Abstract
Introduction
In this study, we investigated the association of migraine with the Variable Number of Tandem Repeats (VNTR), repeated as 27 base pair, gene polymorphism in intron 4 of the endothelial nitric oxide synthase (eNOS) and the insertion/deletion of angiotensin converting enzyme (ACE) gene polymorphisms.
Methods
One hundred and five migraine and ninety seven healthy female control subjects were enrolled in the study. The patients were subdivided as migraine with aura and without aura, and the frequency and severity of migraine headaches were recorded. The eNOS VNTR (eNOS 4 a/b) and ACE insertion/deletion gene polymorphisms (ACE I/D) were assessed by polymerase chain reactions.
Result
The allele and genotype frequencies of eNOS 4 a/b gene polymorphism showed no difference between the migraine and control groups. The genotypic distribution of the ACE I/D gene polymorphism in the migraine group significantly differed from that in the control group. The DD and ID genotype increased the risk of migraine as much as 2.571 (95% CI-1.138–5.811) and 4.453 (95% CI-2.006–9.883) compared to the II genotype. The same increased risk sustained for both genotypes in the migraine with aura subgroup, but only the ID genotype remained as the risk factor in the migraine without aura subgroup (OR-3.750, 95% CI-1.493–9.420). No association of gene polymorphisms with migraine frequency and severity was observed.
Conclusion
Our findings support the relationship between migraine and the ACE I/D gene polymorphism. However, no association was found between migraine and the eNOS 4 a/b gene polymorphism.
Keywords: Migraine, genetic polymorphism, nitric oxide synthase, angiotensin converting enzyme
ÖZET
Giriş
Bu çalışmada migren ile endotelyal nitrik oksit sentaz (eNOS) geni intron 4’de, 27 bazlık tekrarlardan oluşan Ardışık Kopya Sayısı Tekrarları (VNTR) ve anjiyotensin dönüştürücü enzim (ADE) genindeki insersiyon/delesyon polimorfizmlerinin ilişkisi araştırıldı.
Yöntemler
Çalışmaya 105 migren başağrısı olan ve 97 sağlıklı kadın birey alındı. Migren hastaları auralı ve aurasız olmak üzere iki gruba ayrılırken, migren atak sıklığı ve şiddeti kaydedildi. eNOS VNTR (eNOS 4a/b) ve ADE insersiyon/delesyon polimorfizmleri (ADE I/D) polimorfizmleri polimeraz zincir reaksiyonu yöntemi ile belirlendi.
Bulgular
eNOS 4 a/b gen polimorfizminin alel ve genotip sıklıkları migren ile kontrol grubu arasında farklılık göstermedi. ADE I/D gen polimorfizminin migren grubunda genotipik dağılımı kontrol grubundan anlamlı olarak farklı bulundu. DD ve ID genotiplerinin II genotipine göre migren olasılığını 2,571 (%95 CI-1,138–5,811) ile 4,453 (%95 CI-2,006–9,883) oranında artırdığı saptandı. Aynı risk artışı auralı migren alt grubunda her iki genotip için sürerken, aurasız migren grubunda sadece ID genotipi için korundu (OR-3,750, %95 CI-1,493–9,420). Migren sıklığı ve şiddeti ile gen polimorfizmleri arasında ilişki gözlenmedi.
Sonuç
Çalışmamız ADE I/D gen polimorfizmi ile migren ilişkisini desteklemiştir. Ancak eNOS 4 a/b gen polimorfizmi ile migren arasında ilişki gösterilememiştir.
