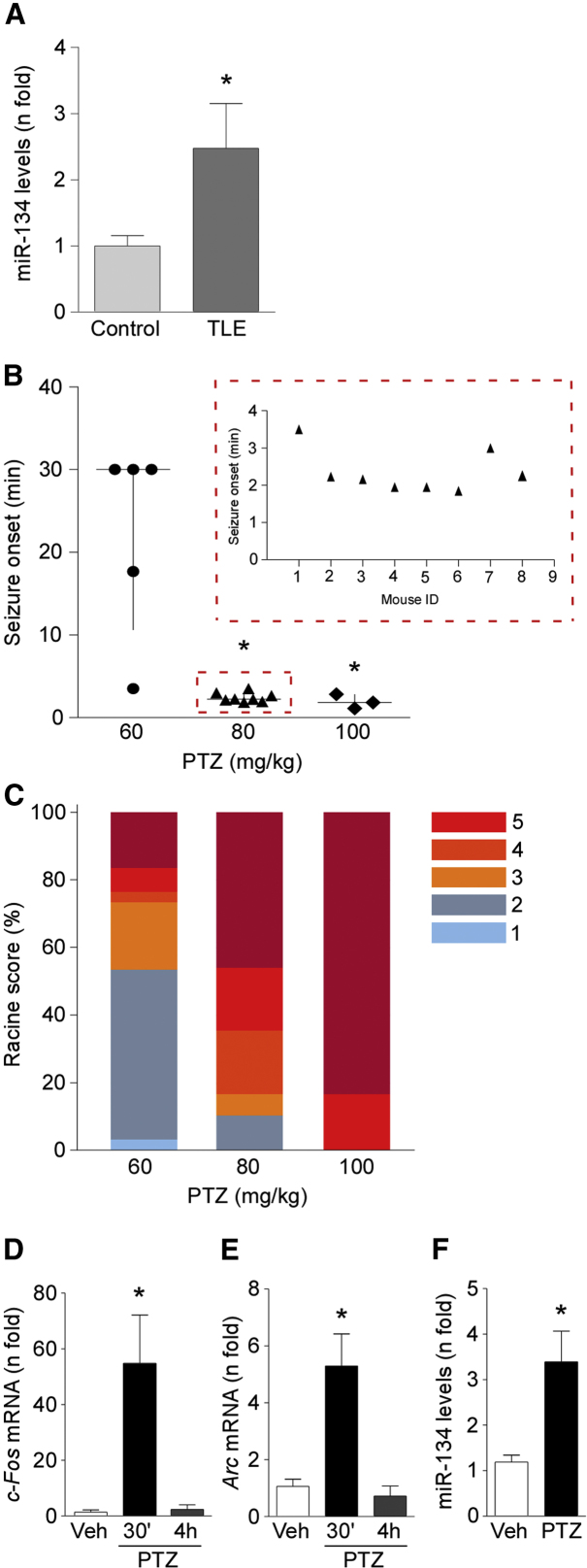
We first sought to extend the clinical relevance of targeting miR-134 in epilepsy. For this, we analyzed levels of miR-134 in the surgically obtained hippocampus from adults with pharmacoresistant TLE and we compared findings to autopsy hippocampal samples from people who died of causes unrelated to neurological disease. These samples were used previously to analyze expression of other genes,31 and clinical data for both groups are briefly summarized in Tables S1 and S2. There were no between-group differences in patient age, and each group included males and females. We detected higher levels (p = 0.037) of mature miR-134 in the TLE specimens compared to autopsy samples (Figure 1A). This difference is unlikely to be an artifact of post mortem delay, since miR-134 levels did not decrease in simulated autopsy experiments lasting up to 12 hr.19
Figure 1.
Increased miR-134 Levels in Human TLE Hippocampus and after PTZ-Induced Seizures in Mice
(A) Graph shows higher levels of mature miR-134 in TLE specimens compared to autopsy control samples (n = 12/group; p = 0.037). (B) Graph shows the seizure onset after challenge with different doses of PTZ (n = 3–8/group; p = 0.0061). Dotted box inset shows individual data for seizure onset in mice treated with 80 mg/kg PTZ. (C) Racine scale readings for the lowest, intermediate, and highest doses (60, 80, and 100 mg/kg) of PTZ (n = 3–8/group; p = 0.014, adjusted for clustering within animal). (D and E) Graphs show mRNA levels of the molecular markers of hyperexcitability c-Fos (D) and Arc (E) 30 min and 4 hr after PTZ injection (n = 3–5/group; p = 0.0373 and p = 0.0125 for c-Fos and Arc, respectively). (F) Increased miR-134 expression in mouse hippocampus 30 min after PTZ injection in comparison with vehicle control (saline) (n = 3–5/ group; p = 0.0266). All error bars shown as means ± SEM. Veh, vehicle. *p < 0.05.
Increased miR-134 Levels after PTZ-Induced Seizures in Mice
We began by establishing dosing parameters in the mouse PTZ model. Generalized tonic-clonic seizures were first reliably elicited at a dose of 60 mg/kg in C57BL/6J mice (Figure 1B). A dose of 80 mg/kg elicited generalized tonic-clonic seizures in all mice and with a more consistent and shorter latency than with the 60 mg/kg dose (Figure 1B). A dose of 100 mg/kg also produced rapid-onset tonic-clonic seizures in all mice but was associated with high mortality (Figure 1B and data not shown). Ordinal logistic regression analysis was used to explore the dose dependence of the PTZ-induced convulsions. PTZ dose was significantly associated with Racine scores (Figure 1C). A dose of 80 mg/kg was selected for further experiments.
To obtain molecular evidence for PTZ-induced seizure activity in the model, we measured expression of activity-regulated genes in the hippocampus. Transcript levels of FBJ osteosarcoma oncogene (Fos), a calcium-dependent marker of neuronal activity and activity regulated cytoskeletal-associated protein (Arc), another immediate early gene responsive to intense neuronal activity, were strongly increased in the hippocampus 30 min, but not at 4 hr, after PTZ-induced seizures (Figures 1D and 1E). These data are consistent with PTZ inducing short-lasting seizure activity. There was no evidence of irreversible neuronal injury in tissue sections from mice after PTZ, as assessed by Fluoro-Jade B (FJB) and NeuN (RNA binding protein, fox-1 homolog [C. elegans] 3) staining (data not shown).
Expression of miR-134 is known to increase in various in vitro and in vivo seizure models.15, 19, 20, 21, 22 We therefore investigated whether PTZ-induced convulsions alter miR-134 levels in mice. TaqMan miRNA assays showed that levels of mature miR-134 were significantly increased in the hippocampus, but not in the cortex, 30 min after PTZ-induced generalized tonic-clonic seizures in mice (Figure 1F and data not shown).
Silencing miR-134 Protects against PTZ-Induced Seizures in Mice
We next explored the consequences of silencing miR-134 on PTZ-induced seizures. For these studies, we performed i.c.v. injection of antagomirs targeting miR-134 (Ant-134) in mice.19, 22 Previous work has established a dose of these antagomirs that reduces hippocampal miR-134 levels by over 90% by 24 hr after injection.19 Accordingly, we tested PTZ responses 24 hr after injection of Ant-134 and compared them to responses in mice that received a scrambled antagomir (Scr).
