Abstract
Several previous studies have been reported to examine the association between Vitamin D receptor (VDR) gene Fok I polymorphism and susceptibility to prostate cancer (PCa), however the results remain inconclusive. To provide a relatively comprehensive account of the association, we searched PubMed, Embase, CNKI, and Wanfang for eligible studies and carry out this meta-analysis. A total of 27 case-control studies with 10,486 cases and 10,400 controls were included. In the overall analysis, Fok I polymorphism was not significantly associated with the susceptibility to PCa. Subgroup analyses showed that significantly association was existed in Caucasian population, the subgroup of population-based controls and the stratified group with advanced tumor.These results indicate that the VDR Fok I polymorphism might be capable of causing PCa susceptibility and could be a promising target to forecast the PCa risk for clinical practice. However further well-designed epidemiologic studies are needed to confirm this conclusion.
Keywords: Fok I, prostate cancer, vitamin D receptor, polymorphisms, meta-analysis
INTRODUCTION
Prostate cancer (PCa) is now thought to be one of the most commonly diagnosed malignant tumors in old men throughout the world, and the second cause of cancer in males. It accounted for approximately 233,000 (27%) new cases and 30,000 deaths in the United States in 2014 [1]. The global incidence of PCa has increased annually. The etiology of PCa is largely unknown. Several factors have been suggested to be strongly associated with the increased risk, including ethnic origin, family history, hormonal status, dietary structure and age [2].
Low levels of vitamin D are considered to be a risk factor for PCa [3]. In vitro experiments suggested that vitamin D inhabits the growth and differentiation of prostate cancer cells, promotes cell apoptosis. It can also inhabit the invasion, metabolism and angiogenesis of tumor cell [3]. A clinical trial of PCa patients showed that calcitriol, analogue of vitamin D can significantly reduce the prostate specical antigen (PSA) level, and improve the patients survival rate [4].
The anticancer effect of vitamin D is activated mainly through the vitamin D receptor (VDR) [5]. 1,25-Dihydroxy vitamin D3 (1,25(OH)2D3), the active form of vitamin D, binds to VDR and form a heterodimer complex, which subsequently binds to the vitamin D response element and down-regulate the transcription of numerous genes that stimulating the cell growth and differentiation [6].
Several single nucleotide polymorphisms (SNPs) of VDR gene were reported to be associated with risk of PCa [7]. Fok I variant (rs10735810) located in exon 2 of VDR gene is one of the most extensively studied SNPs [8]. It could result in a frame-shift mutation in the expression of VDR. It has been reported that f allele results in three amino acids longer VDR than the F allele, and extensive researches indicate that f allele is less effective than the F allele in transcription activity and transactivation of the 1,25(OH)2D3 signal [8]. Recent studies have shown that Fok I polymorphism might accelerate the progression of PCa. However, the results are disputable and contradictory [9, 10], as it might be underpowered for individual study. Therefore, we performed this meta-analysis to draw a more precise conclusion based on the published literature.
RESULTS
Characteristics of studies included in this meta-analysis
A total of 277 potentially relevant studies were identified following the searching strategy. 27 studies [2, 6, 7, 9, 10, 12-32] were finally included in this meta-analysis according to the inclusion criteria (Figure 1). Publication years ranged from 1999 to 2015, the number of cases varied from 28 to 1,518, and the number of controls varied from 56 to 1,432 (Table 1). The distribution of genotype frequency in the control groups was in accordance with the HWE for almost studies, except two studies [9, 15. in which source of controls was hospital-based. As a result, data for our meta-analysis were available from 27 studies with a total of 10,468 cases and 10,400 controls. The eligible studies were assessed by the NOS. Each of the studies scored morethan 4, which suggested that all of them are of high quality researches (Table 1).
Figure 1. Study flowchart for the process of selecting the final 27 studies.
