Abstract
Background
Studies on the pathomechanism of colorectal cancer (CRC) expansion indicate a significant role of metalloproteinases and their inhibitors in the extracellular matrix. The results of the analysis of a profile of transcriptional activity of genes encoding metalloproteinases were the basis of the hypothesis indicating changes in the expression of genes encoding MMP9, MMP28, and TIMP1 as an additional diagnostic and prognostic marker of CRC.
Material/Methods
The material consisted of samples obtained from resected tumors and healthy tissue samples from 15 CRC patients (aged 46–72 years) at clinical stages (CSs) I and II–IV.
Gene expression analysis was done using microarrays. Microarray data analysis was done using the GeneSpring 11.5 platform. The results were validated using the qRT-PCR technique.
Results
We found high levels of expression of MMP9 at each CS, as well as in the tissues at the early stage of CRC. Additionally, we observed high levels of expression of TIMP1 and low levels of MMP28 genes in CS II–IV. No statistically significant differences based on the stage of CRC were observed.
Conclusions
MMP9 gene profile may be a complementary diagnostic marker in CRC. The results suggest a crucial role of MMP9 at the early stage of carcinogenesis in the large intestine. The increase in MMP9 and TIMP1 mRNA concentration and the decrease in MMP28 in the large intestinal tissue may be a confirmation of cancer, but it may not indicate the advance of CRC.
MeSH Keywords: Colorectal Neoplasms, Gene Expression Profiling, Matrix Metalloproteinase 9, Matrix Metalloproteinase Inhibitors, Matrix Metalloproteinases
Background
As the population is aging, both the rate of cancer incidence and the number of cancer-related deaths are constantly increasing. It is also related to colorectal cancer (CRC), which is the most common malignancy of the digestive tract. A significant therapeutic problem experienced by clinicians who treat colorectal patients is connected with high invasiveness of CRC and its ability to form metastases in distant organs. Absence of CRC symptoms, scant symptoms, or symptoms ignored by patients frequently result in treatment at a late advanced stage of the disease. Therefore, early diagnosis is of crucial importance. Recently, much hope has been placed on the constant development of molecular biology techniques in terms of development of early diagnosis and possible treatment options. The most recent diagnostic and therapeutic trends are focused on the molecular basis of CRC. Significantly changed transcriptional gene activity may be a complementing prognostic and diagnostic marker in CRC, and it may be the basis for establishing new therapeutic goals. Studies on the pathomechanism of cancer expansion demonstrate a significant role of extracellular matrix metalloproteinases (MMPs) in this process [1–3]. Their proteolytic activity significantly influences extracellular matrix components (EMCs) through the degradation of its components and regulation of the activity of signal particles [4,5]. Matrix metalloproteinases participate in regulating the ability of cells to differentiate, proliferate, and in the process of apoptosis, adhesion or migration. At some stages, MMPs may also prepare malignant cells for migration, invasion, and dissemination to distant organs. They also participate in malignant transformation itself. However, tissue inhibitors of metalloproteinases (TIMPs) are mainly responsible for controlling the functioning of MMPs, in addition to the other functions they perform. Consequently, their role is to minimize the degradation of EMCs. As a result, MMPs and TIMPs take part in the reconstruction of EMCs, thus influencing transformation of malignant cells and cancer progression.
The aim of the present paper is to analyze a profile of transcriptional activity of genes encoding MMP9, MMP28, and TIMP1, depending on the clinical stage of CRC. The analysis aims at considering them to be potential complementing diagnostic and prognostic markers of CRC.
Material and Methods
The material consisted of samples obtained during the resection of the large intestine. The samples were collected from 15 CRC patients aged 46–72 years. The inclusion criterion was: CRC patients in all stages of the disease undergoing elective classic surgical procedures. The exclusion criteria were: patients reoperated on due to the underlying disease; unclear histological confirmation of CRC; patients with genetic, systemic, or metabolic conditions (with the exclusion of obesity as an isolated condition); and previous radiotherapy or chemotherapy. The study was approved by the Bioethics Commission of the Medical University of Silesia (KNW/0022/KB1/21/I/10). Written informed consent was obtained from all study participants.
