Abstract
Retinoic acid (RA) is a biologically active metabolite of vitamin A (retinol) that serves as an important signaling molecule in orchestrating diverse developmental processes including multiple roles during ocular development. Loss-of-function studies using gene knockouts of RA-synthesizing enzymes encoded by Aldh1a1, Aldh1a2, and Aldh1a3 (also known as Raldh1, Raldh2, and Raldh3) have provided valuable insight into how RA controls eye morphogenesis including corneal development, however it is unclear whether endogenous RA is required for maintenance and regeneration of adult cornea. Here, we investigated the role of Aldh1a genes in the adult cornea using a novel conditional Aldh1a1,2,3-flox/flox;Rosa26-CreERT2 loss-of-function mouse model to determine the biological function of RA. Our findings indicate that loss of RA synthesis results in corneal thinning characterized by reduced thickness of the stromal layer, impaired corneal epithelial cell proliferation, and increased apoptosis. Corneal thinning in Aldh1a-deficient mice was significantly rescued by RA administration, indicating an important role of endogenous RA signaling in adult corneal homeostasis and regeneration. Thus, Aldh1a1,2,3-flox/flox;Rosa26-CreERT2 mice provide a useful model for investigating the mechanistic role of RA signaling in adult corneal maintenance and could provide new insights into therapeutic approaches for controlling corneal repair to prevent vision loss.
Keywords: Corneal stroma, Corneal epithelium, Retinoic acid, ALDH1A, RALDH, Mouse genetic loss-of-function
Retinoic acid (RA) is a small lipophilic signaling molecule derived from vitamin A (retinol). RA regulates diverse biological processes including cellular proliferation, differentiation, and apoptosis throughout embryonic organogenesis including ocular development, although its role in adult tissue maintenance is much less understood. RA serves as a ligand for nuclear RA receptors (RARs) which bind to RA response elements (RAREs) as heterodimers with retinoid X receptors (RXRs) to regulate target genes (Mark et al., 2006). Genetic studies in mouse have shown that RA synthesis is carried out by three retinaldehyde dehydrogenase enzymes encoded by Aldh1a1, Aldh1a2, and Aldh1a3 (also known as Raldh1, Raldh2, and Raldh3), all three conserved in human (Cunningham and Duester, 2015; Niederreither and Dolle, 2008). During embryogenesis, ocular Aldh1a1 and Aldh1a3 expression is limited to the dorsal and ventral retina respectively, where it functions as a paracrine source of RA that diffuses to the perioptic mesenchyme to control anterior eye development (Cunningham and Duester, 2015). A functional role for RA has been very well established during ocular morphogenesis based upon genetic loss-of-function studies on Aldh1a genes and RAR genes (Lohnes et al., 1994; Ghyselinck et al., 1997; Wagner et al., 2000; Mic et al., 2004; Matt et al., 2005; Molotkov et al., 2006; Matt et al., 2008; Kumar et al., 2010). However, the functional role of these enzymes in the adult eye and cornea has not been explored, primarily due to the fact that deletion of Aldh1a2 is lethal early in development (Niederreither et al., 1999), whereas Aldh1a3 null mutants die at birth (Dupé et al., 2003); Aldh1a1 mutants are viable but do not exhibit obvious eye defects (Fan et al., 2003). Recent studies on expression patterns of RA signaling components in the human eye show that ALDH1A enzymes are expressed in adult eye tissues including cornea, conjunctiva, and choroid (Nezzar et al., 2007; Harper et al., 2015). Also, ALDH1A1 expression in the cornea and lens has been proposed to protect inner ocular tissues from ultraviolet radiation and reactive oxygen-induced damage as ALDH1A1 can metabolize reactive aldehydes as well as retinaldehyde (Lassen et al., 2007).