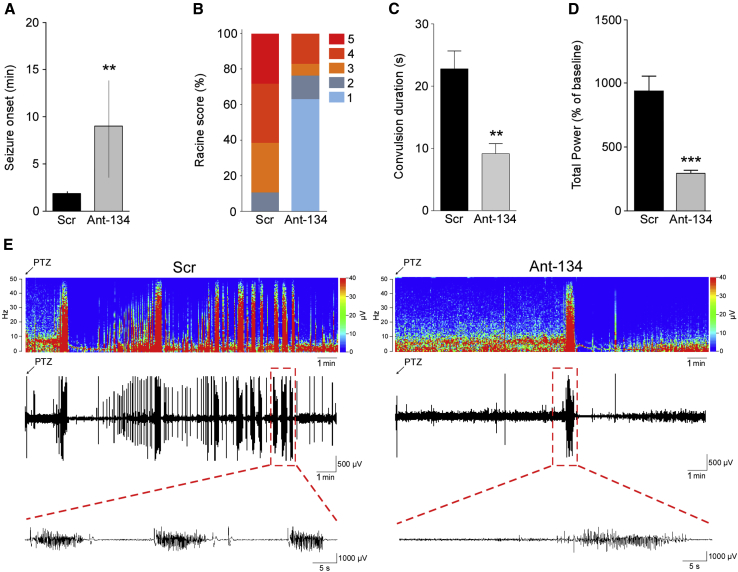
In Scr mice, delay to onset of first tonic-clonic seizure after PTZ injection was similar to that observed before in control mice (Figure 2A, and compare to Figure 1B). Thus, the control antagomir did not alter the normal response to PTZ in the model. The time to first PTZ-induced tonic-clonic seizure was significantly longer in mice injected with Ant-134 compared to Scr-injected animals (Figure 2A). The severity of Racine-scored PTZ-induced seizures was also significantly lower in Ant-134- compared to Scr-injected mice (Figures 2B and 2C). Analysis of electrographically recorded seizure activity determined that electroencephalography (EEG) total power was lower in Ant-134 mice compared to the control group after PTZ (Figure 2D). Representative EEG recordings and frequency-amplitude heatmaps are presented in Figure 2E.
Figure 2.
Silencing miR-134 Protects against PTZ-Induced Seizures in Mice
(A) Latency to the first PTZ-induced tonic-clonic seizure for mice pre-treated with Scramble (Scr) or Ant-134 (n = 3–5/group; p = 0.0079). (B and C) Ant-134 mice display milder convulsive behavior when compared to Scr-injected mice, presented as Racine scores (B; n = 3–5/group; p = 0.01) and duration of generalized tonic-clonic seizures (C; n = 3–5/group; p = 0.01). (D) Graph shows EEG total power, depicting percentage increase from each animal’s own baseline data (n = 3–5/group; p = 0.0004). (E) Representative heatmap showing frequency (in hertz) and amplitude (in microvolts) of EEG recordings over time (in minutes) for Scr-injected (left) and Ant-134-injected (right) mice, followed by the respective EEG traces immediately below. All error bars shown as means ± SEM except in (A), which depicts medians and interquartile ranges. **p < 0.01; ***p < 0.001.
Antagomir Silencing and Suppression of Activity-Regulated Genes
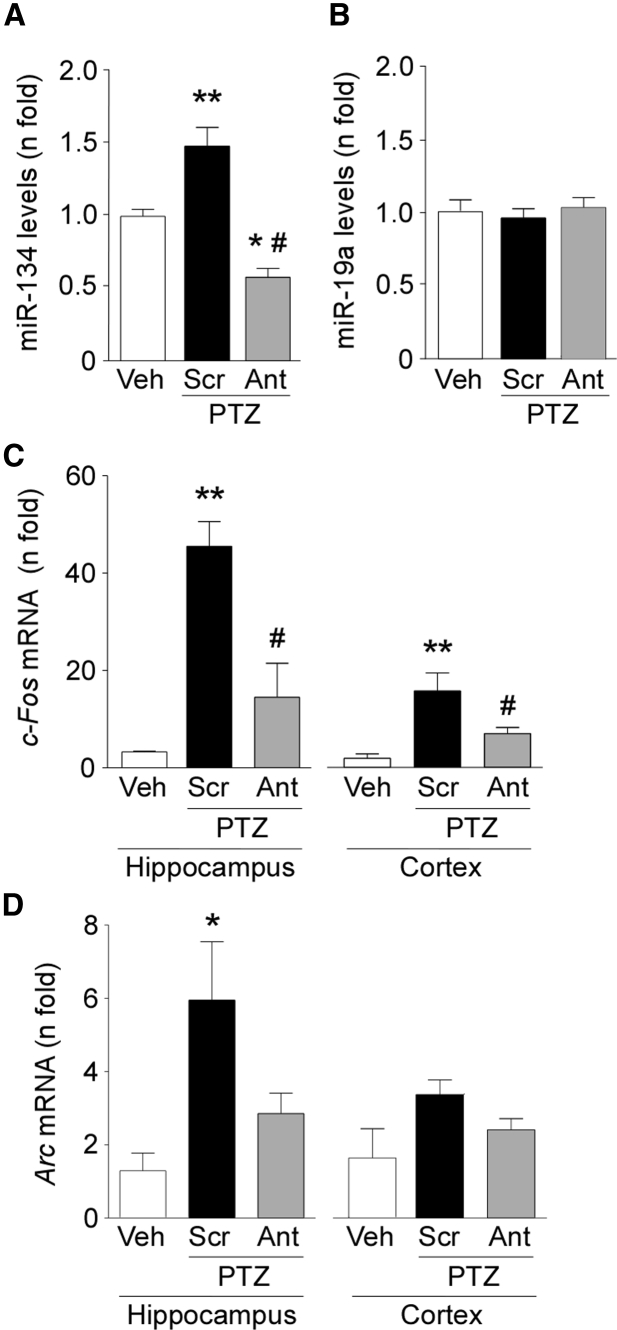
We next analyzed brain tissue samples from Scr- and Ant-134-injected mice for evidence of miRNA silencing and differences in expression of activity-regulated genes. Analysis of the hippocampus obtained after PTZ-induced seizures confirmed that miR-134 expression was lower in Ant-134 pre-treated mice compared to Scr-treated animals (Figure 3A). Ant-134 had no effect on levels of an unrelated miRNA (Figure 3B).
Figure 3.
Effects of Ant-134 on Molecular Markers of Seizures after PTZ
(A) Real-time qPCR analysis showing levels of miR-134 after PTZ-induced seizures. Pre-treatment with Ant-134 effectively silenced miR-134 after PTZ-induced seizures (p = 0.05 and p = 0.01 for Scr and Ant-134, respectively, compared to vehicle [Veh]; p = 0.001 for Ant-134 versus Scr). (B) Expression of an unrelated miRNA, miR-19a, was not affected by Ant-134 (p = 0.8238). (C and D) Graphs present mRNA levels of c-Fos and Arc assessed 30 min after PTZ challenge in hippocampus and cortex of Scr- and Ant-134-injected mice. Data were normalized to RNU19 and β-actin for miRNA and mRNA expression, respectively. For c-Fos, p = 0.0004 (hippocampus) and p = 0.0017 (cortex) for Scr compared to Veh, and p = 0.01 (hippocampus) and p = 0.05 (cortex) for Ant compared to Scr. For Arc, p = 0.032 (hippocampus) when comparing Scr to the vehicle group and p = 0.152 (cortex) (n = 3–5/group). *p < 0.05 (compared to vehicle); **p < 0.01 (compared to vehicle); #p < 0.05 (compared to Scr). All error bars shown as means ± SEM.
Next, we assessed expression of Arc and c-Fos as molecular markers of recent neuronal activity to support the clinically scored behavior and EEG differences in Ant-134 mice. Quantitative real-time PCR analysis revealed increased c-Fos expression in the hippocampus of Scr-injected mice after PTZ-induced seizures (Figure 3C). In contrast, c-Fos levels were lower in Ant-134-injected mice, consistent with reduced seizure severity (Figure 3C). This difference was also apparent in the neocortex from the same animals (Figure 3C). Expression of Arc was increased in the hippocampus of Scr-injected mice that received PTZ. Arc levels were not significantly different in Ant-134 mice compared to Scr-injected animals in the hippocampus and neocortex after PTZ-induced seizures (Figure 3D).
