Abstract
Reductions of cerebral blood flow and gray matter structure have been implicated in early pathogenesis of Alzheimer’s disease, potentially providing complementary information. The present study evaluated regional patterns of cerebral hypoperfusion and atrophy in patients with mild cognitive impairment and healthy older adults. In each participant, cerebral perfusion and gray matter structure were extracted within selected brain regions vulnerable to Alzheimer’s disease using magnetic resonance imaging. Measures were compared between diagnostic groups with/without adjustment for covariates. In mild cognitive impairment patients, cerebral blood flow was significantly reduced in comparison with healthy controls in temporo-parietal regions and the basal ganglia in the absence of local gray matter atrophy. By contrast, gray matter structure was significantly reduced in the hippocampus in the absence of local hypoperfusion. Both, cerebral perfusion and gray matter structure were significantly reduced in the entorhinal and isthmus cingulate cortex in mild cognitive impairment patients compared with healthy older adults. Our results demonstrated partly divergent patterns of temporo-parietal hypoperfusion and medial-temporal atrophy in mild cognitive impairment patients, potentially indicating biomarker sensitivity to dissociable pathological mechanisms. The findings support applicability of cerebral perfusion and gray matter structure as complementary magnetic resonance imaging-based biomarkers in early Alzheimer’s disease detection, a hypothesis to be further evaluated in longitudinal studies.
Keywords: Cerebral blood flow, cerebral blood volume, magnetic resonance imaging, mild cognitive impairment, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is mainly characterized by neuropathological events of amyloid-beta (Aβ) deposition and neurofibrillary tangles,1 which can be detected in early disease stages.2 In addition, vascular pathology has been implicated in early AD pathogenesis.3 Brain vasculature and capillaries change morphology, disrupting normal blood flow and homeostasis between blood supply and neuronal activity, which can lead to neuronal dysfunction.4 Such changes could be associated with vascular risk factors, but results from studies in mouse models of AD and humans also suggest links between Aβ and cerebrovascular deficiencies.5
Reductions of cerebral perfusion are well-documented in patients with AD and mild cognitive impairment (MCI)6 and could become a preclinical biomarker of early disease detection and progression.7,8 Reduced cerebral perfusion and hypometabolism, seen at the MCI stage, seem to affect parietal association areas first, before spreading to temporal regions.6,8,9 By contrast, gray matter atrophy is predominant in medial-temporal regions.10–12 Hence, there appears to be an early dissociation between temporo-parietal hypoperfusion and medial-temporal atrophy.13 Yet, the possibility that magnetic resonance imaging (MRI)-based biomarkers of gray matter structure and cerebral perfusion may provide complementary information in early disease detection remains unclear.
Perfusion imaging sequences can be used to extract different parameters, the most common being cerebral blood flow (CBF) and cerebral blood volume (CBV), indicating the rate of blood delivery to the brain tissue and the fraction occupied by blood, respectively.14 The two measures can be derived simultaneously using dynamic-susceptibility contrast MRI (DSC-MRI).15 The DSC-MRI relies on the passage of a gadolinium-based contrast agent injected intravenously that will flow through the brain vasculature. Applied to MCI patients, a previous study reported changes in CBF prior to CBV in AD progression,11 proposing the former as a more sensitive indicator of perfusion abnormalities.
The present study aimed to systematically evaluate regional reductions of cerebral perfusion (CBV and CBF) using DSC-MRI, and gray matter structure (volume and thickness) using T1-weighted MRI in prodromal AD within areas most affected by the disease. The AD-related regions-of-interest (ROIs) were selected based on previous neuroimaging studies using amyloid, metabolic, structural, and perfusion biomarkers.11,16–18 The MRI-based measures were extracted in the ROIs and compared between MCI patients and healthy control (HC) older adults. Specifically, our first aim was to assess divergence and convergence of regional patterns of hypoperfusion and gray matter atrophy in AD-related regions. Our second aim was to replicate previous findings of CBF being a more sensitive biomarker than CBV to detect perfusion abnormalities in MCI patients.
Material and methods
Participants
Participants (HC and MCI patients) were aged between 50 and 80 years and were native German speakers. The MCI patients were recruited from the memory clinic of the Department of Neurology at the Charité University Hospital, and a Neurology specialist practice in Berlin, Germany. Patients were diagnosed by a board-certified neurologist of the Charité using current criteria of amnestic MCI,19,20 which are as follows: a subjective cognitive complaint and an objective memory impairment measured using standardized subtests of episodic memory (performance at least 1.5 SD below age- and education-specific norm) on the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-Plus) test battery,21 relatively preserved general cognitive functions measured using the Mini-Mental State Evaluation (MMSE),22 no impairment in activities of daily living, and no sign of dementia in accordance with the NINCDS-ADRDA criteria for probable AD.23,24 The MCI sample included single domain amnestic MCI (with isolated episodic memory impairments) and multiple domain amnestic MCI (with episodic memory impairments and deficits in one or more other cognitive domains). The HC older adults were recruited from the general community via advertisement. They had no subjective cognitive complaints, scored at least 26 on the MMSE, and underwent a standardized medical interview and neurological examination.25
Exclusion criteria for both diagnostic groups comprised severe medical, neurological, or psychiatric disease, major brain pathologies identified on MRI scans like territorial stroke or brain tumor, a body mass index (BMI) <18 kg/m2 or >35 kg/m2, and a score above 20 on the Beck’s depression inventory (BDI).26 For the present analysis, only apolipoprotein E (APOE) ɛ4 non-carriers were included in the HC group, to minimize the possibility of AD pathology associated with APOE ɛ4-related genetic risk.17 In the MCI group, APOE ɛ2 carriers were excluded, given the protective role of APOE ɛ2 in AD pathology27 and AD risk.28
The study was approved by the Ethics Committee of the Charité University Hospital, Berlin, Germany and was in accordance with the Declaration of Helsinki. All subjects gave informed written consent before participating in the study.
