Abstract
Background
Expectancy is widely accepted as a key contributor to placebo effects. However, it is not known whether non‐conscious expectancies achieved through semantic priming may contribute to placebo analgesia. In this study, we investigated if an implicit priming procedure, where participants were unaware of the intended priming influence, affected placebo analgesia.
Methods
In a double‐blind experiment, healthy participants (n = 36) were randomized to different implicit priming types; one aimed at increasing positive expectations and one neutral control condition. First, pain calibration (thermal) and a credibility demonstration of the placebo analgesic device were performed. In a second step, an independent experimenter administered the priming task; Scrambled Sentence Test. Then, pain sensitivity was assessed while telling participants that the analgesic device was either turned on (placebo) or turned off (baseline). Pain responses were recorded on a 0–100 Numeric Response Scale.
Results
Overall, there was a significant placebo effect (p < 0.001), however, the priming conditions (positive/neutral) did not lead to differences in placebo outcome. Prior experience of pain relief (during initial pain testing) correlated significantly with placebo analgesia (p < 0.001) and explained 34% of placebo variance. Trait neuroticism correlated positively with placebo analgesia (p < 0.05) and explained 21% of placebo variance.
Conclusions
Priming is one of many ways to influence behaviour, and non‐conscious activation of positive expectations could theoretically affect placebo analgesia. Yet, we found no SST priming effect on placebo analgesia. Instead, our data point to the significance of prior experience of pain relief, trait neuroticism and social interaction with the treating clinician.
Significance
Our findings challenge the role of semantic priming as a behavioural modifier that may shape expectations of pain relief, and affect placebo analgesia.
1. Introduction
Priming is an implicit memory effect based on non‐conscious processes and is theorized to work by spreading activation within associative networks in the brain (Tulving and Schacter, 1990). The literature encompasses a variety of priming types, however, the unifying component of them is that they rest on non‐conscious memory processes. A number of experiments have demonstrated that priming is independent of explicit memory retrieval, as different priming functions have remained intact in amnesic patients (Gabrieli et al., 1990; Verfaellie et al., 1990) and patients with Alzheimer disease (Gabrieli et al., 1994) in spite of severe deficits of explicit memory function. In priming studies, the exposure of one stimulus (the prime) is thought to influence a person's reaction to subsequent stimuli, and thus influence behaviour without the person knowing about the connection between the prime and the measured behaviour (Tulving and Schacter, 1990; Bargh et al., 1996). This effect was demonstrated by Bargh and colleagues in a seminal study (Bargh et al., 1996) where participants were primed with words pertaining to the social stereotype of elderly, using a ‘Scrambled Sentence Test’ (SST). While the sentences did not explicitly include words relating to ‘slowness’, those primed with the stereotype sentences walked more slowly than participants primed with neutral sentences. According to Bargh it is essential that primes are not explicitly related to the experimental task, as the effect can activate explicit memory processes and be biased by demand effects (Bargh and Chartrand, 2014).
Activation of memory networks by means of different semantic primes has been shown to play a role in pain perception (Weiss et al., 2003; Meerman et al., 2011; Richter et al., 2014). However, studies have mainly investigated the effects of negative priming with the hypothesis that such priming increases pain sensitivity. The potential influence of priming on placebo analgesia is largely unknown.
Pain is a complex multi‐modal phenomenon. The perception and processing of painful events is an interplay between incoming nociceptive signals, cognitive (i.e. expectations) and affective factors (i.e. emotional state) (Tracey and Mantyh, 2007). There is a large literature on the impact of cognitive and emotional states on pain (Villemure and Bushnell, 2002; Bushnell et al., 2013). The effect is bidirectional, where positive expectations or a positive emotional state may lead to decreased pain sensitivity, whereas negative expectations or emotional state may lead to increased sensitivity (Zelman et al., 1991).
Placebo analgesia refers to pain reduction that is not attributable to the physical properties of a treatment. Instead, placebo analgesia arises due to psychological factors such as treatment expectations (Colloca and Grillon, 2014). Expectancy is a key contributor to placebo analgesia (Atlas and Wager, 2012), and is commonly regarded as a higher‐order cognitive process that requires conscious awareness (Petrovic and Ingvar, 2002; Wager et al., 2004). However, recent studies from our laboratory (Jensen et al., 2012, 2014, 2015) observed placebo and nocebo effects on pain in response to non‐conscious conditioning with subliminal cues, demonstrating that non‐conscious processes are also involved in placebo analgesia.
A growing amount of evidence suggests that behavioural motivation can be exerted without conscious awareness and suggest an important role of non‐conscious processes for health‐related behaviour (Custers and Aarts, 2010; Sheeran et al., 2013). Hence, people can be primed to become motivated to initiate and exhibit behaviours available in their repertoire, even though they are not aware of the prime or its effect on their motivation and behaviour. As actively changing beliefs and attitudes can be an effective method to cope with chronic pain (Veehof et al., 2011), it is possible that also non‐conscious expectancy processes have a role in behavioural interventions for pain. Examining the role of implicit treatment expectations may thus lead to a better understanding of pain outcomes in behavioural treatment programs.
Previous studies have focused on manipulating pain by inducing negative emotional states, and thus worsened pain by increasing the unpleasantness component of pain perception. However, it is not known whether pain can be modulated through priming that is aimed at enhancing positive cognitive expectations. In order to address this lacuna, we designed a double‐blind placebo experiment aimed at manipulating expectations with semantic priming. The goal was to determine if placebo analgesia could be enhanced by semantic priming of positive expectations. We hypothesized that exposure to positive priming would result in a more positive mindset that would transfer to greater placebo responses, compared to neutral priming.
2. Methods
2.1. The participants
36 healthy participants, 21 women, 15 men (M [mean] age = 25 years, SD [standard deviation] = 7, range = 18–48 years) were recruited through an academic study website (http://www.studentkaninen.se) or via ads on university message boards. Prior to inclusion, participants were informed that the study investigates ‘how pain and learning are related to each other’, and screened for inclusion criteria (age 18–55 years, generally healthy, understand Swedish). All participants were debriefed at the end of the experiment and compensated with 200 SEK (€ 20). The regional ethics committee of Stockholm, Sweden, had approved the study and all participants gave written informed consent. The sample size was determined by a power calculation based on data from an experiment of non‐conscious placebo effects (Jensen et al., 2012), and posited that a sample size of n = 26 was required for a 90% chance to detect an effect at an alpha level of 0.05.