Introduction
Migraine type headache is a disease which affects approximately 12%–18% of the population and leads to significant labour loss. Its etiology and pathophysiology have not been elucidated fully yet. It is thought that genetic factors are involved because of its familial frequency and that environmental factors are involved because of triggering factors including cigarette smoke, parfume odor and open air. Migraine has a complex genetic background and the number of genes which can be considered responsible and genetic predisposition alleles have not been elucidated currently (1,2). Dysfunction of P/Q type calcium channels due to the mutation in the CACNA1A gene has been demonstrated in familial hemiplegic migraine (3). Many gene polymorphisms have been investigated in terms of their relations with migraine and migraine subtypes and different results have been reported as a result of these investigations (4,5,6,7). In patients with genetic predisposition to migraine, neurogenic inflammation with activation of the trigeminovascular system and occurrence of the response of pain is considered as the potential pathophysiological mechanism. Nitric oxide (NO) leads to vasodilatation in this process with relaxation of smooth muscles and has a significant place in the onset of headache (8). In the phase of pain. the level of NO increases in the platelets and exogenous NO worsens headache (9). It has been shown that nitric oxide also increases calcitonin gene-related peptide levels which is a significant mediator of neurogenic inflammation in trigeminal neurons (10).
Nitric oxide synthase enzyme is involved in the production of NO from L-arginine and oxygen molecule. It has been shown that polymorphisms of the genes coding this enzyme affect basal NO levels (11). In one study, it was reported that NOS gene polymorphism was an independent risk factor for migraine with aura (12). while other studies did not support this relation (13,14).
Renin angiotensin system (RAS) is a system which activates vascular reactivity and the relation of ACE and angiotensin 1 gene polymorphisms with migraine is not clear (15,16,17). However. demonstration of the efficiency of ACE inhibitor lisinopril (18) and angiotensin 1 receptor blocker olmesartan (19) in migraine prophylaxis suggests a potential relation between the RAS system and migraine. Angiotensin converting enzyme DD genotype has been reported to increase vascular tonus and lead to smooth muscle hypertrophy, decreased bradykinin level and hypercoagulability (19,20, 21).
In our study. we aimed to investigate the relation between migraine and its subtypes and eNOS 4 a/b and ADE I/D gene polymorphisms.
Method
One hundred five female subjects with migraine and 97 female healthy controls were included in the study. All migraine patients were evaluated by neurologists in Neurology Outpatient clinics. The diagnosis of migraine was made according to the criteria of the International Classification of Headache Disorders, 2nd edition (22). Neurological examination was found to be normal in all patients. The control group was composed of healthy volunteers, healthcare personnel and women who gave birth in the Gynecology and Obstetrics ward. The frequency of headache in migraine patients was recorded as the attack number in one month and pain severity was defined on the visual analog scale. Hypertension, diabetes mellitus, smoking and familial history of migraine were questioned and recorded in the patient and control groups.
The study was approved by the local ethics committee and informed consent was obtained from the subjects.
DNA Isolation
2 ml peripheral blood was collected to tubes containing ethylenediaminetetraacetic acid (EDTA) as anticoagulant. DNA was isolated from the blood which contained EDTA with DNA isolation kit (High Pure PCR Template Preparation Kit, Roche USA).
The purity and amount of DNA was determined according to absorbance values at 260 nm and 280 nm in spectrophotometer. DNAs were examined under UV light after operating in 0.8% agarose gel electrophoresis and staining with EtBR.
eNOS 4 a/b Gene Polymorphism Genotyping
eNOS gene is localized in chromosome 7q35-36 with a length of 21 kilobase. eNOS intron 4 VNTR is repeated for 4 or 5 times. 5 repetitions of this region is genotyped as (b) and 4 repetitions of this region is genotyped as (a).
F:5′-AGGCCCTATGGTAGTGCCTT-3′ and R:5′-TCTCTTAGTGCTGT GGTCAC-3′eNOS 4 a/b primers were used in evaluation of polymorphism (23). 0.5 nmol. 0.2 mM dNTP. 2.5 mM MgCl2, 1X Taq Buffer (75 mM Tris-HCl pH 8.8, 20 mM (NH4)2SO4. 0.01% Tween 20), 0.75 unit Taq DNA polymerase and 100 ng genomic DNA were added from each primer and polymerase chain reaction (PCR) was performed with a total of 15 μl. PCR protocol was started with 1-minute initial denaturation at 94°C. This was followed by 25-second denaturation at 95°C. 35-second binding at 56°C, 40-second lengthening at 72°C and finally 5-minute final lengthening stage at 72°C which were composed of 38 cycles (23). The products were operated in 2.5% agarose gel electrophoresis containing EtBr at 110 volts. They were examined under UV light and polymorphisms were defined (Figure 1).