Neuropsychological testing
Participants were tested for verbal memory using the German version of the auditory verbal learning task (AVLT).29 Scores of the sum and delayed recall AVLT subtests were provided for comparison of HC and MCI groups.
Vascular risk factors
A number of vascular risk factors were selected for sample description. Venous blood samples after overnight fasting were taken to assess markers of short-term fasting glucose levels, long-term glycated hemoglobin (HbA1c), and insulin levels.25 Mean systolic blood pressure was calculated from two measurements taken in sitting position. Lipid parameters were assessed using total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triacylglycerides. In addition, we computed the validated Framingham risk index for cardiovascular disease (CVD, as a combined measure of vascular risk (Appendix e-1).30
Genotyping
The APOE genotype was determined at the laboratory of Prof. Dr. Dan Rujescu (University of Halle, Halle, Germany) following procedures as previously described.31 MCI patients were further dichotomized as APOE ɛ4 carriers (APOE ɛ3/ɛ4 and APOE ɛ4/ɛ4) or non-carriers (APOE ɛ3/ɛ3).
MRI acquisition
Scans were acquired using a 3-Tesla Magnetom Trio (Tim Trio; Siemens AG, Erlangen, Germany) at two different sites. The majority of HC individuals (n = 98) underwent MRI at the Charité Campus Benjamin Franklin; all other participants were examined at the Charité Campus Mitte using identical imaging protocols at both sites.
The high-resolution T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence was acquired with the following parameters: repetition time (TR) = 1900 ms; echo time (TE) = 2.52 ms; 192 sagittal slices; voxel size = 1.0 × 1.0 × 1.0 mm3; flip angle = 9°. For DSC-MRI, participants received 5 mL of the contrast agent Gadobutrol (Gadovist; 1 M; Bayer Schering Pharma AG, Berlin, Germany) followed by 20 mL of saline by a power injector (Spectris; Medrad Inc., Warrendale, PA, USA), both at a rate of 5 mL/s. The DSC T2*-weighted sequence (TR = 1390 ms; TE = 29 ms; 21 axial slices, voxel size = 1.8 × 1.8 × 5.0 mm3; interslice gap = 0.5 mm; flip angle = 60°) yielded perfusion images with high contrast and signal intensity.
MRI processing
T1-weighted image processing
For cortical thickness estimation, T1-weighted images were processed using FreeSurfer (version 5.2, http://surfer.nmr.mgh.harvard.edu).32 In an automated procedure, each T1 scan was bias-field corrected, intensity normalized, and skull stripped using a watershed algorithm. Cortical thickness surface maps were derived by defining the white/gray matter boundary, reconstructing the white/gray matter border surfaces, and estimating pial surfaces.
Perfusion image processing
Perfusion scans were processed using the image analysis software PMA (Perfusion Mismatch Analyzer, ASIST-Japan, version 3.4.0.6, http://asist.umin.jp/index-e.htm copyright). Arterial input functions (AIFs) used for processing the perfusion images were defined in an automated procedure. The automatic algorithm yielded between 7 and 10 AIFs located in both hemispheres. In the few cases in which an automated AIF was located low in the brainstem or in the superior brain slices, it was removed due to possible artifacts and replaced by an AIF defined manually in a major artery. Using the AIFs and standard singular value decomposition (sSVD) with a threshold of 0.2 and a 3 × 3 uniform smoothing kernel, CBV and CBF maps33 were generated through voxel-by-voxel based calculations.
Extraction of MRI measures
Our analyses focused on ROIs preferentially affected in AD. The present ROI-based approach allowed for head-to-head comparisons of perfusion and structural MRI measures between diagnostic groups in the same brain region, as defined in the native space images.