2.2. Procedure
2.2.1. General information
The experiment consisted of 4 steps: (1) Experimenter A performed a pain calibration procedure and a credibility demonstration of the sham analgesic device, (2) Experimenter B performed the priming procedure, (3) Experimenter A tested participants’ pain sensitivity and response to the sham device; both when machine was turned ‘on’ (placebo analgesia) or turned ‘off’ (baseline), (4) questionnaires and debriefing session (see Fig. 1).
Figure 1.
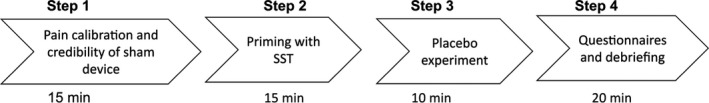
Schematic overview of the experimental procedure.
The experiment was conducted in a hospital environment. The experiment room included a comfortable treatment chair (used during pain testing) and a table with two chairs (used during priming). The room in which the experiment took place was separated into two sections by a screen in order to reduce distraction during the priming task and create separate contexts for the priming procedure and the pain testing. Pain stimuli were delivered using a Thermal Sensory Analyzer with a 3 cm × 3 cm heat probe (Medoc Advanced Medical Systems, Israel). The placebo treatment was administered by means of a sham analgesic device (two small electronic boxes, attached to electrical cords, ‘turned on’ with a beeping sound) and an inactive electrode placed on the skin around the participants’ volar forearm. Experimenter A wore hospital scrubs and a hospital name badge. Experimenter B wore professional attire (Table 1).
Table 1.
Participants’ characteristics and descriptives; mean and SD (±)
| Variable | All participants (n = 36) | Positive priming group (n = 18) | Neutral priming group (n = 18) | p‐Value between |
|---|---|---|---|---|
| Age (years) | 25.33 ± 7.02 | 26.78 ± 7.50 | 23.89 ± 6.39 | 0.222 |
| Women (%) | 58 | 67 | 50 | 0.325 |
| Pain sensitivity (C)a | 47.64 ± 1.22 | 47.78 ± 1.35 | 47.50 ± 1.10 | 0.503 |
| Pain, placebo ‘off’b | 61.29 ± 17.10 | 58.44 ± 17.32 | 64.14 ± 16.88 | 0.325 |
| Pain, placebo ‘on’b | 52.11 ± 16.68 | 49.83 ± 15.53 | 54.39 ± 17.90 | 0.421 |
| Placebo outcomec | 9.18 ± 8.81 | 8.61 ± 6.69 | 9.75 ± 10.70 | 0.704 |
| Priming difficultyd | 31.67 ± 25.21 | 31.39 ± 26.39 | 31.94 ± 24.74 | 0.948 |
| Priming time (min) | 12.03 ± 3.48 | 11.59 ± 4.11 | 12.46 ± 2.77 | 0.465 |
Temperature when pain rating ~60 on a 0–100 Numeric Response Scale (NRS) (0 = no pain, 100 = worst imaginable pain).
Pain rating 0–100 NRS placebo analgesic device on/off.
Difference pain rating placebo ‘off’ and ‘on’.
Difficulty 0–100 NRS, (0 = not at all difficult, 100 = highest difficulty possible).
2.2.2. Step 1 (pain calibration and credibility demonstration)
After giving informed consent, participants were placed in the treatment chair. Experimenter A, (female, physiotherapist, 49 years old) introduced the participants to the pain stimulator. The pain rating scale (0–100 Numeric Response Scale (NRS)) was presented, where 0 was described as ‘not painful at all’ and 100 ‘worst imaginable pain’. In order to find each participants’ subjectively calibrated ‘high pain temperature’, an ascending series of heat pain stimuli were administered to the left forearm. Each pain stimulation lasted for 4 s and started at 40 °C with an increase by one degree per trial up to a maximum of 49 °C. For each participant, the high pain temperature was defined as the temperature that first exceeded 60 on the 0–100 NRS in terms of subjectively experienced pain, or until they reached 49 °C. After the calibration procedure, the sham analgesic device was introduced by saying ‘This is a machine used in our laboratory to lower the sensation of pain. By placing this electrode close to the heat probe, the analgesic device applies a high frequency electrical current which affect nerve fibres and will therefore decrease pain’. The electrode was then placed adjacent to the heat probe and a credibility demonstration was performed: first, each participant's high pain temperature was administered (sham device ‘off’), and then the temperature was surreptitiously lowered by 1.5 °C (sham device ‘on’), lastly, the high pain temperature was administered again (sham device ‘off’). Participants rated each heat stimulation on the 0–100 NRS pain scale. The change in participants’ pain ratings during the credibility demonstration represents each participant's experience of relief (hereafter called ‘prior experience of relief’). After the credibility procedure, the participants were asked about their expectations, by answering the question ‘Based on what you just felt, to what extent do you think this machine may reduce this type of heat‐pain on a scale between 0 and 100, where 0 = no pain relief and 100 = complete pain relief’, as an explicit account of the effectiveness of the machine (hereafter called ‘explicit expectancy’).
2.2.3. Step 2 (priming)
Experimenter A left the room and the participant moved to the priming section of the room. Experimenter B (man, 26 years, doctoral student) performed the priming procedure (randomized to either ‘positive’ or ‘neutral’). This procedure allowed Experimenter A to remain blinded to which priming manipulation the participants had received. The priming manipulation was performed with a SST (Bargh et al., 1996), including sentences that would trigger associations to positive expectations, including words related to positive outcomes in general (for examples see below). The neutral version included similar sentences, but did not include any words that would relate to positive expectations. The SST consisted of 15 scrambled sentences, with six words in each sentence. The order of the sentences was randomized for each participant to exclude any order effects. The SST was explained with the following wording: ‘This language task contains several sentences, and each sentence consists of six words. The words are randomly mixed so that the sentences are grammatically incorrect. Your task is to rearrange the order of the words and make the sentences grammatically correct by using five words for each sentence.’ The participants had a maximum of 15 min to perform the task, and when finished, they were told to knock on the door as a signal for Experimenter B to enter the room and collect the SST. Translated examples of the positive SST include: ‘get you praise today will fine’; ‘exercise relaxation we on now healthy’. Translated examples of the neutral SST include: ‘lunchbox again eats she food from’; the job now year profession ongoing’.