In the adult, vitamin A plays a vital role in ocular surface maintenance and visual function as has been long known from vitamin A deficiency studies (Wolbach and Howe, 1925; Dowling and Wald, 1958). Lack of vitamin A causes xerophthalmia characterized by abnormal differentiation of the ocular surface that results in corneal and conjunctival keratinization, ulceration, epithelial squamous metaplasia, and loss of conjunctival goblet cells, all of which contribute to vision impairment and blindness (Hatchell et al., 1984; Macsai et al., 1998; Sommer, 1998; Combs et al., 1998). Also, retinoic acid treatment has been shown to reverse corneal xerophthalmia and keratinization in vitamin A deficient animal models (Ubels et al., 1984). Much of what is currently known about the role of RA in the adult cornea comes from recent studies suggesting that RA treatment regulates differentiation of corneal epithelial cells to maintain epithelial barrier function (Kim et al., 2012) and to stimulate re-epithelialization of cornea (Keino et al., 2011). RA treatment also increases stromal keratocyte numbers in vitro to promote corneal wound healing (Gouveia et al., 2013), and RA helps reverse corneal epithelial keratinization (Ubels et al., 1987; Johansen et al., 1998). However, little is known about the effect of endogenous RA in maintaining corneal function, and the mechanism of endogenous RA action in the adult eye is not established.
The aim of the present study is to determine the function of endogenous RA in corneal maintenance and regeneration in adult eye using Aldh1a1, Aldh1a2 and Aldh1a3 loss-of-function studies. As germ-line knockouts of Aldh1a2 and Aldh1a3 are lethal (Niederreither et al., 1999; Dupé et al., 2003), we came up with a conditional knockout strategy to investigate the functions of RA-synthesizing enzymes in the adult cornea. We used a novel genetic loss-of-function approach which allows time-controlled knockdown of Aldh1a1, Aldh1a2 and Aldh1a3 in an adult mouse strain carrying homozygous flox alleles for all three genes, referred to here as Aldh1a1,2,3-flox/flox (Vermot et al., 2006; Raverdeau et al., 2012). We generated Aldh1a1,2,3-flox/flox;Rosa26-Cre/ERT2 mice by crossing Aldh1a1,2,3-flox/flox mice with mice carrying the Rosa26-Cre/ERT2 transgene that allows widespread induction of Cre following tamoxifen treatment (Ventura et al., 2007). Heterozygous offspring were back-crossed to Aldh1a1,2,3-flox/flox to obtain adult Aldh1a1,2,3-flox/flox;Rosa26-cre/ERT2 mice, referred to here as Aldh1a1,2,3-f/f;Cre+ mice. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Sanford Burnham Prebys Medical Discovery Institute (Animal Welfare Assurance Number A3053-01) and all animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
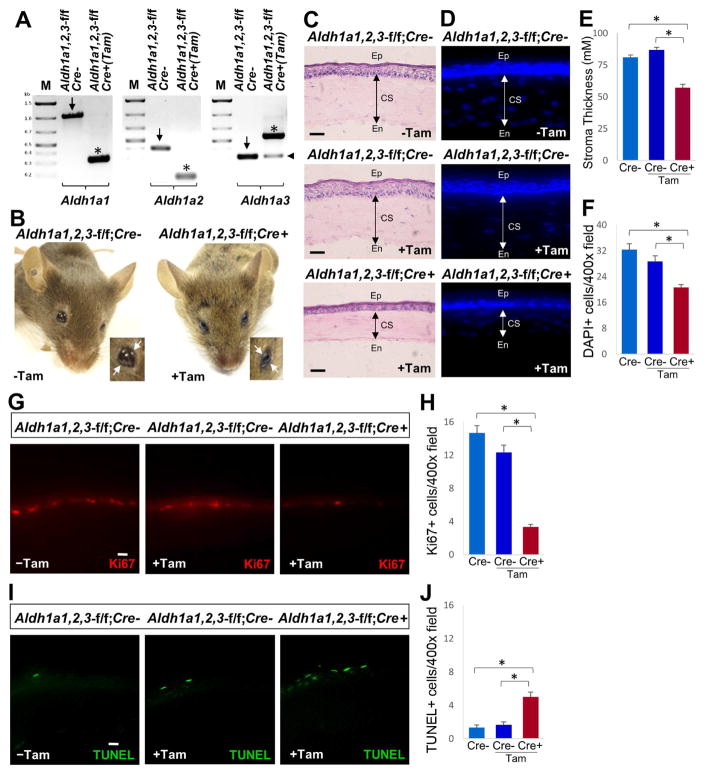
For induction of Cre-mediated recombination, Aldh1a1,2,3-f/f;Cre+ mice carrying the Rosa26-Cre/ERT2 transgene and Aldh1a1,2,3-f/f;Cre− littermates were fed a tamoxifen-containing diet (TD130858, Harlan-Teklad; 0.5 mg tamoxifen per g of diet) for 2–12 weeks starting at the age of 6–8 weeks to analyze inactivation of Aldh1a1, Aldh1a2 and Aldh1a3. In order to assess ablation of Aldh1a genes in the cornea, mice on tamoxifen diet were sacrificed at different time points from 2 to 12 weeks and PCR genotyping of whole cornea was performed using gene specific primers to assess inactivation of all three Aldh1a genes (primers for Aldh1a1, ADX318: ACAGGATCAGGCATCAGGAG, ADX319: GATTCCAGCAAACGGTAGGA; primers for Aldh1a2, ACH91: GTTTCATAGTTGGATATCATAATTTAAACAAGCAAAACC, ACM11:GTGGGACTCTGCCAGAAGTACTTC, ABI118: GTGTTGTGTTCACTCAGTCTC, ACF204:GGCAGCTGTGGAGAACATTTTATATCCATGTTG; primers for Aldh1a3: ACCM223: AAACCAGCACCACCTCCATA, ACCM 224: GACCAGCTTTCCAACCTTCA, ACCM225: AAACAACACAGAACCTCCTT); PCR conditions were previously reported (Vermot et al., 2006; Raverdeau et al., 2012). We found that after 2, 4, or 8 weeks on the tamoxifen diet, Cre-mediated recombination of Aldh1a genes was not complete (data not shown), but after 12 weeks we observed that recombination of Aldh1a1 and Aldh1a2 flox alleles was essentially complete, whereas for Aldh1a3 excision was only ~80% complete (Fig. 1A). Also, at 12 weeks of tamoxifen treatment (but not at 2, 4, or 8 weeks) we observed that Aldh1a1,2,3-f/f;Cre+ mice displayed symptoms of xerophthalmia (inability to open eyes and consistent eye twitching), whereas Aldh1a1,2,3-f/f;Cre− control mice with or without 12 weeks of tamoxifen treatment did not exhibit these symptoms (Fig. 1B). At 12 weeks of tamoxifen treatment, none of the Aldh1a1,2,3-f/f;Cre+ mice displayed a keratoconus-like phenotype. For histological and immunohistochemical evaluation of cornea, eyes from Aldh1a1,2,3-f/f;Cre+ and control mice were enucleated and prefixed in 4% paraformaldehyde (Sigma-Aldrich) in PBS (pH 7.4), embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and then stored at −80°C. Adult cornea tissues were cryo-sectioned at 10 μm in the central region parallel to the optic nerve (to obtain the correct section plane), postfixed in 4% paraformaldehyde and processed for hematoxylin-eosin (H&E) or immunohistochemical staining. Histological analysis of eyes from adult mice revealed significant corneal thinning characterized by reduced thickness of the stromal layer in the central region of Aldh1a1,2,3-f/f;Cre+ cornea compared to Aldh1a1,2,3-f/f;Cre− control mice treated with or without tamoxifen (Fig. 1C). The corneas of Aldh1a1,2,3-f/f;Cre− control mice with and without 12 weeks of tamoxifen treatment both had normal corneal morphology, demonstrating that tamoxifen treatment does not lead to corneal defects. Stromal thickness measurements were performed in H&E stained cryosections of cornea of Aldh1a1,2,3-f/f;Cre+ and control mice using NIH ImageJ software. Stromal thickness measurements from the central region of the cornea were analyzed by averaging three sampled locations per image. The mean central corneal thickness of tamoxifen-treated adult Aldh1a1,2,3-f/f;Cre+ mice was 66% that of the Aldh1a1,2,3-f/f;Cre− tamoxifen control cornea. No statistically significant difference in the thickness of the stromal layer was observed for Aldh1a1,2,3-f/f;Cre− control mice treated with or without tamoxifen (Fig. 1E). Also, a reduced thickness of the corneal epithelium was observed in tamoxifen-treated adult Aldh1a1,2,3-f/f;Cre+ cornea compared with controls. By quantitating DAPI positive cells in the stroma layer, we observed a significant reduction in the number of keratocytes (Fig. 1D,F). These results indicate that Aldh1a1, Aldh1a2 and Aldh1a3 loss-of-function in the adult eye leads to xerophthalmia and corneal defects characterized by reduced thickness of the corneal stroma and epithelium. As Aldh1a3 was not completely inactivated by Cre-recombination, it is possible that it does not contribute to the maintenance of cornea.
Fig. 1. Ablation of Aldh1a genes leads to corneal defects.
