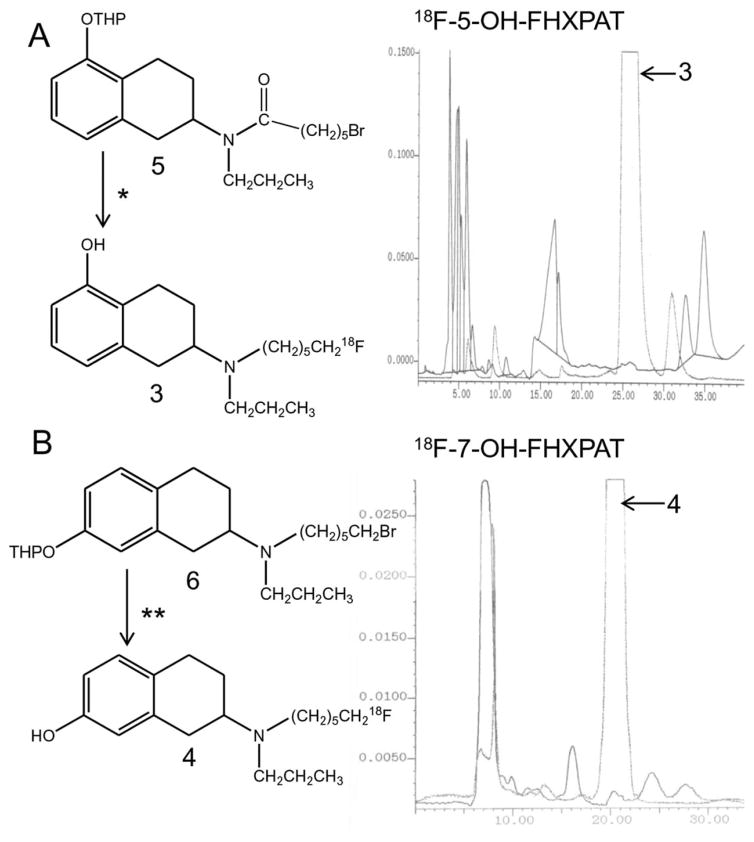
FIGURE 2.
Radiosynthesis of 18F-5-OH-FHXPAT and 18F-7-OH-FHXPAT. (a) Radiosynthesis scheme for 18F-5-OH-FHXPAT and C18 reverse phase HPLC chromatogram showing separation profile of 18F-5-OH-FHXPAT. Reagents *: Brominated precursor 5 was reacted with 1. 18F-fluoride, Kryptofix, potassium carbonate in acetonitrile; 2. Lithium aluminum hydride in tetrahydrofuran; 3. Aqueous HCl in methanol. The predominant radioactive peak at 25–27 min is 18F-5-OH-FHXPAT. (b) Radiosynthesis scheme for 18F-7-OH-FHXPAT and C18 reverse phase HPLC chromatogram showing separation profile of 18F-7-OH-FHXPAT. Reagents **: Brominated precursor 6 was reacted with 1. 18F-fluoride, Kryptofix, potassium carbonate in acetonitrile; 2. Aqueous HCl in methanol. The predominant radioactive peak at 20–22 min is 18F-7-OH-FHXPAT