To determine if the positive and neutral prime words were in fact differentially related to the concept of expectations, the SST was evaluated by an independent group of 36 participants (26 women, M = 30.27, SD = 9.70 years). In order to validate the SST task, 30 sentences (15 positive and 15 neutral) were mixed in one questionnaire. The participants answered to what degree they perceived each sentence to induce positive expectations (belief about something getting better) on a scale from 0 = no specific expectations to 100 = expectations in a completely positive direction. The 15 positive SST sentences were rated significantly more ‘positive’ (M = 56.29, SD = 20.26, NRS) than the 15 ‘neutral’ (M = 19.63, SD = 13.86, NRS), (t = 12.930, df = 35, p < 0.001) sentences, thus validating that the priming task would expose the participants to words that, at least consciously, are deemed different regarding expectations.
2.2.4. Step 3 (placebo experiment)
After the priming procedure, Experimenter A led the participant back to sit in the treatment chair. The heat probe and the sham device were placed on the participant's volar forearm. First, familiarization to the heat probe was performed, in order to remove any surprise effects on pain ratings. Second, the same credibility induction as given in Step 1 was performed to remind the participant of the effectiveness of the sham device. Lastly, a test of each participant's placebo response was performed by administering each participant's high pain temperature while the sham device was turned ‘off’, ‘on’ and then ‘off’ again. The mean of the two ‘off’ stimuli was used to compare with the ‘on’ stimulus. Nota bene, the same high temperature was applied during all three stimulations.
2.2.5. Step 4 (questionnaires and debriefing)
After the experiment, the participants answered a short‐form of the Eynsenck Personality Questionnaire (EPQ12) including 12 items (yes/no answers) regarding trait neuroticism (Eysenck et al., 1985). Also a study‐specific questionnaire was given, including questions about (1) awareness and difficulty of the priming procedure; ‘Could you detect any themes or specific purposes of the language test?, (if yes, the participants were asked to specify their response) and ‘Did you consider the language test to be difficult? (NRS, 0 = not at all difficult to 100 = most possible difficulty)’, (2) credibility of the sham device and; ‘Did you feel that the pain relieving machine was credible?’ (NRS, 0 = not at all credible to 100 = totally credible). (3) credibility of the treating Experimenter (Experimenter A); ‘Did you feel safe and at ease with the experimental leader?’ (NRS, 0 = ‘not safe/at ease at all’ to 100 = ‘completely safe/at ease’); and ‘Did you feel confidence in the experimental leader?’ (NRS, 0 = ‘no confidence at all’ to 100 = ‘highest possible confidence’). Finally, Experimenter A debriefed the participant about the purpose of the SST and that the pain relieving machine was a sham (placebo) device. Participants were offered to withdraw their data from the study if they did not feel comfortable with the deceptive components of the experiment. However, all participants approved of the design and the use their data in the study.
2.3. Statistical analyses
Differences between the priming groups (positive vs. neutral) regarding task duration, difficulty ratings, credibility of the sham device and credibility of the placebo experimenter were analysed by means of two‐sample t‐tests.
The effects of placebo and priming were assessed with a 2 × 2 mixed model analysis of variance (ANOVA) on NRS pain ratings, with device ‘off’ and device ‘on’ as the within‐subject factor and priming (positive vs. neutral) as the between‐subject factor. The effect of gender on placebo outcome was analysed using a multivariate ANOVA (MANOVA). To further assess factors contributing to placebo outcomes, linear multiple regression models were performed with placebo outcome as the dependent variable and pain sensitivity, EPQ12, prior experience of relief and explicit expectancy as independent variables; adjusted for baseline pain (i.e. sham machine ‘off’). The predictors had no problems with collinearity, analysed with multiple ‘tolerance’ and ‘Variance Inflation Factor’ (VIF). For illustrative reasons, correlation analyses were performed using Pearson's r. One placebo response outlier was detected, yet inclusion/exclusion of this individual in the analysis did not change the results and was thus included in all analyses. All statistical analyses were performed in SPSS 22.0. The significance level (α) was set as p < 0.05, two‐tailed. We used the Shapiro–Wilk Test of normality, as it is an appropriate test for small sample sizes (<50 samples). The underlying assumptions for running ANOVA is that the dependent variable should be approximately normally distributed for each category of the independent variable. ANOVA is considered ‘robust’ to violations of normality, meaning that the assumption can be somewhat violated and still provide valid results. Here, the dependent variables used in ANOVAs were: Sham device off and Sham device on. In order to visually determine normality from a graph, we inspected the Q–Q Plots. The data points for the different measures were close to the diagonal line in all our plots; a sign of normal distribution. The underlying assumptions for running regression analysis do not require that the variables need to be normally distributed.
3. Results
3.1. Priming task and credibility ratings
The mean time for performing the priming task, irrespective of priming group, was M = 12.03, SD = 3.48 min. There were no significant differences in the amount of time required to perform the positive priming task (M = 11.59, SD = 4.11 min) compared to the neutral priming task (M = 12.45, SD = 2.77 min), (t = −0.739, df = 34, p = 0.465). The experience of priming task difficulty (NRS 0–100), irrespective of priming group, was M = 31.67, SD = 25.21. There were no significant difference in the experienced difficulty between the positive (M = 31.39, SD = 26.39) and the neutral priming group (M = 31.94, SD = 24.74), (t = −0.065, df = 34, p = 0.948). After the experiment, the participants were presented to the two different SST versions and asked to identify which version they thought they had completed. All participants identified the correct SST, verifying that they had paid attention to the SST sentences during priming.
None of the participants reported any correct perception of the priming content. In a post‐experiment questionnaire, five participants gave a positive answer to the question if they could determine a motive or theme in the priming task, however, none of the answers were related to positive expectations (participants guessed that the SST related to aspects of Swedish grammar).
