Abstract
According to recent reports, systemic treatment of rats with methylpalmoxirate (carnitine palmitoyltransferase-1 inhibitor) decreased peroxidation of polyunsaturated fatty acids in brain tissue. This was taken as evidence of mitochondrial β-oxidation in brain, thereby contradicting long-standing paradigms of cerebral metabolism, which claim that β-oxidation of activated fatty acids has minor importance for brain energy homeostasis. We addressed this controversy. Our experiments are the first direct experimental analysis of this question. We fueled isolated brain mitochondria or rat brain astrocytes with octanoic acid, but octanoic acid does not enhance formation of reactive oxygen species, neither in isolated brain mitochondria nor in astrocytes, even at limited hydrogen delivery to mitochondria. Thus, octanoic acid or l-octanoylcarnitine does not stimulate H2O2 release from brain mitochondria fueled with malate, in contrast to liver mitochondria (2.25-fold rise). This does obviously not support the possible occurrence of β-oxidation of the fatty acid octanoate in the brain. We conclude that a proposed inhibition of β-oxidation does not seem to be a helpful strategy for therapies aiming at lowering oxidative stress in cerebral tissue. This question is important, since oxidative stress is the cause of neurodegeneration in numerous neurodegenerative or inflammatory disease situations.
Keywords: Mitochondria, neuroprotection, lipids, inflammation, energy metabolism
Introduction
According to the commonly held paradigm, the mammalian brain does not really use long-chain fatty acids (LCFA, C12–C18) as fuel in the energy metabolism (for reviews1–3). The only exceptions known so far are specialized mammalian hypothalamic neurons.4 Moreover, neuronal mitochondria have been hypothesized to oxidize fatty acids, when these fatty acids are applied in mixtures together with other substrates.5 Moreover, LCFA exert harmful effects on brain mitochondria, mostly known as depolarization of the electrochemical proton gradient and inhibition of the electron transport chain.3 The latter stimulates the generation of reactive oxygen species (ROS).6 In conclusion, oxidative degradation of glucose is believed to be the main source of the cerebral energy supply, even during short-term starvation.
Nevertheless, we have to state that the minimal use of fatty acids as cerebral fuel is surprising, since LCFA are most rich as hydrogen donors and, in addition, other tissues with high oxygen consumption comparable with the brain, such as liver, heart, and kidney, extensively oxidize LCFA.7 In addition, both isoforms of the carnitine palmitoyltransferase enzymes (CPT1 and CPT2), which are necessary for entry and utilization of cytosolic LCFA-CoA by mitochondria have been detected in the adult rat brain and, patients with CTP2 deficiency show brain defects.8–10 Carnitine palmitoyltransferase-2 (CPT2) catalyzes the formation of LCFA-CoA from LCFA-carnitine inside the mitochondria. Moreover, from the analysis of the metabolite pattern in the intact rat brain supplied with octanoic acid (Oct) by 13C-NMR spectroscopy, it has been postulated that this fatty acid may contribute approximately to 20% of the total cerebral energy generation.11 Surprisingly, removal of the CTP2 in fruit fly Drosophila results in a triglyceride accumulation in the adult brain, a process absent in wild-type fruit fly.12 This finding supports the hypothetical view, according to which the adult brain is able to catabolize LCFA for energy production.
However, mitochondrial β-oxidation is a most prominent source for the generation of ROS.6,13 Generation of superoxide () can take place at the complex I, the acyl-CoA-dehydrogenase, the electron transfer flavoprotein (ETF), the ETF-ubiquinone oxidoreductase, and the complex III. This fact, together with the high content of peroxidizeable polyunsaturated fatty acids and a weak anti-oxidative capacity of the brain tissue (for review see14 and references therein), suggests that mitochondrial β-oxidation of LCFA is a dangerous energy-delivering process in the brain.