Definition of regions-of-interest
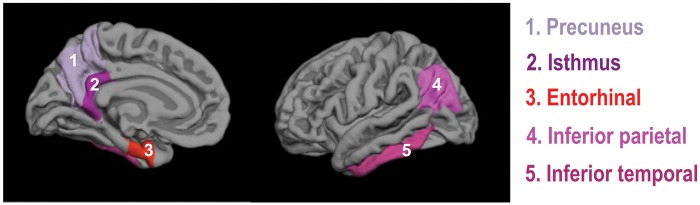
Delineation of cortical and subcortical ROIs from the T1-weighted images was done using the Freesurfer automated ROI parcellation and segmentation procedures based on the Desikan atlas.34 The following AD-related ROIs (Figure 1) were included: precuneus, inferior parietal gyrus, inferior temporal gyrus, isthmus portion of the cingulate gyrus, entorhinal gyrus, hippocampus, as well as basal ganglia (merged from caudate, putamen, and pallidus).
Figure 1.
Representation of the AD-related regions-of-interest. Three-dimensional representation of cortical AD-related regions-of-interest (ROIs). The ROIs are projected on the gray matter surface of the left hemisphere. Subcortical ROIs of the hippocampus and basal ganglia are not shown.
Extraction of cerebral perfusion measures
The FSL software package (version 4.1, www.fmrib.ox.ac.uk/fsl) was used to extract mean perfusion values from the CBV and CBF images for each ROI. The T1-weighted image and the ROIs were first co-registered to the native-space perfusion maps. Using the transformation matrix, the AD-related ROIs were then superimposed on the perfusion maps and the mean value of the non-zero voxels was extracted from the CBF and CBV maps. Perfusion values were normalized using the gray matter cerebellum, referred to as relative (r)CBV and rCBF values. We used the cerebellum based on the fact that perfusion was statistically non differentiable between diagnostic groups in this region (data not shown). This region is typically less affected than cortical areas in AD35 and serves as an established reference region in perfusion imaging.36 A threshold of the 90th percentile was applied before extracting the mean CBF and CBV values in the cerebellum to exclude voxels representing blood vessels or non-physiological artifacts.36
Extraction of gray matter structural measures
Gray matter volume and thickness (when available) were assessed in each ROI using native-space T1-weighted images. Both measures were included since they represent divergent properties. Volume is influenced by both surface area and cortical thickness, but appears to be more closely related to surface area.37 For each ROI, volume was determined using the FSL software and the non-zero voxels combined for the left and right hemisphere. Each volume measure was adjusted by total intracranial volume (ICV), a proxy of head size, via linear regression between gray matter volumes and ICV, i.e. residual approach.38 The ICV-adjusted (residual) volumes were used for statistical analyses. Thickness was extracted for each hemisphere and each given cortical ROI using the FreeSurfer software and combined for left and right hemisphere.
In addition, white matter lesion (WML) volumes, an indicator of cerebrovascular disease, were estimated applying the subcortical FreeSurfer segmentation.39 Acceptable reliability of the procedure using T1-weighted images has been previously demonstrated,40,41 even though it appears to underestimate WML volumes. WML volumes were rank-transformed to address the skewed distribution and adjusted for head size using the residual approach.
Statistical analysis
Statistical analyses were performed using SPSS 20 (IBM). The significance level was set to α = 0.05. Sample characteristics were compared using independent t-tests for parametric variables, chi-square tests for binary variables, and non-parametric (independent samples Mann–Whitney U test) for continuous variables if assumption of normality was not met. The initial multivariate analyses of variance (MANOVA) were performed for each ROI with diagnostic group (HC, MCI) as independent variable and all multimodal MRI measures (rCBV, rCBF, gray matter volume, and cortical thickness) as dependent variables. The multimodal MRI measures were z-transformed to express the same unit. Subsequently, we examined whether relationships between diagnostic group and cerebral perfusion were accounted for by local atrophy (and vice versa) by entering the respective MRI measure as covariate into the multivariate analyses. Results of these MANOVA models are presented separately for perfusion and structural measures in the result section.
Two additional analysis procedures were carried out. First, multivariate analyses of covariance (MANCOVA) models were computed including diagnostic group and covariates of age, sex, depression scores, and WML volumes due to significant group differences or potentially confounding effects. In separate MANCOVA models, we introduced only the Framingham CVD index, as a combined risk factor (supplementary material). Second, we examined effects of genetic risk on the multimodal MRI measures in MCI APOE4 carriers and non-carriers. Exploratory MANOVA models were conducted either including each MCI carrier group compared with HCs or MCI APOE4 carriers versus non-carriers as independent variables and the multimodal MRI measures as dependent variables (supplementary material).
Note, standardized beta coefficients were obtained from all MANOVA/MANCOVA models to provide a standardized effect size for the effect of diagnostic group on each MRI measure. Correction for multiple comparisons was done using false discovery rate (FDR) correction (corrected for the number of tests for each MRI measure).42
Results
Participant characteristics
Participant characteristics are summarized in Table 1. The study included 108 HCs and 43 MCI patients. Diagnostic groups did not differ significantly for sex and education (p’s > 0.2). MCI patients were significantly older (p = 0.017), showed higher depression scores (p = 0.005) and performed significantly worse on the MMSE and the AVLT memory subtests (p’s < 0.05). The measure of cerebrovascular disease (WML volume) was increased in MCI (p = 0.034), while no significant differences were seen in vascular risk factors (p’s > 0.05) and the Framingham CVD risk index (p > 0.05, Appendix e-1).