(A) PCR genotyping of whole cornea for Aldh1a1, Aldh1a2 and Aldh1a3 in untreated Aldh1a1,2,3-f/f;Cre− mice and Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen for 12 weeks; arrows mark the flox allele while asterisks mark the deleted allele that inactivates the gene. In the case of Aldh1a3, the combination of PCR primers results in a band identifying the deleted allele that is larger than the flox allele; for Aldh1a3, Cre-mediated excision is not complete as indicated by an arrowhead next to the band detecting the remaining portion of DNA carrying the flox allele. (B) Aldh1a1,2,3-f/f;Cre+ mutant mice treated with tamoxifen display symptoms of xerophthalmia and inability to open eyes (marked by arrows). (C) Comparison of corneal thickness in hematoxylin/eosin (H&E)-stained sections from adult Aldh1a1,2,3-f/f;Cre− mice with or without tamoxifen treatment and Aldh1a1,2,3-f/f;Cre+ mice with tamoxifen treatment; corneal sectioning can be difficult, however our observation of a consistently thinner cornea in all sections of Cre+ mice treated with tamoxifen indicates that we did not experience significant variations in section quality. (D) DAPI (4′,6-diamidino-2-phenylindole) staining of cell nuclei in central corneal cross-sections to detect stromal keratocytes. (E) Quantitation of corneal stromal thickness in Aldh1a1,2,3-f/f;Cre+ mice with tamoxifen compared with Cre- controls with or without tamoxifen treatment. (F) Quantitation of DAPI+ cells (keratocytes) in corneal stroma. (G) Comparison of Ki67-positive cells in the corneal basal epithelial layer of Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen compared to Cre- controls. (H) Quantitation of Ki67-positive cells in corneal epithelium. (I) Comparison of TUNEL-positive cells in the corneal epithelium of Aldh1a1,2,3-f/f;Cre+ cornea with tamoxifen compared to Cre- controls. (J) Quantitation of TUNEL-positive cells in corneal epithelium. For all panels, scale bar is 20 μm. Number of DAPI+, Ki67+ and TUNEL+ cells per microscopic field was counted at 400X magnification. For each condition examined, data are expressed as mean ± SEM, n = 3 (*, p < 0.05; Student’s t-test, two-tailed). CS, corneal stroma; En, endothelium; Ep, epithelium.
In order to maintain corneal integrity, basal epithelial cells divide and stratify upward to replenish outer epithelial cells that are regularly shed off, providing a dynamic process that is essential for normal vision (Meek and Knupp, 2015). Next, we explored if the loss of Aldh1a function in the adult cornea alters corneal epithelial maintenance. Immunostaining of Ki67, a marker of cell proliferation that is expressed in active cycling cells, was performed on corneal cryosections from Aldh1a1,2,3-f/f;Cre+ and control mice using a Ki67 antibody (RM-9106-R7, Thermofisher). We found that Ki67-positive cells were markedly reduced in the basal layer of the central corneal epithelium in Aldh1a1,2,3-f/f;Cre+ eyes when compared to Aldh1a1,2,3-f/f;Cre− control mice treated with tamoxifen or without the treatment (Fig. 1G,H). This observation indicates that Aldh1a deficiency leads to reduced cell proliferation. To further investigate whether loss of Aldh1a function in Aldh1a1,2,3-f/f;Cre+ cornea leads to apoptosis in the corneal epithelial layer, TUNEL assay was performed on corneal cryosections using the In Situ Cell Death Detection Kit, Fluorescein (Roche) following the manufacturer’s instructions; TUNEL also detects necrosis, however as we did not observe any scarring or clouding of Aldh1a1,2,3-f/f;Cre+ corneas at 12 weeks, it is unlikely that TUNEL staining detected necrotic cells. We observed a drastic increase in TUNEL-positive cells in the central corneal epithelial layer of Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen compared to Aldh1a1,2,3-f/f;Cre− control with or without the tamoxifen administration (Fig. 1I,J). In contrast, we did not observe any Ki67-positive cells or TUNEL-positive cells in the corneal stroma or endothelium in Aldh1a1,2,3-f/f;Cre+ or Aldh1a1,2,3-f/f;Cre− mice, suggesting that dysregulation of corneal stromal-epithelial interactions could be a contributing factor to the corneal thinning phenotype.