Figure 1.
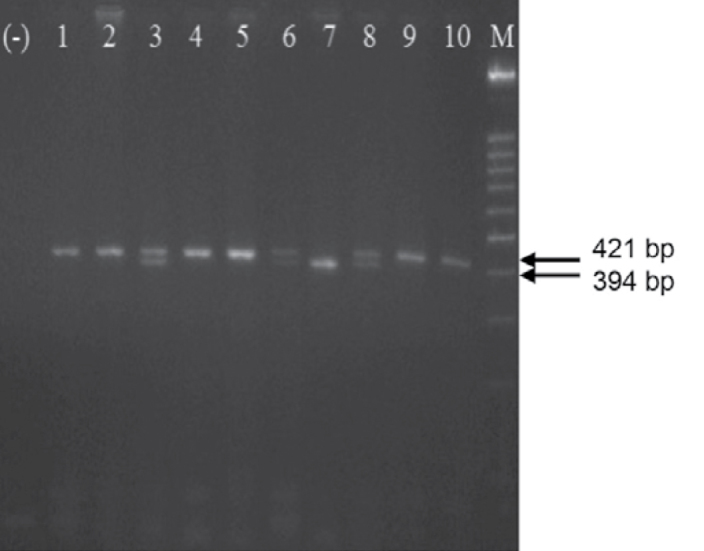
2.5% agarose gel appearance showing eNOS 4 a/b polymorphism stained with EtBr. bb (421 bp): 1, 2, 4, 5, 9 and 10, ba (421 bp ve 394 bp): 3, 6 and 8, aa (394 bp): 7, M: 100 base couple DNA marker and (−): negative control which does not contain DNA
ADE (I/D) gene polymorphism Genotyping
ADE gene is localized on the 17th chromosome (17q 23.3). it has a length of 21 kb and is composed of 26 exons ans 25 introns. ADE (I/D) gene polymorphism is characterized with insertion and deletion of repeated Alu series (274 base couples) in the 16th intron.
PCR method was used to determine ACE (I/D) polymorphism (rs4646994) with DNAs isolated from the patients and controls. In a 15 μl-PCR volume 1X Taq Buffer, 2.5 mM MgCl2, 0.2 mM dNTP, 0.75 unit Taq DNA polymerase, 0.5 nmol from each primer (F:5′-CTGGAGACCACTCCCATCCTTTCT-3′ and R:5′-GACGTGGCCATCACATTCGTCA GAT-3′) and 100 ng DNA were used (24).
PCR protocol; initial 5-minute denaturation at 94°C for 30 cycles;1 minute-denaturation at 94°C, 1 minute-binding at 58°C and 1-minute lengthening at 72°C, finally 7-minute lengthening at 72°C stages were applied (24). The products were operated in 2% agarose gel electrophoresis containing EtBr at 110 volts. They were examined under UV light and polymorphisms were defined (Figure 2).
Figure 2.
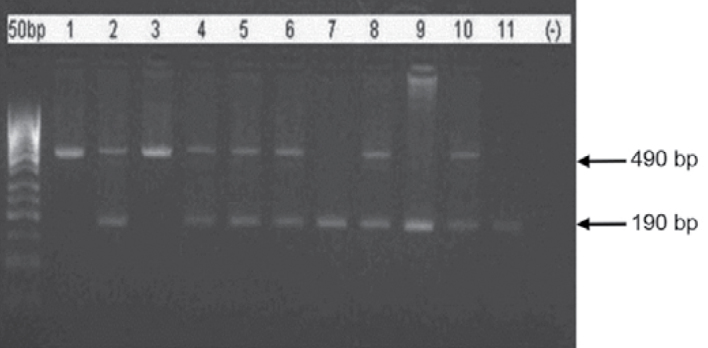
2% agarose gel appearance of PCR products showing ACE (I/D) polymorphism stained with EtBr. DD (190 bp): 7, 9 and 11. ID (490 bp ve 190 bp): 2, 4, 5, 6, 8 and 10. II (490 bp): 1 and 3, 50 bp: 50 base couple DNA marker and (−): negative control which does not contain DNA
Statistical Analysis
The results were expressed as mean ± standard deviation or percentage. Compatibility of the values obtained from the groups with the normal distribution was analysed using one-sample Kolmogrov-Smirnov test. Student’s t-test was used in comparison of the clinical properties of the groups. Allele frequency was analysed with Hardy Weinberg equilibrium test calculating from the genotypes of both groups. Genotype and allele differences were compared using chi-square test and odds ratios and confidence interval values were calculated. The relation of genotype and allele groups with the clinical properties was tested using Pearson’s correlation test. A p value of <0.05 was considered statistically significant. SPSS 19.0 and Stata 12.0 programs were used for statistical analyses.
