Abstract
Focal hyperperfusion after acute ischaemic stroke could be of prognostic value depending upon its spatial localisation and temporal dynamics. Factors associated with late stage (12–24 h) perilesional hyperperfusion, identified using arterial spin labelling, are poorly defined. A prospective cohort of acute ischaemic stroke patients presenting within 4.5 h of symptom onset were assessed with multi-modal computed tomography acutely and magnetic resonance imaging at 24 ± 8 h. Multivariate logistic regression analysis and receiver operating characteristics curves were used. One hundred and nineteen hemispheric acute ischaemic stroke patients (mean age = 71 ± 12 years) with 24 h arterial spin labelling imaging were included. Forty-two (35.3%) patients showed perilesional hyperperfusion on arterial spin labelling at 24 h. Several factors were independently associated with perilesional hyperperfusion: good collaterals (71% versus 29%, P < 0.0001; OR = 5, 95% CI = [1.6, 15.7], P = 0.005), major reperfusion (81% versus 48%, P = < 0.0001; OR = 7.5, 95% CI = [1.6, 35.1], P = 0.01), penumbral salvage (76.2% versus 47%, P = 0.002; OR = 6.6, 95% CI = [1.8, 24.5], P = 0.004), infarction in striatocapsular (OR = 9.5, 95% CI = [2.6, 34], P = 0.001) and in cortical superior division middle cerebral artery (OR = 4.7, 95% CI = [1.4, 15.7], P = 0.012) territory. The area under the receiver operating characteristic curve was 0.91. Our results demonstrate good arterial collaterals, major reperfusion, penumbral salvage, and infarct topographies involving cortical superior middle cerebral artery and striatocapsular are associated with perilesional hyperperfusion.
Keywords: Stroke, perfusion imaging, arterial spin labelling, hyperperfusion, collaterals, topography
Introduction
Grouping of acute stroke patients into specific imaging profiles can allow greater understanding of prognosis and can assist in the selection of patients for specific acute therapies or interventions.1,2 A similar strategy may also be clinically relevant in guiding stroke rehabilitation interventions; however, understanding of specific imaging profiles that are predictive of longer term stroke recovery is limited. Perfusion imaging is a technique now widely used in stroke research and clinical practice. Arterial spin labelling (ASL), a quantitative magnetic resonance imaging (MRI) perfusion measurement technique that harnesses blood water as an endogenous contrast agent, is able to demonstrate hypoperfusion and hyperperfusion patterns in stroke patients.3 Unlike contrast-based perfusion MRI, ASL does not require injectable gadolinium-based contrast agents and shows strong agreement with MRI perfusion measurements.4
Focal hyperperfusion is caused by local or regional increases in cerebral blood flow. Hyperperfusion on ASL perfusion MRI has drawn growing interest lately, a few groups including the authors' have shown that ASL hyperperfusion is associated with haemorrhagic transformation (HT) as well as good or poor clinical outcome depending on the spatial localisation and temporal dynamics. While there is a general consensus that post-operative hyperperfusion following carotid endarterectomy and carotid artery stenting is detrimental and is associated with poor clinical outcomes,5,6 post-ischaemic hyperperfusion may have positive or negative prognostic impact.7–9 In 1996, Marchal et al. noted the phenomenon of hyperperfusion on acute stage perfusion positron emission tomography (PET) images and co-registered with chronic-stage computed tomography (CT) scans in patients with acute middle cerebral artery (MCA) stroke.10 The authors noted that the areas with hyperperfusion were both significantly larger than the final infarct and topographically distinct from the site of final infarction. The hyperperfusion patterns were more commonly found in infarct topographies covering deep MCA regions including striatocapsular territory consistent with lenticulostriate artery mouth occlusion during transient MCA embolism. The authors postulated that spontaneous non-haemorrhagic hyperperfusion may be a marker for favourable tissue outcome.10,11 A recent study using ASL MRI has shown that a select group of patients with ischaemic stroke show focal regions of perilesional hyperperfusion (PLH) on ASL at 24 h and that these patients have better clinical recovery from their initial stroke.8 It is also recognised that focal hyperperfusion early after brain ischaemia can be identified following transient ischaemic attacks and is associated with reversible deficits.12 Extensive studies on animal models have also shown that hyperperfusion is the hallmark of early and efficient recanalisation.13–15 However, there are limited data on the association of PLH with clinical outcomes and more human studies are needed. Moreover, factors associated with late stage (12–24 h) PLH are poorly defined.
Leptomeningeal collaterals are secondary collateral pathways that act as anastomotic channels, towards maintaining blood flow to brain regions distal to an arterial occlusion, in conditions such as acute ischaemic stroke (AIS) where cerebral blood flow is pathologically altered.16,17 They play an important role in sustaining brain viability until reperfusion. Good baseline collaterals are strongly associated with better clinical outcomes in AIS patients. Leptomeningeal collaterals are relatively dense in and around the cortical superior middle cerebral vascular territory. Therefore, arterial collaterals supplying the perilesional areas around the infarct topographies involving cortical superior MCA and lenticulostriate (feeding the striatocapsular region) arterial territories may have a role in focal PLH patterns observed on ASL. To this end, a study on the association of baseline collateral status and the topography of infarcts with late stage (12–24 h) PLH may be useful in understanding the underlying pathophysiological mechanism. In this prospective study, we sought to examine clinical and neuroradiological correlates of late stage (12–24 h) post-ischaemic focal PLH in a group of AIS patients using ASL mapping of brain perfusion. The specific aims of the project were as follows:
To study the association of PLH at 24 h with baseline collateral status.
To identify infarct topographies that associate with PLH at 24 h (identified using MRI ASL blood flow measurement).
To investigate the factors associated with early PLH in these infarct topographies.
Our underlying hypotheses were: (a) that better collateral flow grades will be associated with PLH, (b) that there will be variation in the degree of PLH seen depending on the topography of the ischaemic lesion, and (c) that the infarction in the cortical superior MCA and deep MCA territories such as in striatocapsular region (as per Marchal et al.10) would more commonly show evidence of PLH than other infarct topographies.
Materials and methods
Study design and patient selection
Consecutive acute ischaemic stroke patients admitted to the acute stroke unit at an academic medical centre were prospectively studied applying the following inclusion criteria: (a) presented within 4.5 h of stroke symptom onset, (b) evaluated for the eligibility to receive thrombolysis, (c) age > 18 years, (d) hemispheric stroke, and (d) ASL MRI acquired within 24 h of symptom onset. Patients with lacunar infarctions (small artery occlusion) were excluded. Patients with motion artefacts on ASL source images were also excluded. All patients were managed according to the discretion of the treating stroke physician and based on local guidelines. This study was approved by the Hunter New England Human Research Ethics Committee (Newcastle, NSW) in accordance with the National Statement on Ethical Conduct in Human Research 2007. All patients gave informed consent.
Demographics (age, sex), thrombolytic treatment, and clinical risk factors (hypertension, diabetes, dyslipidaemia, history of smoking, present smoker, atrial fibrillation, depression, and history of stroke or transient ischaemic attack) were assessed in a structured case-record form. Stroke aetiology was categorised for every patient according to the Causative Classification System (CCS) criteria.18 Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) at baseline (immediately before acute CT) and at 24 h. Stroke severity at admission was categorised into mild (for NIHSS = 0–7), moderate (NIHSS = 8–16), and severe (NIHSS = 17 and above).19 Onset to treatment time, the time delay between stroke onset and administration of tPA, was also recorded.