Silencing miR-134 Does Not Alter Acute PPS-Induced Status Epilepticus but Reduces the Later Occurrence of Spontaneous Seizures in the Model
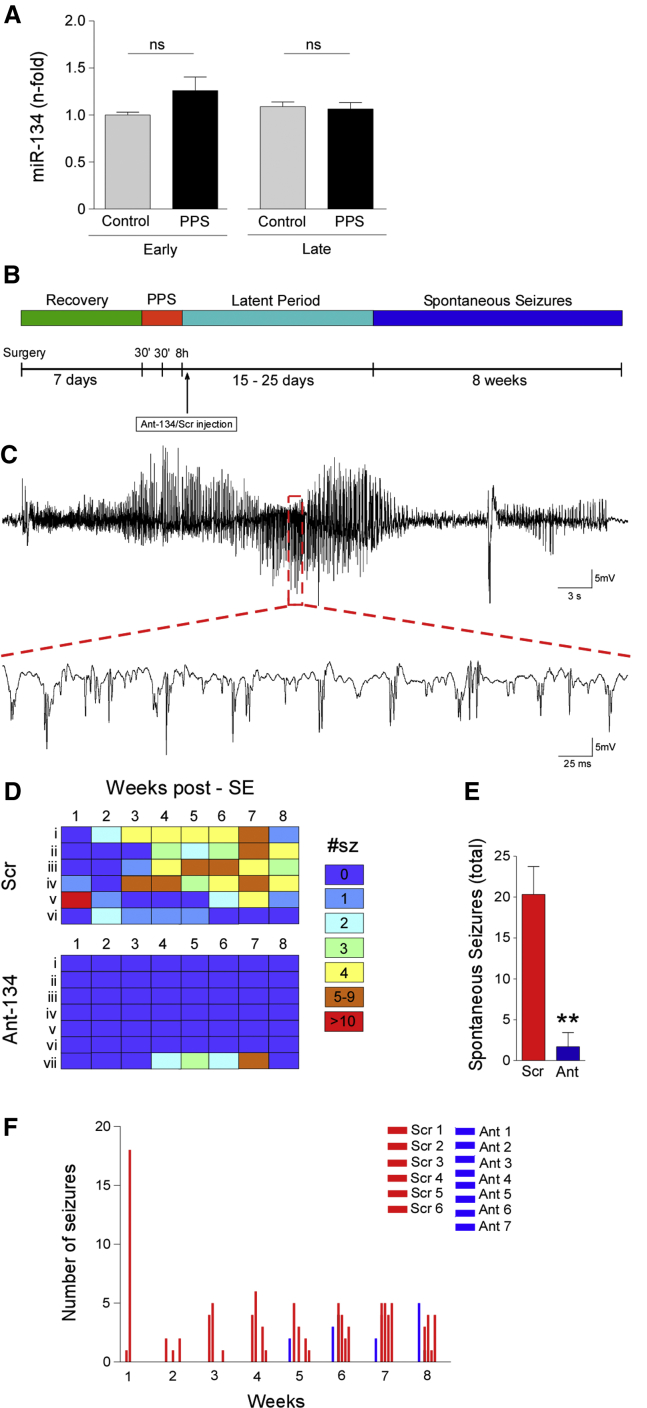
We turned next to the PPS model in rats, first investigating effects on miR-134 levels. TaqMan miRNA assays showed that levels of mature miR-134 were not significantly modified in the hippocampus of rats at two different time points (Figure 4A). Next, we assessed the effect of silencing miR-134 before stimulation on seizure severity during status epilepticus. Rats were pre-treated with Ant-134 or Scr (i.c.v.) 24 hr before the 8-hr stimulation protocol. Analysis of electrographically recorded seizure activity determined that Scr-injected rats presented a mean (± SEM) increase of 1,304.3% ± 94.9% in EEG total power relative to baseline. This was similar to animals that were stimulated but not receiving the oligonucleotide (data not shown). In rats pre-treated with Ant-134, the severity of evoked seizures in the PPS model was similar: EEG total power during seizures was a mean (± S.E.M) of 1,244.6% ± 82.7% (percent of baseline).
Figure 4.
Ant-134 Reduces Spontaneous Seizures after PPS-Induced Status Epilepticus in Rats
(A) miR-134 levels in rat hippocampus at “early” and “late” time points after PPS in comparison with the respective non-stimulated controls (p = 0.3309, n = 3–4/group). (B) Schematic illustrates PPS experimental design. (C) Representative EEG trace of a spontaneous seizure captured in a Scr-injected rat. (D–F) Individual rat (i–vi/vii) weekly seizure counts schematic (D) and graph (F) showing spontaneous seizures during long-term monitoring (8 weeks after the first spontaneous seizure). (E) Spontaneous seizure counts were summed by group. **p < 0.01 (for n = 6–7/group). All error bars shown as means ± SEM. ns, non-significant; SE, status epilepticus.
Despite not seeing an acute anticonvulsant effect of Ant-134 in the PPS model, we were nevertheless interested in whether silencing miR-134 after status epilepticus in the model would alter the emergence of recurrent spontaneous seizures. A key prior finding was that silencing miR-134 after status epilepticus induced by intra-amygdala KA reduced the subsequent occurrence of spontaneous recurrent seizures by over 90%.19 For these studies, rats underwent 8-hr stimulation of the perforant pathway to induce epilepsy and the i.c.v. injection of Ant-134 or Scr was given at the end of stimulation. Rats were then monitored with video-EEG until a first spontaneous seizure was recorded, typically 10–25 days after PPS, and they were then followed for a further 8 weeks (Figure 4B).
Epilepsy developed in all Scr-injected rats over the expected course, with the first spontaneous seizures appearing 1–4 weeks after status epilepticus (Figures 4B and 4C). Scr-injected rats averaged ∼20 spontaneous seizures recorded during the 8-week recording time (Figures 4D and 4E). In contrast, no spontaneous seizures were detected over the 8-week period in six of seven rats injected with Ant-134 after status epilepticus (Figures 4D–4F). Epilepsy emerged in only a single rat treated with Ant-134 (Figures 4D and 4F). The Fisher exact test determined that there was a significant difference in epilepsy development between the groups (p = 0.005). In terms of effect size, Ant-134 prevented 86% of the risk of epilepsy, with a 95% confidence interval (CI) of 12%–98%. An analysis of the duration of spontaneous seizures (electrographically defined) revealed that when spontaneous seizures occurred, they were similar between groups. That is, the average duration of a spontaneous seizure in Scr-treated rats was 53.7 ± 14.4 s (n = 20 seizures, from five rats), which was similar to the duration in epileptic rats in the PPS model that did not receive an i.c.v. injection (49.4 ± 15.9, n = 20 seizures). In the Ant-134 rat that developed epilepsy, the average duration of a spontaneous seizure was 52.3 ± 16.2 s (from n = 12 seizures).
Ant-134 Increases the Delay to Seizure Onset in Ex Vivo Brain Slices Exposed to High K+
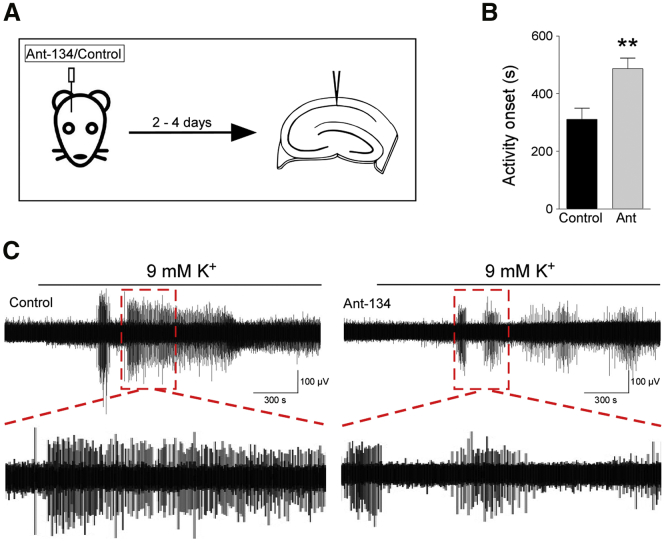
Having observed a disparity between the acute anti-seizure effects of Ant-134 pre-treatment in the PTZ and PPS models, we sought to examine the effect of Ant-134 on naive, non-epileptic tissue and whether the effects were due to long range networks or if local circuitry was sufficient to mediate any seizure protection. We injected Ant-134 in vivo in rats and subsequently prepared ex vivo brain slices (Figure 5A). These slices were then exposed to a 9-mM K+ seizure challenge,30 to determine whether Ant-134 protects against epilepsy in local circuits of the isolated slice in the absence of epileptogenesis.