Table 1. Characteristics and quality assessment of the studies included in this meta-analysis.
| Study ID | Year | Country | Ethnicity | Genotyping method | Source of controls | Total sample size (case/control) | HWE | Quality indicators from NOS |
|---|---|---|---|---|---|---|---|---|
| Atoum | 2015 | Jordan | Asian | TaqMan | PB | 124/100 | Y | 6 |
| Bai | 2009 | China | Asian | PCR-RFLP | HB | 122/130 | Y | 6 |
| Bodiwala | 2004 | UK | Caucasian | PCR-RFLP | HB/BPH | 368/243 | Y | 6 |
| Chen | 2001 | China | Asian | PCR-RFLP | HB | 101/145 | N | 5 |
| Cheteri | 2004 | USA | Caucasian | PCR-RFLP | PB | 552/521 | Y | 6 |
| Chokkalingam | 2001 | China | Asian | PCR-RFLP | PB | 187/302 | Y | 6 |
| Cicek | 2006 | USA | Mixed | PCR-RFLP | PB | 439/479 | Y | 6 |
| Correa-Cerro | 1999 | Germany/France | Caucasian | PCR-RFLP | HB | 118/89 | Y | 6 |
| Hayes | 2005 | Australia | Caucasian | DGGE* | PB | 811/713 | Y | 7 |
| Holick | 2007 | USA | Caucasian | SNPlex | PB | 583/552 | Y | 6 |
| Holt | 2009 | USA | Caucasian | SNPlex | PB | 705/716 | Y | 6 |
| Huang | 2006 | China | Asian | PCR-RFLP | HB/BPH | 416/502 | Y | 6 |
| Jiang | 2013 | China | Asian | PCR-RFLP | PB | 100/108 | Y | 6 |
| John | 2005 | USA | Caucasian | TaqMan | PB | 425/437 | Y | 6 |
| Li | 2007 | USA | Caucasian | PCR-RFLP | PB | 1010/1432 | Y | 8 |
| Luscombe | 2001 | UK | Caucasian | PCR-RFLP | BPH | 209/154 | Y | 6 |
| Mikhak | 2007 | USA | Caucasian | TaqMan | PB | 670/673 | Y | 7 |
| Mishra | 2005 | India | Asian | PCR-RFLP | HB | 147/128 | Y | 6 |
| Oakley-Grivan | 2004 | USA | Mixed | PCR-RFLP | PB | 345/292 | Y | 6 |
| Oh | 2013 | Korea | Asian | IGGGS# | BPH | 272/173 | Y | 6 |
| Rowland | 2013 | USA | Mixed | TaqMan | PB | 1518/1070 | Y | 7 |
| Ruan | 2009 | China | Asian | PCR-RFLP | BPH | 100/100 | Y | 5 |
| Rukin | 2007 | UK | Caucasian | Pyrosequencing | BPH | 430/320 | Y | 6 |
| Tayeb | 2004 | UK | Caucasian | PCR-RFLP | BPH | 28/56 | Y | 6 |
| Torkko | 2008 | USA | Caucasian | TaqMan | PB | 585/761 | Y | 6 |
| Yang | 2004 | China | Asian | PCR-RFLP | PB | 80/96 | Y | 5 |
| Yousaf | 2014 | Pakistani | Asian | PCR-RFLP | HB | 41/108 | N | 6 |
Abbreviations: HWE, Hardy-Weinberg equilibrium; PB, population-based; HB, hospital-based; BPH, Benign Prostate Hyperplasia; RFLP, restriction fragment length polymorphism; DGGE, denaturing gradient gel electrophoresis; IGGGS, Illumina Golden Gate genotyping system
Meta-analysis results
The results of overall analysis are showed in Table 2 and Figure 2. The pooled results indicated that Fok I polymorphism is not associated with the PCa risk in the overall populations (ff vs. FF: OR=1.07, 95%CI=0.98-1.16, p=0.131; Ff vs. FF: OR=1.03, 95%CI=0.97-1.10, p=1.05; Ff/ff vs. FF: OR= 1.04, 95%CI= 0.98-1.10, p=0.173; ff vs. FF/Ff: OR=1.04, 95%CI=0.96-1.12, p=0.318; f vs. F allele: OR=1.03, 95%CI=0.99-1.07, p=0.138). (Table 2).