Imaging acquisition
In accordance with our routine stroke imaging protocol, all the patients underwent non-contrast CT (NCCT), CT perfusion (CTP) with dynamic CT angiography (dCTA) at baseline. Follow-up MRI including diffusion-weighted imaging (DWI) and ASL perfusion MRI was also obtained at 24 ± 8 h. CTA was also obtained at 24 h for measuring the adequacy of reperfusion. All the CT scans were obtained using a 320-detector row 640-slice cone beam multi-detector CT (Aquilion One, Toshiba Medical Systems). NCCT was followed by dCTA and CTP which were acquired simultaneously in two 60 s series. CTP/CTA was obtained in the axial plane before and after the intravenous bolus injection of contrast agent (50 ml of Ultravist 370; Bayer HealthCare, Berlin, Germany) injected at a rate of 6 ml/s chased by 50 ml of saline (acquisition parameters: 120 kV, 128 mA s, scanning coverage (SC = 240 mm) and scanning width = 5 mm). After a delay of 7 s post-contrast administration pulsed full rotation scan with 19 time points was acquired over 60 s with a total pulse image acquisition time of 9.5 s. In order to examine the extracranial vessels, CTA of the extracranial segment was also acquired using bolus tracking with 50 ml of contrast injected at 6 ml/s chased by 50 ml of saline. The total radiation exposure was 5.5–6.0 mSev.
Following baseline CT examination, follow-up MR imaging was performed at 24 ± 8 h based on routine stroke imaging protocol, that included an axial isotropic DWI spin-echo echo-planar imaging sequence, time-of-flight MR angiography, and whole brain perfusion-weighted imaging (PWI) with bolus-tracking dynamic susceptibility contrast-PWI and axial T2-weighted echo planar sequence, on a 3T MRI (Magnetom Verio; Siemens Verio, Erlangen, Germany) with a 32-channel receive-only head coil.20 ASL was performed using a pulsed sequence with a 32 channel receive-only head coil, using quantitative perfusion imaging with a single subtraction, with thin-slice TI1 periodic saturation (Q2TIPS) technique,8,21 having following image parameters: TR 2500 ms, TI1 500 ms, TI1s 1500 ms, inversion time (TI2) 1700 ms, FOV 240 × 240 mm, matrix 64 × 64. This acquired nine slices at 8 mm thickness with 28 repetitions for a scan time of 4:02 min. Images were inspected for the presence of artefacts.
Imaging analysis
All CTP/CTA and perfusion MR data were de-identified prior to analysis, following which both CTP and MR perfusion maps, including mean transit time and cerebral blood volume were generated with MIStar software (Apollo Medical Imaging Technology, Melbourne, Australia), which uses a deconvolution algorithm (using single value deconvolution with delay and dispersion correction) to process the data.22 An arterial input function and venous outflow function was semiautomatically selected from the non-stroke (contralateral) hemisphere MCA/ACA and sagittal sinus, respectively. Previously validated thresholds were applied in order to measure the volume of the acute perfusion lesion (relative delay time (DT) ≥ 3 s) and acute infarct core (relative CBF≤30%).23 Penumbral volume was calculated from the volume of the perfusion lesion (DT threshold ≥ 3 s) minus the volume of the infarct core (relative CBF threshold < 30% within the DT ≥ 3 s lesion). The choice of DT ≥ 3 s as the threshold was based on recent studies.23,24 In addition to the extent of the penumbra in terms of acute penumbral volume, we also recorded presence/absence of acute penumbra for statistical analyses.
ASL imaging data were processed using MIStar as per the protocol described in our previous work.8 PLH was defined as hyperperfusion pattern observed on ASL-MRI as a local CBF of > 130%, surrounding the lesion compared to contralateral healthy tissue (in areas of penumbral salvage), within the acutely hypoperfused area but topographically separate from the 24 h DWI lesion.7,8
Neuroradiological evaluation
Any infarction on the DWI was assigned to either one or multiple arterial territories depending upon its topographical location, i.e. anterior (ACA), middle (cortical superior and inferior divisions) (MCA), posterior cerebral (PCA), lenticulostriate (covering the striatocapsular region) (SCI), anterior choroid, and thalamic artery (see Supplementary Information (SI), Figure 1). Presence/absence of any infarct in a given arterial territory was assigned as 1/0. For example, for a large cortical MCA infarct involving multiple arterial territories including cortical superior MCA and striatocapsular areas, both cortical superior MCA and striatocapsular were assigned 1. Infarcts were also classified into distinct (involving one territory) and multi-territorial (involving multiple territories) topographies. The extent of infarction in a given arterial territory (see Supplementary Figure 1) on DWI image was determined based on whether the infarct covers >80% of arterial territory (‘complete’) or it covers <80% of arterial territory (‘partial’). Watershed infarcts occur in the border zones between major cerebral arterial territories.25 Watershed territory infarcts were classified into: (a) external watershed infarction (EWI): border zone of ACA/MCA and MCA/PCA, and (b) internal watershed infarction: border zone between lenticulostriate perforators and the deep penetrating cortical branches of the MCA or at the border zone of deep white matter branches of the MCA and the ACA.
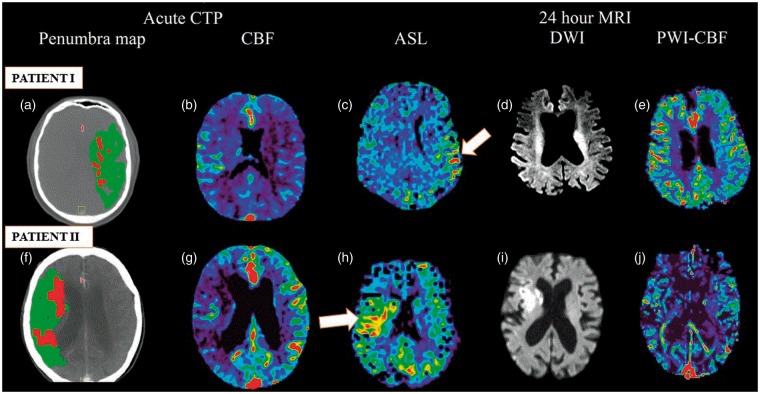
Figure 1.
Multimodal imaging (acute CTP and follow-up 24 h MRI) showing perilesional hyperperfusion (PLH) on ASL at 24 h. Patient I (upper row): CTP demonstrated acute left MCA territory infarct with a small core and large penumbra (a), and decreased rCBF (b). Post-thrombolysis, patient showed excellent recanalisation. PLH patterns were observed on ASL-MRI at 24 h (c). Area of restricted diffusion was observed on follow-up DWI-MRI (d). PWI-CBF at 24 h is also depicted (e). Patient II (bottom row): Acute CTP shows large penumbra (f) and decreased rCBF (g) over a large part of the right MCA territory indicating a large area of hypoperfused but likely viable tissue (penumbra). Post-thrombolysis, the patient showed excellent response to therapy. The regions that were previously hypoperfused showed PLH (white arrow) at 24 h on ASL (h). DWI-MRI (i) demonstrated an area of restricted diffusion. Hyperperfusion patterns are not clearly evident on PWI-CBF (j). Both patients showed good collaterals on baseline CTA.
ASL: arterial spin labelling; CBF: cerebral blood flow; CT: computed tomography; CTA: computed tomographic angiography; CTP: computed tomographic perfusion imaging; DWI: diffusion-weighted imaging; MCA: middle cerebral artery; MIP: maximum intensity projection; PWI: perfusion-weighted imaging.
