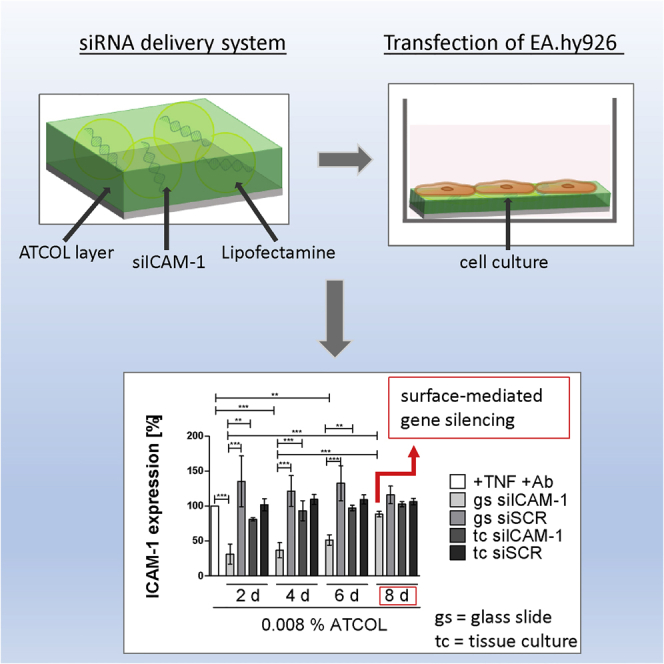
Abstract
In the last decades, many efforts have been made to counteract adverse effects after stenting atherosclerotic coronary arteries. A breakthrough in better vascular wall regeneration was noted in the new era of drug-eluting stents. A novel personalized approach is the development of gene-eluting stents promising an alteration in gene expression involved in regeneration. We investigated a coating system consisting of the polymer atelocollagen (ATCOL) and a specific small interfering RNA (siRNA) for intercellular adhesion molecule-1 (ICAM-1) found on the surface of defective endothelial cells (ECs). We demonstrated very high cell viability, in which EA.hy926 grew on 0.008% or 0.032% ATCOL layers. Additionally, hemocompatibility assays proved the biocompatibility of this coating. The highest transfection efficiency with EA.hy926 was achieved with 5 μg siRNA immobilized in ATCOL after 2 days. The release of fluorescent-labeled siRNA was about 9 days. Long-term knockdown of ICAM-1 was analyzed by flow cytometry, revealing that the coating with 0.008% ATCOL and 5 μg siICAM-1 provoked gene silencing up to 8 days. 5′-RNA ligase-mediated rapid amplification of cDNA ends PCR (RLM-RACE-PCR) demonstrated the specificity of our established ATCOL gene-silencing coating, meaning that our coating is well suited for further investigations in in vivo studies. Herein, we would like to demonstrate that our ATCOL is well-suited for better artery wall regeneration after stent implantation.
Keywords: atherosclerosis, siRNA transfection, local delivery, siICAM-1, gene knockdown, atelocollagen
Graphical Abstract
Introduction
Cardiovascular diseases (CVDs) have long been challenging in modern medicine. This group of diseases accounts for 17.3 million deaths per year and leads the mortality statistics on a global scale.1 Although age-adjusted mortality rates have decreased throughout the last decades, CVD and especially coronary heart disease (CHD) account for about 20% of deaths in Europe annually and remain highly prioritized fields in medical research.2, 3 Since CHD is mainly caused by atherosclerotic plaques, it is reasonable to tackle the main risk factors for atherosclerosis (namely, hypertension, diabetes mellitus, hyperlipidemia, obesity, smoking, and lack of physical activity) in a secondary preventive therapy.4, 5 The prognosis of CHD can be greatly improved by lifestyle changes (e.g., weight loss, cessation of smoking) and by a variety of drugs (e.g., angiotensin-converting enzyme [ACE] inhibitors, antiplatelet therapy, β blockers).4, 6, 7, 8 However, in many cases, intervention in the form of surgery (e.g., coronary artery bypass surgery [CABG] or percutaneous transluminal coronary angioplasty [PTCA], with or without stent insertion) is unavoidable.9 While overall mortality with balloon angioplasty did not differ from mortality with CABG, rates of restenosis and reintervention were considerably higher.10, 11, 12 Balloon angioplasty was soon augmented by use of bare metal stents (BMSs). Unfortunately, in-stent restenosis and stent thrombosis (ST) may occur.13, 14 Stent implantation is seen as a trauma to the intimal structure triggering an inflammatory reaction.15 Endothelial cells (ECs) in the stented area respond to local inflammation through elevated levels of surface-bound adhesion molecules, which facilitate the migration of leukocytes into the intima. The inflammatory reaction mediated through the migrating leukocytes causes a proliferation of intimal smooth muscle cells (SMCs) leading to narrowed vessel lumen.15, 16 Furthermore, overexpression of adhesion molecules promotes a chronification of the inflammatory process.17 Drug-eluting stents (DESs) were introduced to inhibit neointimal hyperplasia and, hence, slow restenosis of the stented lumen. DESs are essentially BMSs coated with a polymer that releases an antiproliferative agent.18 The agents as well as the stent design have changed over the course of time, forming two generations of DESs: first-generation (using sirolimus or paclitaxel) and second-generation (using everolimus or zotarolimus) DESs. Both generations show a significant reduction of target-location revascularization compared to BMSs. However, long-term mortality rates remain similar and the risk of late ST and late target-location revascularization persists.19, 20, 21, 22 Inhibition of re-endothelialization could be responsible for late restenosis.23
There are new approaches in medical research targeting the mechanism of restenosis on a molecular level to prevent an overexpression of adhesion molecules and thus chronic inflammation. A favorable and elegant method to lower the expression of adhesion molecules is gene silencing, also called gene knockdown. 20- to 25-nucleotide-long RNA strands are used, which are complementary to the mRNA of the target protein. This leads to so-called RNAi: mRNA binds the artificial RNA molecule, leading to the degradation of the double-stranded complex and inhibiting the biosynthesis of the target protein. According to their function, these RNA molecules are called small interfering RNAs (siRNAs).24 However, naked siRNAs have a fairly short half-life and are prone to degradation by endo- and exonucleases.25, 26 There are two main working points through which the half-life can be extended: (1) through alteration of the siRNA on a molecular level (e.g., modification of the backbone)26 or (2) through the use of a carrier substance preventing degradation or binding the negatively charged siRNA.27 A potent carrier to do so is atelocollagen (ATCOL). ATCOL is basically a modified type I collagen. It is obtained by enzymatic treatment of bovine type I collagen with pepsin leading to the detachment of the telopeptides (N- and C-terminal ends of the collagen molecule), which influence the immunogenicity of the molecule immensely.28, 29 Combined with the natural biocompatibility of collagen, this modification creates a well-tolerated molecule that has many applications in modern medicine (e.g., as a hemostatic agent, bone cartilage substitute, etc.).30, 31 Referring to its use as carrier, there have been several studies demonstrating the usefulness of ATCOL in the delivery of nucleic acids.31, 32, 33, 34, 35 Furthermore, ATCOL has proven to be a viable option in gene silencing, increasing the efficacy of siRNA transduction and offering protection against nucleases.29 This is mostly achieved through the very stable complexation of the two agents (mostly through hydrogen bonding).36 Another siRNA carrier is the cationic lipid Lipofectamine 2000 (Invitrogen), which is able to form nanoparticles with nucleic acids due to opposite charges. The negatively charged siRNA causes complexation with the cationic lipid. Consequently, the positively charged siRNA/Lipofectamine 2000 complex can be included in the cell because the electrostatic repulsion of the cell membrane will no longer have an effect.37 Lipofectamine 2000 is known as a very potent transfection reagent resulting in high transfection efficiencies.
In this study, we focused on developing a bioactive stent coating using siRNA that is complementary to intercellular adhesion molecule-1 (ICAM-1 or CD54). ICAM-1 is prominent on ECs during inflammatory reactions and part of the inflammatory cascade mentioned above and is thus a promising target to break the “vicious cycle” of restenosis. To find an agreeable concentration of ATCOL, we prepared different ATCOL layers and tested cell growth and hemocompatibility. Our siRNA was immobilized in ATCOL layers by drying them on glass slides, followed by cultivation with an immortalized EC line (EA.hy926). The release of embedded fluorescent-labeled siRNA was tested for long-term efficacy with a fluorescent reader. Afterward, we assessed transfection efficiency using Alexa Fluor 488 (AF 488)-labeled siRNA by flow cytometry. In a second approach, we used ICAM-1 siRNA in a similar setup to evaluate gene knockdown of the ICAM-1 protein in a short- and long-term assay by flow cytometry.