Table 1.
Participant characteristics.
| Group | HC (n = 108) | MCI (n = 43) | p value | ||
|---|---|---|---|---|---|
| Male:Female, No. | 56:52 | 23:20 | 1.000 | ||
| Age (years) | 63.0 (7.2) | 57.0–69.0 | 66.3 (8.2) | 60.0–74.0 | 0.017* |
| Education (years) | 16.2 (3.1) | 14.0–18.0 | 15.4 (3.5) | 13.0–18.0 | 0.177 |
| BDI | 5.8 (4.5) n = 105 | 2.0–9.0 | 8.8 (6.1) n = 42 | 4.0–13.0 | 0.005* |
| APOE genotype, No. | |||||
| 2/2 | 1 | # | |||
| 2/3 | 24 | # | |||
| 2/4 | 0 | # | |||
| 3/3 | 83 | 20 | |||
| 3/4 | # | 18 | |||
| 4/4 | # | 5 | |||
| Vascular factors | |||||
| Glucose (mg/dl) | 91.8 (8.7) | 86.5–97.0 | 93.8 (8.5) | 89.0–98.0 | 0.190 |
| HbA1c (%) | 39.8 (3.1) | 38.0–42.0 | 38.8 (2.5) | 37.0–41.0 | 0.053 |
| Insulin (mU) | 8.8 (4.2) | 6.3–10.0 | 9.3 (12.3) | 5.3–9.3 | 0.739 |
| Systolic blood pressure (mm Hg) | 137.0 (14.9) n = 107 | 125.5–145.5 | 140.0 (18.2) n = 41 | 126.0–151.0 | 0.310 |
| Total cholesterol (mg/dl) | 215.9 (33.0) | 189.5–238.0 | 221.7 (35.3) | 200.0–240.0 | 0.338 |
| HDL (mg/dl) | 63.4 (16.7) | 52.5–72.0 | 65.6 (13.2) | 54.0–72.0 | 0.446 |
| LDL (mg/dl) | 131.5 (30.3) | 105.0–151.0 | 140.1 (30.7) | 120.0–165.0 | 0.122 |
| Triglyceride (mg/dl) | 110.1 (42.7) | 78.5–136.0 | 104.7 (56.8) | 65.0–132.0 | 0.523 |
| WML volume (mm3) | 1925.1 (1588.9) | 1042.0–2310.1 | 3341.4 (3876.8) | 1193.6–3412.0 | 0.034* |
| Cognition | |||||
| MMSE | 29.1 (1.1) | 29.0–30.0 | 28.0 (1.8) | 27.0–29.0 | 0.000* |
| AVLT, sum | 52.1 (9.9) | 44.0–59.0 | 44.0 (11.5) | 36.0–52.0 | 0.000* |
| AVLT, delayed recall | 10.3 (3.5) | 8.0–13.0 | 7.4 (4.3) | 4.0–11.0 | 0.000* |
Mean (standard deviation) and interquartile range is provided for each variable, if available. P-values from two-tailed independent sample t-tests, chi-square test (for sex), and Mann–Whitney U test (for WML volumes) are reported. Asterisks indicate significance at p < 0.05, # excluded data.
APOE: apolipoprotein E; BDI: Beck’s depression inventory; Hbac1: glycosylated hemoglobin; HC: healthy control people; MCI: mild cognitive impairment patients; MMSE: Mini-mental state examination; WML: white matter lesion; AVLT: auditory verbal learning and memory test.
Perfusion differences between MCI and HC
With regard to perfusion measures, MANOVA models showed significantly reduced perfusion (p’s < 0.05, FDR corrected, Table 2, Figure 2) in MCI patients compared with HCs in the following AD-related ROIs: precuneus (rCBF), inferior parietal (rCBF), inferior temporal (rCBF), isthmus cingulate (rCBV, rCBF), entorhinal (rCBF), and the basal ganglia (rCBV, rCBF), but not in the hippocampus. Adjusting for local gray matter structure did not attenuate the observed effects (data not shown), except for the rCBF reduction in the entorhinal cortex (p = 0.071, adjusted for thickness).
Table 2.
Effects of diagnostic group on perfusion and structural MRI measures.
| ROI | Cerebral blood volume rCBV |
Cerebral blood flow rCBF |
Gray matter volume |
Cortical thickness |
||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Precuneus | −0.045 | 0.580 | −0.242 | 0.003* | −0.049 | 0.550 | −0.034 | 0.679 |
| Inferior parietal | −0.078 | 0.344 | −0.277 | 0.005* | −0.019 | 0.814 | −0.005 | 0.952 |
| Inferior temporal | −0.060 | 0.466 | −0.195 | 0.016* | −0.014 | 0.869 | 0.002 | 0.982 |
| Isthmus | −0.152 | 0.062 | −0.239 | 0.003* | −0.012 | 0.885 | −0.181 | 0.026 |
| Entorhinal | −0.025 | 0.760 | −0.181 | 0.027* | −0.105 | 0.200 | −0.254 | 0.002* |
| Hippocampus | 0.011 | 0.898 | 0.001 | 0.989 | −0.291 | 0.000* | NA | NA |
| Basal ganglia | −0.278 | 0.001* | −0.305 | 0.000* | 0.025 | 0.759 | NA | NA |
Effects of diagnostic group (HC = 108, MCI = 43) for cerebral blood volume, cerebral blood flow, gray matter volume, and thickness in regions-of-interest (ROIs). Standardized beta coefficients and p-values are reported. Asterisks indicate significance at p < 0.05, FDR corrected. Negative beta coefficients indicate hypoperfusion or gray matter atrophy in MCI patients.