For static collateral grading, we reviewed CTA axial source images and the multiplanar reformats in the sagittal and coronal planes.26 Collateral circulation status was assessed from the CTA data based on the degree of reconstitution of the MCA up to the distal end of its occlusion and was divided into ‘good’ or ‘poor/reduced’ as described in the protocol used by Miteff et al.27 The exact location of the thrombus or clot (M1 proximal (M1P), M1 distal (M1D), M2, M3, or internal carotid artery (ICA)) was determined. Furthermore, the precise location of the thrombus was also recorded using three-dimensional volume rendering of dCTA. We defined proximal clot as any thrombus/occlusion in M1P or ICA. Any thrombus/occlusion in M1D, M2, or M3 was classified as a distal clot. Clinical, CT, and MRI imaging data were assessed for the following metrics: (a) aetiological classification based on CCS, (b) extent of infarction in the territory of cerebral artery (classified into complete (>80% territory) and partial (<80% territory), (c) presence of minor and major petechial infarctions (defined by minor: < 5 mm diameter, and major > 5 mm diameter), (d) watershed territory infarction, (e) presentation of HT,28 (f) reperfusion status, and (g) collateral grading.27 These images were read by consensus by two experienced readers (SB & CL). All neuroradiological assessment was done blind to the ASL findings.
Outcome measures
Acute HT was defined by hypointensity on -MRI located within the infarction zone as described elsewhere.29 HT was further classified based on extent and severity as per the protocol used in the European-Australasian Acute Stroke Study (ECASS II) trial in the following categories: diffuse haemorrhagic (petechial (HI1) or confluent (HI2)) infarction and parenchymal haemorrhage (PH1 or PH2, defined as space occupying effect).30 At 24 h a repeat CTA was performed and assessed by an independent blinded reviewer for the adequacy of angiographic reperfusion using thrombolysis in the cerebral infarction (TICI) score. TICI is a widely used angiographic score in endovascular stroke studies. Major reperfusion was assessed as modified TICI (mTICI) grade 2b or 3 (defined as tissue reperfusion in ≥50% of the occluded artery territory).31 Partial reperfusion was defined as the mTICI grade of 2a (defined as tissue reperfusion in <50% of the occluded artery territory). The mTICI grade of 0–1 was identified as no reperfusion defined either by the presence of no anterograde flow or the flow past the initial occlusion but with no tissue perfusion.
The modified Rankin Scale (mRS) was used to assess clinical outcome in terms of functional status at three months; a score of 0–2 represented a good outcome and a score of 3 and above indicated poor outcome. The early neurological change was categorised as early neurological improvement (ENI) or early neurological deterioration (END). ENI was defined as a gain of NIHSS ≥8 within the first 24 h or NIHSS < 2 at 24 h.32,33 END was defined as ≥4-point deterioration on 24 h NIHSS.34
Final infarct volume (DWI lesion) at 24 h was calculated. The difference between the acute CTP (PWI lesion) volume and the 24 h DWI lesion volume was used to assess penumbral salvage.8,35 Patients with any degree of penumbral salvage were identified as ones with smaller 24 h DWI lesion volume than the acute perfusion lesion volume.8
Case presentation
Two cases demonstrating the use of multi-modal (CT & MRI) imaging in patients with right and left MCA territory infarct, respectively, showing PLH on ASL-MRI at 24 h, are shown in Figure 1.
Statistical analysis
All the statistical analyses were performed using STATA (Version 10, 2001; College Station, TX, USA). Numerical values given are the means (±standard deviation) or medians (interquartile range) for age, mRS scores, NIHSS at admission, and NIHSS at 24 h scores as appropriate. For ordinal or continuous data, Mann–Whitney (Wilcoxon rank-sum) test was used. Nominal data were analysed with the Pearson's chi-squared (χ2) and the two-tailed Fisher's exact test. We compared two groups of patients stratified by hyperperfusion status (hyperperfusion versus no hyperperfusion). Group differences were considered significant at values of P < 0.05. Univariate logistic regression analysis was used to test associations between covariates and 24 h PLH. Multivariate analysis was also performed to study the association with the long-term clinical outcome (at 90 days). Results of logistic regression are reported as odds ratios (ORs). Only those variables with P < 0.1 were tested in the subsequent multivariate regression analysis. Before fitting the multivariate model, we also tested the multicollinearity using the variance inflation factor (VIF): a common rule of thumb is that VIFs > 5 identify strongly collinear pairs.36 If two or more covariates were found to be collinear, we retained the variable that was more strongly associated with the PLH to ensure a stable model. A multivariate regression model, based on a backwards, the stepwise approach was used; only the most important ‘explanatory’ variables were retained to arrive at the most parsimonious model. Comparison between the various multivariate regression models was made using model selection statistics (including the Akaike information criterion (AIC) and the Bayesian information criterion (BIC)). Multivariate normality (of the regression model) was also checked using the Doornik–Hansen test. The sensitivity, specificity, and overall rate of correct classification for each model were estimated using classification statistics using a cut-off (positive outcome threshold) of 0.5. The goodness of fit using Pearson χ2 test and a number of covariate patterns were calculated for each multivariate model. Finally, the receiver operating characteristic (ROC) curve for the regression model was plotted, and the area under the curve (AUC) was computed to evaluate the predictive ability.
Results
Baseline characteristics
The demographics, risk factors, and other clinical characteristics of the overall study population, stratified by hyperperfusion status, are detailed in Table 1. In total, 119 patients (mean age = 71 ± 12.5 years; the number of males/females = 54 (45.38%)/65 (54.6%)) with hemispheric ischaemic stroke and 24 h ASL imaging data were included in the study, out of which 42 (35.3%) patients showed PLH within 24 h. Thirty-five patients with small artery occlusion/lacunar infarcts were excluded from the study. One hundred patients (84%) received intravenous recombinant tissue plasminogen activator (rtPA) as early thrombolytic therapy. The median NIHSS score on admission was 14 points (interquartile range (IQR) = 8). The results of the topographic classification of infarcts are also given in Table 1. Distinct watershed infarcts were observed in 26 (21.8%) patients, with the majority of them having EWI (n = 24). Thirty-seven (31%) patients had infarcts in border zone areas as an extension of territorial infarcts.
Table 1.
List of baseline characteristics stratified by perilesional hyperperfusion (PLH).