Figure 5.
Ant-134 Delays Epileptiform Activity Onset in Ex Vivo Slices in Response to 9 mM K+
(A) Schematic of experimental design. Rats were injected with Ant-134 or control 2–4 days prior to ex vivo slice preparation. Local field potential recordings were made in hippocampal CA1. (B) Bar chart shows that epileptiform activity onset was delayed by Ant-134 (**p = 0.002, α = 0.025; statistical power = 0.87, Mann-Whitney U test; control, n = 9 slices from 3 rats; Ant-134, n = 11 slices from 3 rats). (C) Representative traces of epileptiform activity elicited by 9 mM K+ in slices from control or Ant-134-treated rats. All error bars shown as means ± SEM.
Onset of activity was delayed in Ant-134-treated slices by 176 s relative to the control (Figures 5B and 5C). This effect was statistically significant (Mann-Whitney U test, p = 0.002, α = 0.025, power = 0.87). We also compared the power of epileptiform activity and duration of seizure-like events in these data; these effects were not statistically significant (power: t test, p = 0.70; duration: Mann-Whitney U test, p = 0.067). This finding suggests that Ant-134 can confer seizure resistance in naive tissue and thus mediate its effect even in the absence of epileptogenesis. In addition, preservation of the seizure resistance in isolated slices indicates that at least some of the effects of Ant-134 are due to local changes in activity.
Discussion
The present study provides important additional evidence of the anticonvulsant and disease-modifying effects of LNA-based antagomirs targeting miR-134 in animal models of epilepsy. Our study shows that Ant-134 is an anticonvulsant in an acute chemoconvulsant model (PTZ in mice) and Ant-134 had anti-epileptogenic activity in the perforant pathway stimulation model of epilepsy in rats. Ant-134 also confers resistance to epileptic activity in naive tissue, reflected as a delay to activity onset following a seizure challenge in ex vivo brain slices. We also report that miR-134 levels are elevated in the hippocampus of patients with pharmacoresistant TLE. The therapeutic potential of targeting miR-134 in established epilepsy will require additional studies, but our findings may encourage further research or pre-clinical development of this miRNA-based treatment for epilepsy.
Despite the recent introduction of two additional AEDs (perampanel and brivaracetam), new drug development in epilepsy faces significant challenges.1, 2, 32 New targets are required that can treat pharmacoresistant patients or have disease-modifying effects that are not served by current therapies. miRNAs have emerged as potential targets of the future via their influence on neuronal microstructure, gliosis, neuroinflammation, and ion channels.8, 33 Silencing miR-134 has produced the most convincing anti-seizure effects to date, reducing status epilepticus in pre-treatment paradigms in the intra-amygdala KA and pilocarpine models and exerting potent long-term effects on spontaneous seizure development.19, 22 This miRNA is also an attractive target, since studies show increased levels of miR-134 within the temporal lobe of adult and pediatric patients with intractable epilepsy.19, 20 We report here that miR-134 is also elevated in the hippocampus of adults with pharmacoresistant TLE, although there are potential confounders with comparing resected material from patients to autopsy control samples.34 The present study has answered key questions that could facilitate pre-clinical development by showing that Ant-134 has anticonvulsant effects in non-status epilepticus models and is disease modifying in another species. The first important finding was that pre-treatment with Ant-134 strongly suppressed seizures in the mouse PTZ model. Effects on both clinical behavior, including delaying the time to first convulsive seizure, and electrographic seizure activity were seen. Thus, lowering brain levels of miR-134 can exert anti-excitability effects, and this implies that miR-134 has a role in setting excitability thresholds in the brain. This is thought to be due to regulation of proteins that control dendritic morphology,14, 15 although other targets are known.35 Ant-134 did not fully prevent seizures in the PTZ model; rather, it delayed and minimized their severity, a finding most closely matching the effect of Ant-134 in the pilocarpine model of status epilepticus.22 It may not be possible to fully prevent all seizures using Ant-134 in chemoconvulsant models, but further adjustments to antagomir dose or delivery may yield optimal seizure-suppressive effects. PTZ triggers seizures via GABAergic blockade; this differs from the mechanism by which status epilepticus is induced by KA and pilocarpine, which work by promoting glutamatergic and cholinergic transmission, respectively. This suggests that Ant-134 may be effective for epilepsies of various etiologies, which, in patients, can vary considerably between trauma, stroke, infection, genetic, and other causes.36 This could maximize the potential patient population that could benefit from Ant-134. Our studies also show that PTZ-induced convulsions increase brain levels of miR-134, transcription of which is probably mediated by myocyte enhancer factor 2C.15 We did not observe an increase in miR-134 after perforant pathway stimulation in the rat. This was surprising, although changes to miR-134 levels are not always detected after chemoconvulsive stimuli,37 and we cannot rule out a technical explanation (e.g., the time points selected or a subfield-specific effect that was masked by the use of the whole hippocampus).
Our study included an analysis of the short-term effects of Ant-134 on neuronal activity-regulated genes in the PTZ model. Expression of genes such as c-Fos and Arc is a well-established surrogate marker of recent seizure activity, the protein products of which are involved in coordinating synaptic plasticity and adaptation to excessive neuronal stimulation.38, 39 Unexpectedly, only levels of c-Fos, and not Arc, showed the expected reductions in Ant-134 mice after PTZ seizures. The explanation for this difference between markers is uncertain. It is known that c-Fos induction is calcium dependent, whereas Arc expression can be induced via calcium-independent pathways.38, 39 Therefore, Ant-134 treatment may have reduced seizure severity sufficient to prevent c-Fos induction, such as by minimizing N-methyl-d-aspartate (NMDA) receptor opening, but enough residual neuronal excitation remained to upregulate Arc. These findings provide additional evidence of the scale and nature of seizure suppression by Ant-134 and suggest molecular markers that can distinguish effects of miRNA manipulations on different components of seizure responses.
In the present study, we observed that pre-treatment of rats with Ant-134 did not protect against perforant pathway stimulation-induced status epilepticus. This suggests its potential limitations as an acute anticonvulsant. While there are examples of anticonvulsants producing effects in some but not all models, these findings suggest that Ant-134 may have the most clinical value as an anti-epileptogenic treatment. Previous work showed that injection of Ant-134 after status epilepticus in mice suppressed the later occurrence of spontaneous seizures by ∼90%, evidence of a disease-modifying effect in the intra-amygdala KA model.19 Here, we found nearly identical anti-epileptogenic effects of Ant-134 in a different model in rats. This is the only demonstration, to our knowledge, of any disease-modifying treatment in the PPS model in rats. The PPS technique induces hippocampal sclerosis and recurrent spontaneous seizures,29, 40 and it provides a means to trigger epilepsy without the bias or other issues41 associated with chemoconvulsants. Ant-134 was injected shortly after triggering status epilepticus in order to model a reasonably realistic clinical scenario, such as a patient being treated shortly after an initial precipitating injury. Ant-134 had dramatic and potent effects, with the majority of Ant-134 rats (six of seven, 86%) never developing spontaneous recurrent seizures. Notably, spontaneous seizures in the study with the intra-amygdala KA model did occur in all Ant-134 mice, albeit with dramatically lower seizure frequency.19 This difference may relate to the extent of hippocampal injury or another aspect of the epileptic phenotype, such as the frequency of spontaneous seizures. Thus, presently we see a possible additional therapeutic action of Ant-134, whereby post-treatment is capable of fully preventing epilepsy at least in some models. We cannot, of course, exclude that epilepsy might have developed in some of the Ant-134 rats at some point in the future. However, we recorded for 8 weeks beyond the standard latent period. We further corroborated the anti-seizure effect of Ant-134 by using an acute seizure challenge with high potassium in otherwise healthy (ex vivo) tissue. Inhibitory neurotransmission is retained in this model (in contrast to PTZ) and there is no hippocampal sclerosis (unlike PPS), further suggesting that Ant-134 can be therapeutic in multiple epileptic syndromes with varying mechanisms. We observed a delay in the onset of epileptiform bursts in brain slices that had been pre-treated with Ant-134. This finding suggests that epileptogenesis is not required for Ant-134 to mediate its effects on seizure onset. We did not see any significant changes in the power or duration of epileptiform activity; however, these parameters are highly variable in brain slices, which can obscure potential effects. In addition, we propose that Ant-134 can mediate seizure protection in the hippocampus via effects on local circuitry, because long range connections would likely be severed during the slicing process. The anti-epileptogenic actions of Ant-134 may be independent of the etiology of the initial precipitating injury, although this will require further testing (e.g., in models of trauma or infection-induced epilepsy). In addition, upregulation of miR-134 is not essential for Ant-134 to produce anti-epileptogenic effects, since miR-134 levels were not altered after PPS-induced status epilepticus. Thus, epilepsy can be dramatically suppressed in two different in vivo models, and one in vitro, in two different species. This provides strong support for and is an argument for further development of Ant-134 for epilepsy treatment or prevention.