Results
The properties of the migraine and control groups included in the study are shown in (Table 1). Both groups were matched in terms of age. Presence of hypertension and diabetes mellitus did not show difference between the two groups.
Table 1.
Characteristics of the patients with migraine and control subjects
| Migraine (n=105) | Control (n=97) | p | |
|---|---|---|---|
| Age | 38.07±9.78 | 35.69±7.35 | 0.053 |
| Hypertension (%) | 18.1 | 15.6 | 0.776 |
| Diabetes mellitus (%) | 3.0 | 5.3 | 0.487 |
| Smoking (%) | 34.3 | 15.0 | 0.004 |
| Familial history of migraine (%) | 52 | 18.5 | 0.004 |
Genotype and allele frequencies of ACE and eNOS polymorphisms are shown in (Table 2 and 3). respectively. ACE genotype distributions showed marked difference in the migraine group compared to the controls (p<0.001). Angiotensin converting enzyme DD and ID genotypes increase the risk of migraine by 2.5-fold and 4.5-fold compared to II genotype. respectively (Table 2). The same increased risk rates were also observed in the migraine with aura group and only ID genotype increased the risk by 3, 75-fold compared to II genotype in the migraine without aura group. Allele distributions did not show significant difference between the migraine and control groups (Table 2).
Table 2.
Angiotensing converting enzyme allele and genotype frequency and odds ratios
| D | I | p | DD | ID | II | p | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Migraine group (%) | 61.4 | 38.6 | 0.06 | 34.3 | 54.3 | 11.4 | 0.001a | DD vs. ID | 0.577 (0.306–1.090) |
| DD vs II | 2.571 (1.138–5.811) | ||||||||
| ID vs II | 4.453 (2.006–9.883) | ||||||||
| Control group (%) | 52.5 | 47.5 | 36.1 | 33 | 30.9 | ||||
| Migraine with aura group (%) | 63.3 | 36.7 | 0.36 | 35.6 | 55.6 | 8.9 | 0.006b 0.777c |
DD vs. ID | 0.585 (0.266–1.289) |
| DD vs II | 3.429 (1.033–11.375) | ||||||||
| ID vs II | 5.859 (1.824–18.824) | ||||||||
| Migraine without aura group (%) | 60.0 | 40.0 | 33.3 | 53.3 | 13.3 | 0.014d | DD vs. ID | 0.571 (0.274–1.193) | |
| DD vs II | 2.143 (0.825–5.563) | ||||||||
| ID vs II | 3.750 (1.493–9.420) |
Migraine-control groups,
Migraine with aura-control groups,
Migraine with aura-migraine without aura groups,
Migraine without aura-control groups,
OR: Odds ratio, CI: Confidence interval
Table 3.
Endothelial nitric oxide synthase allele and genotype frequency
| a | b | p | aa | ab | bb | p | |
|---|---|---|---|---|---|---|---|
| Migraine group (%) | 17.6 | 82.4 | 0.22 | 5.7 | 23.8 | 70.5 | 0.106a |
| Control group (%) | 17.0 | 83.0 | 1.0 | 31.9 | 67.1 | 0.49b | |
| Migraine with aura group (%) | 15.6 | 84.4 | 80.9 | 6.7 | 17.8 | 75.5 | 0.446c |
| Migraine without aura group (%) | 19.1 | 80.9 | 5.0 | 28.3 | 66.7 | 0.293d |
Migraine-control groups,
Migraine with aura-control groups,
Migraine with aura-migraine without aura groups,
Migraine without aura-control groups
Endothelial NOS genotype and allele distributions were found to be similar in the migraine and healthy groups (Table 3).