| All patients | PLH | No PLH | ||
|---|---|---|---|---|
| n = 119 | n = 42 | n = 77 | Pa | |
| Number of patients | 119 | 42 (35.29) | 77 (64.71) | |
| Male | 54 (45.38) | 16 (38.1) | 38 (49.35) | 0.255 |
| Female | 65 (54.62) | 26 (61.9) | 39 (50.65) | 0.255 |
| Age, in years (mean ± SD) | 70.9 ± 12.45 | 69.7 ± 13.9 | 71.5 ± 11.6 | 0.4377 |
| Median baseline NIHSS (IQR) | 14 [8] | 13 [8] | 14 [7] | 0.3054 |
| Time to tPA (in mins); mean ± SD | 158.7 ± 81 | 161.9 ± 74.8 | 156.9 ± 85 | 0.5593 |
| OTT (in min); mean ± SD | 163.5 ± 84.29 | 163.6 ± 80 | 163.4 ± 86.8 | 0.8694 |
| Acute core; median (IQR) | 13.5 [24] | 15.95 [20.4] | 9.2 [24.3] | 0.1569 |
| Acute penumbra, ml; median (IQR) | 47.9 [63.5] | 46.35 [84.7] | 53.1 [62] | 0.2613 |
| Presence of acute penumbra | ||||
| Yes | 96 (80.67) | 36 (85.71) | 60 (77.92) | 0.342 |
| No | 23 (19.33) | 6 (14.29) | 17 (22.08) | 0.342 |
| Occlusion on admission CTA | ||||
| Yes | 73 (61.34) | 28 (66.67) | 45 (58.44) | 0.434 |
| No | 46 (38.66) | 14 (33.33) | 32 (41.56) | 0.434 |
| Clot location | 0.517 | |||
| Proximal thrombus (ICA + M1P) | 37 (31.09) | 13 (30.95) | 24 (31.17) | 1.000 |
| Distal thrombus (M1D + M2 + M3) | 40 (33.61) | 16 (38.10) | 24 (31.17) | 0.543 |
| ICA | 22 (18.49) | 6 (14.29) | 16 (20.78) | 0.464 |
| M1 proximal (M1P) | 20 (16.8) | 9 (21.43) | 11 (14.29) | 0.32 |
| M1 distal (M1D) | 16 (13.45) | 5 (11.9) | 11 (14.29) | 0.786 |
| M2 and M3 | 24 (20.17) | 11 (26.19) | 13 (16.88) | 0.241 |
| Treatment factors | ||||
| Thrombolysed | 100 (84.03) | 37 (88.10) | 63 (81.82) | 0.441 |
| Risk factors | ||||
| Hypertension | 106 (89.08) | 38 (90.48) | 68 (88.31) | 1.000 |
| Diabetes | 37 (31.09) | 13 (30.95) | 24 (31.17) | 1.000 |
| Dyslipidaemia | 54 (45.38) | 16 (38.1) | 38 (49.35) | 0.2555 |
| Present smoker (n = 119) | 25 (21.01) | 10 (23.81) | 15 (19.48) | 0.640 |
| Past smoker (n = 119) | 50 (42.02) | 20 (47.61) | 30 (38.96) | 0.438 |
| AF | 69 (57.98) | 26 (61.9) | 43 (55.84) | 0.564 |
| Depression | 12 (10.08) | 4 (9.52) | 8 (10.39) | 1.000 |
| History of stroke/TIA (n = 153) | 20 (16.81) | 6 (14.29) | 14 (18.18) | 0.798 |
| Aetiology (CCS classification) | ||||
| Large artery atherosclerosis | 64 (53.78) | 27 (64.29) | 37 (48.05) | 0.124 |
| Cardioembolic | 54 (45.38) | 15 (35.71) | 39 (50.65) | 0.128 |
| Collateral grade | ||||
| Good | 53 (44.54) | 30 (71.43) | 23 (29.87) | <0.0001* |
| Poor/reduced | 66 (55.46) | 12 (28.57) | 54 (70.13) | <0.0001* |
| Stroke topography (any infarction in given territory) | ||||
| ACA | 9 (7.56) | 3 (7.14) | 6 (7.79) | 1.000 |
| Complete | 2 (1.68) | 1 (2.38) | 1 (1.30) | 1.000 |
| Partial | 7 (5.88) | 2 (4.76) | 5 (6.49) | 1.000 |
| Absent | 110 (92.44) | 39 (92.86) | 71 (92.21) | 1.000 |
| Cortical superior MCA | 66 (55.46) | 32 (76.19) | 34 (44.16) | 0.001* |
| Complete | 41 (34.45) | 18 (42.86) | 23 (29.87) | 0.164 |
| Partial | 25 (21.01) | 14 (33.33) | 11 (14.29) | 0.019* |
| Absent | 53 (44.54) | 10 (23.81) | 43 (55.84) | 0.001* |
| Striatocapsular | 55 (46.22) | 30 (71.43) | 25 (32.47) | <0.0001* |
| Complete | 35 (29.41) | 13 (30.95) | 22 (28.57) | 0.835 |
| Partial | 20 (16.81) | 17 (40.48) | 3 (3.90) | <0.0001* |
| Absent | 64 (53.78) | 12 (28.57) | 52 (67.53) | <0.0001* |
| Inferior division MCA | 92 (77.31) | 29 (69.05) | 63 (81.82) | 0.168 |
| Complete | 30 (25.21) | 8 (19.05) | 22 (28.57) | 0.279 |
| Partial | 62 (52.10) | 21 (50) | 41 (53.25) | 0.848 |
| Absent | 27 (22.69) | 13 (30.95) | 14 (18.18) | 0.168 |
| Anterior choroidal artery | 28 (23.53) | 9 (21.43) | 19 (24.68) | 0.822 |
| Complete | 11 (9.24) | 2 (4.76) | 9 (11.69) | 0.324 |
| Partial | 17 (14.29) | 7 (16.67) | 10 (12.99) | 0.593 |
| Absent | 91 (76.47) | 33 (79.57) | 58 (75.32) | 0.822 |
| PCA | 17 (14.29) | 4 (9.52) | 13 (16.88) | 0.412 |
| Complete | 4 (3.36) | 0 | 4 (5.19) | 0.296 |
| Partial | 13 (10.92) | 4 (9.52) | 9 (11.69) | 1.000 |
| Absent | 102 (85.71) | 38 (90.48) | 64 (83.12) | 0.412 |
| Watershed Infarcts (EWI or IWI) | ||||
| External watershed infarcts (EWI) | 24 (20.17) | 7 (16.67) | 17 (22.08) | 0.634 |
| Internal watershed infarcts (IWI) | 2 (1.68) | 1 (2.38) | 1 (1.30) | 1.000 |
| Both EWI and IWI | 0 | |||
| Infarcts in border zone (extension of territorial infarcts) | 37 (31.09) | 15 (35.71) | 22 (28.57) | 0.535 |
ACA: anterior cerebral artery; AChA: anterior choroid artery; AF: atrial fibrillation; IQR: interquartile range; MCA: middle cerebral artery; NIHSS: National Institute of Health Stroke Scale Score; OTT: onset to treatment time; PCA: posterior cerebral artery; SCI: striatocapsular, thalamic; SD: standard deviation; TIA: transient ischaemic attack.
Continuous or ordinal variables are shown as mean ± SD or median (IQR), as appropriate. Mann–Whitney test was used to compare continuous or ordinal variables. Nominal data were analysed with the Pearson's chi-squared (χ2) and the two-tailed Fisher's exact test. CCS classification method was used to assess the causative aetiological mechanism underlying the stroke event. Infarct topographies are defined as complete, partial, or no infarction (absent) in that territory. Collateral grading was classified as either good or reduced.
Figures in parentheses are percentages or inter-quartile range (IQR).
P < 0.05 as the threshold for statistical significance.
Uncorrected for multiple comparisons.
PLH versus non-PLH
Good collateral grades at baseline were more common in PLH patients (71% versus 30%; P < 0.0001). Analysis of infarct topography and PLH status demonstrated an association with cortical superior division MCA (76.2% versus 44.2%; P = 0.001) and/or SCI territories (71.4% versus 32.5%; P < 0.0001). Chronic petechial infarction patterns did not differ in proportion between PLH and non-PLH patients. There was a tendency towards higher rates of PLH in patients with large artery atherosclerosis aetiology (64.3% versus 48%, P = 0.124).
In terms of clinical outcome (see Table 2), PLH patients demonstrated significantly higher rates of major reperfusion (81% versus 48%, P < 0.0001), ENI (58.5% versus 37.7%, P = 0.034), and HT (16.7% versus 1.35%, P = 0.003) at 24 h. PLH patients also showed the significantly higher proportion of occurrence of penumbral salvage (76.2% (32/42) versus 46.8% (36/77), P = 0.002). Moreover, mean penumbral salvage volume was significantly greater in PLH patients (22.2 ml versus 3.8 ml, P = 0.028). PLH group showed a tendency towards lower 24 h median NIHSS scores (6 versus 7.5, P = 0.085) and a higher proportion of good clinical outcome at 90 days (61.9% versus 52%, P = 0.338). Non-PLH patients showed significantly higher rates of nil reperfusion at 24 h (39% versus 12%, P = 0.002).
Table 2.