The material consisted of CRC and healthy tissue samples. The samples were collected from CRC from the external margin to exclude the presence of necrotic tissues in the specimens. Control samples were obtained from the macroscopically healthy intestine, from the part of the specimen maximally distant from the lesion and at least 5 cm distant from the margin considered to be healthy. The tumor tissue was divided into 2 parts. One part was referred for standard histological assessment and the other for molecular analysis, as in the case of the control. The tissue collection was done immediately after the resection of the intestinal fragment, thus limiting to the minimum the effect on cancer. The tissues were dissected using classic surgical techniques only. No electrical devices or ultrasound were used. Prior to the molecular analysis, the material was stored in RNAlater™ (Qiagen) at the temperature of −80°C to prevent sample degradation.
Twenty-eight samples were obtained (15 CRCs and 13 controls). In accordance with the 7th edition of the AJCC/UICC staging system of CRC, cancer tissues represented different stages of the disease: 3 Clinical Stage I (CSI), 5 Clinical Stage II (CSII), 3 Clinical Stage III (CS III), and 4 Clinical Stage IV (CSIV). The genetic profile of cancerous tissues was analyzed. The profile was compared to the genetic profile of the control tissues. The samples with CRC were divided into 2 groups: “low stage of cancer” LSC (CSI) and “high stage of cancer” HSC (CSII–CSIV).
Methods of molecular analysis
The molecular analysis was started by extracting total RNA from the obtained fragments of the large intestine. In further stages of the study, RNA was the array for the assessment of intestinal transcriptome, using expression microarrays HG-U133A (Affymetrix®) and validation of the array experiment with qRT-PCR, based on mRNA concentration profiles: MMP9, MMP28, TIMP1, and the control of endogenous GAPDH and β-actin.
RNA purification and analysis
Total RNA from the sample of the large intestine was isolated with a total RNA isolation kit (Total RNA Prep Plus, A&A Biotechnology). Next, the extracted RNA was purified and digested with DNase I, using columns of an RNase Minikit (Qiagen) in accordance with the manufacturer’s instructions. Qualitative assessment of the obtained RNA extracts was performed using 1% agarose gel electrophoresis, stained with ethidium bromide. Additionally, the degree of total RNA integrity was assessed based on the RNA Integrity Number (RIN) parameter – rRNA ratio (28s/18s).
RNA concentration was assessed spectrophotometrically at a wavelength of 260 nm, using Gene Quant II. The total RNA was the array for transcriptome assessment using expression microarray HG-U133A (Affymetrix®) and the number of mRNA copies in μg of the total RNA assessed using qRT-PCR (validation of the array experiment).
Transcriptome assessment with expression microarray HG-U133A (Affymetrix®)
Large intestine transcriptomes were assessed with mRNA expression microarrays, using HG-U133A? (Affymetrix®, CA). The isolated total RNA was the array for the synthesis of marked cRNA (biotinylated complementary RNA), the synthesis of which was performed using the 3′ IVT Express Kit. The obtained particles were hybridized with HG-U133A microarray. At the next stage, the microarrays were washed and marked by immunofluorescence using the Fluidics Station 450 and the Hybridization Wash and Stain Kit. Next, fluorescence intensity of the transcriptomes was read using the GeneChip Scanner 3000 7G and the Affymetrix® GeneChip® Command Console® Software (AGCC) software. Experiment quality control was done at the subsequent stages of transcriptome assessment, starting with the quality assessment using 1% agarose gel electrophoresis of total RNA after extraction from intestinal samples, and of reverse transcription products (cDNA), transcription (cRNA), and cRNA after fragmentation and immediately before the preparation of the hybridization cocktail.
Validation of the array experiment with qRT-PCR
Validation of the array experiment results was done with qRT-PCR, which allowed precise assessment of diagnostic and prognostic values of the determined changes in mRNA concentration of MMP9, MMP28, and TIMP1. Starters synthesized by Oligo IBB PAN were used for amplification. The qRT-PCR reaction was conducted using the SYBR Green Quantitect RT-PCR Kit and the Opticon™ DNA Engine Sequence Detector. The number of mRNA copies in 1 μg of the total RNA extract was determined based on the standard curve made for commercially available DNA specimens of the β-actin gene. For each test, negative control (without RNA array) and endogenous control (of mRNA of β-actin and GAPDH genes) were done. The specificity of the qRT-PCT reaction was assessed based on electrophoretic separation of amplimers at 6% polyacrylamide gel stained with silver salts with size marker pBR322/HaeIII and Tm value determined based on the amplimer melting curve, for each qRT-PCR product. The qRT-PCR reaction was performed in a reaction mix prepared with the use of a robot integrated with ABI PRISM™ 877 thermal cycler, containing 1× Tth.
