Abstract
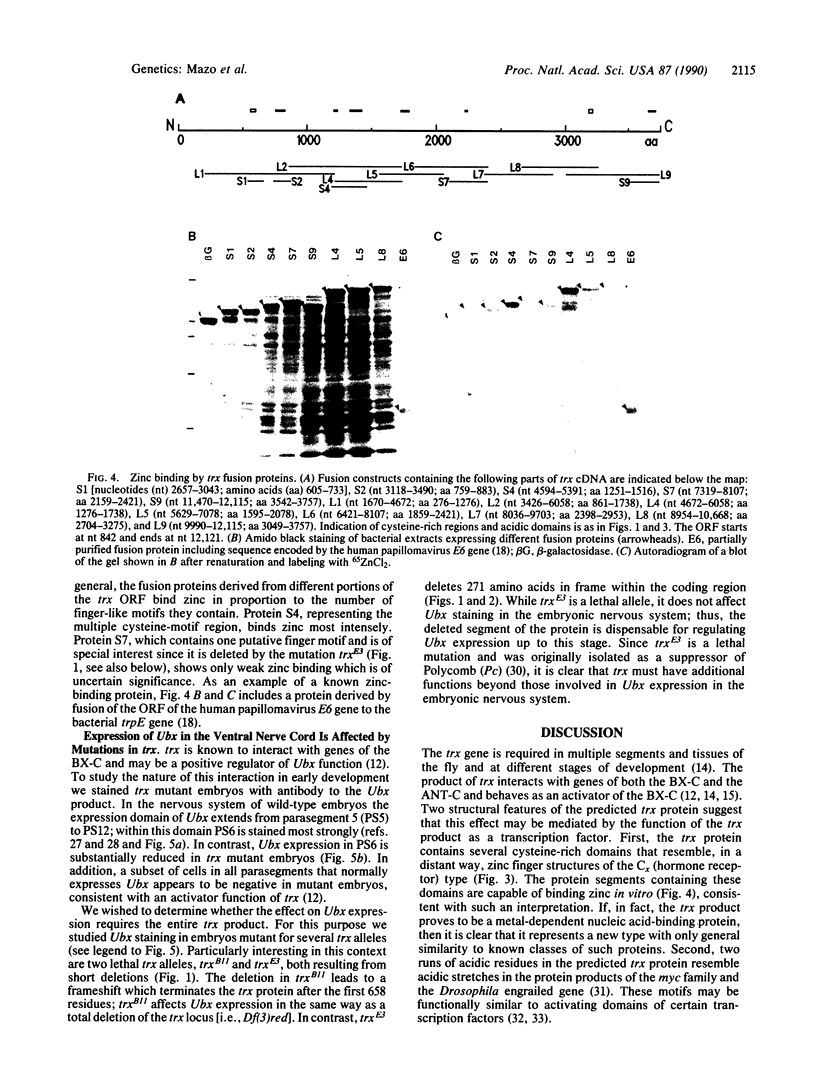
The trithorax (trx) gene functions in segment determination in Drosophila through interaction with genes of the bithorax complex and Antennapedia complex. Genetic evidence suggests that trx may be considered a positive regulator of homeotic genes. Sequencing of cDNAs corresponding to the entire trx transcription unit revealed the existence of an unusually long open reading frame encoding 3759 amino acids. The main features of the predicted trx protein are several cysteine-rich regions which can be folded into zinc finger-like domains. Cysteine-rich portions expressed from trx cDNAs in Escherichia coli are capable of zinc binding in vitro, suggesting a possible function for the trx product as a metal-dependent DNA-binding protein. Analysis of trx mutant embryos with antibody to the Ultrabithorax (Ubx) gene product showed decreased staining in parasegment 6 of the ventral nerve cord of late embryos. However, expression of Ubx was not affected in embryos carrying the lethal mutation trxE3, in which one of the putative zinc finger-like domains of the trx protein is deleted. This differential effect of the E3 mutation suggests that trx exhibits other function(s) besides those involved in the regulation of Ubx expression in the ventral nerve cord of the embryo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. S., Lowy D. R., Schiller J. T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989 Mar;63(3):1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy P. A., Helfand S. L., Hogness D. S. Segmental distribution of bithorax complex proteins during Drosophila development. Nature. 1985 Feb 14;313(6003):545–551. doi: 10.1038/313545a0. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Laymon R. A., McCutcheon M. A., Riley P. D., Scott M. P. The localization and regulation of Antennapedia protein expression in Drosophila embryos. Cell. 1986 Oct 10;47(1):113–122. doi: 10.1016/0092-8674(86)90372-7. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., Scott M. P. Localization of the fushi tarazu protein during Drosophila embryogenesis. Cell. 1985 Nov;43(1):47–57. doi: 10.1016/0092-8674(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Digan M. E., Haynes S. R., Mozer B. A., Dawid I. B., Forquignon F., Gans M. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev Biol. 1986 Mar;114(1):161–169. doi: 10.1016/0012-1606(86)90392-1. [DOI] [PubMed] [Google Scholar]
- Duncan I. Control of bithorax complex functions by the segmentation gene fushi tarazu of D. melanogaster. Cell. 1986 Oct 24;47(2):297–309. doi: 10.1016/0092-8674(86)90452-6. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A. Genetic control of wing disc development in Drosophila. Ciba Found Symp. 1975;0(29):161–182. doi: 10.1002/9780470720110.ch8. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Levine M., Gehring W. J. Regulation of Antennapedia transcript distribution by the bithorax complex in Drosophila. Nature. 1984 Jan 19;307(5948):287–289. doi: 10.1038/307287a0. [DOI] [PubMed] [Google Scholar]