List of clinical outcomes stratified by perilesional hyperperfusion (PLH).
| All patients | PLH | No PLH | ||
|---|---|---|---|---|
| n = 119 | n = 42 | n = 77 | Pa | |
| 24 h core volume (in ml); median (IQR) | 22.4 [61.1] | 16.2 [33.1] | 35.3 [63.7] | 0.1413 |
| Median 24 h NIHSS (IQR) (n = 153) | 7 (11) | 6 (8) | 7.5 (13) | 0.0850 |
| Any penumbral salvage | ||||
| Yes | 68 (57.14) | 32 (76.19) | 36 (46.75) | 0.002* |
| No | 51 (42.86) | 10 (23.81) | 41 (53.25) | 0.002* |
| Penumbral salvage volume (in ml) | ||||
| Median (IQR) | 9.9 [91.9] | 22.75 [68.9] | −6.8 [103.4] | 0.0277* |
| Mean ± SD | 10.33 ± 65.87 | 22.23 ± 71.65 | 3.83 ± 62.01 | 0.0277* |
| Reperfusion status | 0.002* | |||
| Major reperfusion | 71 (59.66) | 34 (80.95) | 37 (48.05) | <0.0001* |
| Incomplete reperfusion | 15 (12.61) | 3 (7.14) | 12 (15.58) | 0.252 |
| No reperfusion | 35 (29.41) | 5 (11.9) | 30 (38.96) | 0.002* |
| HT | 8 (6.9) | 7 (16.67) | 1 (1.35) | 0.003* |
| HI1 or HI2 | 7 (6.03) | 7 (16.67) | 0 (0) | 0.001* |
| PH1 or PH2 | 1 (0.86) | 0 | 1 (1.35) | 1.000 |
| Major petechial (>5 mm) | 94 (81.03) | 33 (78.57) | 61 (82.43) | 0.629 |
| Minor petechial (<5 mm) | 109 (93.97) | 40 (95.24) | 69 (93.24) | 1.000 |
| ENI (ΔNIHSS ≥ 8 or ΔNIHSS < 2) | 53 (44.92) | 24 (58.54) | 29 (37.66) | 0.034* |
| END (ΔNIHSS≤−4) | 5 (4.24) | 0 (0) | 5 (6.49) | 0.162 |
| Median 90 days mRS (IQR) | 2 (3) | 2 (2) | 2 (4) | 0.1775 |
| Good (mRS = 0–2) | 66 (55.46) | 26 (61.9) | 40 (51.95) | 0.338 |
| 0 | 23 (19.33) | 8 (19.05) | 15 (19.48) | 1.000 |
| 1 | 23 (19.33) | 11 (26.19) | 12 (15.58) | 0.224 |
| 2 | 20 (16.81) | 7 (16.67) | 13 (16.88) | 1.000 |
| Bad (mRS = 3–6) | 53 (44.54) | 16 (38.1) | 37 (48.05) | 0.338 |
| 3 | 15 (12.61) | 6 (14.29) | 9 (11.69) | 0.775 |
| 4 | 13 (10.92) | 5 (11.90) | 8 (10.39) | 0.769 |
| 5 | 14 (11.76) | 4 (9.52) | 10 (12.99) | 0.768 |
| 6 | 11 (9.24) | 1 (2.38) | 10 (12.99) | 0.094 |
END: early neurological deterioration; ENI: early neurological improvement; HT: haemorrhagic transformation; mRS: modified Rankin score.
The clinical outcome at 90 days was assessed using mRS (score range: 0–6), where an mRS score of 0–2 corresponds to a good outcome.
Figures in parentheses are percentages or interquartile range (IQR).
P < 0.05 as the threshold for statistical significance.
Uncorrected for multiple comparisons.
Association with PLH
The results of univariate logistic regression analysis examining the association with the PLH (OR and levels of significance) are shown in Table 3. Neither demographic factors (age, sex), stroke severity at admission, thrombolytic treatment, or other clinical risk factors differed significantly between patients with and without PLH. Good collateral status strongly associated with 24 h PLH. Patients with good baseline collateral grades constituted 71% of PLH patients. Patients who developed PLH had a higher prevalence of infarctions in cortical superior MCA and striatocapsular territories. In logistic regression analysis, the OR for the occurrence of PLH was 5.5 in infarcts with partial lesions in cortical superior MCA territory and 24.6 in infarcts with partial lesions in the striatocapsular territory. Patients who received thrombolytic treatment were 1.6 times more likely to show PLH at 24 h. Large artery atherosclerotic patients were two times more likely to show PLH.
Table 3.
Odds ratios (95% confidence intervals) and P-value (only with P≤0.1 shown) for the variables associated with perilesional hyperperfusion (PLH) at 24 h using bivariate logistic regression analysis.
| Variable | % of patients with hyperperfusion within 24 h | OR (95% confidence interval) | P > |z| |
|---|---|---|---|
| Baseline characteristics | |||
| Stroke severity at admission (unit increase) | 0.97 (0.9, 1.03) | 0.285 | |
| Thrombolytic treatment | 88.1 | 1.6 (0.55, 4.93) | 0.375 |
| Collateral grade (n = 151) | |||
| Good | 71.4 | 5.87 (2.56, 13.44) | <0.0001* |
| Poor/reduceda | 28.6 | 1 | |
| Aetiology (CCS classification) | |||
| Large artery atherosclerosis | 64.3 | 1.94 (0.9, 4.2) | 0.092 |
| Cardioembolic | 35.7 | 0.54 (0.25, 1.17) | 0.120 |
| Stroke topography (any infarction in given territory) | |||
| Infarction in cortical sup MCA territory | |||
| Present | 76.2 | 4.05 (1.75, 9.38) | 0.001* |
| Absenta | 23.8 | 1 | |
| Extent of cortical sup MCA infarction | 0.0032* | ||
| Complete | 42.9 | 3.37 (1.34, 8.48) | 0.010* |
| Partial | 33.3 | 5.47 (1.92, 15.6) | 0.001* |
| Absenta | 23.8 | 1 | |
| Infarction in striatocapsular territory | |||
| Present | 71.4 | 5.2 (2.29, 11.83) | <0.0001* |
| Absenta | 28.6 | 1 | |
| Extent of striatocapsular infarction | <0.00001* | ||
| Complete | 31 | 2.56 (1.01, 6.49) | 0.047* |
| Partial | 40.5 | 24.56 (6.19, 97.47) | <0.0001* |
| Absenta | 28.6 | 1 | |
| Clinical outcomes | |||
| NIHSS at 24 h (unit increase) | 0.92 (0.87, 0.98) | 0.014* | |
| Reperfusion status | 0.0035* | ||
| Major reperfusion | 80.95 | 5.33 (1.85, 15.34) | 0.002* |
| Partial reperfusion | 7.14 | 01.58 (0.32, 7.76) | 0.572 |
| No reperfusion | 11.9 | 1 | |
| Penumbral salvage | |||
| Yes | 76.19 | 3.64 (1.57, 8.44) | 0.003* |
| No | 23.81 | 1 | |
| Penumbral salvage volume (in ml) | 1.004 (0.998, 1.01) | 0.147 | |
| HT | 16.7 | 14.6 (1.73, 123.3) | 0.014* |
| HT classification | |||
| HI1 or HI2 | 16.7 | Predicts PLH perfectly. | |
| PH1 or PH2 | 0 | 1 | |
| ENI | 58.5 | 2.34 (1.08, 5.07) | 0.032* |
| mRS at 90 days | 0.86 (0.7, 1.05) | 0.132 | |
| Good (mRS = 0-2) | 61.9 | 1.5 (0.69, 3.2) | 0.297 |
| Bad (mRS 3–6) | 38.1 | 0.67 (0.31, 1.43) | 0.297 |
| Mortality (mRS = 6) | 2.38 | 0.16 (0.02, 1.32) | 0.09 |
HT: haemorrhagic transformation; mRS: modified Rankin Score; SCI: striatocapsular; sup MCA: cortical superior division middle cerebral artery.
Continuous or ordinal variables are shown as mean ± SD or median (IQR). Figures in parentheses are an interquartile range.
Used as reference category.
P < 0.05 as the threshold for statistical significance.