The safety of targeting miR-134 using antagomirs has not yet been explored in depth. Antagomirs targeting a liver-expressed miRNA have been given to humans and were found to be well tolerated and effective against hepatitis C in clinical trials.42 There are concerns, obviously, that because Ant-134 can alter dendritic spines, it could have effects on cognition.17 Studies to date have not identified such problems, with natural exploratory behavior and performance in a hippocampus-dependent task found to be normal in mice a few days after i.c.v. injection of Ant-134.19, 22 However, more demanding tests of cognition or learning will need to be performed. Another factor that argues for general safety is that mice and rats have been recorded for at least 2 months after brain injections of Ant-134. Nevertheless, pre-clinical development will necessitate an extensive battery of toxicity and safety testing.
Work to date on Ant-134 satisfies certain recommendations for testing and evaluating novel therapies for epilepsy, with seizure-suppressive effects demonstrated in five different models and two different species.1, 2, 43, 44 There are, however, additional seizure models that could be used during any future pre-clinical development. This includes kindling, a popular model that has been successful in identifying AEDs with novel mechanisms of action.45 Ant-134 has not been tested in female rodents. While this is not an essential step before pre-clinical development, sex differences in miRNA inhibitor responses may be worth investigating in future studies and sexual dimorphism in miRNA expression has been reported, although not for miR-134.46, 47
It will also be of value to understand how Ant-134 produces its potent seizure-suppressive effects. The most likely mechanism is upregulation of one or more proteins due to de-repression of a miR-134 target. Previous work has shown that miR-134 is uploaded into the RNA-induced silencing complex after seizures, which facilitates targeting of mRNAs, and in vitro studies suggest that the neuroprotective effects of Ant-134 are due to de-repression of LIM domain kinase 1.19 Ant-134 treatment also increases dendritic spine volume and changes spine number in the hippocampus,19, 22 which could influence excitability and would also be consistent with a mechanism involving protection of miR-134 targets such as LIM domain kinase 1 or pumilio RNA-binding family member 2.15 Altered spine volume and number is consistent with our observation of a robust local effect of suppressing miR-134 on delaying epileptiform activity, although additional effects on long range connections cannot be ruled out. We must also consider that the acute seizure-suppressive effects and long-term anti-epileptogenesis effects may arise from different mechanisms. Ultimately, in vivo studies will be necessary to determine the anti-seizure mechanism of Ant-134 (e.g., by analyzing gene expression networks, quantifying the proteome, and applying techniques to validate antagomir-miRNA targeting such as polysome shift assays).48
What are the next steps for translation or pre-clinical development? An obvious question is whether and how antagomirs could be given to patients. Their large size precludes their passing an intact blood-brain barrier (BBB).49 Recent work has explored methods to circumvent the BBB, including conjugating antagomirs with brain-penetrating peptides50 or administering antagomirs via intranasal injection.19, 51 The BBB may also be open after certain epilepsy-precipitating injuries52 or it can be physically breached, such as through use of ultrasound.53 Another question is whether Ant-134 injection into already epileptic animals could alter the frequency or duration of spontaneous seizures or attendant hippocampal pathology. Success with that approach could provide novel treatment opportunities for the population of drug-resistant patients for whom surgery or other alternatives to AEDs are either not possible or not effective currently.
In summary, the present study provides important additional validation of the potent seizure-suppressive and possibly disease-modifying effects of Ant-134 in two different animal models and one brain slice model. The effectiveness of Ant-134 and the potency of seizure suppression suggest a target for drug development in epilepsy that could provide additional effects beyond those provided by any currently marketed drug for epilepsy.
Materials and Methods
Human Brain Tissue Samples
This study was approved by the Ethics (Medical Research) Committee of Beaumont Hospital of Dublin (no. 05/18) and written informed consent was obtained from all patients. The control (autopsy) hippocampus (n = 12) was obtained from 12 individuals. Autopsy control tissue was obtained from the University of Maryland Brain and Tissue Bank. Fresh frozen specimens were from individuals without known neurological disease (Table S1). Patients (n = 12) were referred for surgical resection of the temporal lobe by an epileptologist (N.D.) following neurological assessment, video-EEG recording, and MRI/neuroimaging. Each patient was determined to have medically intractable TLE with a history of recurring seizures (Table S2). All patients were taking anticonvulsant medication prior to surgery. Patients underwent left or right temporal lobe resection. The hippocampus was obtained and a portion of each specimen was frozen in liquid nitrogen and stored at −70°C until use. A pathologist (M.A.F.) assessed the hippocampus as part of the pathologic evaluation, addressed the degree of hippocampal sclerosis, and described other pathologic changes.
Animals
All animal experiments were performed in accordance with the European Communities Council Directive (2010/63/EU). Procedures in mice were approved by the Research Ethics Committee of the Royal College of Surgeons in Ireland (REC-842), under license from the Ireland Health Products Regulatory Authority (AE19127/001). Procedures in rats were performed under authorization of the Philipps University Bioethics Committee and were approved by the local regulation authority (Regierungspräsidium Gieβen), or they were performed according to the UK Animals (Scientific Procedures) 1986 Act (PPL 70-7684) and approved by local ethical review (University College London). Male adult C57BL/6J mice (20–25 g) and male Sprague-Dawley rats (325–350 g or 200–300 g; all from Harlan) were used in all studies. Animals were housed in on-site barrier-controlled facilities having a 12-hr/12-hr light/dark cycle with ad libitum access to food and water.
PTZ Model of Generalized Tonic-Clonic Seizures in Mice
Mice were equipped for EEG recordings under surgical anesthesia (isoflurane; 5% induction, 1%–2% maintenance) in a mouse-adapted stereotaxic frame. Body temperature was maintained within the normal physiological range with a feedback-controlled heat pad (Harvard Apparatus). After a midline scalp incision, partial craniectomies were performed and animals had skull-mounted recording electrodes placed and fixed with dental cement. A second cohort of mice had EEG electrodes and a guide cannula implanted to allow i.c.v. injections.19 This cannula was placed on the dura mater with the following coordinates from the bregma: anterior-posterior (AP), +0.3 mm; lateral (L), +0.9 mm; and ventral (V), −2.0 mm. After recovery from surgery, mice were connected to the lead socket of a swivel commutator, which was connected to a Grass TwiN digital EEG system. Baseline recordings were obtained, after which mice were injected (intraperitoneally [i.p.]) with different doses of PTZ (60, 80, or 100 mg/kg). Lorazepam (8 mg/kg, i.p.) was administered after the first tonic-clonic seizure to reduce morbidity and mortality. Mice were followed for 30 min or 4 hr after PTZ administration for the appearance of seizures by electrographic and behavioral methods. After the observation period, mice were deeply anesthetized (pentobarbital) and transcardially perfused, and their brains were removed for analysis of gene expression or histological assessment. The second cohort of mice received i.c.v. injection of 0.12 nmol/2 μL 3′-cholesterol-tagged locked nucleic acid oligonucleotide targeting miR-134 (Ant-134) or its respective control, a non-targeting scrambled version of the antagomir (Scr; 0.12 nmol/2 μL) (Exiqon A/S), as previously described.19, 22 Twenty-four hours later, mice were connected to an EEG system and a baseline recording was obtained. Next, mice were injected with PTZ (80 mg/kg, i.p.). Lorazepam (8 mg/kg, i.p.) was administered after the first tonic-clonic seizure to reduce morbidity and mortality. These mice were followed up for 30 min after PTZ administration for the appearance of seizures. After this period, mice were deeply anesthetized (pentobarbital overdose) and transcardially perfused (PBS) for further assessments.