Curiously, it has been reported that the systemic application of CPT1-inhibitor methyl palmoxirate (MPL) to rats, decreased the level of non-enzymatically formed oxidized metabolites from polyunsaturated fatty acids.15
Challenged by these claims, conjectures and hypotheses, we revisited this question by studies of brain mitochondria and astrocytes isolated from rat brain. Here we carried out the first study in this direction. We wanted to examine the question of whether fatty acids could stimulate the β-oxidation-associated generation of ROS in brain. To minimize an increase in ROS production by detrimental activities of non-esterified LCFA, we applied octanoic acid as substrate of the β-oxidation. Compared with LCFA, this medium-chain length fatty acid has even in the high micromolar concentration range only a negligible uncoupling activity, a minimal inhibitory effect on the electron transport within the respiratory chain, and octanoic acid does not attack biological membranes by lytic activity.16–20 The use of octanoic acid is advantageous because it rapidly permeates in the non-esterified form the membranes of cells and mitochondria. Consequently, it is not possible that octanoic acid could stimulate the ROS generation by mechanisms that are independent of β-oxidation (e.g. inhibition of the electron transport).
We state that the ROS measurements presented and discussed here with the usage of isolated mitochondria or rat brain astrocytes do not support recently proposed therapeutic concepts,15 which resulted in the claim that inhibition of β-oxidation in the mammalian brain diminished oxidative stress.
Materials and methods
Reagents
Pyruvate, glutamate, malate, Oct, palmitic acid (Pal), ADP, antimycin A (AA), paraquat (PQ), horseradish peroxidase, and fatty acid-free bovine serum albumin were from Sigma-Aldrich Chemie GmbH (Sternheim, Germany). l-Octanoylcarnitine (OctC) and l-palmitoylcarnitine (PalC) were from Larodan Fine Chemicals (Malmö, Sweden). Amplex Red and MitoSOX Red were from Invitrogen (Eugene, OR, USA).
Preparation of astrocytes in culture and brain mitochondria
In vivo experiments with animals were not done. For that reason, ARRIVE guidelines (guidelines for Animal Research: Reporting in Vivo Experiments) are not applicable for this work. Primary astrocytes-enriched cell cultures from rats were obtained according to a previously published method.21 All animal procedures have been approved by the ethics committee of the State Sachsen-Anhalt (Germany) and are in accordance with the European Communities Council Directive (86/609/EEC). We used rats WU (Wistar; Unilever) from Charles River Laboratories.
Newborn animals (female or male) were used for the preparation of astrocytes. All efforts were made to minimize the number of animal used. For cellular experiments, the cells were used between day 10 and 13 in culture. Afterward, the cells were plated in 96-well plates at various densities per well. Three days after plating, astrocytes were treated with substrates or effectors and, after a further 15-min incubation time period, MitoSOX Red-related fluorescence was monitored as described below. The Hanks Balanced Salt Solution (HBSS) incubation buffer (145 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 20 mM HEPES, and 25 mM glucose, pH 7.4) was supplemented with octanoic acid (0.2 mM).
Mitochondria were prepared from the brain and liver tissues of rats as recently described.22 Rats were fed ad libitum, had free access to water, and were kept on a 12: 12 h light: dark cycle, routinely at normal house temperature (24℃). Adult female rats (8–12 weeks old) were routinely used for the preparation of brain mitochondria. Protein concentrations in the mitochondrial stock suspension were determined by the biuret method using bovine serum albumin as standard.
Monitoring superoxide and H2O2
was monitored with MitoSOX Red, which reacts with matrix-released to 2-hydroxyethidium.23 Briefly, MitoSOX Red (5 µM) was added to the wells and, thereafter the fluorescence was measured at 510 nm excitation and 579 nm emission wavelengths. Time-dependent monitoring of the MitoSOX Red-related fluorescence was done using a microplate reader fluorimeter (Tecan Austria GmbH, Salzburg, Austria) at 25℃. With astrocytes, the MitoSOX Red-related fluorescence was strictly linear during the monitoring time of 60 min. For quantification, the fluorescence increase per 30 min was used. With brain mitochondria, the increase of the MitoSOX Red-related fluorescence did show a saturation kinetics increase during the monitoring time of 25 min. Thus, the initial rates were used for quantification.