Logistic regression analysis also showed significant association of HT with PLH. In particular, the presence of any developing haemorrhagic infarctions (HI1 or HI2) predicted PLH perfectly. In terms of reperfusion status at 24 h, PLH was significantly associated with major reperfusion. PLH patients were two times more likely to demonstrate ENI within 24 h with 58.5% of PLH patients showing ENI. Moreover, PLH patients were significantly associated with lower 24 h NIHSS scores per unit increase. The presence of any penumbral salvage was associated with PLH. However, a unit increase in penumbral salvage volume did not show significant association with PLH. PLH patients were 1.5 times more likely to have good clinical outcomes at 90 days. However, the association failed to reach statistical significance.
Multivariate regression analysis
Covariates or baseline characteristics with p≤0.1 (any infarction in cortical superior division MCA or SCI territory, collateral status, HT, NIHSS at 24 h, ENI, reperfusion status, penumbral salvage) and other clinically important (OR > 1.5) variables (thrombolytic treatment and large artery atherosclerosis) were tested for collinearity (Supplementary Information, Table 1). No strong multicollinearity was detected.
Among the baseline characteristics, good collaterals, infarction in cortical superior MCA territory, and infarction in SCI territory were strongly associated with PLH (see Model 1, Table 4). In reduced model, large artery atherosclerosis and thrombolysis were eliminated because of them not being significantly associated. Multivariate modelling was done by fitting a model with all potential (non-collinear) covariates (Model I).
Table 4.
Stepwise-backwards multivariate logistic regression analysis showing the association of baseline characteristics/covariates with PLH at 24 h.
| OR (95% CI) | P > |z| | |
|---|---|---|
| Model I: Baseline characteristics association with PLH (Model parameters: n = 119; dof = 4; pseudo R2 = 0.2767; area under the ROC curve = 0.85; goodness of fit (Pearson χ2) test: P = 0.1458; AIC = 119.8; BIC = 130.9; number of covariate patterns = 8; sensitivity = 66.7%; specificity = 89.61%; PPV = 77.8%; NPV = 83.13%; overall rate of correct classification = 81.5%). | ||
| Good collaterals | 8.4 (3.1, 22.5) | <0.0001* |
| Infarction in cortical superior MCA territory | 3.7 (1.3, 10.6) | 0.013* |
| Infarction in striatocapsular territory | 4.2 (1.6, 11.1) | 0.004* |
| Model II covariates association with PLH (model parameters: n = 116; dof = 8; pseudo R2 = 0.4284; area under the ROC curve = 0.91; goodness of fit (Pearson χ2) test: P = 0.1724; AIC = 102.81; BIC = 124.84; number of covariate patterns = 39; sensitivity = 69.1%; specificity = 91.9%; PPV = 82.86%; NPV = 83.95%; overall rate of correct classification = 83.6%). | ||
| Good collaterals | 5.05 (1.62, 15.7) | 0.005* |
| Infarction in cortical superior MCA territory | 4.7 (1.4, 15.7) | 0.012* |
| Infarction in striatocapsular territory | 9.45 (2.63, 33.96) | 0.001* |
| HT | 7.95 (0.52, 122.3) | 0.137 |
| Reperfusion status | 0.0357* | |
| Major reperfusion | 7.5 (1.6, 35.1) | 0.01* |
| Incomplete reperfusion | 3.7 (0.4, 30.2) | 0.227 |
| No reperfusion | 1a | |
| Penumbral salvage | ||
| Yes | 6.64 (1.8, 24.49) | 0.004* |
| Noa | 1 | |
A/BIC: Akaike/Bayesian information criteria; dof: degrees of freedom; ENI: early neurological improvement; HT: haemorrhagic transformation; NPV: negative predictive value; PPV: positive predictive value; ROC: receiver–operator characteristic curve.
Used as reference category.
P < 0.1 for enter and stay criteria. The two multivariate models differed in terms of the particular covariates/factors included.
P < 0.05 as the threshold for statistical significance.
Among the covariates, independent predictors of 24 h PLH selected by stepwise logistic regression were collateral grade, infarction in cortical superior MCA territory, infarction in the striatocapsular territory, reperfusion status, HT, and penumbral salvage. Results of the stepwise backwards multivariate logistic regression are shown in Table 4, Model II. Multivariate regression analysis revealed that stroke severity at admission, thrombolysis, large artery atherosclerosis, NIHSS at 24 h, and ENI did not appear to influence the PLH.
In the reduced model, several factors were positively associated with PLH (Model II): good collaterals, infarction in SCI territory, infarction in cortical superior MCA territory, major reperfusion, and penumbral salvage. HT showed a trend towards association with increasing PLH rates; however, this did not reach statistical significance. The lower AIC and BIC values indicated that the reduced model fitted the data better than the Model I (AIC = 102 versus 120; BIC = 125 versus 131). The overall rate of correct classification of the reduced model was estimated to be 84%, with 92% specificity and 69.1% sensitivity. The ROC area (AUC) of approximately 0.91 indicated excellent discrimination/accuracy for the model. Finally, the independent predictors of PLH were good collaterals, major reperfusion at 24 h, penumbral salvage, infarction in cortical superior MCA territory, and infarction in the striatocapsular territory.
Association with 90 days clinical outcome
Among PLH patients, 62% showed good clinical outcome at 90 days. There was a tendency towards good clinical recovery in PLH patients. However, in this cohort, the PLH did not demonstrate a significant association with 90 days clinical outcome. In a multivariate analysis, following variables were found to be independently associated with clinical outcome at 90 days: NIHSS at admission, ENI, and core volume.
Discussion
In this study, we sought to examine the clinical and radiological correlates of post-acute (12–24 h) focal PLH in a large sample of AIS patients using non-invasive ASL mapping of brain perfusion. We found that PLH is independently associated with good collaterals, cortical superior division MCA stroke, striato-capsular infarction and occurrence of major reperfusion at 24 h. The previously recognised associations using PET and ASL between PLH and more favourable clinical outcomes may imply that there is longer lived penumbra in some patients, and its survival, presumably via later reperfusion, is supported by the presence of PLH. Alternatively, if PLH is occurring in tissue without residual ischaemia, the hyperperfusion may be resulting in other, yet to be defined, protective mechanisms. Whatever the underlying mechanism, the presence of PLH correlates with favourable clinical trajectory. To the best of our knowledge, there are none or limited prior studies that have investigated the link between infarct topography, collateral status, and PLH at 24 h. Moreover, clinical application of ASL in stroke population is still very limited.
The principal finding of this study was that PLH, defined on ASL-MRI within 12–24 h of the stroke onset, was significantly associated with good baseline collateral vessel status measured using CTA at the time of initial baseline acute stroke imaging. Baseline collateral status is now recognised to be important in the evaluation and treatment of cerebral ischaemia.32,33 The presence and a higher grade of collateral supply through leptomeningeal sources correlate with the presence of smaller final infarct volume,37 improved recanalisation rates, and favourable functional outcomes in patients undergoing endovascular revascularisation therapy.27,38–40 Therapeutically effective recanalization in a setting of poor baseline collaterals may result in increased rates of HT,38,41 thereby leading to worsening of neurological status by restricting effective reperfusion. Our results show that collateral flow accurately predicts 24 h PLH patterns, which have been previously shown to be associated with a reduction in neurological severity at 24 h, penumbral survival until reperfusion, and better clinical outcomes at 90 days,8 in AIS. From a therapeutic standpoint, we postulate that patients with the good collateral flow may have infarcts associated with hyperperfusion in perilesional areas. Moreover, good collaterals may be sustaining penumbral survival until reperfusion. This mechanism may be responsible for driving a favourable clinical trajectory. However, in this study, we acknowledge that we did not show causality between collateral status and PLH.