Table 2. Results of the association between Fok I polymorphism and PCa risk in the whole population.
| Comparison | Studies | Overall effect | Heterogeneity | Public bias | ||||
|---|---|---|---|---|---|---|---|---|
| OR | Z-score | p-value | I2 | P-value | Begg's test | Egger's test | ||
| ff vs FF | 27 | 1.07 [0.98-1.16] | 1.51 | 0.131 | 14% | 0.255 | 0.087 | 0.118 |
| Ff vs FF | 27 | 1.03 [0.97-1.10] | 1.05 | 0.296 | 0% | 0.809 | 0.402 | 0.866 |
| ff+Ff vs FF | 27 | 1.04 [0.98-1.10] | 1.36 | 0.173 | 0% | 0.475 | 0.133 | 0.322 |
| ff vs FF+Ff | 27 | 1.04 [0.96-1.12] | 1 | 0.318 | 13% | 0.274 | 0.227 | 0.138 |
| f vs F | 27 | 1.03 [0.99-1.07] | 1.48 | 0.138 | 27% | 0.102 | 0.027 | 0.101 |
Figure 2. Forest plots to estimate the association of VDR Fok I polymorphism with PCa in the subgroup analysis of ethnicity.
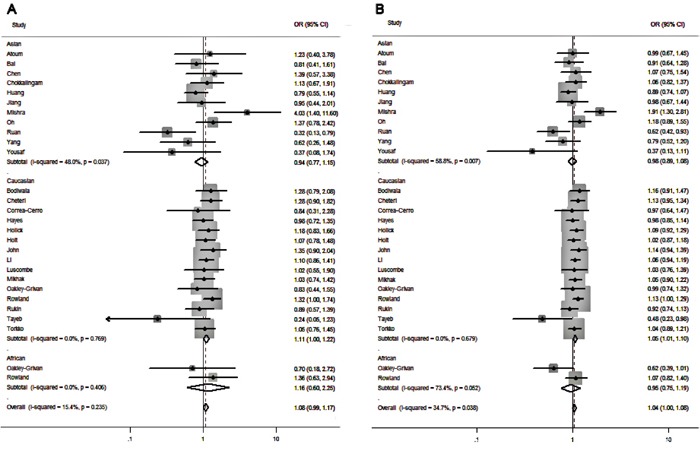
A. Homozygote model (ff vs. FF). B. Allelic frequency model (f vs. F allele).
For the subgroup analysis of ethnicity stratification. Significantly increased risk of PCa was detected in Caucasian populations in the comparison of homozygote model (ff vs. FF: OR=1.107, 95%CI=1.005-1.219, p=0.04), dominant model (Ff/ff vs. FF: OR=1.079, 95%CI=1.010-1.152, p=0.024) and allele-frequency genetic model (f vs. F allele: OR=1.054, 95%CI=1.006-1.103, p=0.026)(Table 3 & Figure 2). However, when 11 studies conducted in Asian populations and 2 studies in African populations were analyzed, no significant associations were found between Fok I polymorphism and the susceptibility to PCa (Table 3).
Table 3. Results of the association between Fok I polymorphism and PCa risk in different ethnicities.
| Comparison | Studies | Overall effect | Heterogeneity | Public bias | ||||
|---|---|---|---|---|---|---|---|---|
| OR | Z-score | p-value | I2 | P-value | Begg's test | Egger's test | ||
| Asian | ||||||||
| ff vs FF | 11 | 0.940 [0.771-1.150] | 0.58 | 0.561 | 48% | 0.037 | 0.876 | 0.901 |
| Ff vs FF | 11 | 1.032 [0.880-1.210] | 0.39 | 0.696 | 18% | 0.276 | 0.721 | 0.819 |
| Ff/ff vs FF | 11 | 1.003 [0.864-1.166] | 0.04 | 0.964 | 43% | 0.063 | 0.213 | 0.635 |
| ff vs FF/Ff | 11 | 0.944 [0.797-1.117] | 0.67 | 0.501 | 41% | 0.078 | 0.876 | 0.95 |
| f vs F | 11 | 0.983 [0.892-1.082] | 0.36 | 0.722 | 59% | 0.007 | 0.213 | 0.637 |
| Caucasian | ||||||||
| ff vs FF | 15 | 1.107 [1.005-1.219] | 2.06 | 0.04 | 0% | 0.769 | 0.138 | 0.034 |
| Ff vs FF | 15 | 1.070 [0.998-1.147] | 1.9 | 0.058 | 0% | 0.973 | 0.488 | 0.562 |
| Ff/ff vs FF | 15 | 1.079 [1.010-1.152] | 2.25 | 0.024 | 0% | 0.915 | 0.488 | 0.176 |
| ff vs FF/Ff | 15 | 1.057 [0.969-1.152] | 1.24 | 0.214 | 0% | 0.694 | 0.276 | 0.089 |
| f vs F | 15 | 1.054 [1.006-1.103] | 2.23 | 0.026 | 0% | 0.679 | 0.428 | 0.06 |
| African | ||||||||
| ff vs FF | 2 | 1.165 [0.603-2.249] | 0.45 | 0.65 | 0% | 0.406 | 1 | - |
| Ff vs FF | 2 | 0.861 [0.646-1.148] | 1.02 | 0.309 | 73% | 0.055 | 1 | - |
| Ff/ff vs FF | 2 | 0.899 [0.673-1.173] | 0.83 | 0.405 | 75% | 0.045 | 1 | - |
| ff vs FF/Ff | 2 | 1.215 [0.633-2.330] | 0.58 | 0.559 | 0% | 0.554 | 1 | - |
| f vs F | 2 | 0.945 [0.751-1.189] | 0.48 | 0.631 | 73% | 0.052 | 1 | - |
For the stratified analysis of source of controls. We found that Fok I polymorphism could significantly increase the risk of PCa in the subgroup of population-based controls in homozygote model (ff vs. FF: OR=1.112, 95%CI=1.011-1.223, p=0.029) and allele-frequency genetic model (f vs. F allele: OR=1.005-1.099, p=0.03) (Table 4 & Figure 3). Meanwhile, no significantly increased risk was observed in the subgroups of hospital-based or BPH controls (Table 4).