Figure 2.
Effects of diagnostic group on perfusion and structural MRI measures. Effects of diagnostic group (HC, MCI) on cerebral blood volume (first bar), cerebral blood flow (second bar), gray matter volume (third bar), or cortical thickness (fourth bar) for AD-related regions-of-interest (ROIs). Negative standardized beta coefficients reflect biomarkers reductions in MCI patients; statistical significance is indicated (*p < 0.05, FDR corrected, unadjusted analyses; +p < 0.05, FDR corrected, when adjusting analyses for age, sex, depression scores, and WML volumes). Upper panel: ROIs with significant hypoperfusion in the absence of significant gray matter reduction in MCI patients. Lower panel: ROIs with significant gray matter reduction and the presence/absence of significant hypoperfusion in MCI patients.
Structural differences between MCI and HC
With regard to measures of gray matter structure (volume and thickness), MANOVA models showed significant gray matter reductions (p’s < 0.05, FDR corrected, Table 2, Figure 2) in MCI patients compared with HCs in the hippocampus (volume) and entorhinal region (thickness), but not in the precuneus, basal ganglia, inferior parietal, or the inferior temporal regions. At a more liberal statistical threshold (p < 0.05, uncorrected), significant cortical thinning was present in the isthmus cingulate (p = 0.026). Adjustment for local perfusion did not attenuate the observed effects on gray matter volume or thickness (data not shown).
Additional statistical analyses
Additional MANCOVA models were carried out, adjusting for sex, age, depression scores, and WML volumes. All significant effects of diagnostic group on the multimodal MRI measures were maintained (p’s < 0.05, FDR corrected, Table 3). Moreover, significant cortical thinning in the isthmus cingulate (p = 0.010) was detected in MCI patients compared with HCs. After adjustment for CVD risk scores significant effects of diagnostic group on the multimodal MRI measures were maintained (p’s < 0.05, FDR corrected, Table e-1).
Table 3.
Adjusted effects of diagnostic group on perfusion and structural MRI measures.
| ROI | Cerebral blood volume rCBV |
Cerebral blood flow rCBF |
Gray matter volume |
Cortical thickness |
||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| Precuneus | −0.092 | 0.297 | −0.276 | 0.001* | −0.052 | 0.548 | −0.017 | 0.842 |
| Inferior parietal | −0.154 | 0.081 | −0.272 | 0.001* | 0.049 | 0.562 | 0.021 | 0.804 |
| Inferior temporal | −0.104 | 0.244 | −0.269 | 0.002* | 0.013 | 0.880 | −0.011 | 0.897 |
| Isthmus | −0.182 | 0.037 | −0.285 | 0.001* | −0.068 | 0.436 | −0.221 | 0.010* |
| Entorhinal | −0.063 | 0.475 | −0.208 | 0.019* | −0.087 | 0.319 | −0.236 | 0.005* |
| Hippocampus | 0.032 | 0.718 | 0.017 | 0.845 | −0.212 | 0.005* | NA | NA |
| Basal ganglia | −0.247 | 0.003* | −0.294 | 0.000* | −0.038 | 0.661 | NA | NA |
Effects of diagnostic group (HC = 105, MCI = 42) for cerebral blood volume, cerebral blood flow, gray matter volume, and thickness in regions-of-interest (ROIs). Statistical models were adjusted for sex, age, depression, and WML volumes. Standardized beta coefficients and p-values are reported. Asterisks indicate significance at p < 0.05, FDR corrected. Negative beta coefficients indicate hypoperfusion or gray matter atrophy in MCI patients.
Exploratory MANOVA models examined differences in the multimodal MRI measures in MCI APOE ɛ4 carriers and non-carriers. Similar patterns of temporo-parietal hypoperfusion and medial-temporal atrophy were observed in both MCI groups in comparison with HCs. However, these effects were significant only in the MCI APOE ɛ4 carriers, but not in the MCI non-carriers (p’s < 0.05, FDR corrected, Tables e-2 and e-3, respectively). In MCI APOE ɛ4 non-carriers, we observed a significant rCBF reduction in the basal ganglia (p < 0.05, FDR corrected, Table e-3) and, at a more liberal statistical threshold, a reduction of hippocampal volume (p < 0.05, uncorrected, Table e-3). There were no significant differences in the multimodal MRI measures between APOE ɛ4 carriers and non-carriers in MCI patients (data not shown).