Perforant Pathway Stimulation Model of Epilepsy in Rats
Under isoflurane (5% induction, ∼3% maintenance) anesthesia, bipolar stainless-steel stimulating electrodes (NEX-200; Rhodes Medical Instruments) were positioned in the angular bundles of the perforant pathway and custom unipolar recording electrodes (crafted from 796000; A-M Systems) were lowered into the dorsal dentate gyrus. Electrode locations were determined by optimizing the potentials evoked by low-frequency PPS. Electrodes and ground screws were connected to miniature wireless transmitters (FT20; Data Sciences International) that were implanted subcutaneously on the animal’s flank. Dental cement connected the electrodes to anchor screws and the skull. Plastic connectors (GS09PLG; Ginder Scientific) joined the electrodes with stimulation/recording equipment. The PPS protocol utilized a paradigm designed to evoke and maintain hippocampal seizure activity throughout the stimulation, but not convulsive status epilepticus,29 which consisted of continuous, bilateral 2-Hz paired-pulse stimuli, with a 40-ms interpulse interval, plus a 10-s train of 20 Hz single-pulse stimuli delivered once per minute, generated by an S88 stimulator (Grass Instruments). All pulses (0.1-ms duration) were delivered at 20–24 V, as this voltage reliably evokes granule cell discharging without tissue-damaging hydrolysis.29, 40 The current associated with these voltages was typically between 15 and 30 μA. Stimulation evoked population spikes with amplitude of 5–10 mV. No samples displayed any signs of hydrolysis (e.g., holes around the stimulating electrodes). As described previously, stimulation for 30 min (on 2 consecutive days) required only isoflurane to terminate seizures.29 Eight-hour stimulation on the third day did not induce convulsive status epilepticus and, therefore, did not require pharmacological termination. All animals survived the 8-hr stimulation. Rats were randomly divided in three cohorts to assess the following: (1) miR-134 expression after PPS (24 hr, 4 days, and 14 days); (2) the effect of Ant-134 on PPS-induced status epilepticus, in which rats were pre-treated with Ant-134 or Scr (0.36 nmol/6 μL, i.c.v.; Exiqon A/S) 24 hr before the 8-hr stimulation; and (3) the effect of Ant-134 on epileptogenesis, in which rats were injected with 0.36 nmol/6 μL (i.c.v.) of Ant-134 or Scr (Exiqon A/S) immediately after 8-hr stimulation. Five rats were excluded from this study due to practical issues (e.g., blocked cannula). For statistical purposes, we pooled samples from 24 hr and 4 days after PPS as an “early” time point, while 14-day samples were considered as the “late” time point.
Behavioral and EEG Analysis
Mouse EEG data were analyzed and quantified using LabChart 8 software (AD Instruments). PTZ-induced seizures were defined as high-amplitude (>2× baseline) high-frequency (>5 Hz) polyspike discharges lasting >5 s. For statistical purposes, we assigned a cut-off time of 1,800 s for those animals that did not present seizures during the observation period. From the EEG recordings, we calculated the delay to first seizure, the total power, and the percentage of total power/spectral bands, as previously described.19, 54 EEG total power was plotted as the percentage of baseline recording (each animal’s EEG power post-seizure compared to its own baseline EEG). Clinical behavior was scored using the following adapted Racine scale: score 0, no seizures observed; score 1, rhythmic mouth and facial movement; score 2, rhythmic head nodding (bobbing); score 3, forelimb clonus; score 4, rearing and bilateral forelimb clonus; and score 5, rearing and falling (stay fallen on rear side; tonic-clonic seizures). Behavior was scored by an observer blind to treatment. The highest score reached during every 5 min during the 30 min after PTZ was recorded.
For studies in rats, EEG activity during and after PPS was amplified and recorded digitally at 2 kHz utilizing a PowerLab 16/35 and LabChart software versions 7 and 8 (AD Instruments). Spontaneous granule cell layer activity was recorded continuously (24 hr/day) and stored digitally and automatically in 3-hr epochs. Each day, we analyzed the preceding 24 hr of recordings, as well as all events with amplitudes exceeding 120% of average baseline. Potential seizures were related to behavior on the time-stamped video recordings. Continuous (24 hour/7 day) video monitoring utilized Edimax 7010W infrared cameras. Video files were captured at 12–15 frames/sec and were time-stamped for integration with the electrophysiological data using SecuritySpy surveillance software (Ben Software) and stored digitally. Confirmed seizures were scored according to the Racine scale.
Epileptiform Activity Induced by 9 mM K+ in Ex Vivo Rat Brain Slices
Rats were anesthetized with isoflurane (5% induction,∼2% maintenance as appropriate) and mounted in a stereotaxic frame. We injected Ant-134 (0.12 nmol) in 1 μL TE buffer (Life Technologies) at coordinates relative to the bregma: AP, −0.92 mm; medial-lateral (ML), +1.3 mm, and dorsal-ventral (DV), −3.3 mm to target the lateral ventricle. To allow the rats to fully recover from the analgesic drugs and to coincide with the maximal anti-epileptic effect seen in vivo, slices were prepared 2–4 days following stereotaxic surgery.19 Rats were anesthetized briefly with inhalational isoflurane and heavily with i.p. sodium pentobarbital and transcardially perfused with ice-cold sucrose artificial cerebrospinal fluid (aCSF; in mM: 205 sucrose, 10 glucose, 2.5 KCl, 0.1 CaCl2, 5 MgCl2, 26 NaHCO3, and 1.2 NaH2PO4.H2O; osmolarity, 295 mOsm). The brain was quickly dissected into ice-cold sucrose aCSF and transferred to a Campden 700 SMZ slicer (Campden Instruments). Slices (400 μm) were prepared in the horizontal orientation between the bregma at approximately −7.1 and – 5.1 mm. For storage, slices were submerged in oxygenated normal aCSF (in mM: 125 NaCl, 10 glucose, 3 KCl, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 1.25 NaH2PO4.H2O; osmolarity, 290 mOsm) and allowed to recover at room temperature for at least 60 min prior to recordings.
Slice recordings were made using a membrane chamber (Scientific Systems Design), a modified submerged chamber,55 which improves oxygen supply to the tissue and supports greater epileptiform bursts than conventional submerged recording chambers. Slices were continually perfused with aCSF, heated to 32°C, at a flow rate of ∼16 mL/min. For local field potential (LFP) recordings, borosilicate glass recording micropipettes (resistance, 3–4 MΩ) were placed into hippocampal CA1b stratum pyramidale and epileptiform activity was induced by raising the perfusing KCl concentration to 9 mM. Recordings were continued for 30 min.