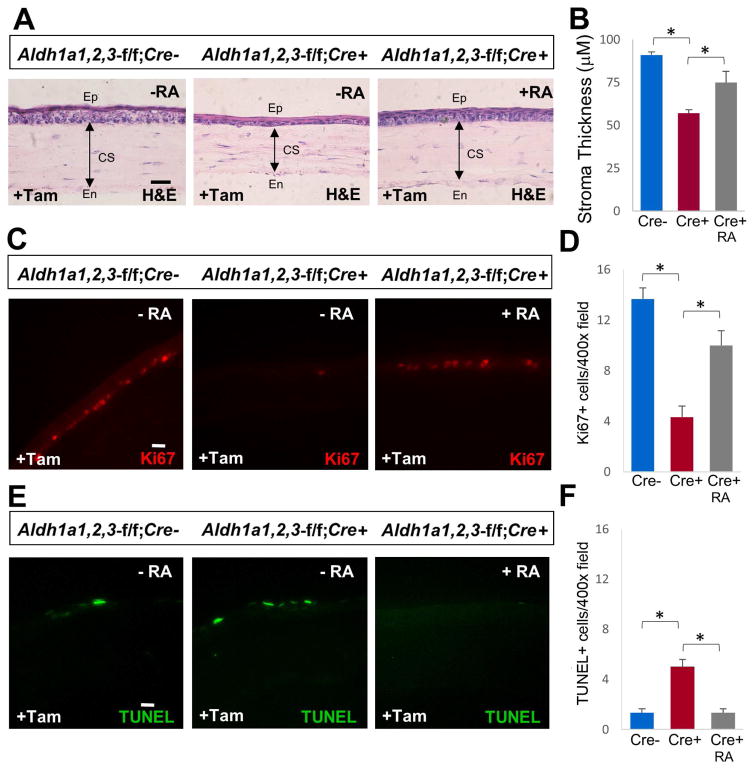
Developmental defects observed in Aldh1a2−/− and Aldh1a3−/− knockout mouse embryos can be rescued with dietary RA supplementation to further understand the role of these RA-synthesizing enzymes during later stages of development (Molotkov et al., 2006). To examine if the corneal defect observed in Aldh1a1,2,3-f/f;Cre+ mice upon induction of Cre by tamoxifen treatment is due to loss of RA, we treated Aldh1a1,2,3-f/f;Cre+ mice with exogenous RA to evaluate if RA treatment can rescue the corneal thinning phenotype. RA treatment of adult mice was performed by dietary supplementation of all-trans-RA doses previously demonstrated to provide an amount of RA in the normal physiological range (Molotkov et al., 2006). All-trans-RA (Sigma-Aldrich) was dissolved in ethanol, diluted in corn oil then mixed with powdered standard mouse chow to provide a final concentration of 0.25 mg RA per g diet. Mice were provided fresh chow containing RA twice a day. For RA rescue experiments, Aldh1a1,2,3-f/f;Cre+ mice were treated with tamoxifen for 12 weeks to stimulate corneal defects followed by RA treatment for 5 weeks. Aldh1a1,2,3-f/f;Cre+ and Aldh1a1,2,3-f/f;Cre− control mice treated with tamoxifen but not RA were used as controls. We found that Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen and RA showed significant rescue of the corneal thinning phenotype compared to Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen but not RA (Fig. 2A,B); Aldh1a1,2,3-f/f;Cre− mice treated with tamoxifen but not RA were also used as a control to observe normal corneal morphology. We examined whether RA rescue of the corneal thinning phenotype observed in Aldh1a1,2,3-f/f;Cre+ mice may be due to a restoration of corneal epithelial proliferation. Interestingly, Aldh1a1,2,3-f/f;Cre+ mice treated with both tamoxifen and RA exhibited a normal level of Ki67-positive cells in the basal corneal epithelial layer compared to Aldh1a1,2,3-f/f;Cre+ with tamoxifen but without RA treatment (Fig. 2C,D). Also, in contrast to Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen but not RA that shows a higher number of TUNEL-positive cells in the corneal epithelium, Aldh1a1,2,3-f/f;Cre+ mice treated with both tamoxifen and RA showed a drastic reduction of TUNEL-positive cells comparable to Aldh1a1,2,3-f/f;Cre− control mice treated with tamoxifen but not RA (Fig. 2E,F). These results indicate that 5 weeks of RA treatment partially rescues the corneal thinning phenotype in Aldh1a-deficient mice, suggesting that RA plays an important role in mediating the proliferative function of corneal basal epithelial cells for maintaining corneal transparency and normal vision.