Results
Cell Viability of EA.hy926 on ATCOL Surfaces
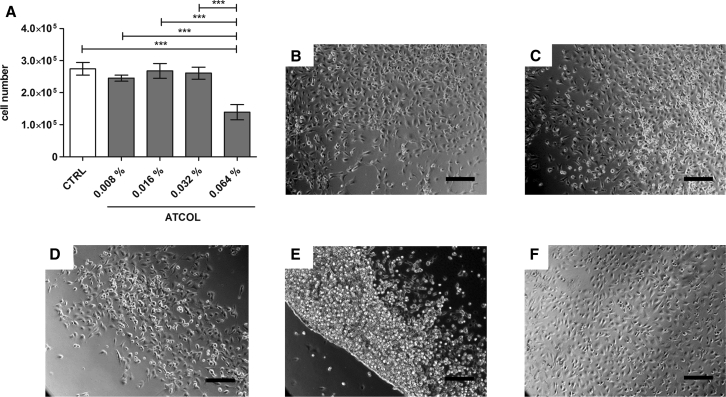
Different concentrations of ATCOL coatings were tested for their influence on cell number, morphology, and adherence of EA.hy926. The evaluation of cell number measurement with a CASY cell counter (Schärfe System) showed no significant alterations in EA.hy926 cell numbers, which were cultivated on 0.008%, 0.016%, and 0.032% ATCOL layers (Figure 1A). A significant cell number reduction of 50% EA.hy926 cultivated on 0.064% ATCOL was seen compared to control glass slides and the other layers. These results could be visually confirmed by microscopic images after 24 hr (Figures 1B–1F) and 48 hr (data not shown) of cultivation. While control slides and concentrations of 0.008%, 0.016%, and 0.032% ATCOL demonstrated adherence of EA.hy926 evenly, the layer with 0.064% ATCOL provoked formation of a boundary line by cells (Figure 1E). Additionally, cells grew mainly one above the other and were round shaped and not fully spread. This phenomenon is also slightly visible with 0.032% and 0.016% ATCOL. In the following experiments, we focused on three concentrations of ATCOL due to excellent cell adhesion and no reduction in cell number: 0.008%, 0.016%, and 0.032%.
Figure 1.
Cell Viability of EA.hy926 Cultured on ATCOL Layers
(A) Cell number analysis of EA.hy926 by CASY measurement. 75,000 cells were seeded onto different ATCOL-coated glass slides in a 24-well plate and cultivated for 48 hr. After cell detachment, the cell number was measured. An uncoated glass slide served as a control. Each bar represents the mean ± SEM (n = 3). ***p < 0.001. (B–F) Microscopy images of EA.hy926 cultivated on different ATCOL layers after 24 hr of cultivation: (B) EA.hy926 on 0.008% ATCOL, (C) EA.hy926 on 0.016% ATCOL, (D) EA.hy926 on 0.032% ATCOL, (E) EA.hy926 on 0.064% ATCOL, and (F) glass slide as a control. Scale bars, 200 μm. CTRL, control.
Release of siRNA AF 488
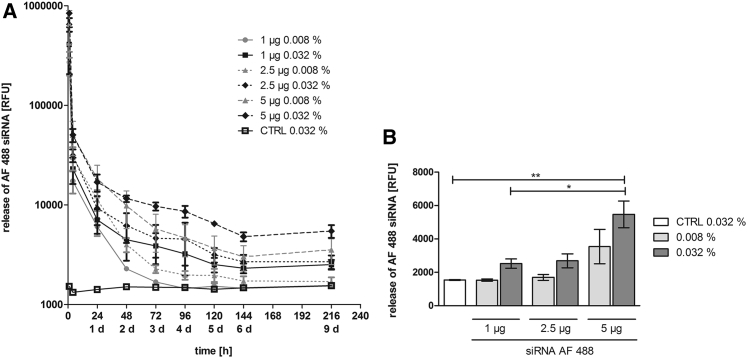
The release of immobilized siRNA from ATCOL was observed over a period of 216 hr (9 days). The release was tested with fluorescently labeled siRNA by measuring the supernatant with a fluorescent reader. Within the first 4 hr of incubation, we detected the highest release of siRNA in the supernatant in all tested samples (Figure 2A). The release rate was nearly the same in all samples until the 24-hr time point of the experiment. Afterward, the 0.008% ATCOL layer demonstrated a faster release than the 0.032% ATCOL layer. Here, 0.008% ATCOL layers with 1 μg and 2.5 μg siRNA showed the lowest release values after 9 days (Figure 2B). The 1-μg 0.008% ATCOL coating provoked a decrease up to the 0.032% ATCOL control (without siRNA) at day 4. The same amounts of siRNA embedded in 0.032% ATCOL showed higher release values than 0.008% ATCOL after 9 days. At this time point, the highest release was observed with 5 μg siRNA in 0.032% ATCOL layers with 5,467 relative fluorescence units (RFU) followed by 5 μg siRNA in 0.008% ATCOL. No significant differences in release kinetics could be determined between the two different ATCOL coatings (0.008% and 0.032%) during the duration of the experiment.
Figure 2.
Release of siRNA AF 488 Particles from ATCOL Coatings
(A) Release kinetics of siRNA AF 488 incorporated in ATCOL layers. Glass slides were coated with either 0.008% or 0.032% ATCOL combined with 1, 2.5, or 5 μg siRNA AF 488. Coated slides were set in a 24-well plate and incubated in PBS at 37°C. Supernatants were collected and measured with a fluorescence reader at 485-nm excitation and 535-nm emission wavelengths at different time points. Each bar represents the mean ± SEM (n = 3). (B) RFU of released siRNA of ATCOL layers after 9 days. Final values of release kinetics in (A) are shown in a column diagram for improved distinction of different ATCOL samples. CTRL, control. *p < 0.05; **p < 0.01.
Transfection Efficiency of siRNA AF 488 Mediated by ATCOL Coatings
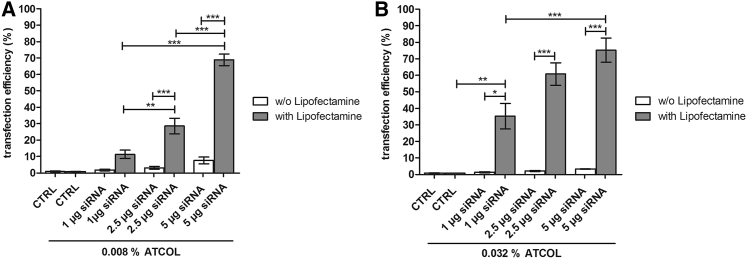
Because ATCOL is used as a transfection reagent in many gene delivery studies,29, 31, 32, 34, 35, 36 we tested the potential of 0.008% and 0.032% ATCOL coatings for transfection efficiency in EA.hy926 with 1, 2.5, and 5 μg siRNA AF 488. After 48 hr of cultivation, the percentage of transfected cells was very low in both ATCOL concentrations but correlated with increased siRNA amounts. The highest transfection efficiency was reached with the combination of 0.008% ATCOL and 5 μg siRNA, with 7.6% positive cells (Figure 3A); 0.032% and 5 μg siRNA showed 3.4% transfected cells (Figure 3B). Considering these unsatisfying transfection efficiencies, we decided to optimize the transfection process by adding Lipofectamine 2000. Here, a remarkable significantly higher amount of transfected cells was observed. The use of Lipofectamine 2000 led to 68.9% positive cells with 0.008% ATCOL and 5 μg siRNA (Figure 3A). The highest transfection efficiency (75.2%) was achieved with 0.032% ATCOL and 5 μg siRNA (Figure 3B). Comparing the two different ATCOL concentrations, the coating with 0.032% ATCOL resulted in generally higher transfection efficiencies than did that with 0.008% ATCOL, especially with 1 μg and 2.5 μg siRNA.
Figure 3.
Transfection Efficiency after ATCOL-Mediated Transfection of siRNA AF 488 with or without Lipofectamine 2000
(A) 75,000 EA.hy926 cells were cultivated on 0.008% ATCOL with incorporated siRNA complexed or noncomplexed with Lipofectamine 2000. Transfection efficiency was analyzed by flow cytometry after 48 hr of cultivation. (B) 75,000 EA.hy926 cells were cultivated on 0.032% ATCOL with siRNA complexed or noncomplexed with Lipofectamine 2000 and then treated as mentioned previously. Coatings without siRNA and Lipofectamine 2000 served as a control. Each bar represents the mean ± SEM (n = 3, or n = 6 for 0.032% ATCOL with Lipofectamine 2000). *p < 0.05; **p < 0.01; ***p < 0.001. CTRL, control.