The mean rating of confidence in the pain relieving machine was high, as participants rated its efficacy (0–100 NRS) to be M = 67.78, SD = 22.59. There were no significant differences in credibility ratings between the positive (M = 71.33, SD = 19.30) and the neutral priming group (M = 64.22, SD = 25.51), (t = −0.9443, df = 34, p = 0.352).
All participants rated a high degree of feeling ‘safe and at ease’ with the experimenter who performed the pain calibration and placebo administration (0–100 NRS) (M = 97.61, SD = 5.27) as well as high professional credibility (M = 95.97, SD = 6.53).
3.2. Placebo outcome
Overall, the participants’ pain ratings were significantly lower when the sham analgesic device was turned ‘on’ (M = 52.11, SD = 16.68) compared to ‘off’ (M = 61.29, SD = 17.01), F(1,34) = 38.06, p < 0.001, η2 = 0.528 (Fig. 2). The magnitude of the differences in means was M = 9.18, 95% Confidence Interval (CI) = 6.19–12.16.
Figure 2.
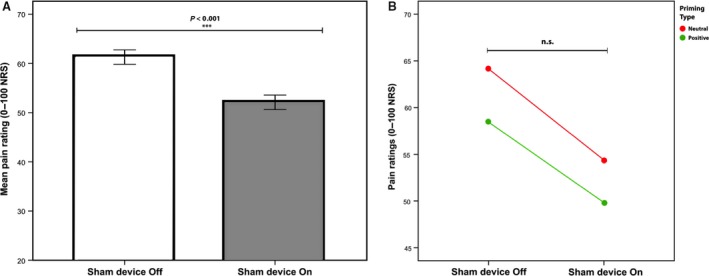
Pain ratings during placebo treatment. (A) Bars represent the average within‐subject pain ratings during sham analgesic device turned ‘on’ (placebo) and ‘off’ (baseline). Participants rated pain intensity on a 0–100 Numerical Response Scale (NRS). Error bars represents 2 intrasubject standard errors. Significance indicated with asterisk. (B) Across priming groups, there was a significant drop in pain ratings when the sham analgesic device was ‘on’, however, there was no interaction between priming condition (positive/neutral) × sham device (‘on’/‘off’). The downward shift in pain ratings for the positive priming group, represented in the image, was not significant.
3.3. Effect of priming on placebo outcome
The mean placebo pain reduction in the positive priming group was M = 8.61, SD = 6.69, and in the neutral priming group M = 9.75, SD = 10.75. There was no significant effect of priming type on overall pain ratings, F(1, 34) = 0.886, p = 0.353, η2 = 0.025. Moreover, there was no significant interaction between priming type and placebo responses, F(1, 34) = 0.146, p = 0.704, η2 = 0.004, as priming did not have differential effects on pain ratings when the sham device was either ‘on’ or ‘off’ (Fig. 2). The participants’ ratings of priming difficulty, or time spent performing the priming task, did not affect placebo outcomes in any of the priming groups.
3.4. Effect of pain sensitivity on placebo outcome
The mean temperature representing the participants’ calibrated high pain (~60 of 100 NRS) was 47 °C and ranged from 45 to 49 °C. There was no correlation between placebo outcome and participants’ pain sensitivity (r = −0.257, p = 0.248), using a regression model where placebo outcome was the dependent variable, adjusting for baseline pain (i.e. machine ‘off’). The model explained 12.9% of variance in placebo outcome, yet this was not significant (R 2 = 0.129, F(2, 33) = 2.452, p = 0.102). In addition, pain sensitivity did not predict placebo outcome (β = −1.417, t(33) = −1.175, p = 0.248), meaning that level of pain sensitivity is not associated with an increase in placebo outcome.
3.5. Effect of EPQ12 on placebo outcome
There was a positive correlation between placebo outcome and EPQ12 ratings (r = 0.334) (Fig. 3). This correlation was significant when tested in a regression model where placebo outcome was the dependent variable, adjusting for baseline pain (i.e. machine ‘off’). The model significantly explained 20.7% of variance in placebo outcome (R 2 = 0.207, F(2, 33) = 2.452, p = 0.022). EPQ12 also significantly predicted placebo outcome, (β = 1.172, t(33) = 2.184, p = 0.036). One extra point on EPQ12 (higher scores on EPQ12 indication high trait neuroticism) was thus associated with an increase in placebo outcome with 1.172 steps (NRS).
Figure 3.
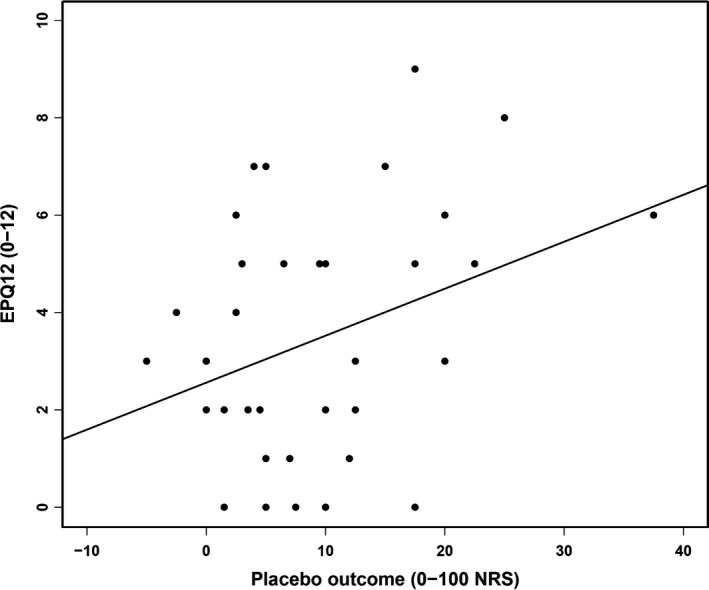
Correlation between trait neuroticism and placebo analgesia. A significant positive correlation was found between EPQ12 ratings (trait neuroticism) and placebo outcome (mean change sham device ‘off’ vs. ‘on’), r = 0.334, p = 0.046.