Discussion
The present study evaluated DSC-MRI perfusion measures (rCBF, rCBV) and gray matter structure (volume and thickness) in regions preferentially affected in AD in a large sample of MCI patients and healthy older adults. Our results indicated a dissociation in regional patterns of hypoperfusion and atrophy in MCI patients, with pronounced hypoperfusion without atrophy in temporo-parietal and basal ganglia areas, and atrophy without hypoperfusion in the hippocampus. Moreover, we confirmed superior sensitivity of rCBF over rCBV to detect perfusion alterations in MCI patients. These findings point toward hypoperfusion as an early event in temporo-parietal regions, detectable even in the absence of local atrophy in these regions. Our results converge with models proposing that cerebral perfusion may be affected first in certain areas,7 however, longitudinal studies will be needed to answer this question. Overall, the present study suggests an added value of CBF imaging over structural imaging and supports applicability of cerebral perfusion and gray matter structure as complementary MRI-based biomarkers in early AD detection.
Perfusion differences between MCI and HC
Statistical analyses detected extended cerebral hypoperfusion, predominantly rCBF reductions, in MCI patients compared with HCs in areas typically affected in AD, except the hippocampus. Such a result converges with previous findings of aberrant perfusion in MCI patients within temporal and parietal regions10,43–45 and the basal ganglia.44 The observed pattern of perfusion abnormalities found in the MCI patients co-localizes with brain hypometabolism, commonly measured using FDG-PET, in prodromal and clinical AD.46,47 Importantly though, no significant local atrophy was found in the precuneus, inferior temporal and parietal regions, as well as the basal ganglia. Temporo-parietal rCBF reductions also remained significant after correcting for local gray matter structure, suggesting associations between cerebral perfusion and cognitive status above and beyond the contribution of gray matter integrity. The result may thus imply an initial dysfunction of neurovascular mechanisms in early AD48 in temporo-parietal and basal ganglia regions.
Our findings further confirm superior sensitivity of local rCBF rather than rCBV to detect hypoperfusion in MCI patients, convergent with a previous study.11 Regional rCBV reductions in MCI patients were significant only in the basal ganglia, with lower effect sizes (beta coefficients) compared with rCBF abnormalities. Both blood flow and volume abnormalities were previously found in AD converters,11,49 highlighting their mutual sensitivity to pathogenic mechanisms. However, rCBF may be more affected by early AD-related pathological mechanisms than rCBV.
Structural differences between MCI and HC
Significant gray matter reductions were seen in the MCI patients compared with HCs in the hippocampus, the entorhinal cortex and the isthmus cingulate (after covariate adjustment), in line with existing findings.50 For the entorhinal cortex, atrophy remained significant after adjustment for local hypoperfusion, while, for the hippocampus, atrophy was found in absence of significant perfusion alterations. Previous findings indicate that gray matter atrophy exceeds mild or moderate metabolic abnormalities in this brain region in AD,47 similar to our results. An earlier study noticed hypoperfusion in the hippocampus of MCI patients51; however, the authors did not examine the degree of local gray matter atrophy. Our findings support observations that gray matter atrophy is localized to medial-temporal regions in MCI patients, where it appears to occur in the absence or independent of local hypoperfusion.
Potential pathological mechanisms underlying hypoperfusion in MCI patients
The observed divergence of temporo-parietal hypoperfusion and medial-temporal atrophy in MCI patients could reflect involvement of partly different pathological mechanisms. Previous findings suggest that CBF reductions may be linked to Aβ pathology, particularly, in early stages of the disease.16 Comparing MCI patients with increased (cortex-wide) Aβ deposition to Aβ-free healthy older individuals, hypoperfusion was found in the patient group in inferior temporal and parietal areas,16 comparable with our findings. Similarly, in a mixed sample of healthy older adults and MCI patients, global Aβ deposition was recently found to correlate with posterior parietal hypometabolism.52 However, no significant CBF reduction was reported in a group of Aβ-positive/tau-negative non-demented older people,53 highlighting the possibility that Aβ alone might not be sufficient to cause cerebral hypoperfusion. In our MCI cohort, CBF alterations appeared to be mainly driven by APOE e4 carriers, although we failed to detect significant perfusion differences between APOE e4 carriers and non-carriers in this group. Nevertheless, the observation converges with a previous report,54 which further indicated that APOE e4 mainly contributed to reductions in global CBF via mechanism that were not limited to Aβ deposition. Medial-temporal atrophy, as seen in the hippocampus and entorhinal regions, has been linked to tau pathology in AD patients,50,55 thought to emerge through at least partly independent pathological pathways.56 Given that local pathological processes may affect distant functionally connected brain regions, it is also possible that medial-temporal gray matter damage impairs temporo-parietal perfusion.57 Finally, multimodal deficits (hypoperfusion and atrophy) as observed in entorhinal and isthmus cingulate regions, might point toward an early intersection of pathological mechanisms in these regions.58
Cerebrovascular pathology and vascular risk factors may also be involved in neurovascular dysfunction and atrophy.3 In the present study, we could not identify a major role of vascular pathology. There were no significant differences between MCI patients and HCs in a number of vascular risk factors and adjustment for increased WML load found in MCI patients or the Framingham CVD risk index did not modify our results. It therefore appears that vascular pathology was not the major factor underlying alterations in perfusion and gray matter structure as shown by the MCI patients. However, pathological pathways potentially related our findings will need to be clarified in future longitudinal studies that combine MRI-based and PET-based imaging markers of neuropathology.