Quantitative Real-Time PCR
Total RNA was isolated from brain samples using Trizol (Invitrogen), as described previously.19, 54 For mRNA qPCR, 1 μg total RNA was used to generate cDNA by reverse transcription using the SuperScript III reverse transcriptase enzyme (Invitrogen). Standard PCR was performed using 2 μL total cDNA. The PCR amplification was performed for 50 cycles on a Thermocycler (Applied Biosystems). Quantitative real-time PCR was performed using a LightCycler 1.5 (Roche Diagnostics) in combination with QuantiTect SYBR Green PCR kit (QIAGEN) as per the manufacturer’s protocol with 25 μM of primer pairs used. Specific primers for each gene assayed were purchased from Sigma and sequences used were as follows: actin, beta (Actb) (forward [F], ggttggccttagggttcagg; reverse [R], gggtgtgatggtgggaatgg); c-fos (F, cattcagaccacctcgacaa; R, ggaattaacctggtgctgga); and Arc (F, agcagcagacctgacatcct; R, gtgatgccctttccagacat). Data were normalized to Actb and relative mRNA transcript levels were quantified using the ΔΔCT method. For miRNA, 250 ng RNA was reverse transcribed using stem-loop specific primers for mmu-miR-134 (Applied Biosystems) and real-time quantitative PCR was carried out on a 7900HT Fast Real-Time System (Applied Biosystems) using TaqMan miRNA assays. Expression of RNU19 was used for normalization. A relative fold change in expression of miR-134 was determined using the ΔΔCT method.19, 56 Specific miRNA assays were obtained from ThermoFisher Scientific for miR-134 (ID 001186), miR-19a (ID 000395), and RNU19 (ID 001003). Data were normalized to RNU19 and relative miRNA expression levels were quantified using the ΔΔCT method.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism and Stata Release 14 software, and a p value < 0.05 was considered significant. Latencies to tonic-clonic seizures were analyzed by the Kruskal-Wallis test, followed by the nonparametric Dunn multiple comparison test when indicated. Data are presented as medians and interquartile ranges. Racine scores were analyzed by ordinal logistic regression and adjusted for clustering within animal. Total time spent in seizures, mean amplitude, frequency, total power of EEG recordings, and mRNA levels were analyzed by the Student t test or one-way ANOVA followed by the Bonferroni test, depending on the experimental design. PPS model data were analyzed by the Fisher exact test. Ex vivo brain slice data were analyzed using the Mann-Whitney U test. Three comparisons were made from the same data and α (significance level) was consequently reduced to 0.025 for these experiments. Data are expressed as means ± SEM.
Author Contributions
C.R.R., L.F.A.S., B.A.N., K.S., G.M., A. S-R., L.C., V.N., S.B., and S.S. conducted experiments and analyzed data; R.M.C. performed statistical analyses; M.A.F., D.F.O., and N.D. provided human samples and data; C.R.R., S.S., R.J.P., F.R., and D.C.H. designed experiments; C.R.R. and D.C.H. wrote the paper and all co-authors contributed to data interpretation, review, and editing of the final paper.
Conflicts of Interest
The Royal College of Surgeons in Ireland filed for a patent on the inhibition of microRNA-134 for the treatment of seizure-related disorders and other neurologic injuries. The authors declare no competing financial interests.
Acknowledgments
The authors thank Eva Jimenez-Mateos and Tobias Engel for advice on technical aspects. The authors acknowledge funding from the Health Research Board Ireland (HRA-POR-2013-325 to D.C.H.) and Science Foundation Ireland (13/IA/1891 and 11/TIDA/B1988 to D.C.H.) and fellowships from the Brazilian National Council for Scientific and Technological Development (CNPq; to L.F.A.S.), the Royal Society (to S.S.), the Irish Research Council (to C.R.R.), CURE (Citizens United for Research in Epilepsy) (to B.A.N. and D.C.H.), and the European Union Seventh Framework Programme (FP7/2007–2013; under grant agreement 602130). They also gratefully acknowledge the NIH NeuroBioBank and the University of Maryland Brain and Tissue Bank for autopsy material. The role of the University of Maryland Brain and Tissue Bank is to distribute tissue, and therefore, it cannot endorse the studies performed or the interpretation of results.
Footnotes
Supplemental Information includes two tables and can be found with this article online at http://dx.doi.org/10.1016/j.omtn.2016.11.002.
Supplemental Information
References
- 1.Löscher W., Klitgaard H., Twyman R.E., Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug Discov. 2013;12:757–776. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- 2.Barker-Haliski M., Friedman D., White H.S., French J.A. How clinical development can, and should, inform translational science. Neuron. 2014;84:582–593. doi: 10.1016/j.neuron.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Pitkänen A., Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 4.Vezzani A., French J., Bartfai T., Baram T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg E.M., Coulter D.A. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat. Rev. Neurosci. 2013;14:337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson M.R., Behmoaras J., Bottolo L., Krishnan M.L., Pernhorst K., Santoscoy P.L., Rossetti T., Speed D., Srivastava P.K., Chadeau-Hyam M. Systems genetics identifies Sestrin 3 as a regulator of a proconvulsant gene network in human epileptic hippocampus. Nat. Commun. 2015;6:6031. doi: 10.1038/ncomms7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClelland S., Brennan G.P., Dubé C., Rajpara S., Iyer S., Richichi C., Bernard C., Baram T.Z. The transcription factor NRSF contributes to epileptogenesis by selective repression of a subset of target genes. eLife. 2014;3:e01267. doi: 10.7554/eLife.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattani A.A., Allene C., Seifert V., Rosenow F., Henshall D.C., Freiman T.M. Involvement of microRNAs in epileptogenesis. Epilepsia. 2016;57:1015–1026. doi: 10.1111/epi.13404. [DOI] [PubMed] [Google Scholar]
- 9.Kosik K.S. The neuronal microRNA system. Nat. Rev. Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 10.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 11.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 13.Tan C.L., Plotkin J.L., Venø M.T., von Schimmelmann M., Feinberg P., Mann S., Handler A., Kjems J., Surmeier D.J., O’Carroll D. MicroRNA-128 governs neuronal excitability and motor behavior in mice. Science. 2013;342:1254–1258. doi: 10.1126/science.1244193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schratt G.M., Tuebing F., Nigh E.A., Kane C.G., Sabatini M.E., Kiebler M., Greenberg M.E. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 15.Fiore R., Khudayberdiev S., Christensen M., Siegel G., Flavell S.W., Kim T.K., Greenberg M.E., Schratt G. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swann J.W., Al-Noori S., Jiang M., Lee C.L. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J. The dendritic spine story: an intriguing process of discovery. Front. Neuroanat. 2015;9:14. doi: 10.3389/fnana.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez-Mateos E.M., Bray I., Sanz-Rodriguez A., Engel T., McKiernan R.C., Mouri G., Tanaka K., Sano T., Saugstad J.A., Simon R.P. miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am. J. Pathol. 2011;179:2519–2532. doi: 10.1016/j.ajpath.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez-Mateos E.M., Engel T., Merino-Serrais P., McKiernan R.C., Tanaka K., Mouri G., Sano T., O’Tuathaigh C., Waddington J.L., Prenter S. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 2012;18:1087–1094. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng J., Omran A., Ashhab M.U., Kong H., Gan N., He F., Yin F. Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J. Mol. Neurosci. 2013;50:291–297. doi: 10.1007/s12031-013-9953-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang X.M., Jia R.H., Wei D., Cui W.Y., Jiang W. miR-134 blockade prevents status epilepticus like-activity and is neuroprotective in cultured hippocampal neurons. Neurosci. Lett. 2014;572:20–25. doi: 10.1016/j.neulet.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Mateos E.M., Engel T., Merino-Serrais P., Fernaud-Espinosa I., Rodriguez-Alvarez N., Reynolds J., Reschke C.R., Conroy R.M., McKiernan R.C., deFelipe J., Henshall D.C. Antagomirs targeting microRNA-134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine-induced status epilepticus. Brain Struct. Funct. 2015;220:2387–2399. doi: 10.1007/s00429-014-0798-5. [DOI] [PubMed] [Google Scholar]