Our findings also show that two infarct topographies, involving cortical superior MCA and striatocapsular territories, are associated with increased rates of PLH patterns. This is in agreement with the previous study by Marchal et al. where they also found that PLH was more commonly seen in patients with small-sized infarcts, located deep in the MCA territory where there is survival of overlying or adjacent cortex, consistent with lenticulostriate artery mouth occlusion during transient MCA embolism.10,42–45 We postulate that the presence of good collaterals is probably an important determinant (possibly a pre-requisite) of the development of the PLH patterns seen on ASL-MRI supported by the knowledge: when there are excellent leptomeningeal collaterals, the cortex is spared and hence infarcts develop only in SCI territory.46,47 The PLH patterns being more prevalent in cortical superior division MCA as opposed to inferior division MCA are in keeping with the recognised denser collateralisation via leptomeningeal communications from ACA to MCA compared to PCA to MCA.48,49 The cortical superior division MCA is potentially fed by the leptomeningeal arteriolar anastomoses (including end-to-end anastomoses, end-to-side connections, and azygos variants) from the ACA, whereas the inferior division MCA is fed by collaterals arising from the PCA.47,49–51
In our acute stroke cohort, major reperfusion was significantly associated with PLH. This is corroborated by the previous studies that linked the presence of relative hyperperfusion of the ipsilateral hemisphere, ranging from 20% to 44%, observed in complications of the carotid revascularisation such as carotid endarterectomy, to the reperfusion.5 Recanalisation post t-PA could have been the driver of major reperfusion. Our results also show a significant association between PLH and ENI. This is consistent with previous studies by Marchal et al.10,52 Moreover, we found that the PLH patients demonstrated a tendency to favourable long-term clinical recovery (64%). Our findings suggest that late stage reperfusion can be harmless, and the fact that PLH may be a marker of favourable tissue outcome concurs with Marchal et al.10,11
We found a strong association between PLH and HT (as expected, since hyperperfusion is a marker of reperfusion of a severely ischaemic tissue with vascular damage), but not with PH1–PH2 (although only one of those occurred). The non-association of PLH with PH1–PH2 could be explained in terms of the small number of HT in our AIS cohort. Owing to the small sample size, it is very well possible that we did not find occurrences of PH1–PH2 due to low power. This may be a matter for future investigation. Multivariate modelling also demonstrated a trend towards higher PLH rates in patients presenting with HT, a subgroup of our cohort who are eight times more likely to show HT than their non-PLH counterparts. Our results are supported by a recent study also showing a strong link between postischaemic hyperperfusion detected by ASL-MRI and HT.9 Using multivariate logistic regression Yu et al. demonstrated that hyperperfusion was an independent risk factor for HT and that hyperperfusion patients were approximately three times as likely to experience HT compared with patients without hyperperfusion. However, the study included patients presenting with hyperperfusion both within (lesional) as well as around (peri-lesional) DWI lesions. In this study, we also found that patients receiving intravenous thrombolytic treatment were more likely to develop PLH; however, the association was not significant.
Our study revealed that ASL imaging can be used to capture post-ischaemic PLH patterns in AIS patients. Our incidence of PLH (42/119; 35.3%) seen in topographically distinct areas from the site of infarction corresponds well with the previous studies.10,53,54 This is especially appropriate given the known limitations of ASL for reduced sensitivity to regions of low perfusion (underestimation of tissue perfusion) in the presence of prolonged arterial transit delays. The shortening of, or avoidance of prolonged, arterial transit time enables easy detection of hyperperfusion on ASL. In an interesting study by Zaharchuk et al., arterial transit artefacts on ASL was used to identify collaterals in patients with Moyamoya disease for angiographic validation.55 Assessment of lesion topography, collateral status, reperfusion, and post-ischaemic hyperperfusion patterns using multimodal imaging can provide additional metrics for patient selection towards intervention strategies and prognosis in acute settings. It may also be useful in guiding appropriate recovery intervention strategies; a therapeutic approach with a focus on infarct topography and collateral status may improve the long-term clinical outcomes, and hence impact the quality of life for treated patients. This may have implications for prognostication in hyper-acute settings and patient selection towards identifying potential recovery targets for long-term therapy and rehabilitation. However, further research is warranted to better evaluate the utility of this imaging-based approach.
Limitations
There may be a selection bias in this study as observed in the high rate of thrombolysis in our cohort's case mix. Endovascular (intra-arterial) treatment is not available at our facility. Therefore, we may not be able to generalise our results to patients receiving intra-arterial therapy. Additionally, it is unknown when patients reperfused exactly while all patients had a relatively variable time of onset from stroke and the majority received rtPA; we cannot be certain how long after rtPA infusion that reperfusion occurred. This may mean that some patients reperfused significantly later than others, thereby limiting their ability to develop hypoperfusion due to poor reperfusion despite the appearance of successful reperfusion 24 h later. However, we consider this variability as strength to our study considering its similitude to the heterogeneity of case mix encountered clinically. Moreover, the large sample size used in this study may reduce the effect of some of the limitations. Nevertheless, future studies on even larger sample size may provide additional insights to our findings. The ASL method used in our study is based on a pulsed sequence with TI1 of 500 ms and TI2 of 1700 ms, resulting in a post-labelling delay of 1200 ms, which is short especially for stroke patients. We acknowledge that this may be relatively ‘old’ with regard to other recent studies on similar lines. As such, the difficulty in differentiation of delayed arterial transit effect from hyperperfusion could possibly have caused confounding effects. It would also be interesting to assess whether hyperglycaemia is associated with PLH. This could potentially be subject of future investigation.