Table 4. Results of the association between Fok I polymorphism and PCa risk in different source of controls.
| Comparison | Studies | Overall effect | Heterogeneity | Public bias | ||||
|---|---|---|---|---|---|---|---|---|
| OR | Z-score | p-value | I2 | P-value | Begg's test | Egger's test | ||
| Population-based | ||||||||
| ff vs FF | 15 | 1.112 [1.011-1.223] | 2.19 | 0.029 | 0% | 0.958 | 0.434 | 0.186 |
| Ff vs FF | 15 | 1.051[0.983-1.124] | 1.45 | 0.148 | 0% | 0.809 | 0.202 | 0.126 |
| Ff/ff vs FF | 15 | 1.064 [0.998-1.133] | 1.9 | 0.058 | 0% | 0.811 | 0.174 | 0.053 |
| ff vs FF/Ff | 15 | 1.074 [0.984-1.171] | 1.6 | 0.109 | 0% | 0.935 | 0.773 | 0.367 |
| f vs F | 15 | 1.051 [1.005-1.099] | 2.17 | 0.03 | 0% | 0.833 | 1.108 | 0.016 |
| Hospital-based | ||||||||
| ff vs FF | 6 | 0.931 [0.711-1.219] | 0.52 | 0.062 | 52% | 0.063 | 0.452 | 0.524 |
| Ff vs FF | 5 | 1.088 [0.866-1.337] | 0.81 | 0.42 | 47% | 0.11 | 0.806 | 0.419 |
| Ff/ff vs FF | 6 | 1.045 [0.862-1.268] | 0.45 | 0.653 | 59% | 0.033 | 0.452 | 0.999 |
| ff vs FF/Ff | 6 | 0.910 [0.718-1.152] | 0.79 | 0.432 | 46% | 0.103 | 1 | 0.642 |
| f vs F | 6 | 0.992 [0.871-1.129] | 0.13 | 0.897 | 69% | 0.006 | 1 | 0.973 |
| BPH | ||||||||
| ff vs FF | 7 | 0.941 [0.982-1.159] | 0.55 | 0.584 | 48% | 0.071 | 0.548 | 0.077 |
| Ff vs FF | 7 | 1.030 [0.861-1.231] | 0.32 | 0.748 | 0% | 0.678 | 0.23 | 0.025 |
| Ff/ff vs FF | 7 | 1.001 [0.846-1.183] | 0.01 | 0.994 | 26% | 0.231 | 0.368 | 0.037 |
| ff vs FF/Ff | 7 | 0.928 [0.955-1.107] | 0.85 | 0.394 | 35% | 0.159 | 0.368 | 0.196 |
| f vs F | 7 | 0.972 [0.875-1.081] | 0.52 | 0.604 | 54% | 0.042 | 0.368 | 0.102 |
Figure 3. Forest plots to estimate the association of VDR Fok I polymorphism with PCa in the subgroup analysis of source of controls.
