Abstract
The NMDA antagonist memantine preferentially inhibits extrasynaptic NMDA receptors, which are overactivated upon stroke and thought to disturb neuroplasticity. We hypothesized that memantine enhances post-ischemic neurological recovery, brain remodeling, and plasticity. C57BL6/j mice were exposed to intraluminal middle cerebral artery occlusion. Starting 72 hours post-stroke, vehicle or memantine (4 or 20 mg/kg/day) were subcutaneously delivered over 28 days. Neurological recovery, perilesional tissue remodeling and contralesional pyramidal tract plasticity were evaluated over 49 days. Memantine, delivered at 20 but not 4 mg/kg/day, persistently improved motor-coordination and spatial memory. Secondary striatal atrophy was reduced by memantine. This delayed neuroprotection was associated with reduced astrogliosis and increased capillary formation around the infarct rim. Concentrations of BDNF, GDNF, and VEGF were bilaterally elevated by memantine in striatum and cortex. Anterograde tract tracing studies revealed that memantine increased contralesional corticorubral sprouting across the midline in direction to the ipsilesional red nucleus. In the contralesional motor cortex, the NMDA receptor subunit GluN2B, which is predominantly expressed in extrasynaptic NMDA receptors, was transiently reduced by memantine after 14 days, whereas GluN2A and PSD-95, which preferentially co-localize with synaptic NMDA receptors, were increased after 28 days. Our data suggest the utility of memantine for enhancing post-acute stroke recovery.
Keywords: Brain plasticity, brain remodeling, cerebral angiogenesis, focal cerebral ischemia, neurological recovery
Introduction
Subsequent to ischemic stroke, the extracellular release of glutamate results in excessive stimulation of NMDA receptors, which induces excitotoxic brain injury.1–3 Indeed, studies in rodents repeatedly showed that the deactivation of NMDA receptors reduces ischemic brain damage.2,3 Unfortunately, the clinical translation of NMDA antagonists to human stroke patients failed,4,5 which has been attributed to a number of reasons including (i) narrow therapeutic time windows of early NMDA antagonists, (ii) cognitive side effects, (iii) underdosing in order to prevent cognitive side effects and (iv) lack of experimental studies with observation time windows that exceed the acute stroke phase.
In ischemic stroke, the abrupt increase of extracellular glutamate results in the activation of extrasynaptic glutamate receptors in addition to synaptic ones, which promotes neuronal death pathways and impedes neuronal plasticity.3 In contrast to high-affinity NMDA antagonists, the uncompetitive low-affinity antagonist memantine, which is clinically used for the treatment of Alzheimer’s disease,6,7 preferentially inhibits extrasynaptic NMDA receptors.8–10 In the acute stroke phase, the delivery of memantine has previously been shown to reduce ischemic brain injury in rats and mice.1,11,12
Interestingly, in two more recent studies, memantine reduced behavioral deficits without affecting brain injury, when delivered up to 2 h post-stroke.13,14 Based on these studies, we asked whether memantine, in addition to its neuroprotective actions, may also have restorative effects in the post-acute stroke phase. To clarify this question, we herein exposed mice to transient intraluminal middle cerebral artery (MCA) occlusion (MCAO), administering memantine at different doses (4 or 20 mg/kg/d) starting 72 h post-stroke, evaluating consequences for motor recovery, cognitive performance, peri-infarct tissue remodeling, and contralesional pyramidal tract plasticity over up to 49 d. We delivered memantine at 72 h post-stroke, since acute brain injury has already maturated at this time-point in this MCAO model.15,16 The 20 mg/kg/d dose was selected, since it induces neuroprotection in the acute stroke phase.12,13 With the 4 mg/kg/d dose, we aimed to characterize memantine’s dose–response relationship.
Materials and methods
Legal issues, animal housing, randomization, and blinding
All animal experiments were performed with local government approval (animal experimentation committee of the Bezirksregierung Düsseldorf) in accordance to E.U. guidelines (Directive 2010/63/EU) for the care and use of laboratory animals in compliance with ARRIVE guidelines. Experiments were strictly randomized. The experimenter performing the animal experiments and histochemical studies (Y. W.) was fully blinded at all stages of the study by another researcher (E. S. -M.) preparing the vehicle and memantine solutions. These solutions received dummy names (solutions A, B, and C or solutions A and B, depending on whether vehicle and two doses of memantine [4 or 20 mg/kg/d] or vehicle and only a single dose of memantine [20 mg/kg/d] was administered). Randomization was achieved animal-wise by always administering solutions in the order A, B or C and A or B, respectively. Animal groups were unblinded after termination of the study. Animals were kept in a regular 12 h:12 h light/ dark cycle in groups of five animals per cage. Behavioural tests and animal surgeries were always performed in the morning hours throughout the study.
Statistical planning
Statistical planning was done by a sample size calculator (https://www.dssresearch..com/KnowledgeCenter/toolkitcalculators/samplesizecalculators.aspx). For behavioral tests, specifically Rotarod and tight rope tests (subsequently evaluated in animal set 1), we postulated an effect size of 25% of the mean value, which with an expected standard deviation of 30% of the mean value required a sample size of 18 animals/ group, given that the alpha error was 5% and the beta error (1—statistical power) was 20%. For histochemical studies (subsequently performed in animal set 2), we expected an effect size of 30% of the mean value, which with a standard deviation of 30% of the mean value demanded a sample size of 12 animals/ group (alpha error again 5%, beta error again 20%). For ELISA and Western blots (performed in animal set 3), we postulated an effect size of 50% of the mean value, which with a standard deviation of 30% of the mean value required a sample size of four animals/group (alpha error again 5%, beta error again 20%).
Memantine delivery and animal groups
After in depth with discussion with Dr. Christopher Parsons (Department of Pharmacology, Merz Pharmaceuticals, Frankfurt, Main, Germany), we decided to use a subcutaneous delivery strategy for memantine in this study using miniosmotic pumps, given that miniosmotic pumps allowed to achieve most stable plasma memantine levels. Memantine is highly stable in miniosmotic pumps at 37℃, as previously demonstrated in rats over observations times of up to three weeks (unpublished data from Merz Pharmaceuticals).