Fig. 2. RA rescues the corneal thinning phenotype in Aldh1a-deficient mice.
(A) Comparison of corneal stroma thickness in hematoxylin/eosin (H&E)-stained sections of Aldh1a1,2,3-f/f;Cre+ mice treated with tamoxifen only or treated with both tamoxifen and RA; Aldh1a1,2,3-f/f;Cre− mice treated with tamoxifen only were used as a control. (B) Quantitation of corneal stromal thickness. (C) Comparison of Ki67-positive cells in basal corneal epithelial layer. (D) Quantitation of Ki67-positive cells in corneal epithelium. (E) Comparison of TUNEL-positive cells in corneal epithelium. (F) Quantitation of TUNEL-positive cells in corneal epithelium. For all panels, scale bar is 20 μm. Number of DAPI+, Ki67+ and TUNEL+ cells per microscopic field was counted at 400X magnification. For each condition examined, data are expressed as mean ± SEM, n = 3 (*, p < 0.05; Student’s t-test, two-tailed). CS, corneal stroma; En, endothelium; Ep, epithelium.
In this study, we found that mice with targeted deletions of RA-generating enzymes encoded by Aldh1a genes exhibit xerophthalmia and corneal thinning defects. Adult vitamin A deficiency has been implicated in abnormal corneal structure and function, and has been suggested to contribute to the onset and progression of corneal diseases such as xerophthalmia (McLaren and Frigg, 2001) and keratoconus (Mutch and Richards, 1939). However, a requirement for the endogenous vitamin A metabolite RA in corneal maintenance has not previously been reported. Corneal stromal thinning and disorganization are common hallmarks of vitamin A deficiency, but the functional role of endogenous RA in corneal stromal biology has not been established. Our results indicate that RA deficiency leads to reduced corneal stromal thickness and directly affects cell proliferation and apoptosis in the corneal epithelium. Over the years, several corneal modulators of stromal-epithelial interactions such as cytokines and growth factors have been implicated in normal corneal maintenance, regeneration and wound healing. Our finding on the endogenous role of RA in the adult cornea provides further insight into signaling mechanisms that may lead to alternative corneal therapies, perhaps the ability to convert human pluripotent stem cells to corneal cells for tissue engineering, transplant therapy, and clinical applications. It will be interesting to further explore how RA affects stromal-epithelial interactions and how deficiency of RA modulates stromal keratocyte homeostasis to affect the integrity of the stromal layer and result in corneal degeneration and disease. Further studies using our Aldh1a1,2,3-flox/flox;Rosa26-creERT2 mouse model will allow us to better understand the mechanistic action of RA signaling in corneal maintenance, regeneration and wound healing.
Highlights.
Retinoic acid (RA) synthesis by ALDH1A enzymes is essential for corneal maintenance.
Aldh1a genetic loss-of-function in adult eye results in corneal thinning.
RA treatment of Aldh1a-deficient mice rescues the corneal thinning phenotype.
RA deficiency reduces cell proliferation & increases apoptosis in corneal epithelium.
Acknowledgments
We thank the Animal Resources Core Facility at Sanford Burnham Prebys Medical Discovery Institute for maintaining mice line. This work was funded by National Institutes of Health grant GM062848 (G.D.).
Footnotes
Disclosure
The authors report no conflicts of interest.