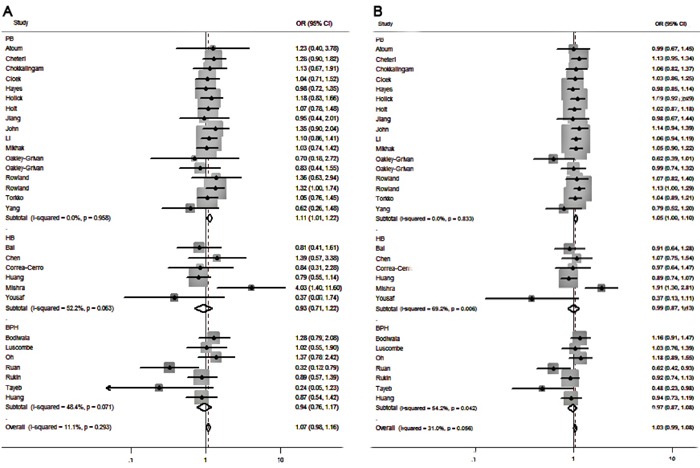
A. Homozygote model (ff vs. FF). B. Allelic frequency model (f vs. F allele).
In the stratified analysis by genotyping method, there was no significant association in different subgroups, which were stratified into TaqMan, PCR-RFLP, SNPlex and other subgroups. As showed in Table 5, the pooled outcome showed that the genotyping methods reported in the included studies are both effective and applicative. Among the 27 studies included in our meta-analysis, there were two studies that deviated from HWE in the controls [9], we conducted a subgroup analysis. When the 2 studies excluded, another result obtained, which is similar to the overall analysis (The result was not given).
Table 5. Results of the association between Fok I polymorphism and PCa risk in different genotyping method.
| Comparison | Studies | Overall effect | Heterogeneity | Public bias | ||||
|---|---|---|---|---|---|---|---|---|
| OR | Z-score | p-value | I2 | P-value | Begg's test | Egger's test | ||
| PCR-RFLP | ||||||||
| ff vs FF | 17 | 1.014 [0.895-1.148] | 0.21 | 0.83 | 36% | 0.068 | 0.077 | 0.182 |
| Ff vs FF | 16 | 1.063 [0.970-1.165] | 1.3 | 0.192 | 0% | 0.611 | 0.192 | 0.565 |
| Ff/ff vs FF | 17 | 1.051 [0.964-1.146] | 1.13 | 0.257 | 27% | 0.149 | 0.053 | 0.18 |
| ff vs FF/Ff | 17 | 0.983 [0.822-1.189] | 0.3 | 0.766 | 23% | 0.188 | 0.149 | 0.176 |
| f vs F | 17 | 1.020 [0.960-1.083] | 0.63 | 0.526 | 49% | 0.012 | 0.019 | 0.127 |
| TaqMan | ||||||||
| ff vs FF | 5 | 1.155 [0.989-1.349] | 1.82 | 0.068 | 0% | 0.8 | 1 | 0.822 |
| Ff vs FF | 5 | 1.018 [0.914-1.134] | 0.33 | 0.74 | 8% | 0.364 | 0.806 | 0.785 |
| Ff/ff vs FF | 5 | 1.047 [0.946-1.159] | 0.88 | 0.377 | 0% | 0.676 | 1 | 0.854 |
| ff vs FF/Ff | 5 | 1.131 [0.981-1.305] | 1.69 | 0.09 | 4% | 0.385 | 0.806 | 0.891 |
| f vs F | 5 | 1.056 [0.983-1.136] | 1.49 | 0.137 | 0% | 0.934 | 0.806 | 0.989 |
| SNPlex | ||||||||
| ff vs FF | 2 | 1.120 [0.866-1.416] | 0.95 | 0.343 | 0.00% | 0.702 | 1 | - |
| Ff vs FF | 2 | 1.003 [0.846-1.188] | 0.03 | 0.976 | 0% | 0.532 | 1 | - |
| Ff/ff vs FF | 2 | 1.031 [0.983-1.102] | 0.37 | 0.712 | 0.00% | 0.509 | 1 | - |
| ff vs FF/Ff | 2 | 1.118 [0.902-1.386] | 1.02 | 0.309 | 0.00% | 0.884 | 1 | - |
| f vs F | 2 | 1.047 [0.935-1.171] | 1.48 | 0.138 | 0% | 0.57 | 1 | - |
| Others | ||||||||
| ff vs FF | 3 | 1.013 [0.802-1.280] | 0.11 | 0.913 | 0% | 0.475 | 1 | 0.607 |
| Ff vs FF | 3 | 0.995 [0.828-1.195] | 0.06 | 0.956 | 0% | 0.803 | 0.296 | 0.175 |
| Ff/ff vs FF | 3 | 0.994 [0.837-1.182] | 0.06 | 0.95 | 0% | 0.656 | 0.296 | 0.49 |
| ff vs FF/Ff | 3 | 0.989 [0.822-1.189] | 0.12 | 0.904 | 1% | 0.365 | 1 | 0.362 |
| f vs F | 3 | 0.944 [0.889-1.110] | 0.11 | 0.91 | 1% | 0.366 | 1 | 0.637 |
A subgroup analysis based on the tumor stages was also conducted to delineate the association in more detail. As presented in Table 6 and Figure 4, the pooled results from 6 studies showed that Fok I polymorphism is associated with the advanced tumor in homozygote model (ff vs. FF: OR=1.210, 95%CI=1.020-1.437, p=0.029) and allele-frequency genetic model (f vs. F allele: OR=1.085, 95%CI=1.000-1.178, p=0.05). Meanwhile, no significant difference in the genetic variants was detected between localized tumor cases or controls.