- 23.Gambari R., Fabbri E., Borgatti M., Lampronti I., Finotti A., Brognara E., Bianchi N., Manicardi A., Marchelli R., Corradini R. Targeting microRNAs involved in human diseases: a novel approach for modification of gene expression and drug development. Biochem. Pharmacol. 2011;82:1416–1429. doi: 10.1016/j.bcp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Christopher A.F., Kaur R.P., Kaur G., Kaur A., Gupta V., Bansal P. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016;7:68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conde J., Artzi N. Are RNAi and miRNA therapeutics truly dead? Trends Biotechnol. 2015;33:141–144. doi: 10.1016/j.tibtech.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox K.S., Dixon-Salazar T., Sills G.J., Ben-Menachem E., White H.S., Porter R.J., Dichter M.A., Moshé S.L., Noebels J.L., Privitera M.D., Rogawski M.A. Issues related to development of new antiseizure treatments. Epilepsia. 2013;54(Suppl 4):24–34. doi: 10.1111/epi.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitkänen A., Nehlig A., Brooks-Kayal A.R., Dudek F.E., Friedman D., Galanopoulou A.S., Jensen F.E., Kaminski R.M., Kapur J., Klitgaard H. Issues related to development of antiepileptogenic therapies. Epilepsia. 2013;54(Suppl 4):35–43. doi: 10.1111/epi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White H.S. Animal models for evaluating epileptogenesis. In: Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V., editors. Jasper’s Basic Mechanisms of the Epilepsies. Fourth Edition. NCBI; 2012. [Google Scholar]
- 29.Norwood B.A., Bumanglag A.V., Osculati F., Sbarbati A., Marzola P., Nicolato E., Fabene P.F., Sloviter R.S. Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J. Comp. Neurol. 2010;518:3381–3407. doi: 10.1002/cne.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traynelis S.F., Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J. Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Pacheco A., Diaz-Hernandez M., Arribas-Blázquez M., Sanz-Rodriguez A., Olivos-Oré L.A., Artalejo A.R., Alves M., Letavic M., Miras-Portugal M.T., Conroy R.M. Transient P2X7 receptor antagonism produces lasting reductions in spontaneous seizures and gliosis in experimental temporal lobe epilepsy. J. Neurosci. 2016;36:5920–5932. doi: 10.1523/JNEUROSCI.4009-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strzelczyk A., Klein K.M., Willems L.M., Rosenow F., Bauer S. Brivaracetam in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert Rev. Clin. Pharmacol. 2016;9:637–645. doi: 10.1586/17512433.2016.1156529. [DOI] [PubMed] [Google Scholar]
- 33.Henshall D.C. MicroRNA and epilepsy: profiling, functions and potential clinical applications. Curr. Opin. Neurol. 2014;27:199–205. doi: 10.1097/WCO.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roncon P., Zucchini S., Ferracin M., Marucci G., Giulioni M., Michelucci R., Rubboli G., Simonato M. Is autopsy tissue a valid control for epilepsy surgery tissue in microRNA studies? Epilepsia Open. 2016 doi: 10.1002/epi4.12023. Published online November 9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaughwin P., Ciesla M., Yang H., Lim B., Brundin P. Stage-specific modulation of cortical neuronal development by Mmu-miR-134. Cereb. Cortex. 2011;21:1857–1869. doi: 10.1093/cercor/bhq262. [DOI] [PubMed] [Google Scholar]
- 36.Thomas R.H., Berkovic S.F. The hidden genetics of epilepsy-a clinically important new paradigm. Nat. Rev. Neurol. 2014;10:283–292. doi: 10.1038/nrneurol.2014.62. [DOI] [PubMed] [Google Scholar]
- 37.Kretschmann A., Danis B., Andonovic L., Abnaof K., van Rikxoort M., Siegel F., Mazzuferi M., Godard P., Hanon E., Fröhlich H. Different microRNA profiles in chronic epilepsy versus acute seizure mouse models. J. Mol. Neurosci. 2015;55:466–479. doi: 10.1007/s12031-014-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheng M., Greenberg M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 39.Bramham C.R., Alme M.N., Bittins M., Kuipers S.D., Nair R.R., Pai B., Panja D., Schubert M., Soule J., Tiron A., Wibrand K. The Arc of synaptic memory. Exp. Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer M., Kienzler-Norwood F., Bauer S., Rosenow F., Norwood B.A. Removing entorhinal cortex input to the dentate gyrus does not impede low frequency oscillations, an EEG-biomarker of hippocampal epileptogenesis. Sci. Rep. 2016;6:25660. doi: 10.1038/srep25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sloviter R.S. Experimental status epilepticus in animals: what are we modeling? Epilepsia. 2009;50(Suppl 12):11–13. doi: 10.1111/j.1528-1167.2009.02363.x. [DOI] [PubMed] [Google Scholar]
- 42.Janssen H.L., Reesink H.W., Lawitz E.J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A.J., Patick A.K., Chen A., Zhou Y. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 43.Simonato M., Löscher W., Cole A.J., Dudek F.E., Engel J., Jr., Kaminski R.M., Loeb J.A., Scharfman H., Staley K.J., Velíšek L., Klitgaard H. Finding a better drug for epilepsy: preclinical screening strategies and experimental trial design. Epilepsia. 2012;53:1860–1867. doi: 10.1111/j.1528-1167.2012.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobow K., Auvin S., Jensen F., Löscher W., Mody I., Potschka H., Prince D., Sierra A., Simonato M., Pitkänen A. Finding a better drug for epilepsy: antiepileptogenesis targets. Epilepsia. 2012;53:1868–1876. doi: 10.1111/j.1528-1167.2012.03716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klitgaard H., Matagne A., Gobert J., Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 46.Pak T.R., Rao Y.S., Prins S.A., Mott N.N. An emerging role for microRNAs in sexually dimorphic neurobiological systems. Pflugers Arch. 2013;465:655–667. doi: 10.1007/s00424-013-1227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma S., Eghbali M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014;5:3. doi: 10.1186/2042-6410-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Androsavich J.R., Sobczynski D.J., Liu X., Pandya S., Kaimal V., Owen T., Liu K., MacKenna D.A., Chau B.N. Polysome shift assay for direct measurement of miRNA inhibition by anti-miRNA drugs. Nucleic Acids Res. 2016;44:e13. doi: 10.1093/nar/gkv893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Straarup E.M., Fisker N., Hedtjärn M., Lindholm M.W., Rosenbohm C., Aarup V., Hansen H.F., Ørum H., Hansen J.B., Koch T. Short locked nucleic acid antisense oligonucleotides potently reduce apolipoprotein B mRNA and serum cholesterol in mice and non-human primates. Nucleic Acids Res. 2010;38:7100–7111. doi: 10.1093/nar/gkq457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roshan R., Shridhar S., Sarangdhar M.A., Banik A., Chawla M., Garg M., Singh V.P., Pillai B. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA. 2014;20:1287–1297. doi: 10.1261/rna.044008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.T., Jeon D., Chu K., Jung K.H., Moon J., Sunwoo J., Park D.K., Yang H., Park J.H., Kim M. Inhibition of miR-203 reduces spontaneous recurrent seizures in mice. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-9901-7. Published online May 10, 2016. [DOI] [PubMed] [Google Scholar]
- 52.Gilad R., Lampl Y., Eilam A., Boaz M., Loyberboim M. SPECT-DTPA as a tool for evaluating the blood-brain barrier in post-stroke seizures. J. Neurol. 2012;259:2041–2044. doi: 10.1007/s00415-012-6445-2. [DOI] [PubMed] [Google Scholar]
- 53.Carpentier A., Canney M., Vignot A., Reina V., Beccaria K., Horodyckid C., Karachi C., Leclercq D., Lafon C., Chapelon J.Y. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016;8:343re2. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 54.Engel T., Sanz-Rodgriguez A., Jimenez-Mateos E.M., Concannon C.G., Jimenez-Pacheco A., Moran C., Mesuret G., Petit E., Delanty N., Farrell M.A. CHOP regulates the p53-MDM2 axis and is required for neuronal survival after seizures. Brain. 2013;136:577–592. doi: 10.1093/brain/aws337. [DOI] [PubMed] [Google Scholar]
- 55.Hill M.R., Greenfield S.A. The membrane chamber: a new type of in vitro recording chamber. J. Neurosci. Methods. 2011;195:15–23. doi: 10.1016/j.jneumeth.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 56.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.