The statistical analysis of the results
The statistical analysis of the array experiment results was started with global normalization of all microarrays accepted for the comparative analysis using the RMA-express software. Next, hierarchical clustering with Statistica v. 9.0 (StatSoft, Tulsa, OK) was used to assess the stage of differentiation of the intestinal transcriptomes. During the next stage of the comparative analysis of transcriptome groups, we used GeneSpring GX 11.5 (Agilent Technologies) for the analysis of array experiment results.
Statistical significance was p<0.05. For each parameter analyzed, the most significant elements of the descriptive statistics were determined, including mean, standard deviation, median, extreme values (minimal and maximal), and lower (25%) and upper (75%) quartiles. The hypothesis on the compliance of the distribution of a given parameter with the normal distribution was verified using the Shapiro-Wilk test. In the case of the normal distribution of a given parameter, differences between groups that were statistically significant in reference to particular variables were verified at the first stage using one-way analysis of variance (ANOVA), and then the nature of particular relationships was determined with the post hoc Tukey test. Comparisons between the 2 groups were made using the t test for independent samples. In the case of lack of compliance between the distribution of a given parameter and the normal distribution, comparisons between the 2 groups were made using the Mann-Whitney U test. To increase the probability of obtaining the correct results, the Benjamini-Hochberg correction was used.
Validation of the array experiment results was performed with the qRT-PCR technique, which allowed a precise assessment of the diagnostic and prognostic values of the changes in mRNA of MMP9 and MMP28, as well as TIMP1.
Results
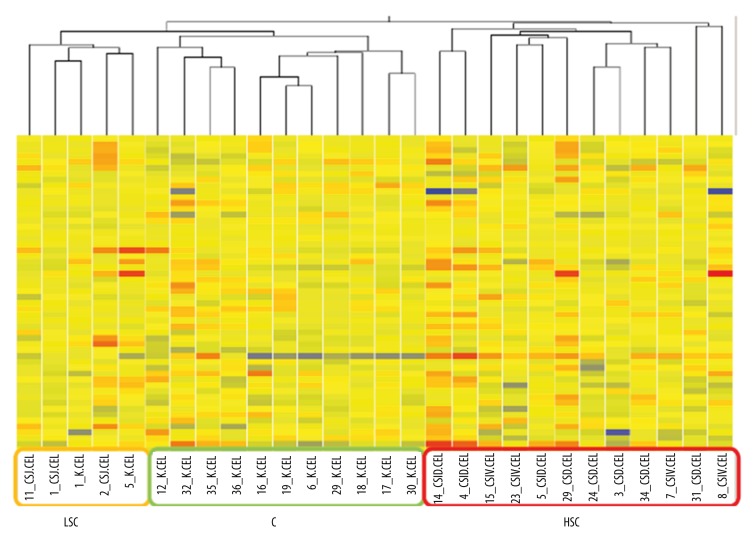
The assessment of the differentiation of transcriptome groups characteristic of the samples from the healthy large intestine and CRC was started with the array experiment. After the initial acceptance of microarrays for the comparative analysis carried out in accordance with the instructions of the microarray producer (Affymetrix®) and result normalization with the RMA method, transcriptome clustering was done with the use of the hierarchical clustering method (Figure 1).
Figure 1.
The result of microarray hierarchical clustering (HG-U133A – Affymetrix®) – normalized mRNA fluorescence signals in transcriptomes. The following numbers – sample number, Clinical Stage (CS) I–IV – stage of colorectal cancer, C – control; LSC – low stage of cancer, HSC – high stage of cancer.
The obtained results demonstrate that all transcriptomes are clustered in 3 main groups, differentiated by the stage of CRC: C – control group – normal large intestine samples, LSC – CRC samples in CSI and HSC – CRC samples in CSII, CSIII, and CSIV.