Table 6. Results of the association between Fok I polymorphism and PCa risk in different tumor stage.
| Comparison | Studies | Overall effect | Heterogeneity | Public bias | ||||
|---|---|---|---|---|---|---|---|---|
| OR | Z-score | p-value | I2 | P-value | Begg's test | Egger's test | ||
| Advanced | ||||||||
| ff vs FF | 6 | 1.210 [1.020-1.437] | 2.18 | 0.029 | 26% | 0.24 | 0.26 | 0.278 |
| Ff vs FF | 6 | 1.023 [0.904-1.158] | 0.36 | 0.715 | 0% | 0.832 | 0.707 | 0.112 |
| Ff/ff vs FF | 6 | 1.070 [0.952-1.202] | 1.13 | 0.259 | 0% | 0.564 | 0.452 | 0.164 |
| ff vs FF/Ff | 6 | 1.194 [1.022-1.395] | 2.23 | 0.026 | 5% | 0.388 | 0.26 | 0.412 |
| f vs F | 6 | 1.085 [1.000-1.178] | 1.96 | 0.05 | 19% | 0.292 | 0.26 | 0.271 |
| Localized | ||||||||
| ff vs FF | 5 | 1.002 [0.817-1.229] | 0.02 | 0.984 | 0% | 0.628 | 0.462 | 0.482 |
| Ff vs FF | 5 | 1.031 [0.891-1.193] | 0.41 | 0.679 | 0% | 0.902 | 0.462 | 0.28 |
| Ff/ff vs FF | 5 | 1.024 [0.892-1.175] | 0.34 | 0.737 | 0% | 0.768 | 0.462 | 0.384 |
| ff vs FF/Ff | 5 | 0.980 [0.814-1.179] | 0.22 | 0.828 | 0% | 0.731 | 0.462 | 0.512 |
| f vs F | 5 | 1.006 [0.913-1.108] | 0.12 | 0.903 | 0% | 0.595 | 0.806 | 0.437 |
Figure 4. Forest plots to estimate the association of VDR Fok I polymorphism with PCa in the subgroup analysis of tumor stage.
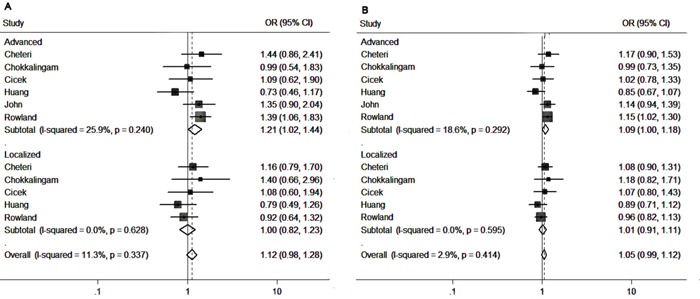
A. Homozygote model (ff vs. FF). B. Allelic frequency model (f vs. F allele).
Heterogeneity
There was no significant between-study heterogeneity in all the comparison models in the overall analysis (ff vs. FF: p=0.131, I2=14%), Ff vs. FF: p=0.105, I2=0%; Ff/ff vs. FF: p=0.173, I2=0%; ff vs. FF/Ff: p=0.318, I2=13%; and f vs. F allele: p=0.138, I2=27%) (Table 2). Thus, fixed-effects estimates would be more appropriate for data analysis.