No relations was observed between genotype and allele groups and hypertension, diabetes, smoking, familial history of migraine and frequency and severity of migraine attacks (p>0.05 for all).
Discussion
The genetic structure of an individual may vary according to his/her ethnic and racial characteristic. Therefore, gene polymorphism studies performed in different countries including individuals with different ethnic and racial structure may not give the same results. In our study, we examined the individuals residing in Trakya, Edirne region as the study groups, Since Edirne region has not allowed immigrants with a high rate yet and intensive population changes have not occurred, it has maintained its demographic properties. In our study we examined two different gene polymorphisms and found that ACE gene polymorphism was found with a higher rate in DD and ID genotypes compared to II genotype in the migraine group. In examination of the subgroups, the significant difference of the same groups was maintained in the migraine with aura group, while only ID genotype was noted to increase the risk of migraine significantly compared to II genotype in the migraine without aura group. If expressed reversely, II genotype was found to be a protective genotype for migraine in all groups. eNOS gene polymorphism did not show difference compared to the healthy control group in the migraine group and its subgroups.
There are both national and international studies investigating angiotensin converting enzyme gene polymorphism in migraine patients. In the study performed by Alaşehirli et al. (25) which was published recently, it was found that ACE gene polymorphism was not different between migraine patients and healthy controls residing in Gaziantep region. In another study performed in Istanbul region, it was found that II genotype was found with a significantly lower rate in the migraine group and ACE activity was high in individuals with DD genotype which was compatible with our study (26). Similarly, DD and ID genotypes were found to be risk factors in the migraine with aura group in Japan (15). In another study, DD genotype was found to pose a risk only in the migraine without aura group in subgroup analysis (16). In this study, it was also found that migraine attack frequency was higher in individuals with DD genotype. In our study, we found no relation between genotypes and migraine attack frequency and time. In contrast to our study, no relation was shown between migraine and ACE gene polymorphism in a study performed in Norwegians (17). In contrast to all this information, Lin et al. (27) reported that DD genotype was found with a lower rate and had protective characteristic for migraine in male patients with migraine.
ACE found in the circulation provides transformation of angiotensin I to angiotensin II and leads to emergence of bradykinin. Angiotensin II which is a potent vasoconstrictor and bradykinin which a vasodilatator are involved in vascular remodeling. ACE levels and activity in the tissues and circulation are under strict genetic control. In ACE gene deletion polymorphism, the activity of ACE in the circulation is increased (28). Studies supporting the positive relation of D allele and DD genotype with lacunar infarcts and coronary artery disease have been published (29,30). This relation is thought to be related with hypercoagulability (19), decreased bradykinin level, increased vascular tonus and smooth muscle hypertrophy (20,21). The relation between ACE and migraine pathogenesis is not clear yet. It has been found that ACE plays a catabolic role in degradation of opioid peptides (31) and the circadian changes of ACE activity is observed with a lower rate in migraine patients (32).
Our study is the first study investigating the relation between eNOS polymorphism and migraine in our country and did not show a relation between them. Endothelial NOS is an enzyme which provides nitric oxide synthesis from the endothelial cells. It has two different isotypes called neuronal (nNOS) and inducible NOS (iNOS). Many studies have shown that both iNOS and eNOS gene polymorphisms are not related with migraine (13,14). However, (a) allele in iNOS gene polymorphism was found with a higher rate in the migraine with aura group compared to the migraine without aura group in one study (33). Similarly, Borroni et al. (12) reported that Asp/Asp genotype increased the risk of migraine with aura by 3-fold compared to migraine without aura. It has been reported that eNOS activity is decreased in aspartate genetic variation of endothelial NOS (34).
The limitations of our study included the facts that our study groups were composed of limited number of subjects and included only female patients. Our results have the property of being regional data. They will provide a more significant outcome when evaluated in a meta-analysis with different results obtained in other studies. We think that demonstration of the relation between gene polymorphisms and migraine will affect therapeutic planning and drug choice.