The next stage of the analysis included a comparison of LSC and HSC transcriptomes with the control group. The analysis was started with the characteristics of the dispersion of fluorescence signals of 44 mRNA of metalloproteinases and their inhibitors in the transcriptome groups, which was based on the descriptive statistics (median and interquartile range) (Figure 2). The obtained results indicate that mRNA concentration profile in the transcriptome groups depends on the stage of CRC.
Figure 2.

Box plot, illustrating the dispersion of fluorescence signals of 44 mRNA of metalloproteinases and their inhibitors in transcriptome groups of the large intestine, with different stage of CRC (min–max. 25–75%, median). C – control; LSC – low stage of cancer, HSC – high stage of cancer.
To determine whether the observed differences were statistically significant, concentration profiles of 44 mRNA of metalloproteinases and their tissue inhibitors in healthy intestinal tissues (C) and in LSC and HSC were compared using ANOVA. The obtained results indicated that out of 44 mRNA assessed by the oligonucleotide microarray method (HG-U133A, Affymetrix®), 11 mRNA differentiate the compared groups with the assumed p<0.05.
mRNA heat maps of metalloproteinases and their inhibitors were simultaneously generated for 11 ID mRNA (GeneSpring GX), demonstrating an mRNA concentration profile differentiating CRC from the control. Compared to the mean mRNA concentration in transcriptomes (yellow), particular metalloproteinases were characterized by varied transcription activity. In some cases, an increase in transcription activity was observed with the increase in the stage of CRC. Metalloproteinase 28 was an exception; the transcription activity decreased with an increase in the stage of CRC.
We also used a test that increased the precision of ANOVA – the post hoc Tukey test – indicating which of 11 mRNA selected in the variance analysis (ANOVA) as the particles differentiating the compared transcriptome groups could be used to differentiate the defined groups of large intestine samples. The analysis indicates that out of 11 selected mRNA, 2 differentiate healthy intestinal tissue samples (C) from LSC, 9 differentiate control samples (C) from HSC, and 5 differentiate LSC from HSC.
Based on these initial analyses (one-way ANOVA combined with post hoc Tukey), 2 genes were extracted that differentiate the following: a macroscopically healthy tissue (C) from a cancerous tissue in both LSC and HSC. The next stages of the comparative analysis included the selection of mRNA differentiating healthy intestine samples and CRC in LSC (C vs. LSC) and healthy intestinal samples and CRC in HSC (C vs. HSC) and CRC in LSC and CRC in HSC. The nonparametric Mann-Whitney U test with the Benjamini-Hochberg correction was used. Validation of the array experiment results was done using the qRT-PCR technique (Table 1).
Table 1.
The results of the comparative analysis using the Mann-Whitney U test, indicating the differentiation level of the transcription activity for MMP28, MMP9 and TIMP1 in CRC samples determined by qRT-PCR.
| mRNA | p value Mann-Whitney U test |
|||
|---|---|---|---|---|
| MMP28 | MMP9 | TIMP | ||
| qRT-PCR | C vs. LSC | NC | 0.0370 | NC |
| C vs. HSC | 0.035 | 0.0002 | 0.0149 | |
| LSC vs. HSC | NC | NC | NC | |
| Microarrays HG-U133A | C vs. LSC | NC | 0.0011063776 | NC |
| C vs. HSC | 1.4701724E-5 | 1.2181828E-6 | 7.554613E-8 | |
| LSC vs. HSC | NC | NC | NC | |
C – control group; LSC – low stage of cancer; HSC – high stage of cancer; NC – no change.
The results of the analyses indicate that the profile of the expression of genes encoding MMP9, MMP28, and TIMP1 differentiates the cancerous tissue from the healthy intestine. However, the profile does not demonstrate statistically significant differences depending on the stage of CRC. We did not confirm the result of the one-way ANOVA combined with the post hoc Tukey analysis in relation to mRNA of MMP28.