- Harding K., Wedeen C., McGinnis W., Levine M. Spatially regulated expression of homeotic genes in Drosophila. Science. 1985 Sep 20;229(4719):1236–1242. doi: 10.1126/science.3898362. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. A clonal analysis of the requirement for the trithorax gene in the diversification of segments in Drosophila. J Embryol Exp Morphol. 1985 Oct;89:349–365. [PubMed] [Google Scholar]
- Ingham P. W. Genetic control of the spatial pattern of selector gene expression in Drosophila. Cold Spring Harb Symp Quant Biol. 1985;50:201–208. doi: 10.1101/sqb.1985.050.01.026. [DOI] [PubMed] [Google Scholar]
- Ingham P. W., Ish-Horowicz D., Howard K. R. Correlative changes in homoeotic and segmentation gene expression in Krüppel mutant embryos of Drosophila. EMBO J. 1986 Jul;5(7):1659–1665. doi: 10.1002/j.1460-2075.1986.tb04409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham P. W., Martinez-Arias A. The correct activation of Antennapedia and bithorax complex genes requires the fushi tarazu gene. Nature. 1986 Dec 11;324(6097):592–597. doi: 10.1038/324592a0. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., Poole S. J., Wright D. K., O'Farrell P. H. Sequence conservation in the protein coding and intron regions of the engrailed transcription unit. EMBO J. 1986 Dec 20;5(13):3583–3589. doi: 10.1002/j.1460-2075.1986.tb04686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison J. A., Tamkun J. W. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Mozer B. A., Dawid I. B. Cloning and molecular characterization of the trithorax locus of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1989 May;86(10):3738–3742. doi: 10.1073/pnas.86.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Denell R. E. Homoeosis in Drosophila: The Lethal Syndrome of the Regulator of bithorax (or trithorax) Locus and Its Interaction with Other Homoeotic Loci. Genetics. 1987 Jul;116(3):389–398. doi: 10.1093/genetics/116.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn A. The ash-1, ash-2 and trithorax genes of Drosophila melanogaster are functionally related. Genetics. 1989 Mar;121(3):517–525. doi: 10.1093/genetics/121.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. A., Lehmann R. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell. 1986 Oct 24;47(2):311–321. doi: 10.1016/0092-8674(86)90453-8. [DOI] [PubMed] [Google Scholar]
- White R. A., Wilcox M. Protein products of the bithorax complex in Drosophila. Cell. 1984 Nov;39(1):163–171. doi: 10.1016/0092-8674(84)90202-2. [DOI] [PubMed] [Google Scholar]
- Zink B., Paro R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature. 1989 Feb 2;337(6206):468–471. doi: 10.1038/337468a0. [DOI] [PubMed] [Google Scholar]