Footnotes
Conflict of interest: The authors reported no conflict of interest related to this article.
Çıkar çatışması: Yazarlar bu makale ile ilgili olarak herhangi bir çıkar çatışması bildirmemişlerdir.
References
- 1.Gardner K. The genetic basis of migraine: Howmuch do we know? Can J Neurol Sci. 1999;26:37–43. doi: 10.1017/s0317167100000184. [DOI] [PubMed] [Google Scholar]
- 2.Montagna P. Molecular genetics of migraine headaches: A review. Cephalalgia. 2000;20:3–14. doi: 10.1046/j.1468-2982.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 3.Raskin NH, Gren MW. Migren ve Diğer Başağrıları. In: Rowland Lewis P., editor; Baslo B, Gürses C, translators and editors. Merritt’s Neurology içinde, 11. baskı. İstanbul: Güneş Tıp Kitapevleri; 2008. pp. 981–990. [Google Scholar]
- 4.Schürks M, Rist PM, Kurth T. Sex hormone receptor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia. 2010;30:1306–1328. doi: 10.1177/0333102410364155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schürks M, Rist PM, Kurth T. 5-HTTLPR polymorphism in the serotonin transporter gene and migraine: a systematic review and meta-analysis. Cephalalgia. 2010;30:1296–1305. doi: 10.1177/0333102410362929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schürks M, Rist PM, Kurth T. STin2 VNTR polymorphism in the serotonin transporter gene and migraine: pooled and meta-analyses. J Headache Pain. 2010;11:317–326. doi: 10.1007/s10194-010-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schürks M, Rist PM, Kurth T. MTHFR 677C>T and ACE D/I polymorphisms in migraine: a systematic review and meta-analysis. Headache. 2010;50:588–599. doi: 10.1111/j.1526-4610.2009.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akerman S, Williamson DJ, Kaube H, Goadsby PJ. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br J Pharmacol. 2002;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeb L, Reuter U. Nitric oxide in migraine. CNS Neurol Disord Drug Targets. 2007;6:258–264. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- 10.Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci. 2006;23:2057–2066. doi: 10.1111/j.1460-9568.2006.04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW, Smits P. The Glu298Asp polymorphism of the NOS3 gene as a determinant of the baseline production of nitric oxide. J Hipertens. 2002;20:2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Borroni B, Rao R, Liberini P, Venturelli E, Cossandi M, Archetti S, Caimi L, Padovani A. Endothelial nitric oxide synthase (Glu298Asp) polymorphism is an independent risk factor for migraine with aura. Headache. 2006;46:1575–1579. doi: 10.1111/j.1526-4610.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 13.Toriello M, Oterino A, Pascual J, Castillo J, Colás R, Alonso-Arranz A, Ruiz-Alegría C, Quintela E, Montón F, Ruiz-Lavilla N. Lack of Association of Endothelial Nitric Oxide Synthase Polymorphisms and Migraine. Headache. 2008;48:1115–1119. doi: 10.1111/j.1526-4610.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonçalves FM, Martins-Oliveira A, Speciali JG, Luizon MR, Izidoro-Toledo TC, Silva PS, Dach F, Tanus-Santos JE. Endothelial nitric oxide synthase haplotypes associated with aura in patients with migraine. DNA Cell Biol. 2011;30:363–369. doi: 10.1089/dna.2010.1152. [DOI] [PubMed] [Google Scholar]
- 15.Kowa H, Fusayasu E, Ijiri T, Ishizaki K, Yasui K, Nakaso K, Kusumi M, Takeshima T, Nakashima K. Association of the insertion/deletion polymorphism of the angiotensin I-converting enzyme gene in patients of migraine with aura. Neurosci Lett. 2005;374:129–131. doi: 10.1016/j.neulet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Paterna S, Di Pasquale P, D’Angelo A, Seidita G, Tuttolomondo A, Cardinale A, Maniscalchi T, Follone G, Giubilato A, Tarantello M, Licata G. Angiotensin-converting enzyme gene deletion polymorphism determines an increase in frequency of migraine attacks in patients suffering from migraine without aura. Eur Neurol. 2000;43:133–136. doi: 10.1159/000008151. [DOI] [PubMed] [Google Scholar]