Knockdown of ICAM-1
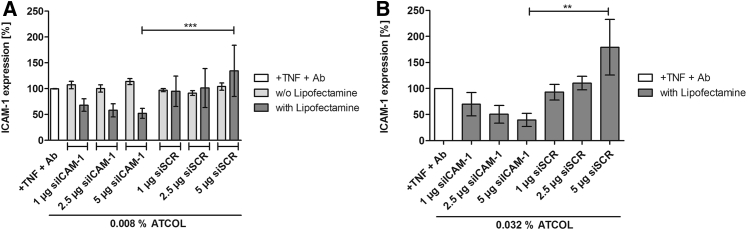
In our study, knockdown of ICAM-1 is defined as the percent removal of the ICAM-1 receptor on EA.hy926 caused by RNAi. Here, siICAM-1 is able to bind to the complementary mRNA and to degrade it. First, ICAM-1 knockdown by 0.008% ATCOL and siICAM-1 without Lipofectamine 2000-coated glass slides was tested after 48-hr incubation with EA.hy926. All three amounts of siICAM-1 (1, 2.5, and 5 μg) could not provoke a knockdown (Figure 4A). The values are even slightly higher than the tumor necrosis factor (TNF)-α/antibody (Ab) control. The scrambled siRNA (siSCR) coatings that contained a siRNA that is not complementary to ICAM-1 mRNA mediated no significant change in ICAM-1 expression compared to the TNF/Ab control and the siICAM-1 samples. The decision to use Lipofectamine 2000 for reinforcement of the transfection efficiency resulted in a higher knockdown of ICAM-1. Both concentrations of ATCOL coatings (0.008% and 0.032%) showed an increase in ICAM-1 knockdown with increasing amounts of siICAM-1 (Figure 4). Furthermore, a significant difference between 5 μg siICAM-1 and the control (5 μg siSCR) was observed with both ATCOL concentrations. While the knockdown provoked with 1 μg siICAM-1 was almost the same with the 0.008% (31.7%) and 0.032% (30.3%) ATCOL coatings, values differed with 2.5 μg and 5 μg siICAM-1. Here, the 0.032% ATCOL coating resulted in a higher ICAM-1 knockdown with 49.6% (2.5 μg) and 60.4% (5 μg), compared to 0.008% ATCOL with 41.8% (2.5 μg) and 47.9% (5 μg).
Figure 4.
ICAM-1 Expression after Knockdown with ICAM-1 siRNA with or without Lipofectamine 2000 Mediated by 0.008% and 0.032% ATCOL Transfection Coatings
Glass slides were coated with ATCOL transfection solution and put into a 24-well plate. 75,000 EA.hy926 cells were cultivated on coated glass slides for 48 hr and were then activated with TNF-α. ICAM-1 expression was analyzed by flow cytometry. (A) Coatings consisting of 0.008% ATCOL and 1, 2.5, or 5 μg siICAM-1 with or without Lipofectamine 2000. 0.008% ATCOL coatings with the same amounts of siSCR (scrambled siRNA) and Lipofectamine 2000 served as a control. (B) 0.032% ATCOL coatings with 1, 2.5, and 5 μg siICAM-1 and Lipofectamine 2000. siSCR served as a control. The control (+ TNF + Ab) was set to 100% in (A) and (B). Each bar in (A) and (B) represents the mean ± SEM (n = 3). **p < 0.01; ***p < 0.001.
Long-Term Knockdown of ICAM-1
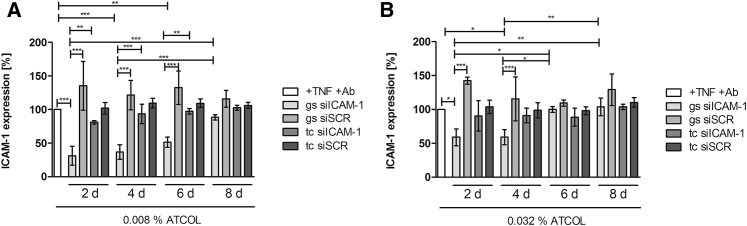
Focusing on a long-term release of siRNA for later medical application, we examined the knockdown of ICAM-1 over a period of 8 days by flow cytometry every second day. Therefore, EA.hy926 cells were seeded onto 0.008% or 0.032% ATCOL transfection coatings (ATCOL/Lipofectamine 2000/siICAM-1) to examine the duration of substrate-mediated transfection. Additionally, we tested the knockdown by conventionally transfected cells to compare and reveal the potential for substrate-mediated knockdown. The highest significant ICAM-1 knockdown with 68.9% was reached with siRNA coatings after second day of incubation in the experiment with 0.008% ATCOL (Figure 5A). Very high knockdown was observed after the fourth and sixth days (63.3% and 48.7%), and 11.6% knockdown on the eighth day. Conventional mediated transfection provoked no significant ICAM-1 knockdown compared to the TNF/Ab control. Moreover, conventional transfected cells showed significantly higher ICAM-1 expression compared to the siICAM-1 coating analyzed at days 2, 4, and 6. Similar results were achieved with conventional transfected cells with 0.032% ATCOL and siICAM-1, where the highest knockdown was reached after 2 days with 10.0% and there was a progressive reduction until the eighth day (Figure 5B). siRNA coating-mediated transfection with 0.032% ATCOL provoked significant knockdown after day 2 (41.1%) and day 4 (41%), ending in no knockdown after the sixth and eighth days.
Figure 5.
ICAM-1 Expression after Long-Term Knockdown of ICAM-1 Examined by Flow Cytometry
Glass slides were coated with ATCOL/siICAM-1 transfection solution for substrate-mediated transfection and laid in a 24-well plate. For conventional transfection in tissue culture, ATCOL/siICAM-1 transfection solution was added to the cell culture medium, where EA.hy926 cells were seeded 1 day prior. After each time point, cells were activated for 14 hr with TNF-α and analyzed by flow cytometry. ICAM-1 expression was calculated after setting the TNF-α control to 100%. Each bar in (A) and (B) represents the mean ± SEM (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001. gs, glass slide; tc, tissue culture.
Specific mRNA Degradation Analyzed by RLM-RACE-PCR
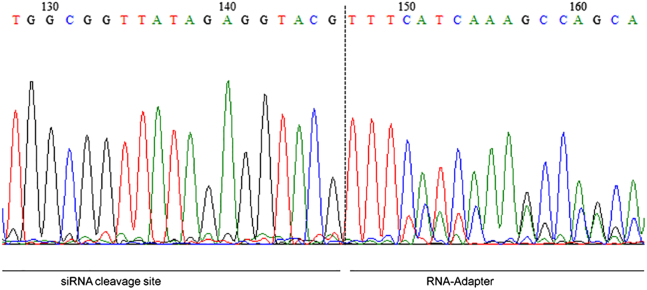
The proof of specific mRNA degradation products by transfection reagent-mediated knockdown is mandatory for specific gene silencing in the RNAi mechanism. Consequently, undesired side effects mediated by the biomaterial or transfection reagent provoking knockdown of the targeted protein mRNA can be excluded. The determination of the ICAM-1 cleavage site by the products of 5′-RNA ligase-mediated rapid amplification of cDNA ends PCR (RLM-RACE-PCR) revealed the correct cleaved mRNA transcript at bp 1,818, based on our expectations (Figure 6). Control coatings such as ATCOL without TNF-α activation, ATCOL, or ATCOL/siSCR with TNF-α activation demonstrated no specific ICAM-1 mRNA cleavage products (data not shown).
Figure 6.
Sequence of 5′-RLM-RACE-PCR Product after Transfecting EA.hy926 with 0.032% ATCOL-Mediated siICAM-1 Coating
After cultivation of 75,000 cells on coated glass slides, RNA was isolated followed by the 5′-RLM-RACE-PCR procedure. The cleavage site of ICAM-1 mRNA was detected at 1,818 bp (128–146) with the RNA adaptor sequence (147–163). Each bar represents the mean ± SEM.