Discussion
Colorectal cancer, the most common cancer of the digestive tract and generally one of the most frequent malignancies in human pathology, is a disease of complex and multi-factorial etiology where the accumulation of genetic and epigenetic changes in the cell plays a crucial role [6–11]. The invasiveness of CRC and the ability of cancerous cells to form metastases in distant organs are one of the most significant therapeutic problems, which result in a number of outcome failures [12]. Matrix metalloproteinases play an active part in that process, degrading EMCs, including basement membranes, which in consequence may result in the infiltration of the parenchyma, adjacent structures, and distant metastases after crossing the blood vessel basement membrane [13].
Currently, CRC treatment is based mainly on radical surgery frequently supported by systemic treatment: chemotherapy and radiotherapy [7,14,15]. The effectiveness of treatment strictly depends on the stage of the disease at diagnosis; faster diagnosis of LSC offers a better prognosis. Together with the increase in the stage of CRC, the probability of successful outcome decreases despite adjunctive therapy. The detection of the disease at its earliest and preferably asymptomatic stage is therefore crucial. Hope is offered by modern techniques that use oligonucleotide microarrays to compare gene expression in cancerous and healthy tissues.
Finding the differences in the expression of genes encoding particular proteins may be helpful in explaining the mechanisms resulting in the occurrence and development of CRC, which in turn is the key to establishing new therapeutic possibilities. Analyzing the problem of solid tumors as a whole, including digestive tract cancers and CRC in particular, more and more reports stress the role of MMPs in CRC formation and progression [3,16–27].
The result of the Mann-Whitney U test with the Benjamini-Hochberg correction used to select mRNA differentiating healthy intestinal samples from CRC in LCS confirmed the result of the analysis of one-way ANOVA combined with the post hoc Tukey analysis only in the case of MMP9. However, the test result comparing healthy intestinal samples with CRC in HSC confirmed the result of ANOVA analysis in MMP9, MMP28, and TIMP1. These results were confirmed using qRT-PCR.
The existing data on the prognostic role of MMPs and their inhibitors as markers of CRC progression are not conclusive. Our study suggests that MMPs and TIMPs may be a substantial element of controlling CRC stage by indicating the complexity of proliferative and migration mechanisms. It seems that an overexpression of MMP9 and TIMP1 and silencing of MMP28 could become a direct independent target of anti-cancer therapy. Because there is a difference in the expression of MMP9 gene in cancerous tissues compared to healthy tissues, the gene demonstrates a diagnostic value; it may serve as an early marker of cancerous transformation due to the changes in its activity as early as at the cancer initiation process. However, the increase in expression of MMP9 encoding gene, the decrease in transcription activity of MMP28, and the increase in transcription activity of TIMP1 gene may be a confirmation of the existence of cancer. Unfortunately, even though it seems that differences in the expression of these genes exist, as shown by the heat map, when LSC is compared with HSC, these differences do not demonstrate statistical significance. Consequently, they cannot be used in differentiating the stage of CRC. In turn, MMP9 seems to take part in a number of key stages of CRC progression, from carcinogenesis, angiogenesis, and the development of primary tumor, to metastases in distant organs. Using immunochemistry techniques, Zeng et al. [28] demonstrated that MMP9 expression correlates with metastases in human CRC, and it is also related to an increase in TIMP1 activity. Furthermore, an increase in mRNA of MMP9 in CRC tissue compared to the concentration in a healthy intestinal tissue increased with disease progression, being a negative prognostic factor for disease-free survival and overall survival [29]. TIMP1 levels in serum and TIMP1 expression in cancerous tissues are associated with poor clinical outcomes in many types of cancer [29,30]. Although a study demonstrated an increase in the activity of genes encoding MMP9 and TIMP1 taking part in tissue reconstruction and regulating the transformation and progression of cancerous cells, no close connection was observed in regulation between MMP9 and TIMP-1 in CRC [30]. In our study, MMP9 and TIMP1 in CRC were overexpressed compared to in healthy tissues, which is similar to the results obtained by Walkiewicz et al. [31]. Their study demonstrated high levels of expression of MMP9 and TIMP1 genes, particularly in the tissues from an early stage of CRC. MMP9 expression was also increased in higher stages of CRC, but at a much lower level, which may also suggest a role of these proteins in the process of initiation of tumor progression.