Publication bias and sensitivity analysis
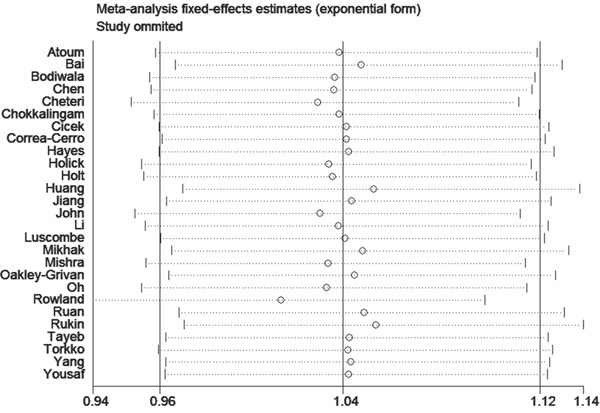
The publication bias of literature assessed with both funnel plots and Egger's test. As shown in Figure 5, it did not reveal any obvious asymmetry in the funnel plots (Figure 5). Moreover, the Egger's test which was used to provide statistical evidence of publication bias suggested that no evidence of publication bias existed in the overall analysis (p=0.118 for ff vs. FF; p=0.866 for Ff vs. FF; p=0.322 for Ff/ff vs. FF; p=0.138 for ff vs. FF/Ff; and p=0.101 for f vs. F allele) (Table 2) and almost the subgroup analyses (Table 3-6). Sensitivity analyses showed that omitting individual study from all the analyses did not affect the pooled ORs significantly, no substantial change was detected, indicating that our results were statistically robust (Figure 6).
Figure 5. Begg's funnel plots to examine piblishcation bias for reported comparisons of VDR gene Fok I polymorphism.
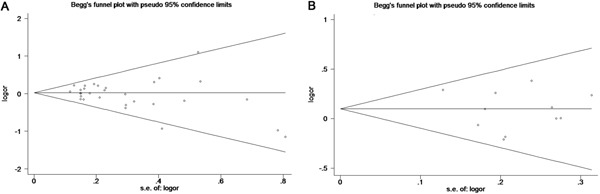
A. Overall comparison for the recessive model (ff vs. FF/Ff). B. Subgroup analysis of tumor stage for the recessive model (ff vs. FF/Ff).
Figure 6. Sensitivity analysis of the comparison in recessive model (ff vs. FF/Ff) in the overall analysis.
DISCUSSION
The VDR gene has earned special attention because an increasing number of studies have revealed that polymorphisms of the VDR gene were associated with the risk of PCa [33]. However, the results across studies have been equivocal [34, 35, 36]. Previous meta-analyses were performed by Xu et al. in 2014, Guo et al. in 2013 and Yin et al. in 2009 [34, 37, 44]. Xu et al. and Yin et al. reported the relationship of cancer risk with several VDR SNPs including Fok I. For the association of Fok I polymorphism with PCa, they included 19 studies and 16 studies, respectively. The shortage of these two studies is that they only performed overall analyses without any detailed subgroup analyses. Guo et al. included 22 stuides and conducted the stratified analyses. But from 2013 to now, some new data appearred, differently from the results of previous meta-analyses [34, 37, 44]. Our study included 10,468 cases and 10,400 controls from 27 independent studies, which is much more than the former three studies. Therefore, the results we obtained might be more stringent and comprehensive.
Our meta-analysis indicated the relationship of VDR gene Fok I polymorphism with the PCa risk is not existed in overall population. It is consistent with the results of previous meta-analyses [34, 37, 44]. But for the subgroup analysis of ethnicity, significant association was found in Caucasians. It is not reported by previous meta-analyses [34, 37, 44]. It suggests that in individuals of Caucasian ethnicity but not of Asians or Africans, the FF genotype and F allele might be protective. Ethnicity is one of the most important biological factors that might influence the function of VDR through gene-gene interaction [38]. The difference might be caused by the discrepancies in racial backgrounds and geography [40]. Besides, different diet structure could play a role in the discrepancies [41]. Our results suggested that the Fok I polymorphism could be a potential biomarker to forecast the PCa risk of Caucasians for clinical practice. Further studies of Asian and African are required.