3.6. Effect of prior experience of pain relief on placebo outcome
There was a significant correlation between placebo outcome and prior experience of pain relief (i.e. difference in pain ratings during credibility manipulation) (r = 0.568, p < 0.001), using a regression model where placebo outcome was the dependent variable, adjusting for baseline pain (machine ‘off’). The model explained 33.9% of variance in placebo outcome (R 2 = 0.339, F(2, 33) = 8.470, p < 0.001). The prior pain relief measure significantly predicted placebo outcome, (β = 0.399, t(33) = 3.507, p < 0.001); i.e. one step on the prior pain relief measure was associated with an increase in placebo outcome with 0.399 steps (NRS).
3.7. Effect of explicit expectancy on placebo outcome
There was a weak correlation between placebo outcome and explicit expectancy ratings (r = 0.256). There was a non‐significant trend, using a regression model where placebo outcome was the dependent variable, adjusting for baseline pain (machine ‘off’). The model explained 15.9% of variance in placebo outcome (R 2 = 0.159, F(2, 33) = 3.117, p = 0.058). The explicit expectancy measure did not predict placebo outcome significantly, (β = 0.126, t(33) = 3.507, p = 0.117). There were no significant differences in explicit expectancy between the positive (M = 36.67, SD = 12.83) and the neutral priming group (M = 32.56, SD = 22.13), (t(33) = 0.682, p = 0.500).
3.8. Effect of gender on placebo outcome and expectancy ratings
A MANOVA, with gender as fixed factor, demonstrated no significant effect of gender on placebo outcome, F(1, 34) = 0.001, p = 0.976, η2 = 0.000, nor on prior experience of pain relief F(1, 34) = 2.136, p = 0.153, η2 = 0.059. There was a significant effect of gender on explicit expectancy F(1,34) = 5.602, p = 0.024, η2 = 0.141; were female participants (n = 21) reported higher explicit expectancy (M = 40.24, SD = 14.96) than male (n = 15) (M = 26.73, SD = 19.3). There was a positive correlation between explicit expectancy and placebo outcome among women (r = 0.476) but not among men (r = 0.122), yet, the difference between these correlations was not statistically significant, z = 1.06, p = 0.289 (Fig. 4).
Figure 4.
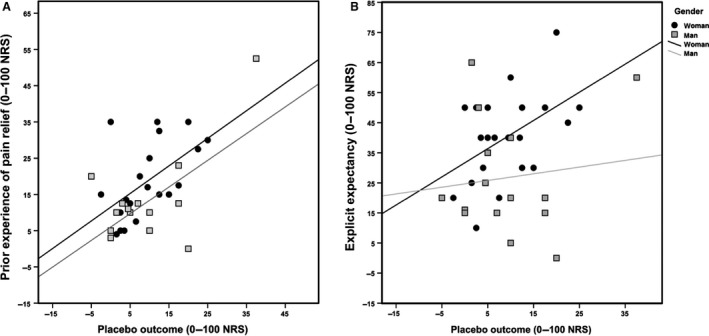
Placebo and expectancy correlations divided by gender. (A) Correlation between prior experience of pain relief and placebo outcome (r = 0.568, p < 0.001). (B). Correlation between pretreatment explicit expectancy and placebo outcome for women (r = 0.476, p = 0.029) and men (r = 0.122, p = 0.664). The difference in correlation between men and women was non‐significant.
4. Discussion
Here, we used a double‐blind randomized experimental design to test if implicit positive expectations, induced by semantic priming, could alter placebo analgesic responses. Previous studies indicate that contextual factors, not directly relating to suggestions of pain relief, can affect placebo analgesia, e.g. through the price of a treatment (Waber et al., 2008; Geuter et al., 2013) or alterations in the patient–clinician relationship (Gracely et al., 1985; Kaptchuk et al., 2008a). In this study the results showed that a sham analgesic device could successfully induce placebo effects. However, there was no difference in placebo effects between the positive and neutral SST priming. Overall, the study showed positive correlations between participants’ prior experience of pain relief and placebo outcome. There was also a correlation between high trait neuroticism and greater placebo outcome. Priming is one of many potential ways to influence behaviour, yet, our study suggests that placebo analgesia is predominantly influenced by prior experience of pain relief and the interaction with a trustworthy clinician, highlighting the role of social interaction in obtaining placebo effects.
Although this study did not find any specific effects of SST priming on placebo analgesia, it provides a unique investigation of the potential effects of semantic priming on placebo effects, using words related to positive expectations. Our study rationale was based on data from previous placebo experiments with varying ‘directness’ of the manipulation. The most direct type of expectancy manipulation is represented in numerous placebo studies, and includes a first‐hand experience of pain relief. The next level, representing less direct manipulations of expectancy, includes a study where one's observation of pain relief in others was enough to create first‐person placebo effects (Colloca and Benedetti, 2009). Hence, positive expectations of pain relief acquired through social observation are thought to mediate placebo analgesia. The most indirect example where researchers aimed at creating expectations of pain relief, is represented by a study (Waber et al., 2008) where false information about the price of ‘analgesic pills’ was provided (with no mention of differences in effectiveness). In that study, expensive placebo pills led to stronger analgesic effects than cheap pills, suggesting that expectations of pain relief can be formed (and affect placebo analgesia) through information that is not directly related to the effectiveness of a treatment. In our study, we wanted to take the indirectness of the expectancy manipulation one step further, by providing an implicit priming task aimed at influencing expectations in a generally positive way, without mentioning pain relief at all.
Priming is well‐established in behavioural science, but in spite of a vast literature on priming effects, studies of semantic priming and pain are scarce. Furthermore, the few published studies on priming and pain include words with negative valence, such as somatic complaints or suggestions of increased pain. Contrary with our study, studies of negative priming and pain sensitivity demonstrate effects on pain tolerance (Meerman et al., 2011) and pain sensitivity (Weiss et al., 2003; Richter et al., 2014). We suggest that priming directed at exacerbating pain, can be obtained through the high salience of words signalling fear or threat, and/or by inducing negative affect. Conversely, positive effects on pain through expectations of pain relief may be more strongly mediated by factors other than semantic primes.