MMP28 is the least well known metalloproteinase. MMP28 is overexpressed during progression of gastric carcinoma and contributes to tumor cell invasion and metastasis [32]. However, the expression of MMP28 is decreased in CRC. Bister et al. [33] using immunohistochemical analyses demonstrated that MMP28 is downregulated during malignant transformation of the colon, which suggests the protective role of MMP28 in this type of cancer. MMP28, as in the case of MMP9, is associated with the activity of the cadherin group, which is the major mediator of cell adhesion. This relationship is very complex in CRC, which results from the changeable activity of genes encoding these mediators [34]. Xiao et al. [35] demonstrated that miRNA-144 (suppressed by the expression of GSPT1) may be the connecting element to regulate the expression of Survivin, thereby inhibiting MMP28.
Conclusions
An increase in MMP9 gene expression may constitute an additional marker in CRC diagnosis, whereas the increase in MMP9 and TIMP1 expression and the decrease in MMP28 in the samples from the large intestine may confirm the existence of a cancerous change, but it does not indicate the stage of CRC. The examined particles may be a possible target in molecular therapy in CRC. However, further basic and clinical studies are needed to confirm the present results.
Footnotes
Source of support: Departmental sources
Conflict of interest
There are no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations. No grants were received for the study and it was supported from the private sources of the authors.
References
- 1.Adachi Y, Yamamoto H, Itoh F, et al. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut. 1999;45:252–58. doi: 10.1136/gut.45.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek MJ. Prognostic role of MMPs in colorectal cancer. J Korean Soc Coloproctol. 2011;27(3):105–6. doi: 10.3393/jksc.2011.27.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Śliwowska I, Kopczyński Z. Matrix metalloproteinases – biochemical characteristics and clinical value determination in breast cancer patients. Contemporary Oncology. 2005;9:327–35. [Google Scholar]
- 4.Worthley DL, Giraud AS, Wang TC. The extracellular matrix in digestive cancer. Cancer Microenvironment. 2010;3:177–85. doi: 10.1007/s12307-010-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagase H, Woessner JF., Jr Matrix Metalloproteinases. J Biol Chem. 1999;31:21491–94. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 6.Groblewska M, Mroczko B, Szmitkowski M. The role of selected matrix metalloproteinases and their inhibitors in colorectal cancer development. Postepy Hig Med Dosw. 2010;64:22–30. [PubMed] [Google Scholar]
- 7.Lian J, Ma L, Yang J, et al. Aberrant gene expression profile of unaffected colon mucosa from patients with unifocal colon polyp. Med Sci Monit. 2015;21:3935–40. doi: 10.12659/MSM.895576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg M, Søreide K. Genetic and epigenetic traits as biomarkers in colorectal cancer. Int J Mol Sci. 2011;1:9426–39. doi: 10.3390/ijms12129426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–47. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 10.Migheli F, Migliore L. Epigenetics of colorectal cancer. Clin Genet. 2012;81(4):312–18. doi: 10.1111/j.1399-0004.2011.01829.x. [DOI] [PubMed] [Google Scholar]
- 11.Lind GE, Danielsen SA, Ahlquist T, et al. Identification of an epigenetic biomarker panel with high sensitivity and specificity for colorectal cancer adenomas. Mol Cancer. 2011;21(10):85. doi: 10.1186/1476-4598-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wideł MS, Wideł M. Mechanisms of metastasis and molecular markers of malignant tumor progression. Colorectal cancer. Postepy Hig Med Dosw. 2006;60:453–70. [PubMed] [Google Scholar]
- 13.Zhang S, Li L, Lin JY, et al. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. 2003;9(5):899–904. doi: 10.3748/wjg.v9.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Oliveira J. Primary colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2009;20(4):49–50. doi: 10.1093/annonc/mdp126. [DOI] [PubMed] [Google Scholar]