- 17.Tronvik E, Stovner LJ, Bovim G, White LR, Gladwin AJ, Owen K, Schrader H. Angiotensin-converting enzyme gene insertion/deletion polymorphism in migraine patients. BMC Neurol. 2008;8:4. doi: 10.1186/1471-2377-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrader H, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor Randomised, placebo controlled, crossover study. BMJ. 2001;322:19–22. doi: 10.1136/bmj.322.7277.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles JA, Jotkowitz S, Byrd LH. Prevention of migraine with olmesartan in patients with hypertension/prehypertension. Headache. 2006;46:503–507. doi: 10.1111/j.1526-4610.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 20.Erdös EG, Skidgel RA. The angiotensin I-converting enzyme. Lab Invest. 1987;56:345–348. [PubMed] [Google Scholar]
- 21.Dzau VJ. Theodore cooper Lecture: tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 22.Headache Classification Sub-Committee of the International Headache Society. International Classification of Headache Disorders, 2nd edn. Cephalgia. 2004;24:1–160. [Google Scholar]
- 23.Wang XL, Sim AS, Badenhap RF, McCredle RM, Wilcken DE. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med. 1996;2:41–45. doi: 10.1038/nm0196-41. [DOI] [PubMed] [Google Scholar]
- 24.Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alaşehirli B, Gür M, Akçalı A, Geyik S, Bülbül B, Sayar D, Yılmaz M, Neyal A, Neyal M. Angiotensin converting enzyme gene ınsetion/deletion polymorphısm in migraine patients. Turk Norol Derg. 2009;15:161–165. [Google Scholar]
- 26.Kara I, Ozkok E, Aydin M, Orhan N, Cetinkaya Y, Gencer M, Kilic G, Tireli H. Combined effects of ACE and MMP-3 polymorphisms on migraine development. Cephalalgia. 2007;27:235–243. doi: 10.1111/j.1468-2982.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin JJ, Wang PJ, Chen CH, Yueh KC, Lin SZ, Harn HJ. Homozygous deletion genotype of angiotensin converting enzyme confers protection against migraine in man. Acta Neurol Taiwan. 2005;14:120–125. [PubMed] [Google Scholar]
- 28.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markus HS, Barley J, Lunt R, Bland JM, Jeffery S, Carter ND, Brown MM. Angiotensin-converting enzyme gene deletion polymorphism. A new risk factor for lacunar stroke but not carotid atheroma. Stroke. 1995;26:1329–1333. doi: 10.1161/01.str.26.8.1329. [DOI] [PubMed] [Google Scholar]
- 30.Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D, Luc G, Bard JM, Bara L, Ricard S, Tiret L, Amouyel P, Alhenc-Gelas F, Soubrıer F. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359:641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 31.Skidgel RA, Erdös EG. Angiotensin converting enzyme (ACE) and neprilysin hydrolyze neuropeptides: a brief history, the beginning and follow-ups to early studies. Peptides. 2004;25:521–525. doi: 10.1016/j.peptides.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Martelletti P, Cugini P, Letizia C, Di Palma L, Battisti P, Granata M, Scavo D, Giacovazzo M. Chronopathology for angiotensin converting enzyme circadian rhythm in migraine. Chronobiologia. 1990;17:59–64. [PubMed] [Google Scholar]
- 33.de O S Mansur T, Gonçalves FM, Martins-Oliveira A, Speciali JG, Dach F, Lacchini R, Tanus-Santos JE. Inducible nitric oxide synthase haplotype associated with migraine and aura. Mol Cell Biochem. 2012;364:303–308. doi: 10.1007/s11010-012-1231-0. [DOI] [PubMed] [Google Scholar]
- 34.Fairchild TA, Fulton D, Fontana JT, Gratton JP, McCabe TJ, Sessa WC. Acidic hydrolysis as a mechanism for the cleavage of the Glu(298)Asp variant of human endothelial nitric-oxide synthase. J Biol Chem. 2001;276:26674–26679. doi: 10.1074/jbc.M103647200. [DOI] [PubMed] [Google Scholar]