Earlier priming studies have demonstrated striking effects on human behaviour (Bargh et al., 1996; Dijksterhuis and van Knippenberg, 1998), however, the robustness of priming results are questioned and there are failed attempts to replicate classic priming studies (Doyen et al., 2012). In order to control for the placebo experimenter's own expectations and hope for priming effects, we employed a double‐blind experimental design. It is well‐established that patients’ expectations influence placebo outcomes, however, the experimenter's expectations may also influence the placebo response (Gracely et al., 1985; Messer and Wampold, 2002). In a priming study with several experimenters, half of the experimenters were led to believe that the participants would walk slower when primed congruently, and the other half were told the opposite (Doyen et al., 2012). The participants walked slower in the group where the experimenters expected the participants to walk slower, indicating that the experimenter's expectations may affect priming results; highlighting the importance of double‐blind designs in priming studies.
For the purpose of our study, we modified an existing method for semantic priming (SST) by including words aimed at activating cognitive associations of positive expectations, and thereby enhance placebo outcomes non‐consciously. As there were no previous attempts to enhance placebo effects this way, the tailored SST was validated in an independent group of participants. The validation procedure confirmed that the two SSTs were perceived as intended, as the positive priming sentences were rated significantly more positive than the neutral sentences. Yet, there is a possibility that the SSTs were different only in participants who were explicitly prompted to rate the perceived expectancy of each sentence (validation). This may not have been the case during the experiment itself, when the participants were unaware of the purpose of the SST.
In line with the goal of the present study, none of the participants reported a correct theme of the positive/neutral priming sentences, indicating that the participants were unaware of the purpose and content of the SST. The questions were asked in order to verify that priming effects were indeed mediated by non‐conscious expectations.
Here, we found a positive correlation between participants’ prior experience of pain relief (based on experienced pain relief during pain testing) and placebo outcomes, which is in line with the known effects of prior experience on placebo analgesia (Jensen et al., 2012, 2015). A placebo‐controlled study in patients with neuropathic pain (Andre‐Obadia et al., 2011) showed that prior experience of pain relief was essential for obtaining placebo effects during sham transcranial magnetic stimulation (TMS). In line with the scope of the present study, Andre‐Obadia et al. suggest that conscious expectancy may have a rather limited effect on placebo effects, compared to the implicit learning obtained through experience. Moreover, in the present study there was no overall correlation between participants’ explicit expectancy ratings and placebo outcome. However, this ‘mismatch’ was true for male but not for female participants, as male participants had a tendency to give lower ratings when asked about the effectiveness of the sham device, compared to the actual pain relief reported during pain testing (prior experience of pain relief). This raises the question if male participants may have underreported their belief in the effectiveness of the sham device, as a result psychosocial factors. Studies have suggested that traditional gender roles influence the verbalization of pain (Sanford et al., 2002; Robinson and Wise, 2003). It is possible that the effect of the opposite sex had an influence on men's explicit expectancy ratings in our study, as the experimenter was a woman. Our study thereby emphasizes the importance of considering the social dynamics and the context in which treatment expectations and pain are reported. In spite of the gender differences in reporting explicit expectancy, there were no overall differences in placebo analgesia between male and female participants. Gender differences in placebo outcome are reported occasionally, however, the results are inconclusive and several studies report interaction effects with, e.g. stress (Enck et al., 2008; Kelley et al., 2009; Aslaksen et al., 2011).
The notion of predicting who will be a placebo responder has intrigued researchers since the advent of systematic placebo investigations (Kaptchuk et al., 2008b), yet, studies have failed to find conclusive evidence for a typical placebo responder (Horing et al., 2014). However, individual studies have linked psychological traits such as dispositional optimism (Geers et al., 2010), empathy (Hunter et al., 2014) and fear of pain (Lyby et al., 2011) to placebo analgesic responses. In one of the earliest investigations of a placebo responder profile, Lasagna et al. (1954) described responders, compared to non‐responders, as being more anxious, self‐centred and had more somatic symptoms. Conversely, non‐responders were described as more rigid and emotionally controlled. The same was found in a study (Wasan et al., 2006) where high neuroticism correlated with high response to placebo injections in patients with discogenic low back pain. In line with these preliminary findings, we found a positive correlation between high trait neuroticism and placebo analgesia. Yet, neuroticism has been used as a possible predictor of placebo outcomes before, without showing any effects (Kelley et al., 2009; Pecina et al., 2013). As the contextual factors are likely to vary considerably between studies, and different trait variables interact with different environmental cues (i.e. patient–clinician relationship) (Kong et al., 2013; Kelley et al., 2014), it is unlikely that one trait is related to placebo response. The interaction between personality traits and environmental cues was investigated in a placebo acupuncture study (Kelley et al., 2009) where the patient–clinician relationship was manipulated (emphatic vs. neutral therapist). The authors found that gender and personality traits (extraversion, agreeableness and openness to experience) influenced placebo responses. However, this was true only in the treatment group with warm and empathetic patient–clinician interactions (Kelley et al., 2009).
In the present study, participants reported feeling very safe and at ease, and rated high confidence in the treating experimenter; indicating that treatment was administered in a ‘safe’ environment. In analogy with Kelley et al. (2009) it is possible that an ‘unsafe’ environment may have rendered different results, so that high trait neuroticism would not correlate with placebo analgesia. A neurotic personality may be defined as anxious and insecure, and is sometimes referred to as ‘emotionally unstable’. Hence, a safe environment may be required in order to succeed with altering expectations in participants with a high level of emotional instability.
A possible reason for the lack of priming effect in this study is the interaction with the placebo experimenter. It is well‐known that the interaction between a patient and clinician has robust effects on treatment outcomes (Bensing and Verheul, 2010; Kelley et al., 2014). Furthermore, we expected the priming effects to be subtle and therefore it is possible that the participants’ interaction with the experimenter may have overridden the effects of priming. In a separate validation procedure, performed before the experiment, we found that the positive priming sentences were perceived as significantly more positive than the neutral sentences. Yet, participants were explicitly asked to rate the positivity of the sentences, and were thus consciously aware of the possibility that sentences had different valence. It would have been preferable to know that the sentences could affect participants non‐consciously before onset of the placebo experiment. Now it is not clear if the participants were properly primed, which could be an alternate explanation for the lack of difference between the positive and neutral priming condition.