- 15.Derwinger K, Gustavsson B. A study of aspects on gender and prognosis in synchronous colorectal cancer; Clinical medicine insights. Oncology. 2011;5:259–64. doi: 10.4137/CMO.S7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst NG, Stocken DD, Wilson S, et al. Elevated serum matrix metalloproteinase 9 (MMP-9) concentration predicts the presence of colorectal neoplasia in symptomatic patients. Br J Cancer. 2007;97:971–77. doi: 10.1038/sj.bjc.6603958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilska M, Roberts PJ, Collan YU, et al. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714–23. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- 18.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 19.Bister VO, Salmela MT, Karjalainen-Lindsberg ML, et al. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig Dis Sci. 2004;49(4):653–61. doi: 10.1023/b:ddas.0000026314.12474.17. [DOI] [PubMed] [Google Scholar]
- 20.Lubbe WJ, Zuzga DS, Zhou Z, et al. Guanylyl cyclase C prevents colon cancer metastasis by regulating tumor epithelial cell matrixmetalloproteinase-9. Cancer Res. 2009;69(8):3529–36. doi: 10.1158/0008-5472.CAN-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masaki T, Matsuoka H, Sugiyama M, et al. Matrilysin (MMP-7) as a significant determinant of malignant potential of early invasive colorectal carcinomas. Br J Cancer. 2001;84(10):1317–21. doi: 10.1054/bjoc.2001.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koskensalo S, Mrena J, Wiksten JP, et al. MMP-7 overexpression is an independent prognostic marker in gastric cancer. Tumour Biol. 2010;31(3):149–55. doi: 10.1007/s13277-010-0020-1. [DOI] [PubMed] [Google Scholar]
- 23.Hong WS, Kang YK, Lee B, et al. Matrix metalloproteinase-2 and -7 expression in colorectal cancer. J Korean Soc Coloproctol. 2011;27(3):133–39. doi: 10.3393/jksc.2011.27.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Łukaszewicz-Zając M, Mroczko B, Szmitkowski M. The significance of metalloproteinases and their inhibitors in gastric cancer. Postepy Hig Med Dosw. 2009;63:258–65. [PubMed] [Google Scholar]
- 25.Vihinen P, Kähäri VM. Matrixmetalloproteinases in cancer: Prognostic markers and therapeutic targets. Int J Cancer. 2002;99(2):157–66. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 26.Heslin MJ, Yan J, Johnson MR, et al. Role of matrix metalloproteinases in colorectal carcinogenesis. Ann Surg. 2001;233(6):786–92. doi: 10.1097/00000658-200106000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murnane MJ, Cai J, Shuja S, et al. Active MMP 2 effectively identifies the presence of colorectal cancer. Int J Cancer. 2009;125(12):2893–902. doi: 10.1002/ijc.24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng ZS, Guillem JG. Distinct pattern of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 mRNA expression in human colorectal cancer and liver metastases. Br J Cancer. 1995;72:575–82. doi: 10.1038/bjc.1995.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20(5):749–55. doi: 10.1093/carcin/20.5.749. [DOI] [PubMed] [Google Scholar]
- 30.Holten-Andersen MN, Nielsen HJ, Sørensen S. Tissue inhibitor of metalloproteinases-1 in the postoperative monitoring of colorectal cancer. Eur J Cancer. 2006;42(12):1889–96. doi: 10.1016/j.ejca.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 31.Walkiewicz K, Kozieł P, Bednarczyk M, et al. Expression of migration-related genes in human colorectal cancer and activity of a disintegrin and metalloproteinase 17. Biomed Res Int. 2016;2016:8208904. doi: 10.1155/2016/8208904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jian P, Yanfang T, Zhuan Z, et al. MMP28 (epilysin) as a novel promoter of invasion and metastasis in gastric cancer. BMC Cancer. 2011;11:200. doi: 10.1186/1471-2407-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bister VO, Salmela MT, Karjalainen-Lindsberg ML, et al. Differential expression of three matrix metalloproteinases, MMP-19, MMP-26, and MMP-28, in normal and inflamed intestine and colon cancer. Dig Dis Sci. 2004;49(4):653–61. doi: 10.1023/b:ddas.0000026314.12474.17. [DOI] [PubMed] [Google Scholar]
- 34.Lorenc Z, Opiłka MN, Kruszniewska-Rajs C, et al. Expression level of genes coding for cell adhesion molecules of cadherin group in colorectal cancer patients. Med Sci Monit. 2015;21(13):2031–40. doi: 10.12659/MSM.893610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao R, Li C, Chai B. miRNA-144 suppresses proliferation and migration of colorectal cancer cells through GSPT1. Biomed Pharmacother. 2015;74:138–44. doi: 10.1016/j.biopha.2015.08.006. [DOI] [PubMed] [Google Scholar]