Hemocompatibility
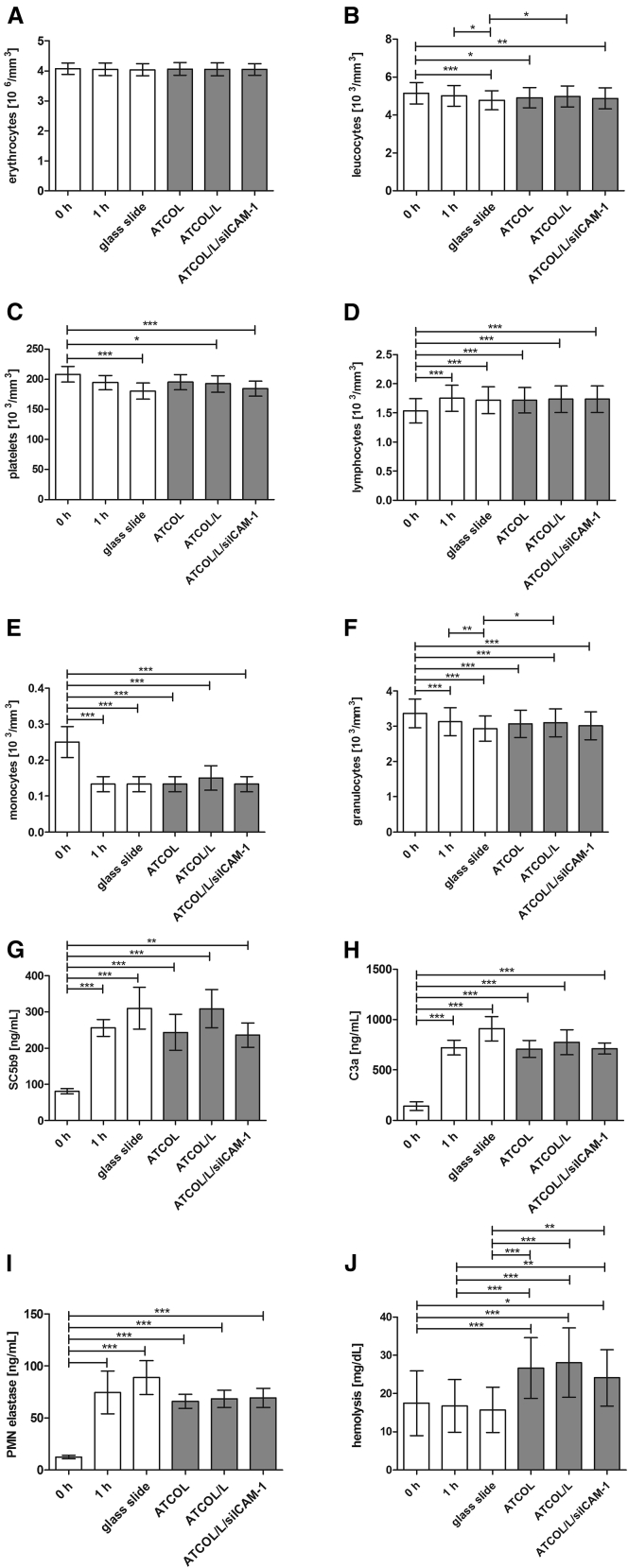
The determination of hemocompatibility is required for all medical devices according to International Organization for Standardization (ISO) standard 10993-4 and applied in our study testing 0.032% ATCOL coatings with incorporated siICAM-1 for later clinical application as a “worst case.” First, cell numbers of blood cells were analyzed before incubation (0 hr) and after 1 hr of incubation with coated glass slides. The 1-hr sample served as a control and standard value. Then, coated glass slide samples were analyzed for hemocompatibility: ATCOL, ATCOL/Lipofectamine 2000, and ATCOL/Lipofectamine 2000/siICAM-1. Herein, the cell number of erythrocytes did not show variations in any sample (Figure 7A). Significant decreases (Figures 7B, 7C, 7E, and 7F) or increases (Figure 7D) of cell numbers were encountered comparing the 0-hr sample with most of the other tested samples. Furthermore, uncoated glass slides provoked a significant reduction of cell numbers in leukocytes and granulocytes compared to 1-hr control and ATCOL/Lipofectamine 2000 samples. However, the 1-hr blood control is crucial for the assessment of hemocompatibility and no significant changes in the cell numbers of leukocytes, platelets, lymphocytes, monocytes, and granulocytes were detected herein.
Figure 7.
Hemocompatibility Analysis of 0.032% ATCOL/siICAM-1-Coated Glass Slides
Coated slides were incubated with fresh human blood at 37°C under gentle shaking. The 0-hr and 1-hr samples served as the control and the baseline, respectively. After incubation, different blood cell numbers and inflammatory parameters were analyzed by a cell counter or ELISAs: (A) erythrocytes × 103 mm−3; (B) leukocytes × 103 mm−3; (C) platelets × 103 mm−3; (D) lymphocytes × 103 mm−3; (E) monocytes × 103 mm−3; (F) granulocytes × 103 mm−3; (G) SC5b9 (ng mL−1); (H) C3a (ng mL−1); and (I) PMN elastase (ng mL−1). Hemolysis was determined by the detection of free hemoglobin with citrate plasma and a reaction kit: (J) hemolysis (mg dL−1). Each bar represents the mean ± SEM (n = 6). *p < 0.05; **p < 0.01; ***p < 0.001.
Furthermore, blood parameters (e.g., polymorphonuclear [PMN]) elastase) and values of the complement system (e.g., SC5b9 and C3a) were analyzed, revealing no significant value changes compared to the 1-hr control (Figures 7G–7I). Further investigations for hemocompatibility analysis were made analyzing hemolysis. All coated glass slides revealed significantly higher amounts of free hemoglobin in plasma (Figure 7J). Nevertheless, the standard value of 40 mg/100 mL plasma was been exceeded.
Discussion
The development of new therapeutic strategies for preventing in-stent restenosis after coronary stent placement is a huge challenge. A promising new personalized therapeutic strategy comprises the application of gene-silencing stents that release specific therapeutic siRNAs to the ECs of the vascular wall for its faster regeneration and inhibition of restenosis. Several studies have evaluated the use of siRNAs against different target proteins involved in atherosclerotic processes.38, 39, 40, 41, 42 For therapeutic siRNA release over a period of time, a biomaterial that supports storage features and controlled release and has no adverse effects to cell viability and hemocompatibility should be chosen. Therefore, we decided to use the biocompatible protein ATCOL, which has low toxicity and immunogenicity.
We evaluated the viability of EA.hy926 cells after seeding them onto different ATCOL layers. Our analysis revealed comparable cell counts between 0.008%, 0.016%, and 0.032% ATCOL coatings and the control. However, a significant reduction in cell number was found on the 0.064% ATCOL surface. We assume that higher ATCOL concentrations result in a softer coating surface. It is well known that substrate stiffness plays a pivotal role in cell adhesion, migration, proliferation, and differentiation.43, 44, 45, 46, 47, 48 The majority of EA.hy926 cells grown on 0.064% ATCOL showed a change in cell adhesion with less pseudopodia and a rounded cell shape. These results coincide with the findings of Nolte et al.,49 in which softer polyelectrolyte multilayers consisting of hyaluronic acid/chitosan caused lower cell viability than the stiffer material (sulfonated polystyrene/polyallylamine hydrochlorite). Additionally, their microscopic images showed the same cell morphology with rounded cells on low rigidity films, like we observed with the 0.064% ATCOL layer.
A gene-eluting stent coating must fulfill the following requirements: biocompatibility, nucleic acid storage, and controlled release. Furthermore, it is beneficial if the coating supports the delivery of the therapeutic siRNA into cells.
Many previous studies have indicated that ATCOL is a highly potential transfection reagent for gene transfer in vitro and in vivo.29, 31, 32, 34, 35, 50, 51 In addition, several opportunities for designing an ATCOL gene carrier exist, including minipellets, gels, sponges, or injectable solutions. In this study, we prepared an air-dried siRNA/ATCOL film for surface-mediated therapeutic siRNA delivery for later medical application. We examined the combination of 0.008% or 0.032% ATCOL with 1, 2.5, and 5 μg naked siRNA AF 488 for transfection efficiency in EA.hy926 and revealed unexpectedly few transfected cells (Figure 3). The knockdown experiment showed no ICAM-1 protein reduction, which further provided evidence that the coating is not able to efficiently transfect EA.hy926 cells (Figure 4A). With regard to the results of Minakuchi et al.29 in preparing 0.008% ATCOL layers with siRNA, our results with 0.008% and 0.032% ATCOL could not achieve transfection efficiencies of 40%–60% (data not shown). Another study by Mu et al.50 showed successful gene silencing with 0.008% ATCOL/siRNA coating by RT-PCR. Due to their same experimental setup with an ATCOL/siRNA transfection layer in well plates, we assume that the preparation may be a focal point for successful incorporation of siRNA. Both groups prepared their transfection solution by mixing equal volumes of diluted ATCOL and siRNA solution. The solution was rotated at 4°C for 20 min and then air-dried. In contrast, we built the coating by similar mixing and static incubation at room temperature for 30 min. Another reason for the different transfection efficiencies may be the cell type intended for transfection.52 Whereas the other investigators used human prostate cancer and human testicular tumor cell lines, we transfected a hybrid cell line consisting of primary human umbilical vein cells and a clone of A549. Nevertheless, our approach of encapsulating siRNA into Lipofectamine 2000 led to significant augmented transfection efficiency in EA.hy926. We revealed that the transfection efficiency is dependent on the siRNA amount and ATCOL concentration. As siRNA quantity increased from 1 μg to 5 μg, we noticed significant increases in transfected cells in both ATCOL concentrations. Transfection efficiency with 5 μg siRNA was comparable between 0.008% (68.9%) and 0.032% (75.2%) ATCOL coatings. Higher values could be achieved by the 0.032% ATCOL coating with 1 and 2.5 μg siRNA compared to the transfection efficiencies of 0.008% ATCOL with the same siRNA amounts (Figure 3).
Gene silencing of cell adhesion molecules (CAMs) in ECs is one key focus of our group as a possible strategy to circumvent and prevent the inflammatory process after stent implantation.53 While DESs elute cytostatic agents for inhibiting excessive cell growth and migration of SMCs, our RNAi approach intends to interfere in this mechanism before the inflammatory machinery due to vessel injury can take place.54, 55 Therefore, we chose to silence the ICAM-1 adhesion molecule of ECs by local release to prevent the leukocyte adhesion cascade.17 The coatings we designed with 0.008% or 0.032% ATCOL with 5 μg siICAM-1 and Lipofectamine 2000 were both able to reduce ICAM-1 significantly in our short-term experiment (Figure 4). Consequently, the transfection efficiency and short-term knockdown results led us to conclude that the amount of 5 μg siRNA embedded in 0.008% or 0.032% ATCOL layers is suitable for ICAM-1 knockdown in EA.hy926. 5′-RLM-RACE-PCR demonstrated the functionality of our substrate-mediated gene-silencing system. We detected the correct cleavage site for ICAM-1 induced by siICAM-1 coating, meaning that siICAM-1 was able to degrade mRNA specifically. The control coatings with siSCR or with ATCOL only did not show typical mRNA degradation products at the cleavage site of ICAM-1. Consequently, we can say that the knockdown of ICAM-1 is due to the specific siRNA we used. To successfully prevent an inflammatory process after stent placement, long-term inhibition of leukocyte-endothelial interaction seems to be of crucial importance against in-stent restenosis.19, 20, 21, 22
The release of fluorescent-labeled siRNA was in line with our knockdown experiments and with our previous studies. The results after 9 days of release revealed that the coating of 0.032% ATCOL with 5 μg siICAM-1 contained the highest siRNA residual amount followed by the combination of 0.008% ATCOL and 5 μg siICAM-1 (Figure 2B). Therefore, we chose these two combinations as delivery coatings in our long-term knockdown experiment. The initial burst release within 4 hr seems to be responsible for the excellent ICAM-1 knockdown using 5 μg siICAM-1 embedded in 0.008% and 0.032% ATCOL (Figure 2A). Although the coating with 5 μg siICAM-1 and 0.032% ATCOL showed higher amounts of siRNA release over the 9-day period compared to the same coating with 0.008% ATCOL, we achieved lower knockdown values especially at days 6 and 8. We assume that a faster release of siRNA within the first days of transfection is mandatory for a high and sustained knockdown of the appropriate protein. Data from our two recent studies revealed the same results (O.K., D. Zengerle, N.P., S. Hossfeld, B.N., A.B., M. Avic-Adali, T.W., C.S., H.P.W., and A.N., unpublished data).53 Comparing the release of coatings with the same siRNA amount but different ATCOL concentrations, we found different release profiles after 24 hr of incubation. Different concentrations of ATCOL are known to influence the density of the collagen matrix and, consequently, the release of molecules. If the collagen concentration is reduced, the collagen matrix shows a lower density leading to a faster escape out of the collagen matrix.28 Our data underline the findings of Sano et al.,28 showing that the coatings with the lower ATCOL concentration (0.008%) released the fluorescent-labeled siRNA faster than the higher concentration (0.032%) after day 1.
Analysis of our long-term knockdown experiments with 5 μg siICAM-1 in either 0.008% or 0.032% ATCOL revealed an effective surface-mediated local knockdown compared to transfection particles in suspension. Moreover, ICAM-1 knockdown was extended when the cells were grown on the coatings. In particular, the 0.008% ATCOL coating demonstrated a considerable difference between substrate-mediated transfection and transfection by particles in suspension, showing significance until transfection day 6. Furthermore, we detected a difference in knockdown values between these two transfection methods on day 8, revealing a substrate-mediated knockdown of ICAM-1 with 11.6% and no knockdown in suspension. In comparison, the 0.032% ATCOL coatings could not provoke such considerable ICAM-1 knockdown values as 0.008% did. But we observed a significant ICAM-1 knockdown after the second and fourth days of incubation by ATCOL substrate and no significant differences after transfection in suspension. Additionally, when comparing both knockdown experiments with 0.008% and 0.032% ATCOL coatings, we found that 0.008% provoked an extended knockdown of ICAM-1 until the end of experiment (day 8), while 0.032% showed gene silencing until day 4. The higher transfection efficiencies and the higher release values of 0.032% ATCOL with 5 μg siICAM-1 compared with the results of the 0.008% ATCOL coating seem to be contradictory to the long-term knockdown results. However, we may find an explanation by taking a closer at the chemistry of our chemicals. Due to the positive charge of ATCOL and Lipofectamine 2000, both chemicals may be in competition with the negatively charged siRNA, especially when the concentration of ATCOL is augmented. Therefore, we expected more naked siRNA in the 0.032% ATCOL coating, which leads to a higher fluorescence signal in transfection efficiency measurement and in the release study. The worse long-term knockdown results may be attributed to the faster degradation of the naked siRNA by nucleases. Because the coating with 5 μg siICAM-1 embedded in 0.008% ATCOL shows better performance than 0.032% ATCOL transfection coating concerning the long-term knockdown of ICAM-1, it would be ideally suited for inhibiting restenosis after stent placement. Furthermore, it is important to emphasize that we expect an even more prolonged gene silencing of ICAM-1 in in vivo experiments than in in vitro experiments, because cell division in tissue culture is much faster than in in vivo ECs. When cells divide, siRNAs are diluted each time and, consequently, the silencing of the protein is diminished.56
The examination of the hemocompatibility of our ATCOL coatings proved that the coating of siICAM-1 and Lipofectamine 2000 embedded in ATCOL is biocompatible in contact with blood, leading to a favorable outlook for stent coating. We observed no significant cell number reduction in erythrocytes, leukocytes, platelets, lymphocytes, monocytes, and granulocytes after incubation with coated glass slides compared with the control (Figures 7A–7F). We revealed significant differences comparing the uncoated or coated glass slides with the nonincubated blood control (0 hr), which is mediated most likely due to an interaction between the incubation tube and the blood cells. The results of our blood cell test indicate that blood cells do not accumulate on the ATCOL/siICAM-1 coating, because no reduction in cell number was observed compared to the 1-hr control. Furthermore, we proved the hemocompatibility of our coating system by testing the complement system values of SC5b9 and C3a and the PMN elastase. We noted a significant increase comparing 0-hr samples to 1-hr samples as described above in all three parameters, but we determined no increase in transfection coating with ATCOL and siICAM-1. Additionally, we found out that none of the coatings provoke hemolysis. In this respect, we suppose that our experiments and results lead the way to a promising new approach in stent development for combating risk after PTCA.
The combination of the biocompatible protein ATCOL with embedded siRNA particles targeting ICAM-1 seems to be a sophisticated therapeutic approach for the intervention in inflammatory processes after stent placement. The high cell viability of EA.hy926 grown on 0.008%–0.032% ATCOL demonstrated the imperative biocompatibility of the polymer. Furthermore, the coating shows good hemocompatibility, proven by no adsorption of blood cells to the coatings and no hemolysis, inflammation, or complement activation. Unexpectedly, ATCOL with naked siRNA was not able to transfect EA.hy926 sufficiently. As a consequence, we added Lipofectamine 2000 to our ATCOL/siRNA coatings and achieved significantly high transfection efficiencies with 68.9% (0.008% ATCOL) and 75.2% (0.032% ATCOL) positive cells. Our experiments show that the coating consisting of 0.008% ATCOL and 5 μg siICAM-1 is suitable for long-term knockdown of ICAM-1 protein up to 8 days. Because of this excellent performance of ATCOL combined with the personalized approach of using therapeutic siRNA, we consider our coating as a transferable system for in vivo applications that should be further investigated.
Materials and Methods
Chemicals for ATCOL Coating
Bovine dermis ATCOLs (3 mg/mL) from Cosmo Bio was diluted under sterile conditions with 5 mM sodium acetate buffer (pH 5.5). Different concentrations of ATCOL were used for coatings: 0.008%, 0.016%, 0.032%, and 0.064%. Sodium acetate was purchased from Sigma-Aldrich and prepared with double distilled water (ddH2O) (Ampuwa; Fresenius Kabi).
siRNAs
The following siRNA (siICAM) was used for gene knockdown: human ICAM-1 with sense strand 5′-GCC UCA GCA CGU ACC-UCU ATT-3′ and antisense 5′-UAG AGG UAC GUG CUG AAG CTT-3′. AF 488-labeled E-selectin siRNA AF 488 was applied to test the transfection efficiency (sense strand 5′-UUG AGU GGU GCA UUC AAC CTT-3′ and antisense 5′-GGU UGA AUG CAC CAC UCA ATT-3′; both from Eurofins MWG Operon). Control nonsense siRNA (siSCR) was supplied by QIAGEN. QIAGEN does not provide the sequences of their nonsilencing siRNAs but ensures that they have no homology to any known mammalian gene. This nonsilencing siRNA is validated by using Affymetrix GeneChip arrays and a variety of cell-based assays and is shown to ensure minimal nonspecific effects on gene expression and phenotype.
Substrate for ATCOL Coatings
The ATCOL coating was prepared on glass slides from Marienfeld, with a dimension of 10 × 10 × 1 mm. The slides were purified by ultrasonication (Bandelin RK 100H Sonorex; Bandelin Electronic) with 2% Hellmanex solution from Hellma before rinsing with ddH2O. Air-dried slides were sterilized in a heating furnace (Binder) at 200°C for 4 hr prior to their use in the ATCOL coating procedure.
Preparation of ATCOL/siRNA Coatings
The different ATCOL concentrations (0.008%, 0.016%, 0.032%, and 0.064%) were prepared by diluting the stock solution with sodium acetate buffer. siRNA (20 μM) was diluted in 0.15 M NaCl (Fresenius Kabi) and either added to Lipofectamine 2000 or not, depending on the experiment. We mixed different amounts of Lipofectamine 2000 with different amounts of siRNA in the following way: 1 μL Lipofectamine 2000 to 1 μg siRNA, 2 μL to 2.5 μg and 3 μl to 5 μg, respectively. Then, the two solutions (diluted ATCOL and siRNA with or without Lipofectamine 2000) were incubated separately for 10 min and then combined, shortly vortexed, and spun down. Within 20 min, complexes were allowed to form at room temperature. Glass slides were covered with 100 μL of the respective coating solution and air-dried under sterile conditions. ATCOL coatings without siRNA and Lipofectamine 2000 served beside uncoated glass slides as a control in flow cytometry.
ATCOL/siRNA-Mediated Transfection of EA.hy926
Coated glass slides were put in a 24-well plate and a maximum of 75,000 EA.hy926 cells in a 50-μL volume were applied on one glass slide. After 30-min incubation time, 1 mL medium was added to each well and cells were cultivated for 48 hr at 37°C. Thereafter, cells were prepared to determine transfection efficiency and knockdown of ICAM-1 protein expression by flow cytometry. The long-term knockdown experiment required adaption of the cell number due to the elongation of cultivation time up to 8 days. The cell number for seeding was reduced from 75,000 EA.hy926 to 30,000 for 4-day cultivation, to 23,000 for 6-day cultivation, and to 15,000 for 8-day cultivation.
To compare the results of the coating-mediated transfection with conventional transfection, cells were seeded 24 hr prior to coating transfection in a 24-well plate. At time point 0 (cultivation of cells on the coating), the same siRNA Lipofectamine 2000 and ATCOL amount that was used for the coating preparation was added to the cells in a solution. After 4 hr of transfection, the complete medium with transfection solution was discarded and cells were washed with cell culture medium. EA.hy926 cells were further cultured with fresh DMEM until the day of flow cytometry analysis. To enable the best cell growth, the medium was changed every second day.
Cultivation of EA.hy926
The immortalized human umbilical vein cell line EA.hy926 (LGC Standards) was used for all cell experiments. Cells were cultured in high-glucose DMEM containing 10% fetal calf serum, 1% penicillin/streptomycin, and 1% l-glutamine (Gibco, Division of Life Technologies).
Release of AF 488-Labeled E-selectin siRNA AF 488
Coatings of two different concentrations of ATCOL, 0.008% and 0.032%, were tested with 1, 2.5, and 5 μg E-selectin siRNA AF 488 to determine their release profile. Coatings were prepared as mentioned previously and the dried coated glass slides were placed in 24-well plates with 1 mL PBS. Slides were incubated under cell culture conditions at 37°C and supernatant was measured at defined time points with the Mithras LB 940 fluorescent reader (Berthold Technologies) at 485-nm excitation and 535-nm emission. Each sample value was represented in a XY scatter chart. Additionally, the last measured fluorescence intensity values were summed to determine the differences between the samples in a bar chart at day 9.
Flow Cytometry
Transfection efficiency or knockdown of ICAM-1 protein expression was determined by flow cytometry after different cultivation times. Transfection efficiency is shown as the relationship of positive (fluorescent) cells to the total cell amount, given in percentages. In our study, cells were transfected by substrate-mediated transfection with AF 488-labeled siRNA. After cultivation, cells were removed from the substrate by washing and detachment. Cells were subsequently fixed with 2.5% paraformaldehyde (PFA). Flow cytometry analysis was performed with 5,000 cells/measurement (FACScan; Becton Dickinson). The green fluorescence (FL1) signal within the cells was detected by a flow cytometer laser that also recorded forward and side scatter for the detection of viable cells. The results were evaluated with CellQuestPro software (Becton Dickinson), and the number of counts was plotted against the logarithmic scale of FL1. The marker was set at control samples ≤ 1 and transfection efficiency of the transfection samples was determined by this gate. This resulted in the transfection efficiency, given in percentages.
In our study, knockdown of ICMA-1 is defined as the percentage removal of the ICAM-1 receptor on EA.hy926 caused by RNAi. Here, siICAM-1 is able to bind to the complementary mRNA and degrade it. Therefore, surface-mediated siICAM-1 transfection was ended after the defined period and cells were stimulated with 5 ng/mL TNF-α (BD Biosciences) for 14 hr to induce ICAM-1 protein expression. Afterward, immunofluorescence staining with PE mouse anti-human CD54 (BD Biosciences) (30 min at 37°C) was prepared, followed by detachment, PFA fixation, and flow cytometry analysis. Here, the number of counts was plotted against the logarithmic scale of FL2. Geometric mean fluorescence was used to evaluate the results. The control treated with TNF-α and stained with Ab was set to 100%, and transfected cells with siICAM-1 and siSCR were assessed in relation to the control. The figures show the ICAM-1 expression after gene knockdown. Knockdown values are the calculated difference between the control at 100% and the ICAM-1 expression values.
Cell Viability: Cell Counting and Inverted Microscopy
Cell compatibility of EA.hy926 cultivated on ATCOL-coated glass slides was tested by a CASY cell counter and inverted microscopy. Different concentrations of ATCOL were examined: 0.008%, 0.016%, 0.032%, and 0.064%, while uncoated glass slides served as a control. 75,000 EA.hy926 cells were seeded onto the coated glass slides and cultivated for 24 and 48 hr, respectively. Cells were then washed and detached for cell counting with a CASY cell counter to distinguish dead cells from living cells, due to their lower resistance in an electronic pulse area analysis. EA.hy926 morphology and cell behavior were visualized with an Axiovert 135 microscope (Zeiss) and images were captured with the corresponding software.
Hemocompatibility
The development of medical devices requires their verification and qualification in respect tobiocompatibility, primarily cytocompatibility (ISO 10993-5), and hemocompatibility (ISO 10993-4). ISO 10993-4 demands at least one test addressing thrombosis/coagulation, hematology, inflammation, and the complement system to determine the compatibility of a medical device with blood.
Therefore, hemocompatibility of different combinations was analyzed with glass slides coated on both sides. Both sides were coated because glass activates blood coagulation. Consequently, we reduced the free glass surfaces, with the exception of their edges. We used 0.032% ATCOL as a worst case in the following sample preparations as coating solution: (1) ATCOL, (2) ATCOL and Lipofectamine 2000, and (3) ATCOL and siICAM-1-Lipofectamine 2000 (5 μg siRNA). Uncoated glass slides with 0-hr and 1-hr blood contact time served as a control and a baseline, respectively. Glass slides were incubated in 14-mL round-bottom tubes (Falcon; Corning Life Sciences) at 37°C under gentle shaking with 13 mL human blood. Six independent blood donors were used for hemocompatibility evaluation. After 1-hr incubation, blood was analyzed with a Micros 60 counter (ABX Diagnostics) for blood cells such as leukocytes, erythrocytes, and platelets. Protein expression of C3a and SC5b9 associated with the complement system was analyzed by ELISAs (both Osteomedical). PMN elastase was tested as a sign of degranulation of leukocytes during an inflammatory reaction (Demeditec Diagnostics). Additionally, β-thromboglobulin expression, associated with activated platelets, was determined by the ASSERA-CHROM β-TG kit (Diagnostica Stago).
Furthermore, a hemoglobin color test (no longer available; Roche) was used to assess coated and uncoated glass slides for their hemolysis capability. In short, defrosted citrated plasma was incubated with a reaction solution (0.6 mM potassium hexacyanoferrate III and 750 nM potassium cyanide) for 3 min at room temperature. Free hemoglobin converts into cyanohemoglobin in the presence of reaction solution and is measured photometrically at 546 nm.
RLM-RACE-PCR
The pivotal point of gene knockdown with siRNA is to prove that RNAi is due to the complementary binding of the siRNA to the respective mRNA and not because of unspecific reactions. In 2004, Soutschek et al.26 established the 5′-RLM-RACE-PCR technique detecting siRNA-mediated mRNA cleavage. Subsequently, Davis et al.57 used this technique to verify siRNA-mediated mRNA cleavage in a human phase I clinical trial. Herein, cleaved mRNA products can be detected, meaning that 5′-RLM-RACE-PCR is especially suitable for applications with siRNA transfection, to confirm the mRNA degradation dependent on the base pair sequence of the siRNA. Coatings of glass slides were produced as mentioned before, with 5 μg siICAM-1, Lipofectamine 2000, and 0.032% ATCOL. 75,000 EA.hy926 cells were cultivated on the coated glass slides for 48 hr. Afterward, cells were stimulated with TNF-α (5 ng/mL) for 14 hr before the RNA was isolated using the Aurum total RNA mini kit (Bio-Rad Laboratories). After quantification of eluted RNA, the RNA was ligated with a 5′-RACE adaptor in presence of T4 RNA ligase, which is commercially available in the First Choice RLM-RACE Kit (Life Technologies). RNA re-isolation was followed by reverse transcription using 200 ng ligated RNA Moloney murine leukemia virus (M-MLV) reverse transcriptase for first-strand cDNA synthesis. Gene-specific primers must be designed for the first-strand synthesis as well as for the outer and inner PCR occurring after first-strand cDNA synthesis. Primers were designed with Primer358 and Primer Premier 5 software (PREMIER Biosoft International) and are shown in Table 1. Outer and inner PCR reactions were conducted via qRT-PCR and contained IQ SYBR Green Supermix (Bio-Rad) performed in triplicate. The reaction mixture contained 400 nM forward and reverse primers with 2 ng cDNA in a total volume of 15 μL.
Table 1.
Primer Sequences for 5′-RLM-RACE-PCR
| 5′-RLM-RACE-PCR | Primer |
|---|---|
| First-strand ICAM-1 | 5′-AGGTACCATGGCCCCAAATG-3′ |
| ICAM-1 RACE 1 | 5′-ACTCTGTTCAGTGTGGCACC-3′ |
| ICAM-1 RACE 2 | 5′-TCTTCCTCGGCCTTCCCATA-3′ |
| ICAM-1 RACE 3 | 5′-TGGCCCCAAATGCTGTTGTA-3′ |
| RNA adaptor (universally) | 5′-GCUGAUGGCGAUGAAUGAACACUGC GUUUGCUGGCUUUGAUGAAA-3′ |
| Outer primer (universally) | 5′-GCTGATGGCGATGAATGAACACTG-3′ |
| Inner primer (universally) | 5′-CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATG-3′ |
Statistical Analysis
All experiments were conducted at least three times independently, except for the hemocompatibility and hemolysis assay in which the blood of six different donors was used. Comparison of the different samples was done by one-way ANOVA and Bonferroni correction as a post-test.
Author Contributions
O.K. designed the experiments and wrote the paper. O.K. and D.N. conducted most of the experiments. N.P. and A.B. provided support to conduct the transfection studies. B.N. provided technical support for the hemocompatibility assays. I.D. performed the hemolysis experiment. T.W., C.S., and H.P.W. supported the work with their clinical expertise and revised the manuscript. A.N. supervised the work.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Laslett L.J., Alagona P., Jr., Clark B.A., 3rd, Drozda J.P., Jr., Saldivar F., Wilson S.R., Poe C., Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012;60(Suppl):S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Roth G.A., Huffman M.D., Moran A.E., Feigin V., Mensah G.A., Naghavi M., Murray C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 3.Nichols M., Townsend N., Scarborough P., Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 2014;35:2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 4.Winniford M.D., Jansen D.E., Reynolds G.A., Apprill P., Black W.H., Hillis L.D. Cigarette smoking-induced coronary vasoconstriction in atherosclerotic coronary artery disease and prevention by calcium antagonists and nitroglycerin. Am. J. Cardiol. 1987;59:203–207. doi: 10.1016/0002-9149(87)90785-5. [DOI] [PubMed] [Google Scholar]
- 5.Wilson P.W. Established risk factors and coronary artery disease: the Framingham Study. Am. J. Hypertens. 1994;7:7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 6.Thompson P.D. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354–2363. doi: 10.1161/CIRCULATIONAHA.104.502591. [DOI] [PubMed] [Google Scholar]
- 7.Smith S.C., Jr., Benjamin E.J., Bonow R.O., Braun L.T., Creager M.A., Franklin B.A., Gibbons R.J., Grundy S.M., Hiratzka L.F., Jones D.W., World Heart Federation and the Preventive Cardiovascular Nurses Association AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 8.Eckel R.H., Jakicic J.M., Ard J.D., de Jesus J.M., Houston Miller N., Hubbard V.S., Lee I.M., Lichtenstein A.H., Loria C.M., Millen B.E., American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(Suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 9.Windecker S., Kolh P., Alfonso F., Collet J.P., Cremer J., Falk V., Filippatos G., Hamm C., Head S.J., Jüni P., Authors/Task Force members 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 10.Henderson R.A., Pocock S.J., Sharp S.J., Nanchahal K., Sculpher M.J., Buxton M.J., Hampton J.R. Long-term results of RITA-1 trial: clinical and cost comparisons of coronary angioplasty and coronary-artery bypass grafting. Randomised Intervention Treatment of Angina. Lancet. 1998;352:1419–1425. doi: 10.1016/s0140-6736(98)03358-3. [DOI] [PubMed] [Google Scholar]
- 11.Hamm C.W., Reimers J., Ischinger T., Rupprecht H.J., Berger J., Bleifeld W. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. German Angioplasty Bypass Surgery Investigation (GABI) N. Engl. J. Med. 1994;331:1037–1043. doi: 10.1056/NEJM199410203311601. [DOI] [PubMed] [Google Scholar]
- 12.RITA-2 Trial Participants Coronary angioplasty versus coronary artery bypass surgery: the Randomized Intervention Treatment of Angina (RITA) trial. Lancet. 1993;341:573–580. [PubMed] [Google Scholar]
- 13.Daemen J., Boersma E., Flather M., Booth J., Stables R., Rodriguez A., Rodriguez-Granillo G., Hueb W.A., Lemos P.A., Serruys P.W. Long-term safety and efficacy of percutaneous coronary intervention with stenting and coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis with 5-year patient-level data from the ARTS, ERACI-II, MASS-II, and SoS trials. Circulation. 2008;118:1146–1154. doi: 10.1161/CIRCULATIONAHA.107.752147. [DOI] [PubMed] [Google Scholar]
- 14.Cutlip D.E., Chauhan M.S., Baim D.S., Ho K.K., Popma J.J., Carrozza J.P., Cohen D.J., Kuntz R.E. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J. Am. Coll. Cardiol. 2002;40:2082–2089. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 15.Kornowski R., Hong M.K., Tio F.O., Bramwell O., Wu H., Leon M.B. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J. Am. Coll. Cardiol. 1998;31:224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 16.Kearney M., Pieczek A., Haley L., Losordo D.W., Andres V., Schainfeld R., Rosenfield K., Isner J.M. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997;95:1998–2002. doi: 10.1161/01.cir.95.8.1998. [DOI] [PubMed] [Google Scholar]
- 17.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 18.Serruys P.W., Kutryk M.J.B., Ong A.T.L. Coronary-artery stents. N. Engl. J. Med. 2006;354:483–495. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]
- 19.Kimura T., Morimoto T., Nakagawa Y., Kawai K., Miyazaki S., Muramatsu T., Shiode N., Namura M., Sone T., Oshima S., j-Cypher Registry Investigators Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–591. doi: 10.1161/CIRCULATIONAHA.111.046599. [DOI] [PubMed] [Google Scholar]
- 20.Bangalore S., Kumar S., Fusaro M., Amoroso N., Attubato M.J., Feit F., Bhatt D.L., Slater J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 21.Fajadet J., Wijns W., Laarman G.J., Kuck K.H., Ormiston J., Münzel T., Popma J.J., Fitzgerald P.J., Bonan R., Kuntz R.E., ENDEAVOR II Investigators Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114:798–806. doi: 10.1161/CIRCULATIONAHA.105.591206. [DOI] [PubMed] [Google Scholar]
- 22.Stettler C., Wandel S., Allemann S., Kastrati A., Morice M.C., Schömig A., Pfisterer M.E., Stone G.W., Leon M.B., de Lezo J.S. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370:937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 23.Douglas G., Van Kampen E., Hale A.B., McNeill E., Patel J., Crabtree M.J., Ali Z., Hoerr R.A., Alp N.J., Channon K.M. Endothelial cell repopulation after stenting determines in-stent neointima formation: effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur. Heart J. 2013;34:3378–3388. doi: 10.1093/eurheartj/ehs240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorsett Y., Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discov. 2004;3:318–329. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- 25.Dowler T., Bergeron D., Tedeschi A.L., Paquet L., Ferrari N., Damha M.J. Improvements in siRNA properties mediated by 2′-deoxy-2′-fluoro-beta-D-arabinonucleic acid (FANA) Nucleic Acids Res. 2006;34:1669–1675. doi: 10.1093/nar/gkl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soutschek J., Akinc A., Bramlage B., Charisse K., Constien R., Donoghue M., Elbashir S., Geick A., Hadwiger P., Harborth J. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 27.Boussif O., Lezoualc’h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B., Behr J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sano A., Maeda M., Nagahara S., Ochiya T., Honma K., Itoh H., Miyata T., Fujioka K. Atelocollagen for protein and gene delivery. Adv. Drug Deliv. Rev. 2003;55:1651–1677. doi: 10.1016/j.addr.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Minakuchi Y., Takeshita F., Kosaka N., Sasaki H., Yamamoto Y., Kouno M., Honma K., Nagahara S., Hanai K., Sano A. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res. 2004;32:e109. doi: 10.1093/nar/gnh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin A.L., Miyata T., Stenzel K.H. Collagen: medical and surgical applications. J. Macromol. Sci. Chem. 1969;3:113–118. [Google Scholar]
- 31.Ochiya T., Nagahara S., Sano A., Itoh H., Terada M. Biomaterials for gene delivery: atelocollagen-mediated controlled release of molecular medicines. Curr. Gene Ther. 2001;1:31–52. doi: 10.2174/1566523013348887. [DOI] [PubMed] [Google Scholar]
- 32.Honma K., Ochiya T., Nagahara S., Sano A., Yamamoto H., Hirai K., Aso Y., Terada M. Atelocollagen-based gene transfer in cells allows high-throughput screening of gene functions. Biochem. Biophys. Res. Commun. 2001;289:1075–1081. doi: 10.1006/bbrc.2001.6133. [DOI] [PubMed] [Google Scholar]
- 33.Hirai K., Sasaki H., Sakamoto H., Takeshita F., Asano K., Kubota Y., Ochiya T., Terada M. Antisense oligodeoxynucleotide against HST-1/FGF-4 suppresses tumorigenicity of an orthotopic model for human germ cell tumor in nude mice. J. Gene Med. 2003;5:951–957. doi: 10.1002/jgm.440. [DOI] [PubMed] [Google Scholar]
- 34.Hanai K., Kurokawa T., Minakuchi Y., Maeda M., Nagahara S., Miyata T., Ochiya T., Sano A. Potential of atelocollagen-mediated systemic antisense therapeutics for inflammatory disease. Hum. Gene Ther. 2004;15:263–272. doi: 10.1089/104303404322886110. [DOI] [PubMed] [Google Scholar]
- 35.Monaghan M., Browne S., Schenke-Layland K., Pandit A. A collagen-based scaffold delivering exogenous microRNA-29B to modulate extracellular matrix remodeling. Mol. Ther. 2014;22:786–796. doi: 10.1038/mt.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svintradze D.V., Mrevlishvili G.M. Fiber molecular model of atelocollagen-small interfering RNA (siRNA) complex. Int. J. Biol. Macromol. 2005;37:283–286. doi: 10.1016/j.ijbiomac.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Dalby B., Cates S., Harris A., Ohki E.C., Tilkins M.L., Price P.J., Ciccarone V.C. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 38.San Juan A., Bala M., Hlawaty H., Portes P., Vranckx R., Feldman L.J., Letourneur D. Development of a functionalized polymer for stent coating in the arterial delivery of small interfering RNA. Biomacromolecules. 2009;10:3074–3080. doi: 10.1021/bm900740g. [DOI] [PubMed] [Google Scholar]
- 39.Li J.M., Newburger P.E., Gounis M.J., Dargon P., Zhang X., Messina L.M. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 2010;17:1279–1287. doi: 10.1038/gt.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Che H.L., Bae I.H., Lim K.S., Song I.T., Lee H., Muthiah M., Namgung R., Kim W.J., Kim D.G., Ahn Y. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012;33:8548–8556. doi: 10.1016/j.biomaterials.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 41.Hlawaty H., San Juan A., Jacob M.P., Vranckx R., Letourneur D., Feldman L.J. Inhibition of MMP-2 gene expression with small interfering RNA in rabbit vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3593–H3601. doi: 10.1152/ajpheart.00517.2007. [DOI] [PubMed] [Google Scholar]
- 42.Shyu K.G., Wang B.W., Kuan P., Chang H. RNA interference for discoidin domain receptor 2 attenuates neointimal formation in balloon injured rat carotid artery. Arterioscler. Thromb. Vasc. Biol. 2008;28:1447–1453. doi: 10.1161/ATVBAHA.108.165993. [DOI] [PubMed] [Google Scholar]
- 43.Janmey P.A., Winer J.P., Murray M.E., Wen Q. The hard life of soft cells. Cell Motil. Cytoskeleton. 2009;66:597–605. doi: 10.1002/cm.20382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo C.-M., Wang H.-B., Dembo M., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhana B., Iyer R.K., Chen W.L.K., Zhao R., Sider K.L., Likhitpanichkul M., Simmons C.A., Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 46.Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 47.Engler A.J., Carag-Krieger C., Johnson C.P., Raab M., Tang H.Y., Speicher D.W., Sanger J.W., Sanger J.M., Discher D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saunders R.L., Hammer D.A. Assembly of human umbilical vein endothelial cells on compliant hydrogels. Cell. Mol. Bioeng. 2010;3:60–67. doi: 10.1007/s12195-010-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nolte A., Hossfeld S., Schroeppel B., Mueller A., Stoll D., Walker T., Wendel H.P., Krastev R. Impact of polyelectrolytes and their corresponding multilayers to human primary endothelial cells. J. Biomater. Appl. 2013;28:84–99. doi: 10.1177/0885328212437610. [DOI] [PubMed] [Google Scholar]
- 50.Mu P., Nagahara S., Makita N., Tarumi Y., Kadomatsu K., Takei Y. Systemic delivery of siRNA specific to tumor mediated by atelocollagen: combined therapy using siRNA targeting Bcl-xL and cisplatin against prostate cancer. Int. J. Cancer. 2009;125:2978–2990. doi: 10.1002/ijc.24382. [DOI] [PubMed] [Google Scholar]
- 51.Takeshita F., Minakuchi Y., Nagahara S., Honma K., Sasaki H., Hirai K., Teratani T., Namatame N., Yamamoto Y., Hanai K. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim T.K., Eberwine J.H. Mammalian cell transfection: the present and the future. Anal. Bioanal. Chem. 2010;397:3173–3178. doi: 10.1007/s00216-010-3821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nolte A., Walker T., Schneider M., Kray O., Avci-Adali M., Ziemer G., Wendel H.P. Small-interfering RNA-eluting surfaces as a novel concept for intravascular local gene silencing. Mol. Med. 2011;17:1213–1222. doi: 10.2119/molmed.2011.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan W., Farah S., Domb A.J. Drug eluting stents: developments and current status. J. Control Release. 2012;161:703–712. doi: 10.1016/j.jconrel.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Sluiter W., Pietersma A., Lamers J.M., Koster J.F. Leukocyte adhesion molecules on the vascular endothelium: their role in the pathogenesis of cardiovascular disease and the mechanisms underlying their expression. J. Cardiovasc. Pharmacol. 1993;22(Suppl 4):S37–S44. [PubMed] [Google Scholar]
- 56.Dykxhoorn D.M., Novina C.D., Sharp P.A. Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 57.Davis M.E., Zuckerman J.E., Choi C.H., Seligson D., Tolcher A., Alabi C.A., Yen Y., Heidel J.D., Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rozen S., Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]