Another possible reason for the lack of priming effects is the time span between the priming procedure and the placebo manipulation. Not only may the time span be problematic per se (time passes and the effect of the prime may be blurred), but time may also introduce competing elements (new environmental cues), which could overshadow a possible priming effect. In this study, we separated the place for priming from the pain testing with a screen wall, in order to reduce influences. However, some parts of the experiment (heat stimulation and demonstration of the sham device) were repeated before testing the placebo outcome. As a result, the effect of priming may have suffered from a combination of elapsing time as well as competing influences from the repeated heat stimulation trial.
One limitation to our study is the use of regression analysis in spite of a relatively small number of participants. The number of subjects for running regressions can be considered low, yet, the regression included only one predictor (and adjustment for baseline pain), which requires less demands on the number of subjects.
5. Conclusion
Priming is one of many ways to influence behaviour, and non‐conscious activation of positive expectations could theoretically affect placebo analgesia. Yet, our study indicates that placebo analgesia was more related to prior experience of pain relief and trait neuroticism; possibly also to ratings of high confidence in a treating clinician.
Author contributions
AR, JY, TK, MI and KJ designed the study. AR, JY and KJ collected the data. AR, JY, IK, TK, MI and KJ analysed the data. AR, JY, TK, IK, MI and KJ prepared the manuscript. All authors have read and approved the paper.
Acknowledgements
The authors wish to thank Moa Pontén and Sara Bengtsson for the valuable help with this study.
Funding sources
This work was supported by the Osher Center for Integrative Medicine at Karolinska Institutet, Sweden.
Conflicts of interest
None declared.
References
- Andre‐Obadia, N. , Magnin, M. , Garcia‐Larrea, L. (2011). On the importance of placebo timing in rTMS studies for pain relief. Pain 152, 1233–1237. [DOI] [PubMed] [Google Scholar]
- Aslaksen, P.M. , Bystad, M. , Vambheim, S.M. , Flaten, M.A. (2011). Gender differences in placebo analgesia: Event‐related potentials and emotional modulation. Psychosom Med 73, 193–199. [DOI] [PubMed] [Google Scholar]
- Atlas, L.Y. , Wager, T.D. (2012). How expectations shape pain. Neurosci Lett 520, 140–148. [DOI] [PubMed] [Google Scholar]
- Bargh, J.A. , Chartrand, T.L. (2014). Studying the mind in the middle: A practical guide to priming and automaticity research In Handbook of Research Methods in Social Psychology, Reis H., Judd C., ed. (New York, NY: Cambridge University Press; ) pp. 253–285. [Google Scholar]
- Bargh, J.A. , Chen, M. , Burrows, L. (1996). Automaticity of social behavior: Direct effects of trait construct and stereotype‐activation on action. J Pers Soc Psychol 71, 230–244. [DOI] [PubMed] [Google Scholar]
- Bensing, J.M. , Verheul, W. (2010). The silent healer: The role of communication in placebo effects. Patient Educ Couns 80, 293–299. [DOI] [PubMed] [Google Scholar]
- Bushnell, M.C. , Ceko, M. , Low, L.A. (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca, L. , Benedetti, F. (2009). Placebo analgesia induced by social observational learning. Pain 144, 28–34. [DOI] [PubMed] [Google Scholar]
- Colloca, L. , Grillon, C. (2014). Understanding placebo and nocebo responses for pain management. Curr Pain Headache Rep 18, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custers, R. , Aarts, H. (2010). The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science 329, 47–50. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis, A. , van Knippenberg, A. (1998). The relation between perception and behavior, or how to win a game of trivial pursuit. J Pers Soc Psychol 74, 865–877. [DOI] [PubMed] [Google Scholar]
- Doyen, S. , Klein, O. , Pichon, C.L. , Cleeremans, A. (2012). Behavioral priming: It's all in the mind, but whose mind? PLoS ONE 7, e29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enck, P. , Benedetti, F. , Schedlowski, M. (2008). New insights into the placebo and nocebo responses. Neuron 59, 195–206. [DOI] [PubMed] [Google Scholar]
- Eysenck, S.B.G. , Eysenck, H.J. , Barrett, P. (1985). A revised version of the psychoticism scale. Personality Individ Differ 6, 21–29. [Google Scholar]
- Gabrieli, J.D. , Milberg, W. , Keane, M.M. , Corkin, S. (1990). Intact priming of patterns despite impaired memory. Neuropsychologia 28, 417–427. [DOI] [PubMed] [Google Scholar]
- Gabrieli, J.D. , Keane, M.M. , Stanger, B.Z. , Kjelgaard, M.M. , Corkin, S. , Growdon, J.H. (1994). Dissociations among structural‐perceptual, lexical‐semantic, and event‐fact memory systems in Alzheimer, amnesic, and normal subjects. Cortex 30, 75–103. [DOI] [PubMed] [Google Scholar]
- Geers, A.L. , Wellman, J.A. , Fowler, S.L. , Helfer, S.G. , France, C.R. (2010). Dispositional optimism predicts placebo analgesia. J Pain 11, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter, S. , Eippert, F. , Hindi Attar, C. , Buchel, C. (2013). Cortical and subcortical responses to high and low effective placebo treatments. NeuroImage 67, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely, R.H. , Dubner, R. , Deeter, W.R. , Wolskee, P.J. (1985). Clinicians’ expectations influence placebo analgesia. Lancet 1, 43. [DOI] [PubMed] [Google Scholar]