In addition, ROS generation was measured as release of H2O2 from isolated mitochondria, using the oxidation of non-fluorescent Amplex Red to the fluorescent resorufin. Fluorescence was monitored by the Perkin-Elmer Luminescence spectrometer LS-50B (excitation at 560 nm, emission wavelengths at 590 nm) in 1 ml cuvette. The cuvette-holder was thermostated (37℃) and connected with a stirring device. Mitochondria (0.4 mg/ml) were kept in incubation buffer (110 mM mannitol, 60 mM KCl, 60 mM Tris-HCl, 10 mM KH2PO4, 0.5 mM EGTA, pH 7.4), supplemented with substrates or effectors, and Amplex Red (5 µM), horseradish peroxidase (2 U/ml), and superoxide dismutase (10 U/ml). The fluorescence signal of resorufin was calibrated by sequential additions of known amounts of H2O2.
Oxygen consumption
Oxygen consumption of mitochondria was measured using an Oxygraph® from Oroboros Instruments (Innsbruck, Austria) at 37℃. For this purpose, isolated mitochondria (0.5 mg of protein per ml) were suspended in the incubation buffer supplemented with 5 mM malate, in the absence and or in the presence of 0.2 mM octanoic acid or l-octanoylcarnitine. Phosphorylating respiration was adjusted by addition of 1 mM ADP.
Statistical analysis
All data are represented as mean ± S.D. of three to five different experiments and preparations. Statistical significance of mean differences was tested by the paired Students t-test as specified in the figure legend using SigmaPlot 11.0 software. The sample size was using three to five separate preparations of mitochondria or astrocytes per incubation condition. No randomization was appropriate. Statistically significant differences are set at #P < 0.05 or 0.03, ##P < 0.01, §P < 0.03, §§P < 0.001, and &P < 0.005. Sample size analysis was done using the GPower software.24 Power of all statistical calculations was higher than 0.8.
Results
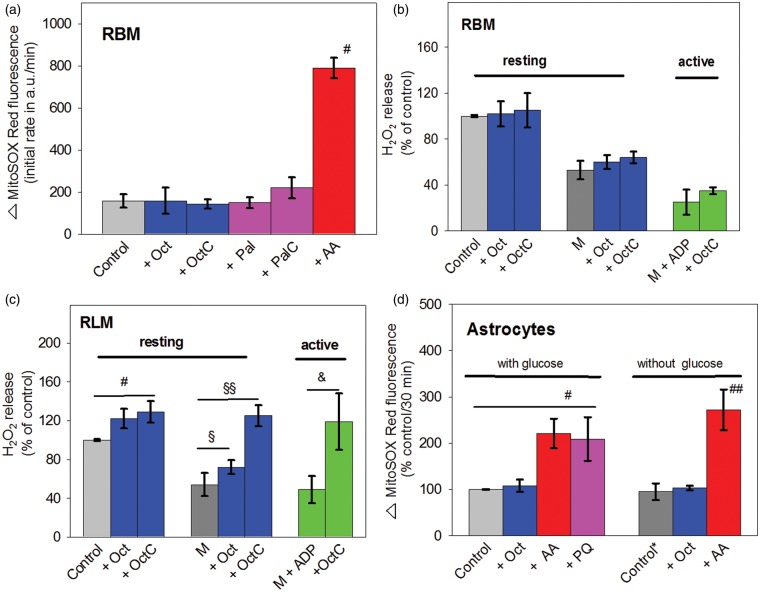
We estimated first the ROS generation by isolated rat brain mitochondria (RBM) respiring in resting state using the dye MitoSOX Red. This dye monitors intramitochondrial . At the beginning, generation by isolated RBM, which were supplied with pyruvate, glutamate plus malate (control), was measured in the absence or presence of octanoic acid or l-octanoylcarnitine (Figure 1(a)). Importantly, octanoic acid or l-octanoylcarnitine did not enhance the MitoSOX Red-linked fluorescence. Similarly, the MitoSOX Red-linked fluorescence did also not respond to the addition of the long-chain palmitic acid or its carnitine-derivative, in contrast to the complex III inhibitor AA, which caused a five-fold stimulation.
Figure 1.
ROS generation by mitochondria (a–c) and astrocytes (d) from brain with octanoate as β-oxidation substrate. (a) RBM (0.1 mg/ml) were suspended in incubation medium (25℃) consisting of 10 mM KH2PO4, 0.5 mM EGTA, 60 mM TRIS, 60 mM KCl, 110 mM mannitol pH 7.4, supplemented with substrates pyruvate, glutamate, and malate (5 mM each). Additions were Oct (0.2 mM), OctC (0.2 mM), Pal (30 nmol/mg), PalC (30 nmol/mg), or AA (5 µM). The initial rates of the increase of the MitoSOX Red-linked fluorescence. Autoxidation of MitoSOX Red was negligible during the incubation period (not shown). The mean values ± SD shown in (a) were obtained from five incubations. #P < 0.05, compared with control (P/G/M). (b,c) In one incubation series, RBM (0.1 mg/ml) or RLM (0.4 mg/ml) were suspended in incubation medium (thermostated to 37℃) and supplied with Oct or OctC, which is comparable with the conditions used in (a). In a second incubation series, mitochondria were suspended in M-containing incubation medium without and with ADP (1 mM). Shown data (mean values ± SD) of the H2O2 release expressed in percent of the control incubation were normalized to 100%. Rates of the H2O2 release measured in the control incubation was 66 ± 14 for RBM and 50 ± 6 pmol/min/mg protein for RLM, respectively. Data obtained under resting conditions (without ADP) were from 5 (b,c), or in active conditions (with ADP) were from 3 (b) and 4 (c) mitochondrial preparations, respectively. (c) #P < 0.03, compared with control (P/G/M), §P < 0.03 as well as §§P < 0.001, compared with control (M), &P < 0.005, compared with control (M + ADP). (d) Astrocytes (1 × 104 cells/well) supplemented with glucose (25 mM)-containing HBSS buffer or glucose-free HBSS buffer, were treated with Oct (0.2 mM), AA (10 µM), or PQ (20 µM). Shown data are the mean values ± SD of 5 (with glucose) or 4 (without glucose) astrocyte preparations, reflecting the increase of the MitoSOX Red-associated fluorescence during a 30-min incubation period. Both controls, obtained either with glucose-containing (control) or glucose-free (control*) medium correspond to 7794 ± 1844 a.u. per 30 min or to 4703 ± 903 a.u. per 30 min, respectively. #P < 0.03, compared with glucose; ##P < 0.01, compared with control.
ROS: reactive oxygen species; Oct: octanoic acid; OctC: l-octanoylcarnitine; AA: Antimycin A; PQ: paraquat; Pal: palmitic acid; PalC: l-palmitoylcarnitine; RBM: rat brain mitochondria; RLM: rat liver mitochondria; HBSS: Hanks Balanced Salt Solution; M, malate.
In addition, mitochondrial ROS generation was also measured as release of H2O2 from RBM, using the Amplex Red/horseradish peroxidase assay. In contrast to MitoSOX Red, the H2O2-sensitive non-fluorescent Amplex Red is extramitochondrially oxidized. Figure 1(b) shows that, irrespective of the absence or presence of octanoic acid or l-octanoylcarnitine, the release of H2O2 is not different from the control value. Next, when RBM were supplied only with the poorly hydrogen-delivering substrate malate (M), the release of H2O2 was only about 50% of that seen in control. More importantly, even with malate, l-octanoylcarnitine did not significantly enhance the release of H2O2 by resting (M + OctC) or actively phosphorylating (M + ADP + OctC) RBM.
In contrast to RBM, rat liver mitochondria (RLM) responded under the same incubation conditions clearly to octanoic acid or l-octanoylcarnitine, particularly when l-octanoylcarnitine was applied (Figure 1(c)). With malate, the addition of octanoic acid or l-octanoylcarnitine increased the H2O2 release to 130% or 225%, respectively. Interestingly, l-octanoylcarnitine stimulates a stronger release of H2O2 release than octanoic acid. There is good reason to attribute this difference to the necessary activation of octanoic acid within the inner mitochondrial compartment. This process is associated with ATP wastage, most likely due to a futile cycle.20 In conclusion, in contrast to l-octanoylcarnitine, octanoic acid converted ATP to AMP in a significant portion in the mitochondrial matrix, thereby limiting the availability of ATP for the activation of octanoic acid to octanoyl-CoA and thus, diminishing the concentration of octanoyl-CoA for degradation by the β-oxidation pathway.
A possible effect of octanoic acid on mitochondrial ROS generation was also examined under in situ conditions using astrocytes (Figure 1(d)). While glucose is known to be the preferred oxidizable substrate for neural cells, astrocytes were provided with glucose-supplemented HBSS medium (control) without and with octanoic acid. In addition, the astrocytes were exposed to AA or PQ. Similar to isolated RBM, AA stimulates generation in astrocytes supplied with glucose or deprived of glucose. Furthermore, a clear stimulation of generation by PQ was found with astrocytes supplied with glucose. The latter stimulation has been attributed to the PQ-mediated electron-transfer from complex III to O2.25 Finally, a possible effect of octanoic acid on the mitochondrial ROS generation was also examined with astrocytes in the absence of glucose supplementation (control*). Remarkably, even with these “starved” astrocytes, octanoic acid did not enhance the fluorescence of MitoSOX Red. Similar results were seen with incubations containing higher concentrations of octanoic acid (0.5 and 1 mM; data not shown). In contrast to octanoic acid, AA stimulates MitoSOX Red-related fluorescence in the absence of glucose.
From the data obtained (Figure 1(d)), we conclude that octanoic acid was without any effect on mitochondrial ROS generation in astrocytes, even then, when they were depleted from oxidizable glucose.
Discussion
Oxidative stress is generally considered to be a major risk factor for the development of neurodegenerative diseases. One striking paradigmatic example is that it has been postulated that oxidative stress underlies the axonal degeneration in adrenoleukodystrophy.14 The major source of oxidative stress is the induction of mitochondrial ROS generation by accumulation of fatty acids within cells, resulting in an impairment of the electron transport in the respiratory chain and/or enhanced degradation of fatty acids by the β-oxidation.
The results obtained in our present study clearly exclude the possibility that supplying isolated brain mitochondria or astrocytes with octanoic acid enhanced the mitochondrial ROS generation. Thus, the data shown in Figure 1 clearly reveal that octanoic acid or l-octanoylcarnitine does not enhance the formation of the oxidized forms of MitoSOX Red (Figure 1(a)) or that of Amplex Red (Figure 1(b)), indicating that the mitochondrial generation or the mitochondrial release of H2O2 were not stimulated. Even when we used “starved” brain mitochondria (supplemented with only malate or malate plus ADP), octanoic acid or l-octanoylcarnitine does not induce the release of H2O2 from RBM. Similarly, addition of octanoic acid or l-octanoylcarnitine to RBM supplemented with malate and ADP did not stimulate phosphorylating respiration, indicating that octanoic acid or l-octanoylcarnitine did not enhance the supply of β-oxidation-linked reducing equivalents to the respiratory chain (data not shown). Consistent results on the ROS generation were found with astrocytes, which were either “fed” with glucose or were “starved” (Figure 1(d)). First, these findings contradict the view, according to that β-oxidation contributes to a significant extent to the cellular ROS generation.15 However, our findings are in line with the reported low mitochondrial enzymatic capacity of the β-oxidation in brain mitochondria.26
In our study, octanoic and palmitic acid were applied (Figure 1(a)). Furthermore, the cytosolic acyl-CoA synthetase has significant specificity only for LCFA, such as palmitic acid.27 This explains why non-esterified octanoic acid permeates the inner mitochondrial membrane and, becomes subsequently activated by matrix-localized acyl-CoA synthetase. The cytosolic palmitoyl-CoA is unable to enter mitochondria. Before being β-oxidized, palmitoyl-CoA has to be converted into the acyl-carnitine ester (PalC) by the CPT1, and after its transmembrane transport via the acylcarnitine/carnitine shuttle, PalC is reconverted into the palmitoyl-CoA thioester inside the mitochondria.
In addition, there is a certain activity in brain to degrade palmitic acid by peroxisomal β-oxidation.28 Finally, LCFA (such as palmitic acid) can exert in the non-esterified form a mild-uncoupling effect, whereas octanoic acid does not exhibit such activity.16,20 Mild-uncoupling might potentially modulate mitochondrial ROS generation. Thus, as mentioned above, we applied octanoic acid as substrate for examining the β-oxidation as enhancer of ROS generation in brain mitochondria.
It could be discussed that a low octanoic acid activation inside the mitochondria limits the β-oxidation-linked ROS generation. Therefore, in separate incubations using RLM, the concentration of octanoic acid was estimated by oxygen uptake measurements, which is sufficient to support a stationary maximal rate of phosphorylation (data not shown). This concentration was 0.2 mM, which was throughout applied in our incubation experiments. In addition, we obtained also with RLM and l-octanoylcarnitine or l-palmitoylcarnitine comparable rates of ROS generation (Figure 1(c)).
It has been reported that brain-specific carnitine palmitoyltransferase-1 (CPT1c) is not able to catalyze the acyl transfer from acyl-CoAs to carnitine and thus, CPT1c should not be involved in the β-oxidation of LCFA.29 Notably, a CPT1c-knockout mouse has reduced body mass and food intake. These findings suggest a role of CPT1c in the regulation of whole-body energy homeostasis. In addition, hypothalamic neurons might be able to degrade to some extent LCFA by β-oxidation. Treatment of primary hypothalamic neurons with palmitate and the compound C75 (trans-tetrahydro-4-methylene-2-octyl-5-oxo-3-furancarboxylic acid), an inhibitor of the fatty acid synthase and stimulator of CPT1, results in a significant ROS generation.4 This ROS generation could stem either from an enhanced mitochondrial β-oxidation and (or) peroxisomal β-oxidation of endogenous LCFA.
What might be a tentative explanation for the discrepancy between the finding reporting that a systemic treatment of rats with the β-oxidation inhibitor MPL decreased lipid peroxidation in brain tissue,15 and our observation that fueling the β-oxidation by octanoic acid in brain mitochondria or astrocytes does not enhance the mitochondrial ROS generation? As a general answer, we refer to our previous comprehensive analysis, where we summarized the experimental literature data suggesting that in brain tissue β-oxidation is only a poor source for the delivery of reducing equivalents to mitochondria.3
More specifically, it can be estimated that an injection of MPL into rats (10 mg/kg body mass) as done,15 results in a MPL concentration in the body water of about 20 µM (body mass 300 g; water content 60%). MPL, a lipophilic derivative of palmitic acid, enriches mostly in the lipid phase of membranes. Furthermore, MPL has a highly reactive oxiran ring, which undergoes reactions with nucleophiles. Thereby, most likely MLP competes with the double-bonds of polyunsaturated fatty acids for reaction with ROS. For that reason, there is a sound basis to hypothesize that MPL reacts with ROS and thus exerts a substantial antioxidative activity per se.
Acknowledgments
We thank H. Goldammer and P. Grüneberg for their expert technical assistance.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors contributions
Both authors made a substantial and almost equal contribution to the concept and design, acquisition of data or analysis and interpretation of data; both drafted the article, revised it critically for important intellectual content, and approved the version to be published.
References
- 1.Wang SP, Yang H, Wu JW, et al. Metabolism as a tool for understanding human brain evolution: lipid energy metabolism as an example. J Hum Evol 2014; 77: 41–49. [DOI] [PubMed] [Google Scholar]
- 2.Speijer D. Oxygen radicals shaping evolution: why fatty acid catabolism leads to peroxisomes while neurons do without it: FADH2/NADH flux ratios determining mitochondrial radical formation were crucial for the eukaryotic invention of peroxisomes and catabolic tissue differentiation. Bioessays 2011; 33: 88–94. [DOI] [PubMed] [Google Scholar]
- 3.Schönfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab 2013; 33: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFadden JW, Aja S, Li Q, et al. Increasing fatty acid oxidation remodels the hypothalamic neurometabolome to mitigate stress and inflammation. PLoS One 2014; 9: e115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panov A, Orynbayeva Z, Vavilin V, et al. Fatty acids in energy metabolism of the central nervous system. Biomed Res Int 2014; 2014 article ID 472459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schönfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 2008; 45: 231–241. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell PR, Wittenberg BA. The oxygen dependency of mammalian tissues. Am J Med 1974; 57: 447–452. [DOI] [PubMed] [Google Scholar]
- 8.Lavrentyev EN, Matta SG, Cook GA. Expression of three carnitine palmitoyltransferase-I isoforms in 10 regions of the rat brain during feeding, fasting, and diabetes. Biochem Biophys Res Commun 2004; 315: 174–178. [DOI] [PubMed] [Google Scholar]
- 9.North KN, Hoppel CL, De Girolami U, et al. Lethal neonatal deficiency of carnitine palmitoyltransferase II associated with dysgenesis of the brain and kidneys. J Pediatr 1995; 127: 414–420. [DOI] [PubMed] [Google Scholar]
- 10.Pierce MR, Pridjian G, Morrison S, et al. Fatal carnitine palmitoyltransferase II deficiency in a newborn: new phenotypic features. Clin Pediatr (Phila) 1999; 38: 13–20. [DOI] [PubMed] [Google Scholar]
- 11.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 2003; 23: 5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz JG, Laranjeira A, Van Huffel L, et al. Glial b-oxidation regulates Drosophila energy metabolism. Sci Rep 2015; 5 article number: 7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perevoshchikova IV, Quinlan CL, Orr AL, et al. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic Biol Med 2013; 61: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galea E, Launay N, Portero-Otin M, et al. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta 2012; 1822: 1475–1488. [DOI] [PubMed] [Google Scholar]
- 15.Chen CT, Trepanier MO, Hopperton KE, et al. Inhibiting mitochondrial beta-oxidation selectively reduces levels of nonenzymatic oxidative polyunsaturated fatty acid metabolites in the brain. J Cereb Blood Flow Metab 2014; 34: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plomp PJ, van Roermund CW, Groen AK, et al. Mechanism of the stimulation of respiration by fatty acids in rat liver. FEBS Lett 1985; 193: 243–246. [DOI] [PubMed] [Google Scholar]
- 17.Sauer SW, Okun JG, Hoffmann GF, et al. Impact of short- and medium-chain organic acids, acylcarnitines, and acyl-CoAs on mitochondrial energy metabolism. Biochim Biophys Acta 2008; 1777: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 18.Zborowski J, Wojtczak L. Induction of Swelling of Liver Mitochondria by Fatty Acids of Various Chain Length. Biochim Biophys Acta 1963; 70: 596–598. [DOI] [PubMed] [Google Scholar]
- 19.Singh AK, Yoshida Y, Garvin AJ, et al. Effect of fatty acids and their derivatives on mitochondrial structures. J Exp Pathol 1989; 4: 9–15. [PubMed] [Google Scholar]
- 20.Schönfeld P, Wojtczak AB, Geelen MJ, et al. On the mechanism of the so-called uncoupling effect of medium- and short-chain fatty acids. Biochim Biophys Acta 1988; 936: 280–288. [DOI] [PubMed] [Google Scholar]
- 21.Hamprecht B, Löffler F. Primary glial cultures as a model for studying hormone action. Methods Enzymol 1985; 109: 341–345. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 2002; 80: 780–787. [DOI] [PubMed] [Google Scholar]
- 23.Robinson KM, Janes MS, Pehar M, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA 2006; 103: 15038–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- 25.Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem 2007; 282: 14186–14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem 1987; 262: 13027–13032. [PubMed] [Google Scholar]
- 27.Aas M, Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta 1968; 164: 157–166. [DOI] [PubMed] [Google Scholar]
- 28.Wanders RJ, van Roermund CW, van Wijland MJ, et al. Studies on the peroxisomal oxidation of palmitate and lignocerate in rat liver. Biochim Biophys Acta 1987; 919: 21–25. [DOI] [PubMed] [Google Scholar]
- 29.Wolfgang MJ, Kurama T, Dai Y, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci USA 2006; 103: 7282–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]