Author contributions
SK: study design, experimental work and manuscript writing, GD: experimental inputs and editing, PD and NG: manuscript editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Combs GF., Jr Emerging relationships of vitamins and cancer risks. Curr Opin Clin Nutr Metab Care. 1998;1:519–23. doi: 10.1097/00075197-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Rev Mol Cell Biol. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Wald G. Vitamin A deficiency and night blindness. Proc Natl Acad Sci USA. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupé V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A. 2003;100:14036–41. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupé V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A. 2003;100:14036–41. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23:4637–48. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck NB, Dupé V, Dierich A, Messaddeq N, Garnier JM, Rochette-Egly C, Chambon P, Mark M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int J Dev Biol. 1997;41:425–47. [PubMed] [Google Scholar]
- Gouveia RM, Connon CJ. The effects of retinoic acid on human corneal stromal keratocytes cultured in vitro under serum-free conditions. Invest Ophthalmol Vis Sci. 2013;54:7483–91. doi: 10.1167/iovs.13-13092. [DOI] [PubMed] [Google Scholar]
- Harper AR, Wiechmann AF, Moiseyev G, Ma JX, Summers JA. Identification of active retinaldehyde dehydrogenase isoforms in the postnatal human eye. PLoS One. 2015;10:e0122008. doi: 10.1371/journal.pone.0122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchell DL, Sommer A. Detection of ocular surface abnormalities in experimental vitamin A deficiency. Arch Ophthalmol. 1984;102:1389–93. doi: 10.1001/archopht.1984.01040031131040. [DOI] [PubMed] [Google Scholar]
- Johansen S, Heegaard S, Prause JU, Rask-Pedersen E. The healing effect of all-trans retinoic acid on epithelial corneal abrasions in rabbits. Acta Ophthalmol Scand. 1998;76:401–4. doi: 10.1034/j.1600-0420.1998.760403.x. [DOI] [PubMed] [Google Scholar]
- Keino H, Watanabe T, Sato Y, Okada AA. Oral administration of retinoic acid receptor-alpha/beta-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52:1548–56. doi: 10.1167/iovs.10-5963. [DOI] [PubMed] [Google Scholar]
- Kim SW, Seo KY, Rhim T, Kim EK. Effect of retinoic acid on epithelial differentiation and mucin expression in primary human corneal limbal epithelial cells. Curr Eye Res. 2012;37:33–42. doi: 10.3109/02713683.2011.620728. [DOI] [PubMed] [Google Scholar]
- Kumar S, Duester G. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2. Dev Biol. 2010;340:67–74. doi: 10.1016/j.ydbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, Piatigorsky J, Duester G, Day BJ, Huang J, Hines LM, Vasiliou V. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. J Biol Chem. 2007;282:25668–76. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–48. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Macsai MS, Agarwal S, Gamponia E. Bilateral corneal ulcers in primary vitamin A deficiency. Cornea. 1998;17:227–9. doi: 10.1097/00003226-199803000-00020. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–80. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupé V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Pellerin I, Dupé V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140–8. doi: 10.1016/j.ydbio.2008.04.039. [DOI] [PubMed] [Google Scholar]
- McLaren DS, Frigg M. Sight and Life Manual on Vitamin A Deficiency Disorders (VADD) Vol. 2001 Basel, Switzerland: Task Force SIGHT AND LIFE; 2001. [Google Scholar]
- Meek KM, Knupp C. Corneal structure and transparency. Progress in Retinal & Eye Research. 2015;49:1–16. doi: 10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270–7. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–10. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch JR, Richards MB. Keratoconus experimentally produced in the rat by vitamin A deficiency. Br J Ophthalmol. 1939;23:381–387. doi: 10.1136/bjo.23.6.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezzar H, Chiambaretta F, Marceau G, Blanchon L, Faye B, Dechelotte P, Rigal D, Sapin V. Molecular and metabolic retinoid pathways in the human ocular surface. Mol Vis. 2007;13:1641–50. [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–8. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nature Rev Genet. 2008;9:541–53. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, Chambon P, Mark M, Ghyselinck NB. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci U S A. 2012;109:16582–7. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A. Xerophthalmia and vitamin A status. Progress in Retinal and Eye Research. 1998;17:9–31. doi: 10.1016/s1350-9462(97)00001-3. [DOI] [PubMed] [Google Scholar]
- Ubels JL, Edelhauser HF, Foley KM, Liao JC, Gressel P. The efficacy of retinoic acid ointment for treatment of xerophthalmia and corneal epithelial wounds. Current eye research. 1985;4:1049–1057. doi: 10.3109/02713688509003350. [DOI] [PubMed] [Google Scholar]
- Ubels JL, Rismondo V, Edelhauser HF. Treatment of corneal xerophthalmia in rabbits with micromolar doses of topical retinoic acid. Curr Eye Res. 1987;6:735–7. doi: 10.3109/02713688709034838. [DOI] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Vermot J, et al. Conditional (loxP-flanked) allele for the gene encoding the retinoic acid-synthesizing enzyme retinaldehyde dehydrogenase 2 (RALDH2) Genesis. 2006;44:155–158. doi: 10.1002/gene.20195. [DOI] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Drager UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–70. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]