- Horing, B. , Weimer, K. , Muth, E.R. , Enck, P. (2014). Prediction of placebo responses: A systematic review of the literature. Front Psychol 5, 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, T. , Siess, F. , Colloca, L. (2014). Socially induced placebo analgesia: A comparison of a pre‐recorded versus live face‐to‐face observation. Eur J Pain 18, 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, K.B. , Kaptchuk, T.J. , Kirsch, I. , Raicek, J. , Lindstrom, K.M. , Berna, C. , Gollub, R.L. , Ingvar, M. , Kong, J. (2012). Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci USA 109, 15959–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, K.B. , Kaptchuk, T.J. , Chen, X. , Kirsch, I. , Ingvar, M. , Gollub, R.L. , Kong, J. (2014). A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cerebral cortex. [DOI] [PMC free article] [PubMed]
- Jensen, K. , Kirsch, I. , Odmalm, S. , Kaptchuk, T.J. , Ingvar, M. (2015). Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc Natl Acad Sci USA 112, 7863–7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk, T.J. , Kelley, J.M. , Conboy, L.A. , Davis, R.B. , Kerr, C.E. , Jacobson, E.E. , Kirsch, I. , Schyner, R.N. , Nam, B.H. , Nguyen, L.T. , Park, M. , Rivers, A.L. , McManus, C. , Kokkotou, E. , Drossman, D.A. , Goldman, P. , Lembo, A.J. (2008a). Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ 336, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk, T.J. , Kelley, J.M. , Deykin, A. , Wayne, P.M. , Lasagna, L.C. , Epstein, I.O. , Kirsch, I. , Wechsler, M.E. (2008b). Do “placebo responders” exist? Contemp Clin Trials 29, 587–595. [DOI] [PubMed] [Google Scholar]
- Kelley, J.M. , Lembo, A.J. , Ablon, J.S. , Villanueva, J.J. , Conboy, L.A. , Levy, R. , Marci, C.D. , Kerr, C.E. , Kirsch, I. , Jacobson, E.E. , Riess, H. , Kaptchuk, T.J. (2009). Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med 71, 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, J.M. , Kraft‐Todd, G. , Schapira, L. , Kossowsky, J. , Riess, H. (2014). The influence of the patient‐clinician relationship on healthcare outcomes: A systematic review and meta‐analysis of randomized controlled trials. PLoS ONE 9, e94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, J. , Spaeth, R. , Cook, A. , Kirsch, I. , Claggett, B. , Vangel, M. , Gollub, R.L. , Smoller, J.W. , Kaptchuk, T.J. (2013). Are all placebo effects equal? Placebo pills, sham acupuncture, cue conditioning and their association. PLoS ONE 8, e67485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna, L. , Mosteller, F. , Von Felsinger, J.M. , Beecher, H.K. (1954). A study of the placebo response. Am J Med 16, 770–779. [DOI] [PubMed] [Google Scholar]
- Lyby, P.S. , Aslaksen, P.M. , Flaten, M.A. (2011). Variability in placebo analgesia and the role of fear of pain–an ERP study. Pain 152, 2405–2412. [DOI] [PubMed] [Google Scholar]
- Meerman, E.E. , Verkuil, B. , Brosschot, J.F. (2011). Decreasing pain tolerance outside of awareness. J Psychosom Res 70, 250–257. [DOI] [PubMed] [Google Scholar]
- Messer, S.B. , Wampold, B.E. (2002). Let's face facts: Common factors are more potent than specific therapy ingredients. Clin Psychol: Sci Pract 9, 21–25. [Google Scholar]
- Pecina, M. , Azhar, H. , Love, T.M. , Lu, T. , Fredrickson, B.L. , Stohler, C.S. , Zubieta, J.K. (2013). Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology, 38, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic, P. , Ingvar, M. (2002). Imaging cognitive modulation of pain processing. Pain 95, 1–5. [DOI] [PubMed] [Google Scholar]
- Richter, M. , Schroeter, C. , Puensch, T. , Straube, T. , Hecht, H. , Ritter, A. , Miltner, W.H. , Weiss, T. (2014). Pain‐related and negative semantic priming enhances perceived pain intensity. Pain Res Manage 19, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M.E. , Wise, E.A. (2003). Gender bias in the observation of experimental pain. Pain 104, 259–264. [DOI] [PubMed] [Google Scholar]
- Sanford, S.D. , Kersh, B.C. , Thorn, B.E. , Rich, M.A. , Ward, L.C. (2002). Psychosocial mediators of sex differences in pain responsivity. J Pain 3, 58–64. [DOI] [PubMed] [Google Scholar]
- Sheeran, P. , Gollwitzer, P.M. , Bargh, J.A. (2013). Nonconscious processes and health. Health Psychol 32, 460–473. [DOI] [PubMed] [Google Scholar]
- Tracey, I. , Mantyh, P.W. (2007). The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. [DOI] [PubMed] [Google Scholar]
- Tulving, E. , Schacter, D.L. (1990). Priming and human memory systems. Science 247, 301–306. [DOI] [PubMed] [Google Scholar]
- Veehof, M.M. , Oskam, M.J. , Schreurs, K.M. , Bohlmeijer, E.T. (2011). Acceptance‐based interventions for the treatment of chronic pain: A systematic review and meta‐analysis. Pain 152, 533–542. [DOI] [PubMed] [Google Scholar]
- Verfaellie, M. , Cermak, L.S. , Blackford, S.P. , Weiss, S. (1990). Strategic and automatic priming of semantic memory in alcoholic Korsakoff patients. Brain Cogn 13, 178–192. [DOI] [PubMed] [Google Scholar]
- Villemure, C. , Bushnell, M.C. (2002). Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain 95, 195–199. [DOI] [PubMed] [Google Scholar]
- Waber, R.L. , Shiv, B. , Carmon, Z. , Ariely, D. (2008). Commercial features of placebo and therapeutic efficacy. JAMA 299, 1016–1017. [DOI] [PubMed] [Google Scholar]
- Wager, T.D. , Rilling, J.K. , Smith, E.E. , Sokolik, A. , Casey, K.L. , Davidson, R.J. , Kosslyn, S.M. , Rose, R.M. , Cohen, J.D. (2004). Placebo‐induced changes in FMRI in the anticipation and experience of pain. Science 303, 1162–1167. [DOI] [PubMed] [Google Scholar]
- Wasan, A.D. , Kaptchuk, T.J. , Davar, G. , Jamison, R.N. (2006). The association between psychopathology and placebo analgesia in patients with discogenic low back pain. Pain Med 7, 217–228. [DOI] [PubMed] [Google Scholar]
- Weiss, T. , Miltner, W.H. , Dillmann, J. (2003). The influence of semantic priming on event‐related potentials to painful laser‐heat stimuli in migraine patients. Neurosci Lett 340, 135–138. [DOI] [PubMed] [Google Scholar]
- Zelman, D.C. , Howland, E.W. , Nichols, S.N. , Cleeland, C.S. (1991). The effects of induced mood on laboratory pain. Pain 46, 105–111. [DOI] [PubMed] [Google Scholar]