Limitations and strengths
Several limitations should be considered when interpreting our findings. First, spatial resolution of the perfusion imaging is rather low, yielding imperfect registration on the perfusion maps. To minimize the impact of this technical limitation, images were inspected visually after ROIs registration on the perfusion map. Although prone to perfusion changes44 and Aβ accumulation,59 frontal areas were excluded, due to distortions in these regions often seen in DSC-imaging.60 Second, imaging data were acquired on the same scanner model, but at two different locations. Given that inter-scanner differences have substantially less impact than the effect of disease in AD61 and that other MRI-specific factors such as scanner platforms and field-strengths introduce more measurement bias,62 it is unlikely that scanner location confounded our results. Third, MCI patients had a higher age and subclinical depression scores compared with the HC group. Still, adjustments for these parameters did not modify our results. Strengths of the study encompass the large and well-characterized sample of HC individuals and MCI patients, with gadolinium-based assessment of cerebral perfusion in all participants.
Conclusion
Our results demonstrate divergent patterns of hypoperfusion and gray matter atrophy in MCI patients: reduced perfusion is present mainly in temporo-parietal and basal ganglia regions, while reductions of gray matter structure occur mainly in medial-temporal regions. Our findings support applicability of perfusion- and structure-based MRI measures as complementary biomarkers in early AD detection, which needs further evaluation in future longitudinal studies.
Supplementary Material

Acknowledgments
The authors thank Dr. Lucia Kerti (NeuroCure Clinical Research Center and Department of Neurology, Charité - Universitätsmedizin Berlin, Germany) and Dr. Sylvia Villeneuve (Centre for Studies on Prevention of Alzheimer’s Disease, Douglas Mental Health University Institute, Montreal, Canada) for help in data collection, analysis or manuscript drafting, respectively.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research work was supported by grants from the Deutsche Forschungsgemeinschaft (Fl 379-8/1, Fl 379-10/1, Fl 379-11/1, and DFG-Exc 257), the Bundesministerium für Bildung und Forschung (FKZ 0315673A, 01EO0801, 01GQ1424A, and 01GQ1420B), the Hans Gerhard Creutzfeldt scholarship, and the NeuroCure Female postdoctoral research fellowship.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Agnes Flöel has obtained consulting fees from Schwabe, Honoraria for oral presentations from Novartis, Böhringer-Ingelheim, Schwabe, and Souvenaid, as well as royalties from the book “Alzheimer-Unabwendbares Schicksal?”
Author's contributions
Miranka Wirth: Analysis or interpretation of data, drafting/revising the manuscript for content, final approval. Alexa Pichet Binette: Analysis or interpretation of data, drafting/revising the manuscript for content, final approval. Peter Brunecker: Analysis or interpretation of data, drafting/revising the manuscript for content, final approval. Theresa Köbe: Analysis or interpretation of data, drafting/revising the manuscript for content, final approval. Veronica Witte: Study concept or design, drafting/revising the manuscript for content, final approval. Agnes Flöel: Study concept or design, drafting/revising the manuscript for content, final approval.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011; 1: a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010; 9: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010; 120: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006; 100: 328–335. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C. Cerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer's dementia. Cell Mol Neurobiol 2003; 23: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin BP, Nair VA, Meier TB, et al. Effects of hypoperfusion in Alzheimer's disease. J Alzheimer's Dis: JAD 2011; 26(Suppl 3): 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. J Alzheimer's Dis: JAD 2014; 42: S411–S419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirao K, Ohnishi T, Hirata Y, et al. The prediction of rapid conversion to Alzheimer's disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage 2005; 28: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer's disease. Ann Nucl Med 2001; 15: 85–92. [DOI] [PubMed] [Google Scholar]
- 10.Luckhaus C, Cohnen M, Fluss MO, et al. The relation of regional cerebral perfusion and atrophy in mild cognitive impairment (MCI) and early Alzheimer's dementia. Psychiatry Res 2010; 183: 44–51. [DOI] [PubMed] [Google Scholar]
- 11.Lacalle-Aurioles M, Mateos-Perez JM, Guzman-De-Villoria JA, et al. Cerebral blood flow is an earlier indicator of perfusion abnormalities than cerebral blood volume in Alzheimer's disease. J Cereb Blood Flow Metab 2014; 34: 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol 1999; 246: 477–485. [DOI] [PubMed] [Google Scholar]
- 13.Alsop DC, Casement M, de Bazelaire C, et al. Hippocampal hyperperfusion in Alzheimer's disease. Neuroimage 2008; 42: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calamante F, Thomas DL, Pell GS, et al. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab 1999; 19: 701–735. [DOI] [PubMed] [Google Scholar]
- 15.Essig M, Shiroishi MS, Nguyen TB, et al. Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol 2013; 200: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson N, Tosun D, Insel PS, et al. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer's disease and mild cognitive impairment. Brain 2014; 137(Pt 5): 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirth M, Madison CM, Rabinovici GD, et al. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci 2013; 33: 5553–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr 2008; 13: 45–53. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56: 303–308. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M, Folstein S, McHugh P. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939–944. [DOI] [PubMed] [Google Scholar]