Conclusion
Post-ischaemic hyperperfusion in acute settings can be of prognostic value depending on the spatial localisation and temporal dynamics. PLH in AIS patients predicts a more favourable recovery in some patients. We have identified that several lesion topographies and collateral status at baseline are associated with PLH. In terms of clinical outcomes, PLH was also found to be associated with penumbral salvage and major reperfusion at 24 h. This study is novel considering its pathophysiological and clinical standpoints. Predicting post-stroke recovery remains a challenge to stroke physicians. However, profiling patients using imaging metrics may enable clinicians to better understand prognosis and design individualised stroke recovery protocols. Further studies are required to replicate our results in a broader case mix of acute stroke and to measure effects of these synergistic factors and to further evaluate the long-term recovery trajectories of the various imaging defined subgroups. This may have practical significance for therapeutic clinical trials of acute stroke. Our study also shows the potential application of non-invasive imaging using ASL in the acute stroke population. ASL at 12–24 h can be useful for pathophysiological investigations.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Hunter Medical Research Institute & the University of Newcastle.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contribution
Contributors SB, MP, AB, and CL conceived and designed the study. SB, AB, and CL collected and analysed the data. SB, CL, and JRA contributed in the data analysis. SB, PS, and CL drafted the article, and all authors (SB, CL, PS, AB, JRA, MN, and MP) contributed towards the patient recruitment, study design, data analysis, drafting, and revision of the article.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.De Georgia M. Extending the time window for stroke treatment: advanced brain imaging +/− bat saliva. Int J Stroke 2009; 4: 94–96. [DOI] [PubMed] [Google Scholar]
- 2.Yoo AJ, Pulli B, Gonzalez RG. Imaging-based treatment selection for intravenous and intra-arterial stroke therapies: a comprehensive review. Exp Rev Cardiovasc Therapy 2011; 9: 857–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deibler AR, Pollock JM, Kraft RA, et al. Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. AJNR 2008; 29: 1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber MA, Gunther M, Lichy MP, et al. Comparison of arterial spin-labeling techniques and dynamic susceptibility-weighted contrast-enhanced MRI in perfusion imaging of normal brain tissue. Invest Radiol 2003; 38: 712–718. [DOI] [PubMed] [Google Scholar]
- 5.Karapanayiotides T, Meuli R, Devuyst G, et al. Postcarotid endarterectomy hyperperfusion or reperfusion syndrome. Stroke 2005; 36: 21–26. [DOI] [PubMed] [Google Scholar]
- 6.Ogasawara K, Sakai N, Kuroiwa T, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 2007; 107: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 7.Bivard A, Krishnamurthy V, Stanwell P, et al. Spectroscopy of reperfused tissue after stroke reveals heightened metabolism in patients with good clinical outcomes. J Cereb Blood Flow Metab 2014; 34: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bivard A, Stanwell P, Levi C, et al. Arterial spin labeling identifies tissue salvage and good clinical recovery after acute ischemic stroke. J Neuroimaging 2013; 23: 391–396. [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Liebeskind DS, Dua S, et al. Postischemic hyperperfusion on arterial spin labeled perfusion MRI is linked to hemorrhagic transformation in stroke. J Cereb Blood Flow Metab 2015; 35: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchal G, Furlan M, Beaudouin V, et al. Early spontaneous hyperperfusion after stroke. A marker of favourable tissue outcome? Brain 1996; 119: 409–419. [DOI] [PubMed] [Google Scholar]
- 11.Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab 1999; 19: 467–482. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann A. Prolonged disturbances of regional cerebral blood flow in transient ischemic attacks. Stroke 1985; 16: 932–939. [DOI] [PubMed] [Google Scholar]
- 13.Lassen NA. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet (London, England) 1966; 2: 1113–1115. [DOI] [PubMed] [Google Scholar]
- 14.Tamura A, Asano T, Sano K. Correlation between rCBF and histological changes following temporary middle cerebral artery occlusion. Stroke 1980; 11: 487–493. [DOI] [PubMed] [Google Scholar]
- 15.Tasdemiroglu E, Macfarlane R, Wei EP, et al. Pial vessel caliber and cerebral blood flow become dissociated during ischemia-reperfusion in cats. Am J Physiol 1992; 263: H533–H536. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 17.Campbell BCV, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke 2007; 38: 2979–2984. [DOI] [PubMed] [Google Scholar]
- 19.Briggs DE, Felberg RA, Malkoff MD, et al. Should mild or moderate stroke patients be admitted to an intensive care unit? Stroke 2001; 32: 871–876. [DOI] [PubMed] [Google Scholar]
- 20.Bivard A, McElduff P, Spratt N, et al. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc Dis (Basel, Switzerland) 2011; 31: 238–245. [DOI] [PubMed] [Google Scholar]
- 21.Luh WM, Wong EC, Bandettini PA, et al. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med 1999; 41: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 22.Parsons MW, Pepper EM, Chan V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol 2005; 58: 672–679. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Han Q, Ding X, et al. Defining core and penumbra in ischemic stroke: a voxel- and volume-based analysis of whole brain CT perfusion. Scientific Rep 2016; 6: 20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L, Bivard A, Levi CR, et al. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke: back-to-back quantitative analysis. Stroke 2014; 45: 1727–1732. [DOI] [PubMed] [Google Scholar]
- 25.D'Amore C, Paciaroni M. Border-zone and watershed infarctions. Front Neurol Neurosci 2012; 30: 181–184. [DOI] [PubMed] [Google Scholar]
- 26.Bhaskar S, Bivard A, Parsons M, et al. Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: associations with collateral status. J Cerebr Blood Flow Metab. Epub ahead of print 10 March 2016. DOI: 10.1177/0271678X16637611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009; 132: 2231–2238. [DOI] [PubMed] [Google Scholar]
- 28.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models, New York: McGraw-Hill/Irwin, 2004. [Google Scholar]
- 29.Kablau M, Kreisel SH, Sauer T, et al. Predictors and early outcome of hemorrhagic transformation after acute ischemic stroke. Cerebrovasc Dis (Basel, Switzerland) 2011; 32: 334–341. [DOI] [PubMed] [Google Scholar]
- 30.Larrue V, von Kummer RR, Muller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001; 32: 438–441. [DOI] [PubMed] [Google Scholar]
- 31.Suh SH, Cloft HJ, Fugate JE, et al. Clarifying differences among thrombolysis in cerebral infarction scale variants: is the artery half open or half closed? Stroke 2013; 44: 1166–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saver JL. Optimal end points for acute stroke therapy trials: best ways to measure treatment effects of drugs and devices. Stroke 2011; 42: 2356–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labeyrie MA, Turc G, Hess A, et al. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke 2012; 43: 2986–2991. [DOI] [PubMed] [Google Scholar]
- 34.Seners P, Turc G, Tisserand M, et al. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke 2014; 45: 2004–2009. [DOI] [PubMed] [Google Scholar]
- 35.Bivard A, Spratt N, Levi C, et al. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain 2011; 134: 3408–3416. [DOI] [PubMed] [Google Scholar]
- 36.O'brien R. A caution regarding rules of thumb for variance inflation factors. Qual Quant 2007; 41: 673–690. [Google Scholar]
- 37.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008; 79: 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon BK, Smith EE, Modi J, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR 2011; 32: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR 2009; 30: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marks MP, Lansberg MG, Mlynash M, et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke 2014; 45: 1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baird AE, Donnan GA, Austin MC, et al. Early reperfusion in the ‘spectacular shrinking deficit’ demonstrated by single-photon emission computed tomography. Neurology 1995; 45: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 43.Lee VH, John S, Mohammad Y, et al. Computed tomography perfusion imaging in spectacular shrinking deficit. J Stroke Cerebrovasc Dis 2012; 21: 94–101. [DOI] [PubMed] [Google Scholar]
- 44.Minematsu K, Yamaguchi T, Omae T. ‘Spectacular shrinking deficit’: rapid recovery from a major hemispheric syndrome by migration of an embolus. Neurology 1992; 42: 157–162. [DOI] [PubMed] [Google Scholar]
- 45.Ringelstein EB, Biniek R, Weiller C, et al. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology 1992; 42: 289–298. [DOI] [PubMed] [Google Scholar]
- 46.Bladin PF, Berkovic SF. Striatocapsular infarction: large infarcts in the lenticulostriate arterial territory. Neurology 1984; 34: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 47.Donnan GA, Bladin PF, Berkovic SF, et al. The stroke syndrome of striatocapsular infarction. Brain 1991; 114: 51–70. [PubMed] [Google Scholar]
- 48.Menon BK, O'Brien B, Bivard A, et al. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 2003; 34: 2750–2762. [DOI] [PubMed] [Google Scholar]
- 50.Liebeskind DS. Intracranial collateral routes and anastomoses in interventional neuroradiology. In: Hurst RW (ed.) Neurointerventional management: diagnosis and treatment. 2nd ed. Boca Raton, FL: CRC Press, 2012, pp.57–86.
- 51.Vander Eecken HM. Morphological significance of leptomeningeal anastomoses confined to the territory of cerebral arteries. Acta Neurol Psychiatr Belgica 1954; 54: 525–532. [PubMed] [Google Scholar]
- 52.Marchal G, Serrati C, Rioux P, et al. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: relation to outcome. Lancet (London, England) 1993; 341: 925–927. [DOI] [PubMed] [Google Scholar]
- 53.Hakim AM, Pokrupa RP, Villanueva J, et al. The effect of spontaneous reperfusion on metabolic function in early human cerebral infarcts. Ann Neurol 1987; 21: 279–289. [DOI] [PubMed] [Google Scholar]
- 54.Heiss WD, Graf R, Lottgen J, et al. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. J Cereb Blood Flow Metab 1997; 17: 388–400. [DOI] [PubMed] [Google Scholar]
- 55.Zaharchuk G, Do HM, Marks MP, et al. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke 2011; 42: 2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.