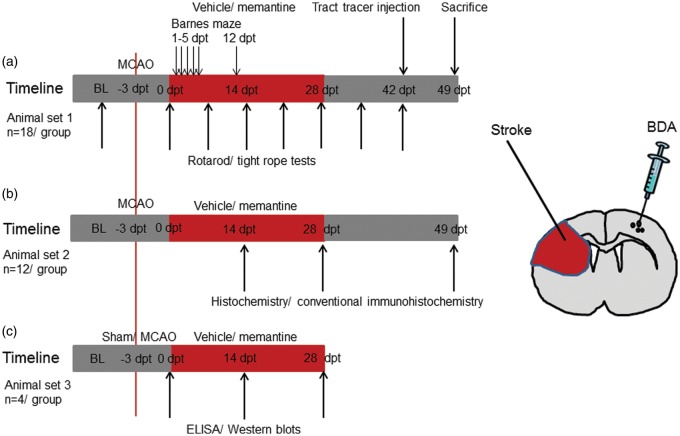
Focal cerebral ischemia was induced in male C57BL6/j mice (8–12 weeks; 23–28 g; Harlan Laboratories, Indianapolis, IN) by 40 min of left-sided MCAO. Seventy-two hours post-stroke, one set of mice (animal set 1) received subcutaneous implantations of miniosmotic pumps (Alzet 2004; Alzet, Cupertino, CA) which were randomly filled with vehicle (normal saline) or memantine (4 or 20 mg/kg/d in normal saline; Merz, Frankfurt, Germany) that were left in place for 28 d (n = 18 animals/group; Figure 1). Animals received detailed assessments of motor-coordination and cognitive deficits, as described below. For evaluation of pyramidal tract plasticity, the anterograde tract tracer biotinylated dextran amine (BDA) was injected into the contralesional motor cortex at 42 d post-treatment onset (dpt). These animals were sacrificed at 49 dpt by transcardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS).
Figure 1.
Experimental procedures and animal groups. Mice submitted to transient middle cerebral artery (MCA) occlusion (MCAO) that were treated with vehicle or memantine (4 or 20 mg/kg/d) starting at 72 h after reperfusion over up to 28 d were used for (a) behavioral tests (Rotarod, tight rope, and Barnes maze tests) and tract tracing studies, (b) histochemistry and conventional immunohistochemistry, and (c) ELISA and Western blots. Numbers of animals evaluated for each group and time-point of animal sacrifice (in days post-treatment onset, dpt) are also shown. BL, baseline.
Another set of mice (animal set 2) was subjected to 40 min of left-sided MCAO, followed by implantation of miniosmotic pumps filled with vehicle or memantine (4 or 20 mg/kg/d) at 72 h post-stroke that were left in place for up to 28 d. These animals were sacrificed at 14 dpt (n = 12 animals/group), 28 dpt (n = 12 animals/group), or 49 dpt (n = 12 animals/group) by transcardiac perfusion with 4% paraformaldehyde in 0.1 M PBS (Figure 1). These animals were used for histochemical and conventional immunohistochemical studies.
Likewise, a third set of mice (animal set 3) was exposed to 40 min of left-sided MCAO, followed by implantation of miniosmotic pumps filled with vehicle or memantine (20 mg/kg/d) at 72 h post-stroke. These animals were sacrificed at 14 dpt (n = 4 animals/group) or 28 dpt (n = 4 animals/group) by transcardiac perfusion with normal saline (Figure 1). Additional animals were subjected to 40 min of left-sided MCAO or sham surgery (n = 4 animals/group). These animals did not receive implantations of miniosmotic pumps. These animals were transcardially perfused with normal saline at 72 h post-stroke or post-sham surgery. These animals were used for enzyme-linked immunosorbance assays (ELISA) and Western blots.
The animal flow within this study is summarized in Supplementary Figure 1. As exclusion criteria, animals were removed from the study when suffering from central respiratory abnormalities (i.e., apneas) or from severe motor handicaps with inappropriate nurturing, resulting in a weight loss > 20%. Excluded animals were replaced by new animals throughout the study.
Focal cerebral ischemia
At all stages of the animal experiments, wounds were carefully instilled with buprenorphine (0.1 mg/kg; Reckitt Benckiser, Slough, UK). For induction of MCAO, animals were anaesthetized with 1.0–1.5% isoflurane (30% O2, remainder N2O). Rectal temperature was maintained between 36.5 and 37.0℃ using a feedback-controlled heating system (Fluovac, Harvard apparatus). During and after MCAO, cerebral blood flow was recorded by laser Doppler flowmetry using a flexible probe attached to the skull overlying the core of the MCA territory. The left common and external carotid arteries were isolated and ligated, and the internal carotid artery was temporarily clipped. A silicon-coated nylon monofilament was introduced through a small incision into the common carotid artery and advanced to the carotid bifurcation for middle cerebral artery occlusion. Reperfusion was initiated by monofilament removal 40 min later. Wounds were carefully sutured and animals returned to their cages. For sham surgery, a midline neck incision was made and the common carotid artery was exposed, but left intact, while laser Doppler flow (LDF) was recorded. For anti-inflammation, animals received daily i.p. injections of carprofen (4 mg/kg; Bayer Vital, Leverkusen, Germany) during the first 3 d post-stroke or sham-surgery.
Miniosmotic pump implantation
Seventy-two hours post-stroke, animals were re-anesthetized with 1.0–1.5% isoflurane (30% O2, remainder N2O). Miniosmotic pumps (Alzet 2004; 0.25 µl/hour) that were filled with vehicle (normal saline) or memantine (4 or 20 mg/kg/d in normal saline) were subcutaneously implanted.17,18 Miniosmotic pumps were removed at 28 dpt in animals with survival times of 49 d.
Behavioral tests
Neurological recovery was analyzed in animal set 1 using the RotaRod, tight rope, and Barnes maze tests. Animals were pre-trained over 1–2 d prior to MCAO in the Rotarod and tight rope tests, ensuring that they were able to stay on the rotating rod over 300 seconds and reach the platform within 60 s.
Rotarod test: The Rotarod, which consists of a rotating drum (Ugo Basile, model 47600, Comerio, Italy), was used to evaluate motor coordination skills. Animals were placed on the accelerating drum. The time until animal dropped off was analyzed.17,18
Tight rope test: In the tight rope test, animals were placed in the middle of a 60-cm-long rope which they grasped with both forepaws. The time until the animals reached the platform in the end of the rope was analyzed. The maximum testing time was 60 s.17,18
Barnes maze: The Barnes maze is composed of a circular platform (92 cm diameter) with 20 equally spaced holes (5 cm diameter, 7.5 cm between holes) along the perimeter 72 cm above the ground that is used for the evaluation of spatial memory. There is a rectangle box 15.5 × 9.5 × 6 cm (length, width, and height) placed under the target hole in which animals may escape. Before and after each test, the platform was cleaned with 70% ethanol. Animals were placed into a cylindrical black start chamber in the middle of the maze, which was lifted 10 seconds later with bright light switched on, allowing the animal to explore the maze for 3 min. Primary errors in finding the target hole, total errors until escaping into the target hole, primary latency until finding the target hole and total latency until escaping into the target hole were recorded.19 Whenever an animal escaped into the target hole, the light was switched off and the animal was allowed to stay in the hole for 1 min. In case an animal did not find the target hole within 3 min, the animal was gently placed in front of it and stimulated to enter into the hole. Following 4 d of training (on 1–4 dpt), the target hole was blocked followed by two testing sessions on 5 and 12 dpt, in which animals were allowed to explore the maze for 90 s. During this retention phase, primary errors, total errors, and primary latency were recorded. In the Barnes maze, each animal was examined four times on occasion of each session. Mean values were calculated for these tests.
Biotinylated dextran amine (BDA) injection
The anterograde tract tracer BDA has previously been used in our group to evaluate pyramidal tract plasticity contralateral to the stroke in mice exposed to focal cerebral ischemia.17,18 At 42 dpt, animals belonging to animal set 1 were re-anesthetized with 1.0–1.5% isoflurane (30% O2, remainder N2O). A cranial bur hole was drilled 0.5 mm rostral and 2.5 mm lateral to the bregma, via which deposits of 10% BDA (MW 10,000; Molecular Probes, Waltham, MA; diluted in 0.01 M PBS at pH 7.2) were placed into the contralesional motor cortex by means of microsyringe injections. A stotal volume of 2.1 µl tracer was administered to each animal, which was injected in three equal deposits located rostrally, medially and caudally of the bur hole inside the motor cortex. For this purpose, the syringe was inserted into the brain at inclination angles of 45°, 90°, and 135° against the midline. Hence deposits were made 1.5 mm rostal, 0.5 mm rostral, and 0.5 mm caudal to the bregma and 1.5 mm lateral to the midline at a depth of 1.0 mm. Seven days after the tracer injection, animals were transcardially perfused with 4% paraformaldehyde in 0.1 M PBS. Brains were removed, post-fixed overnight in 4% paraformaldehyde in 0.1 M PBS and cryoprotected by immersion in increasing sucrose concentrations (5%, 10%, and 30%) over 3 d. Brains were then frozen on dry ice.
Analysis of brain atrophy
Brain atrophy and striatal atrophy were volumetrically evaluated using cresyl violet-stained 20 -µm-thick coronal brain sections that had been collected at millimeter intervals throughout the forebrain (from most rostral pole to most caudal end) from animals sacrificed by transcardiac perfusion with 4% paraformaldehyde (animal set 2).17,20 In coronal sections obtained from the bregma level, corpus callosum atrophy was furthermore analyzed by determining the tissue area covered by the corpus callosum.17,20
Enzyme-linked immunosorbance assay (ELISA)
Tissue samples obtained from the motor cortex or striatum ipsilateral or contralateral to the stroke of animals sacrificed by transcardiac perfusion with normal saline (animal set 3) were homogenized using a lysis buffer that consisted of 1% NP40 containing 50 mM Tris (pH 8.0), 150 mM NaCl and protease inhibitor cocktail and phosphatase inhibitor. In these homogenates, levels of brain-derived neurotrophic factor (BDNF; Promega, Madison, WI), basic fibroblast growth factor (FGF; R&D Systems, Minneapolis, MN), glial cell line-derived neurotrophic factor (GDNF; Promega) and vascular endothelial growth factor (VEGF; R&D Systems) were determined using commercial mouse ELISA kits according to the instructions of the manufacturers.21
Immunohistochemistry for endothelial, astrocytic, and microglial markers
Twenty-µm-thick coronal brain sections obtained from the rostrocaudal level of the bregma of animals sacrificed by transcardiac perfusion with 4% paraformaldehyde (animal set 2) were rinsed three times for 5 min in 0.1 M PBS and immersed in 0.1 M PBS containing 0.3% Triton X-100 (PBS-T) and 10% normal donkey serum for 1 h. Sections were incubated overnight at 4℃ in Alexa Fluor 488-conjugated monoclonal mouse anti-glial fibrillary acidic protein (GFAP) (3656; Cell Signaling, Billerica, MA), polyclonal rabbit anti-ionized calcium binding adaptor protein (Iba)-1 (Wako Chemicals, Neuss, Germany), or monoclonal rat anti-CD31 (557355; BD Biosciences, Heidelberg, Germany) antibody that was detected with Alexa Fluor 594 or Alexa Fluor 488 conjugated secondary antibody in experiments in which unconjugated primary antibody was used. Sections were counterstained with 4′-6-diamidino-2-phenylindole (DAPI). Sections were evaluated under a motorized Zeiss AxioObserver.Z1 inverted epifluorescence microscope equipped with Apotome optical sectioning. GFAP + astrogliosis was evaluated by analyzing the optical density of the perilesional (i.e., parietal cortical) scar tissue 3.0 mm lateral to the bregma/1.5 mm below the brain surface using the Olympus Cell F Program, from which the background optical density in homologous tissue contralateral to the stroke was subtracted. Iba1 + microglia were analyzed in a blinded way by measuring numbers of cells in six defined regions of interests (ROI) of the striatum ipsilateral to the stroke (size: 500 µm × 500 µm; ROI centered 1.5 mm and 2.5 mm lateral to midline/2.5 mm, 3.25 mm and 4.0 mm below brain surface).17,18 Optical sectioning was used for correction of cell overcounts. CD31 + microvessels were evaluated by counting numbers of microvessels intersecting grid lines within these ROI. For Iba1 + cell counts and CD31 + microvessels, mean values were calculated for all ROI. GFAP + astrogliosis, Iba1 + microglial activation and CD31 + microvessel density were finally normalized with brain atrophy as described above.
Immunohistochemistry for BDA
Forty-µm-thick coronal brain sections obtained from animals sacrificed by transcardiac perfusion with 4% paraformaldehyde (animal set 1) were rinsed three times for 10 min each in 50 mM Tris-buffered saline (pH 8.0) containing 0.5% Triton X-100 (TBST). Sections were incubated overnight with avidin–biotin–peroxidase complex (ABC Elite; Vector Laboratories, Burlingame, CA). Stainings were revealed with 3,3′-diaminobenzidine (DAB) (D4418; Sigma, Deisenhofen, Germany) containing 0.4% ammonium sulfate and 0.004% H2O2.
Analysis of corticorubral projections
The location of tracer deposits was checked at the levels of the needle tracks, thus ensuring that the motor cortex had indeed been injected in all animals. To account for variabilities in tracer uptake in different mice, we first evaluated the number of tracer-stained fibres in the pyramidal tract at the level of the red nucleus. For this purpose, two consecutive sections were analyzed, counting the number of fibres crossing the sections in four regions of interest of 2865 µm2 each that had been selected in the dorsolateral, ventrolateral, dorsomedial, and ventromedial portion of the pyramidal tract. By measuring the total area of the pyramidal tract using the Cell Software image system (Olympus) connected to an Olympus BX42 microscope, we calculated the overall number of labelled pyramidal tract fibres, as described.17,18 Corticorubral projections were evaluated at the level of the parvocellular red nucleus (bregma –3.0 to –3.5 mm). A 500 µm long intersection line was superimposed on the brain midline. Along that line those fibers crossing into the ipsilesional hemisphere in direction of the red nucleus were quantified. For each animal, the total number of fibres counted was normalized with the total number of labeled fibers in the pyramidal tract and multiplied by 100, resulting in percent values of fibers crossing the midline. Two consecutive sections were analyzed for each animal, of which mean values were determined.
Western blots
Tissue samples obtained from the motor cortex ipsilateral or contralateral to the stroke of animals sacrificed by transcardiac perfusion with normal saline (animal set 3) were homogenated using lysis buffer as before. Protein concentration was measured using the Bradford method (BioRad, Hercules, CA). Equal amounts of protein were used for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by protein transfer onto Protran BA83 nitrocellulose blotting membranes (GE Healthcare Life Sciences, Freiburg, Germany). Membranes were blocked in 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 0.1% (TBS-T) for 1 h at room temperature, washed three times in TBS-T, and incubated overnight with polyclonal rabbit anti GluN2A antibody (612-401-D89; Rockland, Limerick, PA), polyclonal rabbit anti-GluN2B antibody (AGC-003; Alomone Labs, Jerusalem, Israel), monoclonal mouse anti-PSD95 antibody (124011; Synaptic Systems, Göttingen, Germany), monoclonal mouse anti-tau5 antibody (ab80579; Abcam, Cambridge, UK), polyclonal rabbit anti-β-actin antibody (#4967L; Cell Signaling Technology, Danvers, MA), or monoclonal rabbit anti-GADPH antibody (#2118S; Cell Signaling Technology, Danvers, MA). The membranes were washed three times, incubated in blocking solution containing secondary antibody conjugated with peroxidase, washed three times, and developed using a chemiluminescence kit (RPN2235; GE Healthcare Life Sciences) according to the instructions of the manufacturers. The optical density of each protein was measured using the Image J program in 4 independent blots, which were corrected for protein loading using β-actin or GAPDH blots. Mean values were calculated for these blots, which were used for further data analysis.
Statistical analysis
Behavioral tests were analyzed using two-way repeated measurement analysis of variance (ANOVA), evaluating main effects of treatment and time and interaction effects of treatment × time starting on day 3 post-stroke (i.e., the time-point of minipump implantation). For all tests, significant main effects of treatment or interaction effects of treatment × time at the p < 0.05 level were noted. Two-tailed t-tests were then performed for each time point. Tract tracing studies were evaluated by one-way ANOVA followed by least significant differences (LSD) tests. All other histochemical data were analyzed by two-way ANOVA, evaluating main effects of treatment and time and interaction effects of treatment × time starting at 14 dpt. Wherever a main effect of treatment or interaction effect of treatment × time was present at the p < 0.05 level, two-tailed t tests were performed for each time point. Data were presented in graphs as mean ± standard deviation (SD) (longitudinal comparisons within the same animals) or in box blots as median/mean ± interquartile range (IQR) with minimum and maximum data (all other comparisons between animals or tissue samples). Throughout the study, p values < 0.05 were considered significant.
Results
Post-acute delivery of memantine improves post-ischemic neurological recovery
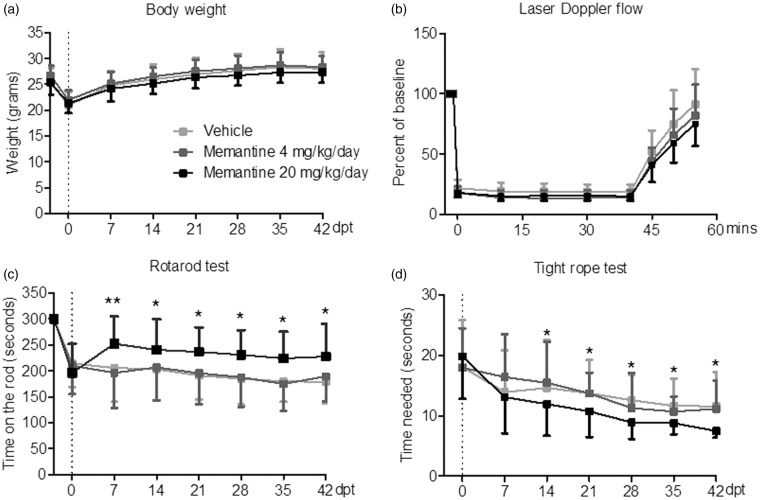
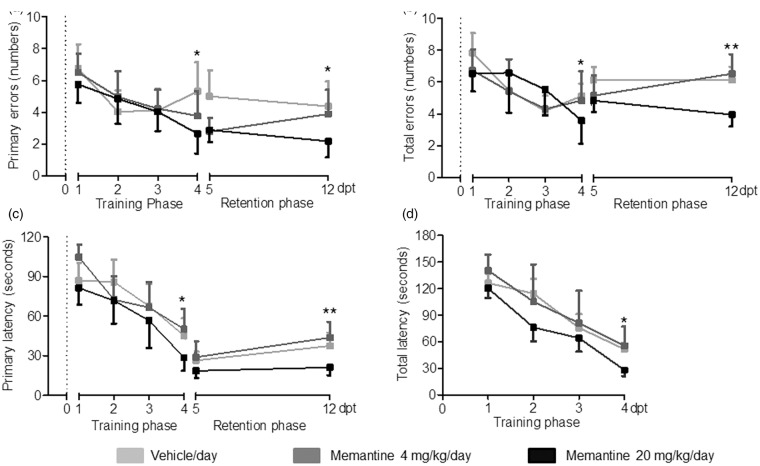
Body weight and laser Doppler flow (LDF) above the core of the MCA territory did not reveal any difference between animals treated with vehicle or memantine (4 or 20 mg/kg/d) (Figure 2a and b). In all groups, LDF decreased to ∼15–20% of baseline during MCAO, followed by a rapid recuperation of blood flow after reperfusion. Rotarod and tight rope tests revealed a clear-defined motor-coordination deficit after MCAO that was attenuated by memantine delivered at 20 mg/kg/d but not 4 mg/kg/d (Figure 2c and d). Interestingly, the improvement of motor-coordination deficits was rapid, being significant already at 7 or 14 dpt. In line with the motor-coordination improvement, spatial memory in the learning and retention phase of the Barnes maze test was better in mice receiving 20 mg/kg/d memantine than vehicle but not in mice receiving 4 mg/kg/d memantine than vehicle (Figure 3). Better performance was similarly noted regarding primary errors in finding the target hole, total errors until escaping in it, primary latency until finding the target hole and total latency until escaping in it.
Figure 2.
Delayed delivery of memantine at 20 mg/kg/d, but not 4 mg/kg/d promotes post-ischemic recovery of motor-coordination deficits. (a) Body weight, (b) LDF recordings above the core of the MCA territory, and (c and d) coordination skills evaluated by RotaRod and tight rope tests in animals exposed to transient MCAO. Vehicle or memantine (4 or 20 mg/kg/d) were subcutaneously administered over 28 d starting 72 h after reperfusion onset. Note the rapid improvement of coordination skills in animals receiving 20 mg/kg/d memantine. LDF recordings (a) and body weight (b) do not differ between groups. Results are means ± SD values (n = 18 animals/ group). Data were analyzed by two-way repeated measurement ANOVA, followed by two-tailed unpaired t tests for individual time-points. *p < 0.05, **p < 0.01 compared with ischemic vehicle.
Figure 3.
Delayed memantine delivery at 20 mg/kg/d enhances post-ischemic spatial memory. (a) Primary errors in finding the target hole, (b) total errors until escaping into the target hole, (c) primary latency until finding the target hole, and (d) total latency until escaping into the target hole of the Barnes maze test in animals exposed to transient MCAO. Vehicle or memantine (4 or 20 mg/kg/d) were subcutaneously administered over 28 d starting 72 h after reperfusion onset. A memory-training phase was followed by a memory retention phase, in which, the target hole was blocked. Results are means ± SD values (n = 18 animals/ group). Data were analyzed by repeated measurement ANOVA, followed by two-tailed unpaired t tests for individual time-points. *p < 0.05, **p < 0.01 compared with ischemic vehicle.
Post-acute memantine delivery promotes peri-infarct tissue remodeling and prevents accumulation of tau sprotein
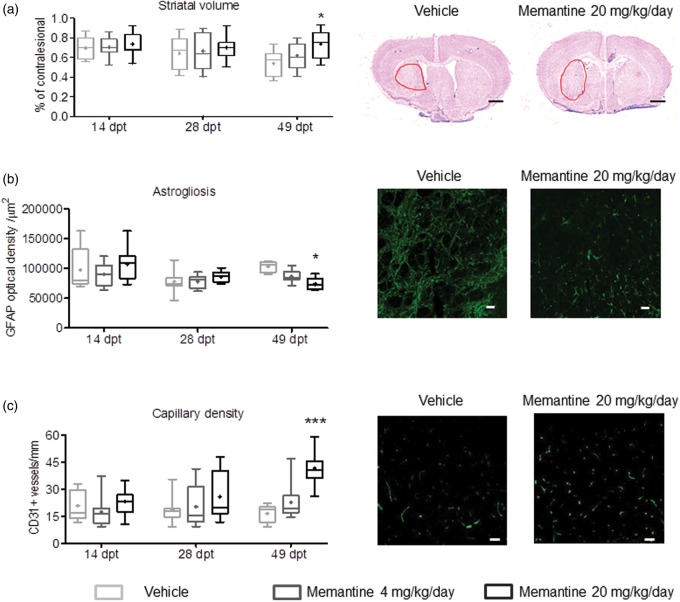
To assess perilesional remodeling in response to memantine, histochemical studies were performed. Cresyl violet stainings revealed localized infarcts restricted to the striatum and most lateral parietal cortex. Notably, the secondary atrophy of the striatum, which represents the core of the MCA territory, was significantly reduced by memantine at 49 dpt (Figure 4a), while whole brain atrophy (Supplementary Figure 2a) and corpus callosum atrophy (Supplementary Figure 2b) where not significantly altered. Peri-infarct astrogliosis, on one hand, evaluated by GFAP immunohistochemistry (Figure 4b), was significantly decreased by memantine at 49 dpt, while capillary density, evaluated by CD31 immunohistochemistry, was significantly increased (Figure 4c). Microglial activation, on the other hand, evaluated by Iba1 immunohistochemistry, was not influenced by memantine (Supplementary Figure 2c). Memantine significantly reduced the accumulation of tau protein in the peri-infarct cortex (Supplementary Figure 3).
Figure 4.
Memantine promotes peri-infarct brain remodeling. (a) Striatal volume, evaluated on cresyl violet-stained brains sections, (b) diffuse astrogliosis, evaluated by GFAP immunohistochemistry, and (c) capillary density, evaluated by CD31 immunohistochemistry, in animals exposed to transient MCAO that were subcutaneously treated with vehicle or memantine (4 or 20 mg/kg/d) starting 72 h after reperfusion onset. Photomicrographs at 49 dpt are also shown. Optical density in (b) was evaluated in the peri-infarct (parietal) cortex, whereas capillary density in (c) was assessed in a total of six ROI within the striatum, as described in the Materials and methods section. Representative microphotographs from these brain regions are shown. Results are medians (lines inside boxes)/means (crosses inside boxes) ± IQR (boxes) and minimum/maximum data (elongation lines) (n = 12 animals/ group). Data were analyzed by two-way ANOVA followed by two-tailed unpaired t tests for individual time-points. *p < 0.05, ***p < 0.001 compared with ischemic vehicle. Bars, 1000 µm (a)/25 µm (b and c).
Post-acute memantine delivery increases growth factor levels in the ipsilesional and contralesional brain hemisphere
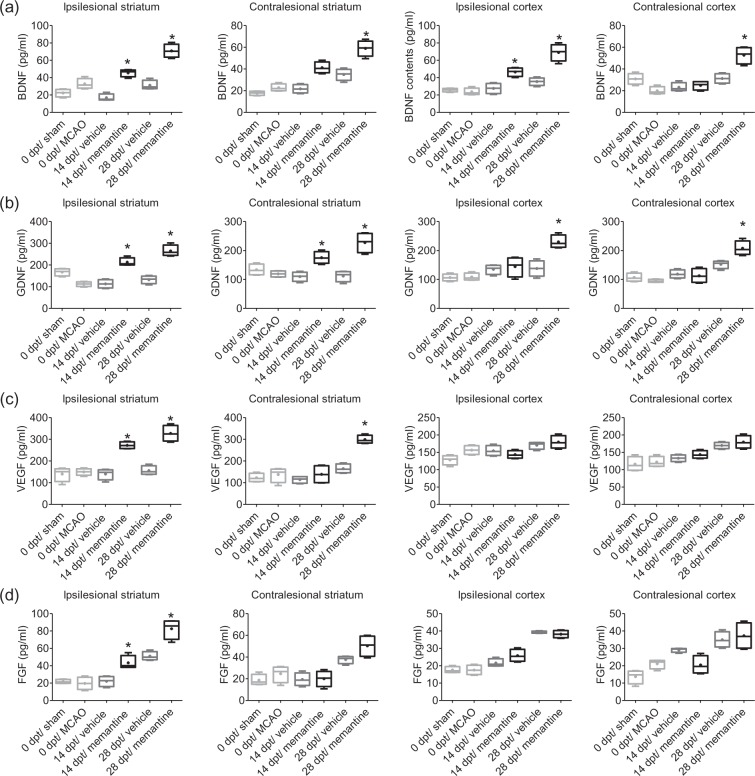
To further characterize restorative responses to post-acute memantine, concentrations of selected growth factors were evaluated in the ipsilesional and contralesional cortex and striatum prior to treatment onset and at 14 and 28 dpt. Interestingly, concentrations of BDNF, GDNF, and VEGF, but not FGF, were significantly increased in the ipsilesional striatum at 14 and 28 dpt and in the ipsilesional cortex, contralesional cortex and contralesional striatum at 28 dpt (Figure 5a–d).
Figure 5.
Memantine elevates growth factor concentrations both ipsilateral and contralateral to the stroke. Concentrations of (a) BDNF, (b) GDNF, (c) VEGF, and (d) FGF in the ipsilesional striatum, contralesional striatum, ipsilesional motor cortex, and contralesional motor cortex, evaluated by ELISA in animals exposed to transient MCAO or sham-surgery that were subcutaneously treated with vehicle or memantine (20 mg/kg/d) starting 72 h after reperfusion onset. Results are medians (lines inside boxes)/means (crosses inside boxes) ± IQR (boxes) and minimum/maximum data (elongation lines) (n = 4 animals/ group). Data were analyzed by two-way ANOVA followed by two-tailed unpaired t tests for individual time-points. *p < 0.05 compared with ischemic vehicle.
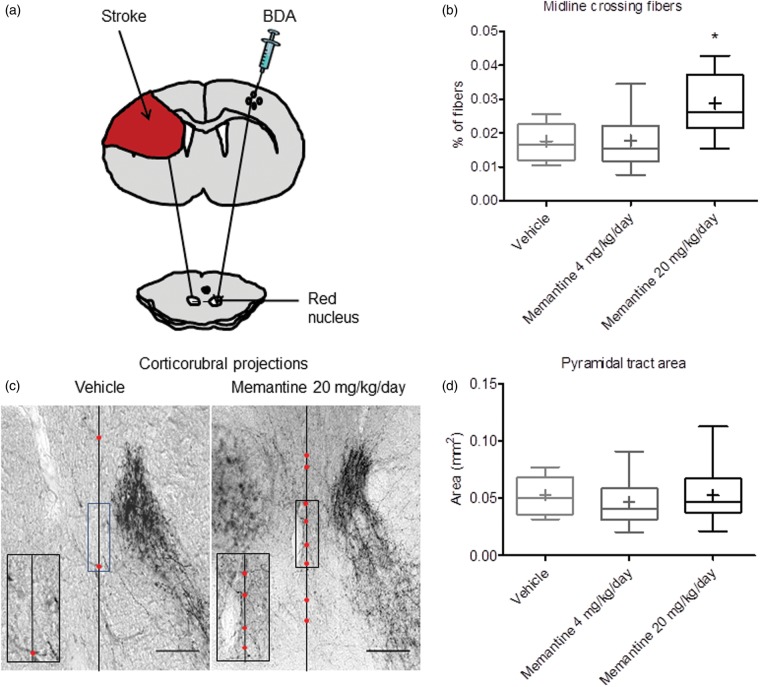
Memantine promotes contralesional corticorubral plasticity
In response to growth factors, namely erythropoietin and VEGF, increased sprouting of contralesional pyramidal tract axons across the midline in direction to ipsilesional midline and brainstem targets has previously been reported.17,18 In view of the elevation of growth factors in the contralesional motor cortex, we asked whether memantine influenced contralesional pyramidal tract plasticity evaluated by the anterograde tract tracer BDA, which we injected into the contralesional motor cortex at 42 dpt (Figure 6a). BDA-stained fibers originating from the contralesional motor cortex crossed the pyramidal tract in order to branch off dorsomedially at mesencephalic levels, terminating as previously described in the contralesional parvocellular red nucleus.17 At this level, we quantified the number of midline-crossing terminal fibers emanating from this fiber bundle in direction to the ipsilesional parvocellular red nucleus and normalized them with the total number of fibers determined in the contralesional pyramidal tract at the same rostrocaudal level. Compared with animals receiving vehicle, animals receiving 20 but not 4 mg/kg/d memantine revealed a significantly higher number of midline crossing fibers (Figure 6b and c). The pyramidal tract area contralesional to the stroke was not altered by memantine (Figure 6d), indicating that memantine did not affect pyramidal tract integrity.
Figure 6.
Memantine promotes contralesional corticorubral plasticity. (a) Midline-crossing fibers originating from the contralesional motor cortex in direction to the ipsilesional parvocellular red nucleus were evaluated by injection of the anterograde tract tracer BDA into the contralesional motor cortex at 42 dpt. (b) Percentage of BDA-labeled midline-crossing fibers after subcutaneous delivery of vehicle or memantine (4 or 20 mg/kg/d). Note the significant increase of midline-crossing fibers in response to 20 mg/kg/d, but not 4 mg/kg/d memantine (representative microphotographs with magnified inlets are shown in (c)). (d) Total area of the contralesional pyramidal tract at the level of the red nucleus, which does not change in response to memantine, indicating the absence of contralesional corticospinal tract degeneration. Results are medians (lines inside boxes)/means (crosses inside boxes) ± IQR (boxes) and minimum/maximum data (elongation lines) (n = 18 animals/group). Data were analyzed by one-way ANOVA followed by LSD tests. *p < 0.05 compared with ischemic vehicle. Bar, 100 µm (B).
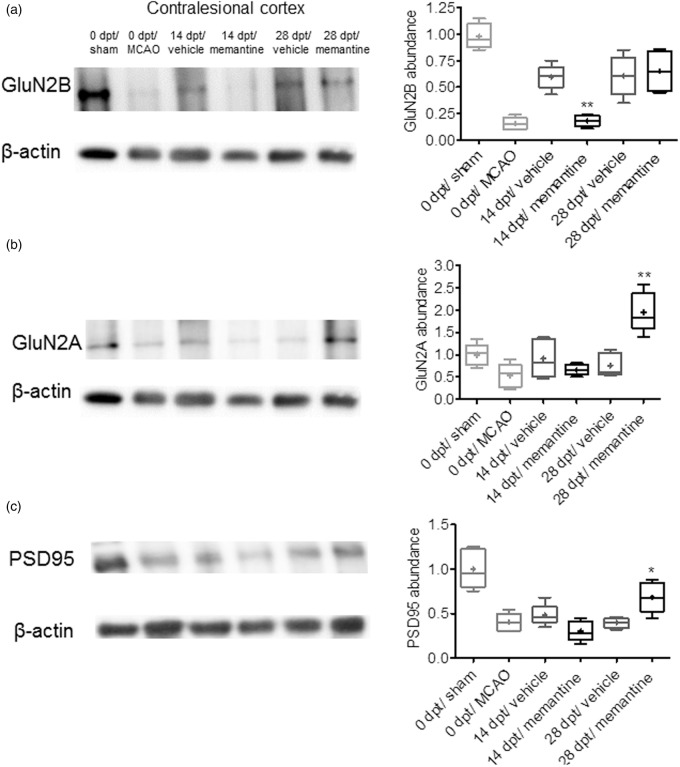
Memantine regulates contralesional motor cortical GluN2B, GluN2A, and PSD95 abundance
In view of the finding that memantine induced lesion-remote brain plasticity, we next examined how memantine at a dose of 20 mg/kg/d influenced the abundance of the NMDA receptor subunits GluN2B, which predominantly localizes to extrasynaptic NMDA receptors targeted by memantine,9 and GluN2A, which is preferentially found in synaptic NMDA receptors,9 as well as of post-synaptic density protein-95 (PSD-95), which upon NMDA receptor binding consolidates synaptic plasticity processes,3 by Western blotting. In the contralesional motor cortex, the overall abundance of GluN2B, GluN2A, and PSD-95 proteins was generally lower at 3 days post-stroke than after sham-surgery (Figure 7a–c; Supplementary Figure 4). Protein abundance was partly restored in vehicle-treated animals at 14 and 28 dpt. Interestingly, memantine reduced GluN2B, but not GluN2A and PSD-95 abundance in the contralesional motor cortex at 14 dpt (Figure 7a–c; Supplementary Figure 4). Conversely, memantine increased GluN2A and PSD-95, but not GluN2B abundance in the contralesional motor cortex at 28 dpt (Figure 7a–c; Supplementary Figure 4).
Figure 7.
Memantine regulates contralesional motor cortical NMDA receptor components. Western blots for (a) the NMDA receptor subunit GluN2B, which predominantly localizes in extrasynaptic NMDA receptors targeted by memantine, (b) GluN2A, which is preferentially found in synaptic NMDA receptors, and (c) PSD-95, which upon NMDA receptor binding consolidates synaptic plasticity processes, in the contralesional motor cortex of animals exposed to transient MCAO or sham-surgery. Note that in response to stroke, all three proteins are reduced at 3 d after reperfusion. Protein abundance is partly restored in vehicle-treated animals at 14 and 28 dpt. Interestingly, memantine reduces GluN2B abundance at 14 dpt and increases GluN2A and PSD-95 abundance at 28 dpt. Representative blots are also shown. Results are medians (lines inside boxes)/means (crosses inside boxes) ± IQR (boxes) and minimum/maximum data (elongation lines) (n = 4 independent blots). Data were analyzed by two-way ANOVA followed by two-tailed paired t tests for individual time-points. *p < 0.05, **p < 0.01 compared with ischemic vehicle.
Discussion
We herein show that the low-affinity NMDA antagonist memantine, which is clinically used in Alzheimer’s disease, induces sustained post-ischemic neurological recovery, when delivered in the post-acute stroke phase starting 72 hours post-stroke. Functional neurological recovery was associated with sustained peri-infarct brain remodeling, i.e., prevention of secondary brain atrophy, reduction of astrogliosis, promotion of capillary formation, reduction of tau accumulation, and elevation of growth factors that was accompanied by the promotion of contralesional corticorubral plasticity. This lesion-remote plasticity was associated with coordinated changes in the abundance of the NMDA receptor components GluN2B, GluN2A, and PSD-95 in the contralesional motor cortex.
We were surprised that memantine enhanced motor-coordination almost immediately within 7–14 dpt. In this respect, memantine differed from studies with growth factor (erythropoietin, VEGF),17,18,22 neural precursor cell,18 and hydroxymethylglutaryl-CoA reductase inhibitor23 delivery, where we previously observed slowly evolving motor-coordination improvements that became detectable only after several weeks using the same MCAO model. Importantly, motor-coordination recovery persisted after the termination of memantine delivery. These findings clearly show that functional neurological improvements were not simply symptomatic. As expected from observations in Alzheimer’s pathology,6,7 memantine enhanced spatial memory acquisition and retention.
Structural neuroprotective effects have previously been described after acute memantine delivery following focal cerebral ischemia in rats and mice.1,11,12 Interestingly, in two more recent studies, memantine reduced behavioral deficits without affecting structural brain injury, when delivered up to 2 h post-stroke,13,14 suggestive that memantine in addition to its neuronal survival promoting effects may have restorative actions after stroke. In one study using photothrombotic stroke in mice,14 memantine decreased peri-infarct astrogliosis and increased capillary density. Considering the early memantine delivery, hidden neuroprotective effects could not completely be ruled out. The evaluation of neuroprotection effects was not objective of this study. Prevention of reactive astrogliosis, promotion of angiogenesis, and prevention of secondary brain atrophy have repeatedly been reported as accompaniments of successful brain remodeling post-stroke.17,18,20,22
Prompted by the observation that memantine increased concentrations of the growth factors BDNF, GDNF, and VEGF in both hemispheres, we studied pyramidal tract plasticity contralateral to the stroke. Growth factors, namely erythropoietin and VEGF, have previously been demonstrated to promote contralesional pyramidal tract plasticity.17,18,22 Indeed, a higher density of BDA-labeled corticorubral fibers originating from the contralesional motor cortex in direction to the ipsilesional red nucleus was noted in memantine-treated mice compared with vehicle-treated mice. Following acute memantine delivery, increased abundance of BDNF and its receptor Trk-B associated with ipsilesional sensory forepaw map remodeling have previously been reported in the ischemic hemisphere.14 By demonstrating changes of growth factors and pyramidal tract plasticity contralateral to the stroke, we now expand these observations, providing evidence that memantine induces profound neuronal rewiring that is not restricted to the lesion rim.
Mechanistically, the restorative actions of memantine may be attributed to its preferential inhibitory effect on extrasynaptic glutamate receptors,8–10 which are strongly activated upon stroke, stimulating neuronal death pathways and impeding neuronal plasticity, while synaptic glutamate receptors have opposite effects in the stroke brain, inhibiting neuronal death pathways and promoting neuronal plasticity.2,3 Arguing in favor of this hypothesis, the NMDA receptor subunit GluN2B, which predominantly localizes to extrasynaptic NMDA receptors,9 was transiently downregulated in the contralesional motor cortex by memantine, whereas GluN2A and PSD-95, which are preferentially accumulated in synaptic NMDA receptors,3,9 were upregulated by memantine. Our observations argue in favor of a facilitation of synaptic as compared with extrasynaptic NMDA receptor signaling, which might explain memantine’s restorative effects post-stroke. After focal cerebral ischemia, overactivation of GluN2B-containing extrasynaptic NMDA receptors has specifically been shown to promote cerebral tau protein accumulation.24 Hence, the downregulation of GluN2B might represent a mechanism via which cerebral tau accumulation was prevented.
In this study, the improvement of neurological recovery, re-regulation of NMDA receptor proteins and elevation of growth factors preceded the prevention of secondary brain atrophy, reduction of astrogliosis, promotion of capillary formation, prevention of cerebral tau accumulation, and promotion of contralesional corticorubral plasticity, indicating that as a consequence of NMDA receptor re-regulation pleiotropic growth factors were induced that promoted brain remodeling and plasticity. According to our data, symptomatic treatments acting on neurotransmitter balance may induce rapid functional neurological improvements, at the same time, promoting trophic responses that allow the induction of structural brain remodeling and plasticity both in the vicinity and at distance to the stroke.
The clear strength of this study is the delivery of an NMDA antagonist that is clinically used in patients suffering from Alzheimer’s disease. By demonstrating that memantine promotes post-ischemic neurological recovery, brain remodeling, and plasticity, we provide a rationale for the delivery of memantine to patients in the post-acute stroke phase. The dose of memantine we used is higher than that delivered in human patients (20 mg per patient per day). Yet, in view of the far larger plasma half-life of memantine in humans (60–100 h) than rodents (2–5 h), memantine plasma levels are very similar (∼0.5–1.0 µM), with brain levels being ∼30–40% lower.25 The main limitation of this study is the use of—compared with humans—relatively simple motor-coordination tests, which were able to document an improvement of stroke recovery with a statistical power (1—beta error) of 80%, given that the alpha error was 5%. Our data open fascinating perspectives for clinical studies, in which NMDA receptor antagonists might be re-evaluated in the post-acute stroke setting.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the German Research Council (HE3173/3-1) and China Scholarship Council (2011614002).
Supplementary Material
Acknowledgements
The authors gratefully acknowledge in depth discussions with Dr. Christopher Parsons, Department of Pharmacology, Merz Pharmaceuticals (Frankfurt, Main, Germany) regarding pharmacological properties of memantine.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
YW and DMH designed the study; YW performed the animal experiments; YW, ESM and TRD conducted the histochemical and molecular biological studies; YW and DMH drafted the manuscript; and all four authors finalized it.
Supplementary information
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Lipton SA. Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders. J Alzheimers Dis 2004, pp. S61–S74. [DOI] [PubMed] [Google Scholar]
- 2.Lai TW, Shyu WC, Wang YT. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol Med 2011; 17: 266–275. [DOI] [PubMed] [Google Scholar]
- 3.Lai Tw, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 2014; 115: 157–188. [DOI] [PubMed] [Google Scholar]
- 4.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 Experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 5.Savitz SI, Schäbitz WR. A critique of SAINT II: wishful thinking, dashed hopes, and the future of neuroprotection for acute stroke. Stroke 2008; 39: 1389–1391. [DOI] [PubMed] [Google Scholar]
- 6.Tan CC, Yu JT, Wang HF, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimer’s Dis 2014; 41: 615–631. [DOI] [PubMed] [Google Scholar]
- 7.Matsunaga S, Kishi T, Iwata N. Memantine monotherapy for Alzheimer's disease: a systematic review and meta-analysis. Plos one 2015; 10 e0123289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordji K, Becerril-Ortega J, Nicole O, et al. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ß production. J Neurosci 201030: 15927–15942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rush T, Buisson A. Reciprocal disruption of neuronal signaling and Aβ production mediated by extrasynaptic NMDA receptors: a downward spiral. Cell Tissue Res 2014356: 279–286. [DOI] [PubMed] [Google Scholar]
- 10.Wu YN, Johnson SW. Memantine selectively blocks extrasynaptic NMDA receptors in rat substantia nigra dopamine neurons. Brain Res 2015; 1603: 1–7. [DOI] [PubMed] [Google Scholar]
- 11.Chen HS, Wang YF, Rayudu PV, et al. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience 1998; 86: 1121–1132. [DOI] [PubMed] [Google Scholar]
- 12.Culmsee C, Junker V, Kremers W, et al. Combination therapy in ischemic stroke: synergistic neuroprotective effects of memantine and clenbuterol. Stroke 2004; 35: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 13.Babu CS, Ramanathan M. Pre-ischemic treatment with memantine reversed the neurochemical and behavioural parameters but not energy metabolites in middle cerebral artery occluded rats. Pharmacol Biochem Behav 2009; 92: 424–432. [DOI] [PubMed] [Google Scholar]
- 14.López-Valdés HE, Clarkson AN, Ao Y, et al. Memantine enhances recovery from stroke. Stroke 2014; 45: 2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata R, Maeda K, Hermann D, et al. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2000; 20(6): 937–946. [DOI] [PubMed] [Google Scholar]
- 16.Hermann DM, Kilic E, Hata R, et al. Relationship between metabolic dysfunctions, gene responses and delayed cell death after mild focal cerebral ischemia in mice. Neuroscience 2001; 104(4): 947–955. [DOI] [PubMed] [Google Scholar]
- 17.Reitmeir R, Kilic E, Kilic U, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain 2011; 134: 84–99. [DOI] [PubMed] [Google Scholar]
- 18.Reitmeir R, Kilic E, Reinboth BS, et al. Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol 2012; 123: 273–284. [DOI] [PubMed] [Google Scholar]
- 19.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 1979; 93: 74–104. [DOI] [PubMed] [Google Scholar]
- 20.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009; 132: 2239–2251. [DOI] [PubMed] [Google Scholar]
- 21.Doeppner TR, Mlynarczuk-Bialy I, Kuckelkorn U, et al. The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 2012; 135: 3282–3297. [DOI] [PubMed] [Google Scholar]
- 22.Herz J, Reitmeir R, Hagen SI, et al. Intracerebroventricularly delivered VEGF promotes contralesional corticorubral plasticity after focal cerebral ischemia via mechanisms involving anti-inflammatory actions. Neurobiol Dis 2012; 45: 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilic E, Reitmeir R, Kilic Ü, et al. HMG-CoA reductase inhibition promotes neurological recovery, peri-lesional tissue remodeling, and contralesional pyramidal tract plasticity after focal cerebral ischemia. Front Cell Neurosci 2014; 8: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu CS, Liu AC, Chen J, et al. Overactivation of NR2B-containing NMDA receptors through entorhinal-hippocampal connection initiates accumulation of hyperphosphorylated tau in rat hippocampus after transient middle cerebral artery occlusion. J Neurochem 2015; 134(3): 566–577. [DOI] [PubMed] [Google Scholar]
- 25.Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology 2007; 53(6): 699–723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.