- 24.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerti L, Witte AV, Winkler A, et al. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 2013; 81: 1746–1752. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Prob Pharmacopsychiatr 1974; 7: 151–169. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010; 67: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994; 7: 180–184. [DOI] [PubMed] [Google Scholar]
- 29.Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest, Hogrefe: Goettingen, 2001. [Google Scholar]
- 30.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–753. [DOI] [PubMed] [Google Scholar]
- 31.O'Dwyer L, Lamberton F, Matura S, et al. Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study. PLoS One 2012; 7: e48895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9: 179–194. [DOI] [PubMed] [Google Scholar]
- 33.Ostergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med: Off J Soc Magn Reson Med/Soc Magn Reson Med 1996; 36: 715–725. [DOI] [PubMed] [Google Scholar]
- 34.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 35.Pickut BA, Dierckx RA, Dobbeleir A, et al. Validation of the cerebellum as a reference region for SPECT quantification in patients suffering from dementia of the Alzheimer type. Psychiatry Res 1999; 90: 103–112. [DOI] [PubMed] [Google Scholar]
- 36.Bozzao A, Floris R, Baviera ME, et al. Diffusion and perfusion MR imaging in cases of Alzheimer's disease: correlations with cortical atrophy and lesion load. AJNR Am J Neuroradiol 2001; 22: 1030–1036. [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010; 53: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 2004; 42: 1394–1413. [DOI] [PubMed] [Google Scholar]
- 39.Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology 2011; 76: 1492–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirth M, Haase CM, Villeneuve S, et al. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 2014; 35: 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsson E, Klasson N, Berge J, et al. White matter lesion assessment in patients with cognitive impairment and healthy controls: Reliability comparisons between visual rating, a manual, and an automatic volumetrical MRI method-the Gothenburg MCI study. J Aging Res 2013; 2013: 198471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc Series B (Method) 1995; 57: 289–300. [Google Scholar]
- 43.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 2005; 234: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexopoulos P, Sorg C, Forschler A, et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer's disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci 2012; 262: 69–77. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 2011; 77: 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chetelat G, Desgranges B, Landeau B, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer's disease. Brain 2008; 131(Pt 1): 60–71. [DOI] [PubMed] [Google Scholar]
- 48.Mazza M, Marano G, Traversi G, et al. Primary cerebral blood flow deficiency and Alzheimer's disease: shadows and lights. J Alzheimer's Dis: JAD 2011; 23: 375–389. [DOI] [PubMed] [Google Scholar]
- 49.Khan UA, Liu L, Provenzano FA, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nat Neurosci 2014; 17: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 2008; 71: 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luckhaus C, Flub MO, Wittsack HJ, et al. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer's dementia by perfusion-weighted magnetic resonance imaging. Neuroimage 2008; 40: 495–503. [DOI] [PubMed] [Google Scholar]
- 52.Altmann A, Ng B, Landau SM, et al. Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain 2015; 138 (Pt 12): 3734–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binnewijzend MA, Benedictus MR, Kuijer JP, et al. Cerebral perfusion in the predementia stages of Alzheimer's disease. Eur Radiol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michels L, Warnock G, Buck A, et al. Arterial spin labeling imaging reveals widespread and Abeta-independent reductions in cerebral blood flow in elderly apolipoprotein epsilon-4 carriers. J Cereb Blood Flow Metab 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villemagne VL, Furumoto S, Fodero-Tavoletti MT, et al. In vivo evaluation of a novel tau imaging tracer for Alzheimer's disease. Eur J Nucl Med Mol Imaging 2014; 41: 816–826. [DOI] [PubMed] [Google Scholar]
- 56.Small SA, Duff K. Linking A beta and Tau in late-onset Alzheimer's Disease: A dual pathway hypothesis. Neuron 2008; 60: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villain N, Desgranges B, Viader F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J Neurosci 2008; 28: 6174–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villeneuve S, Wirth M, La Joie R. Are AD-typical regions the convergence point of multiple pathologies? Front Aging Neurosci 2015; 7: 42: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.La Joie R, Perrotin A, Barre L, et al. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer's disease dementia. J Neurosci 2012; 32: 16265–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varallyay CG, Nesbit E, Fu R, et al. High-resolution steady-state cerebral blood volume maps in patients with central nervous system neoplasms using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J Cereb Blood Flow Metab 2013; 33: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stonnington CM, Tan G, Kloppel S, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. Neuroimage 2008; 39: 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 2009; 46: 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials