Abstract
Setting
For now, hematological markers of inflammatory response have emerged as prognostic factors for patients with cancer. Many articles have confirm that neutrophil to lymphocyte ratio(NLR) and platelet–lymphocyte ratio (PLR) are relate with poor prognosis in various types of tumors.
Objective
To investigate the association between NLR/PLR and progression free survival (PFS), overall survival (OS) and clinicopathologic parameters in lung cancer patients.
Design
We performed relevant searches in PubMed database, Google Scholar, Springer Link. We included retrospective cohort studies that reported hazard ratios with 95% confidence intervals for the NLR or PLR and PFS or OS.
Results
Both high NLR (P < 0.00001) and high PLR (P = 0.01) were significantly predictive of poorer OS. It also demonstrated that elevated NLR predicted poorer PFS (P = 0.0002). High NLR was significantly associated with deeper Invasive of tumor, (P = 0.006) extensive lymph nodetastasis(N2–3) (P = 0.01), poor differentiation (P = 0.0002) and vascular invasion(P = 0.002). There was no evidence of publication bias. Subgroup analysis indicated that little evidence of heterogeneity. However, PLR has no prognostic significance for SCLC.
Conclusions
We provides further evidence in support of elevated NLR and PLR were predictors of poor OS and PFS in patients with lung cancer. Given this, NLR and PLR may be markers to report treatment outcomes.
Keywords: NLR, PLR, OS, clinicopathologic parameters, lung cancers
INTRODUCTION
It is widely believed that systemic inflammatory response is important in monitoring tumor progression and evaluating prognosis in many cancer types and then influence survival outcomes in cancer patients. Many hematological parameters such as neutrophil counts [1], monocyte counts [2], platelet counts [3], which are as components of systemic inflammation factors, neutrophil-lymphocyte ratio (NLR) [4] and platelet lymphocyte ratio [5], have proved to be indicators that have prognostic implications with many types of cancer in many studies. As we all known, Neutrophil–lymphocyte ratio (NLR) can easily get and is an important sign of inflammatory processes. The relevance between high NLR and poor prognosis in many types of cancers such as the breast cancer, kidney cancer, pancreas cancer, and stomach cancer be reported in many researches [1, 6]. Platelet to lymphocyte ratio (PLR), another factor which exerts a very important effect on the pathogenesis of systemic inflammatory response, was also proved to be associated with survival in patients with cancer [7]. Several studies have established that the platelet is supplementary of the role of neutrophils in the blood vessel metastasis [8]. However, the prognostic role of NLR and PLR in lung cancer and their significance in the clinical and pathological features still needs more studies to prove. Given the newly emerging evidence, we conducted a meta-analysis of retrospective cohort studies with the following objectives:(1):to systematically review, summarize and further confirm the prognostic value of NLR and PLR in lung cancer patients(2):to evaluate the impact of NLR and PLR on clinical and pathological features parameters of lung cancer.
RESULTS
Search outcomes
Nineteen studies involving 7,283 patients were included in the meta-analysis. A flow chart showing the study selection is presented in Figure 1. We initially retrieved unique studies. Of these, 143 citations were excluded after duplicated data leaving, 240 citations are left after screening based on abstracts or titles. Of these studies, 10 citations were excluded because of lack of enough data or the cut-offs of NLR or PLR, one citation fail to present the high NLR's survival data. Thus leaving 19 studies for the final analysis. Characteristics of the selected studies are presented in Table 1. All 19 retrospective cohort studies were published between 2012 and 2015. Among them, Nine studies were conducted in the China, three in Turkey, two in Japan, two in Korea, and one in Spain, one in UK, one in USA. The number of participants ranged from81 to 1,238, with a sum of 7,283, including 5,881 with NSCLC1,402 with SCLC. Seven of the studies evaluated both NLR and PLR. The other twelve evaluated only NLR. The cut-off value for HNLR was < 3 in seven studies, 3 ≤ to < 5 in six studies and ≥ 5 in five study. ALL studies evaluated OS outcomes. Eight studies evaluated both OS and PFS outcomes. In eighteen of the enrolled cohorts, HRs and 95% CIs reported directly. All of nineteen cohorts reported these data for NLR analyses. Eight studies directly reported these data for PLR analyses.
Figure 1. Flow chart demonstrating process of study selection.
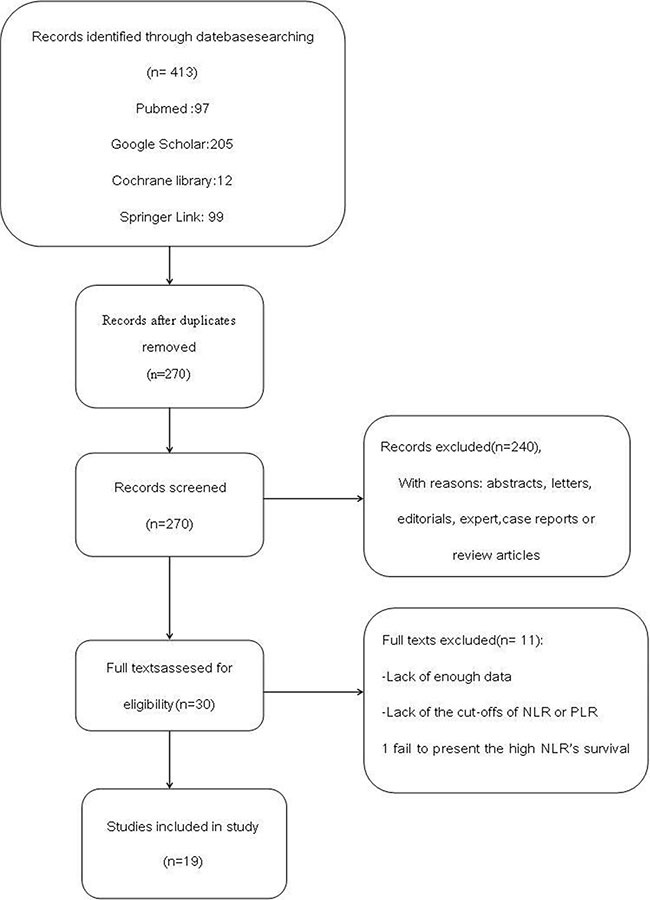
Table 1. Characteristics of all the studies included in the meta-analysis.
| Reference, year | Country | Nos. | HR | Design (period) | No of Patients(M/F) | TNM stage | Histology | NLR cut-off | PLR cut-off | Treatment | Prognosticvalue analyses | Survival outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Youngjoo Lee 2012 [27] | Korea | 6 | U/M | Retrospective | 199 (17/182) | III–IV | NSCLC | 3.25 | NR | C | NLR | OS and PFS |
| S. Cedré 2012 [28] | Spain | 7 | U/M | Retrospective | 171 (143/28) | IV | NSCLC | 5 | NR | C | NLR | OS and PFS |
| Yanwen Yao 2012 [29] | China | 7 | U/M | Retrospective | 182 (63/119) | III–IV | NSCLC | 2.63 | NR | C | NLR | OS and PFS |
| M H Kang 2014 [22] | Korea | 8 | U/M | Retrospective | 187 (162/25) | I–IV | SCLC | 4 | 160 | C | NLR PLR | OS and PFS |
| D J Pinato 2014 [9] | UK | 7 | U/M | Retrospective | 220 (110/110) | I–III | NSCLC | 5 | 300 | S/C | NLR PLR | OS and PFS |
| GuannanWu 2014 [30] | China | 7 | U/M | Retrospective | 366 (246/120) | III–IV | NSCLC | 2.68 | 119.5 | NR | NLR PLR | OS and PFS |
| Mehmet Kos 2014 [16] | Turkey | 7 | U/M | Retrospective | 145 (130/15) | I–IV | NSCLC | NR | 198.2 | S/C | PLR | OS |
| Tiehong Zhang 2014 [31] | China | 7 | U/M | Retrospective | 400 (272/128) | I–II | NSCLC | 2.6 | 200 | S | NLR PLR | OS and DFS |
| Turgut Kacan 2014 [32] | Turkey | 6 | M | Retrospective | 299 (270/29) | I–IV | NSCLC | 5 | NR | NR | NLR | OS |
| Gui-Nan LIN 2014 [33] | China | 7 | U/M | Retrospective | 81 (47/34) | NR | NSCLC | 3.5 | NR | TKIs | NLR | OS and PFS |
| Xinyue Wang 2014 [34] | China | 6 | M | Retrospective | 114 (89/25) | NR | SCLC | 3 | NR | S/C | NLR | OS |
| Katsuhiko Shimizu 2015 [35] | Japan | 7 | U/M | Retrospective | 334 (213/121) | I–III | NSCLC | 2.5 | NR | S | NLR | OS and DFS |
| Jae Eun Choi 2015 [36] | USA | 7 | U/M | Retrospective | 1139 (602/537) | I–III | NSCLC | 5 | NR | S/C | NLR | OS and RFS |
| FahriyeTugba Kos 2015 [37] | Turkey | 6 | U/M | Retrospective | 138 (124/14) | I–IV | NSCLC | 3.24 | NR | S/C | NLR | OS |
| Yusuke Takahashi 2015 [38] | Japan | 7 | U/M | Retrospective | 361 (114/152) | I–III | NSCLC | 2.5 | NR | S | NLR | OS and RFS |
| Hua Zhang 2015 [39] | China | 8 | U | Retrospective | 678 (449/229) | NR | NSCLC | 2.3 | 106 | S | NLR PLR | OS and DFS |
| Hua Zhang 2015(2) [40] | China | 7 | U/M | Retrospective | 1238 (812/426) | I–III | NSCLC | 2.3 | NR | S/C | NLR | OS and DFS |
Chemotherapy, DFS disease-free survival, HR hazard ratio, NR not reported, NLR neutrophil-to-lymphocyte ratio, OS overall survival, PLR platelet-to-lymphocyte ratio, PFS progression free survival, S surgery, TNM tumor, node, metastasis classification system.
Associations between NLR or PLR and prognostic
Significance of lung cancer
Eighteen studies presenting data of 5,881 patients for NLR and OS in lung cancer patients. In both studies, the elevated NLR were expected to have shorter OS with a pooled HR of 1.23 (95% CI: 1.17–1.29; P < 0.00001), However, with significant between-study heterogeneity (I2 53%, P = 0.004), a forest plot of this is shown as Figure 2. And there showed no significant publication bias by using Egger's test (P = 0.075) and Begg's test (P = 0.111). There are seven studies which included 1,298 patients investigated the association between NLR and PFS suggested that PFS was significantly poorer in patients with high NLR (HR = 1.18; 95% CI = 1.08 to 1.29, P = 0.0002) and heterogeneity was observed (P = 0.02, I2 = 70%). Begg's test (P = 0.048), but Egger's test (P = 0.097), showed no significant publication bias. Figure 3 presentes this analysis by the forest plot. Seven studies presenting data on PLR and OS of lung cancer for estimating HR and 95% CI showed a significant survival in patients with high compared to low PLR (HR 1.07, 95% CI 1.01–1.13, P = 0.01), with no significant heterogeneity (I2 = 52%, P = 0.05). Begg's test (P = 0.133) and Egger's test (P = 0.382) also indicated little evidence of publication bias. There is no meta-analysis of PLR and PFS was performed because only two studies reported this data.
Figure 2. Forest plots of HNLR versus LNLR with OS of all patients in all studies.
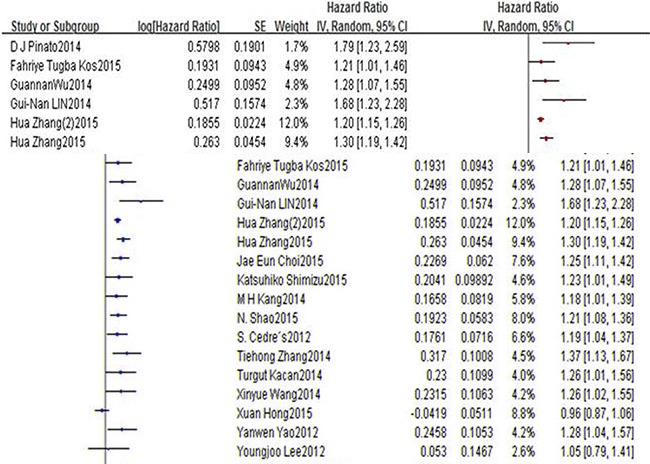
HNLR high neutrophil-to-lymphocyte ratio, LNLR low neutrophil-to-lymphocyte ratio, OS overall survival.
Figure 3. Forest plots of HNLR versus LNLR with PFS of all patients in studies.
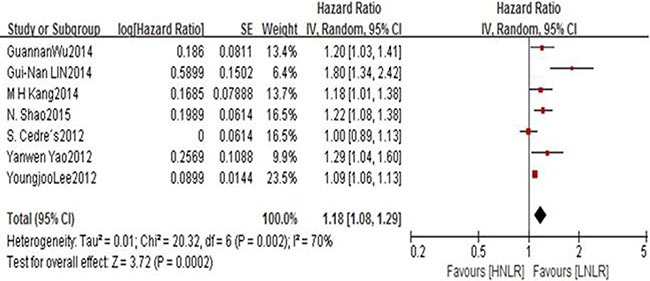
HNLR high neutrophil-to-lymphocyte, LNLR low neutrophil-to-lymphocyte ratio, PFS progression free survival.
Subgroup and sensitivity analyses
The subgroup analyses according to cut-offs for NLR, type of treatment, pathological type and region regarding the effect of NLR on OS is shown in Table 2. The results of most subgroup found little evidence of heterogeneity for high NLR suggested significantly poorer OS. However In subgroup of NLR ≥ 5, the I2 = 80%, p < 0.00001. From the subgroup which include of PLR on OS in NSCLC, we can got prognostic significance of high PLR (HR 1.14, 95% CI 1.06–1.23, P = 0.0002). While, the subgroup of PLR on OS in SCLC has no significance for PLR on OS. (HR 0.98, 95% CI 0.90 – 1.06, P = 0.61) (Figure 4). The differences between two subgroups were statistically significant (P for subgroup difference = 0.005). From the results above, we made a further subgroup analysis which is about the cut-offs for PLR on OS in NSCLC (Figure 5). It showed the cut-off value of PLR is not the source of heterogeneity (P for subgroup Difference = 0.07). Sensitivity analyses investigating the influence by omitting one study at a time and Calculating the combined HRs. Any single study was omitted, the pooled HRS were not substantially affected.
Table 2. Subgroup analyses.
| Subgroup | N | HR (95% CI) P | I2 (%) | P | P for subgroup difference | ||||
|---|---|---|---|---|---|---|---|---|---|
| RE | FE | RE | FE | ||||||
| Cut-offs for NLR | 0.96 | 0.05 | |||||||
| < 3 | 7 | 1.23 (1.19,1.28) | < 0.00001 | 1.23 (1.19,1.28) | < 0.00001 | 0% | 0.65 | ||
| 3–4 | 6 | 1.22 (1.13,1.32) | < 0.00001 | 1.22 (1.13,1.32) | < 0.00001 | 6% | 0.38 | ||
| NLR ≥ 5 | 5 | 1.21 (1.03,1.42) | 0.02 | 1.13 (1.06,1.20) | 0.0003 | 80% | < 0.00001 | ||
| Type of treatment | 0.07 | 0.07 | |||||||
| Chemotherapy | 5 | 1.20 (1.12,1.29) | < 0.00001 | 1.10 (1.04,1.17) | 0.002 | 0% | 0.88 | ||
| surgery | 5 | 1.31 (1.23,1.41) | < 0.00001 | 1.31 (1.23,1.41) | < 0.00001 | 0% | 0.50 | ||
| Pathological type | 0.11 | 0.03 | |||||||
| NSCLC | 14 | 1.24 (1.20,1.28) | < 0.00001 | 1.24 (1.20,1.28) | < 0.00001 | 2% | 0.43 | ||
| SCLC | 4 | 1.15 (1.06,1.25) | 0.0010 | 1.14 (1.07,1.22) | < 0.0001 | 35% | 0.20 | ||
| Region | 0.53 | 0.50 | |||||||
| Easterncountries | 15 | 1.22 (1.17,1.27) | < 0.00001 | 1.21 (1.18,1.25) | < 0.00001 | 23% | 0.20 | ||
| Westren countries | 3 | 1.28 (1.11,1.48)) | 0.0006 | 1.25 (1.15,1.37) | < 0.00001 | 49% | 0.14 | ||
FE fixed-effect model, HR hazard ratio, RE random-effect model, NSCLC non small cell lung cancer, N number, NLR neutrophil-to-lymphocyte ratio, SCLC small cell lung cancer.
Figure 4. Forest plots of HPLR versus LPLR with OS of all patients in studies and subgroups of patients who are NSCLC or SCLC.
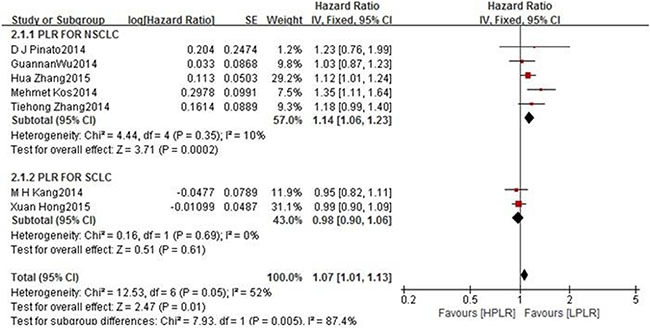
HNLR high neutrophil-to-lymphocyte ratio, LNLR low neutrophil-to-lymphocyte ratio NSCLC non small cell lung cancer, OS overall survival SCLC small cell lung cancer.
Figure 5. Forest plots of PLR ≥ 200 versus PLR < 200 with OS of NSCLC.
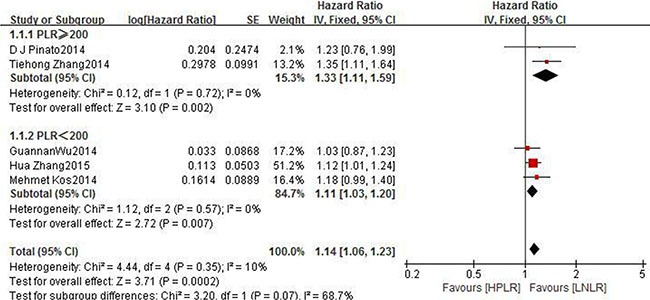
HPLR high platelet-to-lymphocyte ratio, LPLR low platelet-to-lymphocyte ratio, OS overall survival, NSCLC non small cell lung cancer.
Publication bias
There was no evidence of publication bias because of bias exploration funnel plots demonstrated symmetry. Egger's test and Begg's test also validates little publication bias. The associations between NLR and Clinicopathologic Parameters. For the associations between NLR and Clinicopathologic Parameters which are summarized in Table 3, showed that high NLR was significantly associated with deeper Invasive of tumor, (OR 1.54,95% CI 1.13–2.09, P = 0.006) extensive lymph node metastasis(N2-3)(OR 1.47,95% CI 1.09–1.97, P = 0.01), poor differentiation(OR1.72,95% CI 1.30 – 2.29, P = 0.0002))and vascular invasion(OR1.70,95% CI 1.21 – 2.40, P = 0.002). But it has no evidence to support that high NLR was associated with worse tumor stage (OR 0.92,95% CI 0.65–1.32, P = 0.66). The heterogeneity of all studies has no significance with P > 0.05.
Table 3. Associations between NLR and clinicopathologic parameters.
| Parameter | Study no. [references] | No. of patients | OR (95% CI, P) | I2 (P) |
|---|---|---|---|---|
| Invasive tumor (T3–4) | 3 | 719 | 1.54 (1.13–2.09,0.006) | 16% (0.30) |
| Lymph node Metastasis) (N2-3) | 4 | 1243 | 1.47 (1.09–1.97,0.01) | 0% (0.56) |
| Poor differentiation | 4 | 1053 | 1.72 (1.30–2.29,0.0002) | 0% (0.71) |
| Vascular invasion | 2 | 695 | 1.70 (1.21–2.40,0.002) | 61% (0.11) |
| Tumor Stage (IV) | 3 | 686 | 0.92 (0.65–1.32,0.66) | 61% (0.08) |
OR odds ratio.
DISCUSSION
The systematic inflammatory response of cancer-related can be easily embodied by measuring available blood parameters such as NLR, PLR [9]. NLR which is one of markers of systemic inflammation was accepted to be associated with prognosis in different cancers. NLR may present the pro-angiogenic/pro-inflammatory status in tumor tissue [10], and may also show how to balance neutrophils and lymphocytes, and then reflect patients' immune function [11, 12]. Preclinical studies showed that neutrophils may act through TGF-β induced signal pathway, with tumor promoting proliferation of leucocytes [13]. Patients with an elevated NLR exhibit the ratio of neutrophils and lymphocytes and may indirectly suggests poorer lymphocyte-mediated immune response to tumors, therefore, accelerating the process of tumor and prompting with worse prognosis [14]. Cytokines, such as vascular endothelial growth factor (VEGF) and transforming growth factor β, are meaningful in tumor angiogenesis. Platelets were also considered to be the major sources of these cytokines. PLTs could be elevated because of tumors or inflammatory cells releasing inflammatory mediators which can stimulate megakaryocyte release platelets [15]. PLR are reported that is associated with poor prognosis in many kinds of malignant tumors such as the pancreas, esophagus, stomach cancers in many studies [16].
Changes of the NLR and PLR levels has been reported to be predictive markers were consistent with chemotherapeutic efficacy and prognosis, and so NLR and PLR associated with pathological response to neoadjuvant chemotherapy or preoperative chemoradiotherapy in gastric cancer, esophageal and rectal cancers [17–19]. In our subgroup analysis of treatment, as for the NLR’ prognostic significance, there is no difference in surgery or chemotherapy. And we can not highlight NLR's prominent predictiverole in the chemotherapy. So it's needs more studies to clarify NLR and chemotherapy prognostication in lung cancers.
The present meta-analysis of 19 studies comprising 7,283 patients with lung cancers provides further evidence that high NLR was associated with poorer prognostic significance for lung cancer. High NLR compared with the low could predict OS and PFS in patients. In our subgroup analysis and sensitivity analyses associations did not significantly modified by type of treatment, pathological type and region. In addition, it showed the cut – offs of NLR < 5 were statistically worse affected than the patients with the cut – offs of NLR ≥ 5. Furthermore, elevated PLR could also predict OS of lung cancer patients. But, in subgroup analysis, as for SCLC, high PLR was not associated with a poorer prognosis.
All the intrinsic characteristics of tumor cells and the tumor microenvironment can be act to tumor progression and metastasis, which is mainly influenced by inflammatory cells, including neutrophile granulocyte [20].
Immune system promotes tumour vessel regeneration, migration, invasion, and metastasis by raising regulatory T lymphocytes and activation of related modulators such as IL-6 and TNF-α, C reactive protein, induction of neutrophilia, battering down immune system [21]. In our study there was also a significant association between NLR and Clinicopathologic Parameters, such as deeper Invasive of tumor, extensive lymph node metastasis, poor differentiation and vascular invasion. Taking all these into consideration, NLR and PLR are useful prognostic indicator in lung cancer. There are some important strengths in our study. Compared with the previous studies, we have larger sample size and with evidence to provide the association between high NLR and poor Clinicopathologic Parameters. Potential limitations should be concerned too. First we could not exclude uncontrolled or unmeasured risk factors from original studies that have confounded the true association. Second, Small-cell lung cancer (SCLC) accounts for 15–20% of all lung.
cancers and has an overbearing nature with a poor prognosis [22]. The studies we collected was rarely describe the PLR, furthermore the lack of studies showing the relationship between PLR and clinical pathological data. Third all articles are in English and the heterogeneity of some research is relatively large, which may be caused by some unmeasured factors. Finally, platelet, neutrophil and lymphocyte counts would be affected by the patients' basic state, infection, chemotherapy and other related factors [23], the subgroup proved that the heterogeneity of treatment is not significant. But we can not rule out the existence of patients' own inflammatory conditions completely.
MATERIALS AND METHODS
Search strategy
We attempted to report this meta-analysis in accordance with the Meta-Analysis of Observational Studies in Epidemiology guidelines. We conducted a systematic literature search of the PubMed database, Google Scholar, Cochrane databases and Springer link up to December 2015 by using the following search terms. NLR” (or “neutrophil lymphocyte ratio,”) OR “PLR”(or “platelet lymphocyte ratio,”) AND “lung cancer” AND “survival” Reference lists of the retrieved articles were also reviewed. We did not contact authors of the primary studies for additional information.
Inclusion and exclusion criteria
Study selection was based on an initial screen of identified abstracts or titles and a second screen of full-text articles. Studies were included if they met the following criteria:1) studies had to compare survival outcomes in lung cancer patients with high NLR (or PLR) versus low NLR (OR PLR) and report their cut-off values, and 2) availability of a hazard ratio(HR) and 95% confidence interval (CI) or a P value for overall survival (OS) or progression-free survival (PFS). Exclusion criteria were: (1) the full text was not available of quality assessment and data extraction; (2) abstracts, letters, editorials, expert, case reports or review articles; and (3) non-clinical studies or case reports.
Data extraction and quality assessment
All potentially eligible studies reviewed by two investigators independently and they collected data of patients and study characteristics. Conflicts of Data extraction or quality assessment were resolved by discussion and consensus. The inclusion/exclusion criteria and outcome measures are described below. (Table 1)The Newcastle-Ottawa Scale (NOS) was used to assess study quality. The NOS consists of three parameters of quality: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Studies with scores of 6 representing the high quality methodological study. OS and PFS were the primary outcomes of interest. The following details were extracted: (first author, year of publication, country of origin, number of patients included in analysis, disease Stage, histologic type, tumor invasion, lymph node status, metastasis, cut-off defining of peripheral blood NLR (or PLR), and hazard ratios and associated 95% confidence intervals for OS, PFS as applicable. Hazard ratios were extracted preferentially from multivariable analyses where available. If not, we extract hazard ratios from univariate analyses.
Statistical analyses
Extracted data were combined into a meta-analysis using RevMan5.3 analysis software. The primary summary is logarithm of the hazard ratio (HR) with 95% confidence interval (CI) [24]. The data of HR and 95% CIs were obtained directly from individual articles or were calculated from indirect data [24]. Practical methods for incorporating summary time-to-event data into meta-analysis. Meta-analysis of the data was conducted using random-effects model and the fixed-effects model. Publication bias was explored graphically by visual inspection of funnel plots to detect asymmetry and any outliers and was assessed by Egger's I test and Begg's test. When P < 0.05, they were considered to be significantly biased [25, 26]. Heterogeneity was assessed using the square statistic and the I2 value which is a quantitative measure of in consistency across studies. This was graded as low (I2 < 25%), moderate (I2 = 25 to 75%) or high (I2 > 75%). and was considered significant at the P < 0.05 level. We conducted subgroup analyses according to cut-offs for NLR, type of treatment, pathological type and region. All statistical tests were two-sided, P value < 0.05 was considered statistically significant, except where otherwise specified.
CONCLUSIONS
This meta-analysis suggests that the high NLR and PLR is associated with worse survival in lung cancer patients and support a significantly relation between high NLR and deeper Invasive of tumor, extensive lymph node metastasis, poor differentiation and vascular invasion. However, there needs more well-designed and large-scale studies to demonstrate the PLR's value in SCLC and the connection of high PLR with clinicopathologic parameters. High systemic inflammation as measured by NLR and PLR can well assess the poor survival among lung cancer patients in clinical application.
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Pei D, Zhu F, Chen X, Qian J, He S, Qian Y, Shen H, Liu Y, Xu J, Shu Y. Pre-adjuvant chemotherapy leukocyte count may predict the outcome for advanced gastric cancer after radical resection. Biomed Pharmacother. 2014;68:213–7. doi: 10.1016/j.biopha.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Tsai YD, Wang CP, Chen CY, Lin LW, Hwang TZ, Lu LF, Hsu HF, Chung FM, Lee YJ, Houng JY. Pretreatment circulating monocyte count associated with poor prognosis in patients with oral cavity cancer. Head Neck. 2014;36:947–53. doi: 10.1002/hed.23400. [DOI] [PubMed] [Google Scholar]
- 3.Rachidi S, Wallace K, Day TA, Alberg AJ, Li Z. Lower circulating platelet counts and antiplatelet therapy independently predict better outcomes in patients with head and neck squamous cell carcinoma. J Hematol Oncol. 2014;7:65. doi: 10.1186/s13045-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao N, Cai Q. High pretreatment neutrophil-lymphocyte ratio predicts recurrence and poor prognosis for combined small cell lung cancer. Clin Transl Oncol. 2015;17:772–8. doi: 10.1007/s12094-015-1289-8. [DOI] [PubMed] [Google Scholar]
- 5.Kemal Y, Yucel I, Ekiz K, Demirag G, Yilmaz B, Teker F, Ozdemir M. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pacific journal of cancer prevention. 2014;15:2651–4. doi: 10.7314/apjcp.2014.15.6.2651. [DOI] [PubMed] [Google Scholar]
- 6.Forget P, Machiels JP, Coulie PG, Berliere M, Poncelet AJ, Tombal B, Stainier A, Legrand C, Canon JL, Kremer Y, De Kock M. Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20:S650–60. doi: 10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Wu Y, Wang Z, Yao Y, Chen F, Zhang H, Wang Y, Song Y. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer. J Thorac Dis. 2013;5:783–9. doi: 10.3978/j.issn.2072-1439.2013.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013. [DOI] [PMC free article] [PubMed]
- 9.Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–5. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, Staropoli N, Pastina P, Marvaso G, Martellucci I, Guglielmo A, Pirtoli L, Sperlongano P, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther. 2013;14:469–75. doi: 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, Rotundo MS, Ginanneschi C, Martellucci I, Francini E, Cusi MG, Tassone P, Francini G. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial) Clin Cancer Res. 2008;14:4192–9. doi: 10.1158/1078-0432.CCR-07-5278. [DOI] [PubMed] [Google Scholar]
- 12.Lissoni P, Brivio F, Fumagalli L, Messina G, Ghezzi V, Frontini L, Giani L, Vaghi M, Ardizzoia A, Gardani GS. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–40. doi: 10.1177/172460080401900208. [DOI] [PubMed] [Google Scholar]
- 13.Yao ZH, Tian GY, Wan YY, Kang YM, Guo HS, Liu QH, Lin DJ. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139:2117–23. doi: 10.1007/s00432-013-1523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandrakis MG, Passam FH, Perisinakis K, Ganotakis E, Margantinis G, Kyriakou DS, Bouros D. Serum proinflammatory cytokines and its relationship to clinical parameters in lung cancer patients with reactive thrombocytosis. Respir Med. 2002;96:553–8. doi: 10.1053/rmed.2002.1328. [DOI] [PubMed] [Google Scholar]
- 16.Kos M, Hocazade C, Kos FT, Uncu D, Karakas E, Dogan M, Uncu HG, Yildirim N, Zengin N. Prognostic role of pretreatment platelet/lymphocyte ratio in patients with non-small cell lung cancer. Wien Klin Wochenschr. 2015. [DOI] [PubMed]
- 17.Dou X, Wang RB, Yan HJ, Jiang SM, Meng XJ, Zhu KL, Xu XQ, Mu DB. Circulating lymphocytes as predictors of sensitivity to preoperative chemoradiotherapy in rectal cancer cases. Asian Pacific journal of cancer prevention. 2013;14:3881–5. doi: 10.7314/apjcp.2013.14.6.3881. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World journal of surgery. 2012;36:617–22. doi: 10.1007/s00268-011-1411-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Liu ZY, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR, Tao M, Lian L, Li W. Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncology letters. 2015;10:3411–8. doi: 10.3892/ol.2015.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, Koike K, Nishino H, Tajiri H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988–93. doi: 10.1038/bjc.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MH, Go SI, Song HN, Lee A, Kim SH, Kang JH, Jeong BK, Kang KM, Ling H, Lee GW. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111:452–60. doi: 10.1038/bjc.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445–52. doi: 10.1159/000331494. [DOI] [PubMed] [Google Scholar]
- 24.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y, Kim SH, Han JY, Kim HT, Yun T, Lee JS. Early neutrophil-to-lymphocyte ratio reduction as a surrogate marker of prognosis in never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. J Cancer Res Clin Oncol. 2012;138:2009–16. doi: 10.1007/s00432-012-1281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cedres S, Torrejon D, Martinez A, Martinez P, Navarro A, Zamora E, Mulet-Margalef N, Felip E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864–9. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- 29.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–9. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu G, Yao Y, Bai C, Zeng J, Shi D, Gu X, Shi X, Song Y. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac Cancer. 2015;6:275–87. doi: 10.1111/1759-7714.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Jiang Y, Qu X, Shen H, Liu Q, Du J. Evaluation of preoperative hematologic markers as prognostic factors and establishment of novel risk stratification in resected pN0 non-small-cell lung cancer. PLoS One. 2014;9:e111494. doi: 10.1371/journal.pone.0111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kacan T, Babacan NA, Seker M, Yucel B, Bahceci A, Eren AA, Eren MF, Kilickap S. Could the neutrophil to lymphocyte ratio be a poor prognostic factor for non small cell lung cancers? Asian Pacific journal of cancer prevention. 2014;15:2089–94. doi: 10.7314/apjcp.2014.15.5.2089. [DOI] [PubMed] [Google Scholar]
- 33.Lin GN, Peng JW, Liu PP, Liu DY, Xiao JJ, Chen XQ. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol. 2014. [DOI] [PubMed]
- 34.Wang X, Jiang R, Li K. Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell Biochem Biophys. 2014;69:633–40. doi: 10.1007/s12013-014-9845-3. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K, Okita R, Saisho S, Maeda A, Nojima Y, Nakata M. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol. 2015;13:291. doi: 10.1186/s12957-015-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi JE, Villarreal J, Lasala J, Gottumukkala V, Mehran RJ, Rice D, Yu J, Feng L, Cata JP. Perioperative neutrophil:lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: a retrospective study. Cancer Med. 2015;4:825–33. doi: 10.1002/cam4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kos FT, Hocazade C, Kos M, Uncu D, Karakas E, Dogan M, Uncu HG, Ozdemir N, Zengin N. Assessment of Prognostic Value of “Neutrophil to Lymphocyte Ratio” and “Prognostic Nutritional Index” as a Sytemic Inflammatory Marker in Non-small Cell Lung Cancer. Asian Pacific journal of cancer prevention. 2015;16:3997–4002. doi: 10.7314/apjcp.2015.16.9.3997. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi Y, Horio H, Hato T, Harada M, Matsutani N, Morita S, Kawamura M. Prognostic Significance of Preoperative Neutrophil-Lymphocyte Ratios in Patients with Stage I Non-small Cell Lung Cancer After Complete Resection. Ann Surg Oncol. 2015;22:1324–31. doi: 10.1245/s10434-015-4735-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Xia H, Zhang L, Zhang B, Yue D, Wang C. Clinical significance of preoperative neutrophil-lymphocyte vs platelet-lymphocyte ratio in primary operable patients with non-small cell lung cancer. Am J Surg. 2015;210:526–35. doi: 10.1016/j.amjsurg.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu J, Yue D, Zhang B, Wang C. Prognostic Significance of Combination of Preoperative Platelet Count and Neutrophil-Lymphocyte Ratio (COP-NLR) in Patients with Non-Small Cell Lung Cancer: Based on a Large Cohort Study. PLoS One. 2015;10:e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J Exp Med. 2015;236:297–304. doi: 10.1620/tjem.236.297. [DOI] [PubMed] [Google Scholar]