For the source of controls, borderline significant association was found in population-based controls. Possibly some sick population were enrolled in the groups of hospital-based controls and HBP controls, so that these groups could not represent all population [42]. Hence, the results of these groups would be lack of credibility. Our results showed that no difference between the genotyping methods. It suggested that all the genotyping methods applied in the included studies are appropriate to get accurate genotype distribution. As a research reported in 2004, polymorphism would be associated with the tumor stage of PCa [43]. We also performed a stratified analysis by tumor stage. Differently from the previous meta-analyses [44, 45], we found that in the subgroup of advanced tumor stage, ff genotype and f allele might increase the PCa risk. It indicating that Fok I polymorphism could indeed be a risk factor associated with PCa progression.
The heterogeneity between the studies was very low in the overall analysis. It suggested that the results from these studies were suitable to be pooled [46]. Although evidence of heterogeneity existed in some subgroup analyses, the sensitivity analysis indicated that studies contribute to the heterogeneity did not significantly alter the pooled results. It suggested our results were statistically robust.
Several limitations in our meta-analysis should be acknowledged. First, several studies with small sample size included in our analysis might be underpowered to detect the relationship. Second, our results were according to the unadjusted parameters, a more accurate analysis should be performed, in which the outcomes would be adjusted by some related parameters, including age, dietary status, and other important lifestyle factors.
In conclusion, our meta-analysis might be the largest meta-analysis to estimate the association of VDR gene Fok I polymorphism with the risk of PCa. Although no significantly association of Fok I polymorphism with PCa risk was found in overall population, the possibility of an association in specific subpopulations such as Caucasians and the advanced tumor patients could not be ruled out. In the future, large and well-designed studies are required to illustrate the interactions of VDR genetic variants including Fok I polymorphism, environmental factors, life style and PCa.
MATERIALS AND METHODS
Literature and search strategy
The PubMed, Embase, Wanfang and Chinese National Knowledge Infrastructure (CNKI) database searches were conducted for all the eligible papers. The following search terms were used: “VDR/vitamin D receptor” and “prostate cancer/tumor/carcinoma”. Manually searching for the additional studies was conducted according to the references of the original and review reports. The literature search was updated on February, 2016.
Study selection
Retrieved studies screened should meet the following criteria: (i) studies on human beings; (ii) in a case-control or nested case-control design; (iii) investigated the association between VDR gene Fok I polymorphism and PCa risk; (iv) detailed genotype distribution frequency of cases and controls could be obtained or calculated; (v) and received more than four points in the Newcastle-Ottawa Scale (NOS), which was considered to be high quality.
Data extraction
The studies meeting the inclusion criteria were read carefully by two investigators independently (Yansheng Zhao and Lei Wang). The following information was extracted for reaching consensus on all of the items: the first author's name, year of publication, country of origin, ethnicity of study population, genotyping methods, source of controls, and number of cases and controls. The subjects were categorized as Asians, African and Caucasians for ethnicity; TaqMan, PCR-RFLP, SNPlex and other subgroup for genotyping method; population-based, hospital-based and Benign Prostate Hyperplasia (BPH) for the source of controls, respectively. We also divided the clinical stages into a localized group and an advanced group. Any disagreements were resolved by a third reviewer (Geng Zhao).
Statistical analysis
A χ2-test based on the Q statistic was conducted to assess the heterogeneity. The between-study heterogeneity was considered to be significant when I2>50% and p<0.1, and the random effects model was chosen to combine values from studies [11]. Otherwise, for homogeneous studies, the fixed effects model was used. The pooled odds ratios (ORs) together with its 95% confidence intervals (95% CIs) were calculated to evaluate the risk. In addition, subgroup analyses were conducted based on ethnicity, genotyping method, source of controls and clinic stages. Sensitivity analysis was performed to assess the stability of pooled results. Begg's Funnel plot and Egger's test were preformed to assess the potential publication bias. Moreover, Hardy-Weinberg equilibrium (HWE) of controls was reexamined by us with the goodness-of-fit χ2-test. All analyses were performed using STATA package version 11.0 (Stata Corp, College Station, TX, USA).
Acknowledgments
Conceived and designed the experiments: Shaosan Kan and Xiaoqiang Li. Extracted data: Yansheng Zhao, Geng Zhao and Lei Wang. Performed the data analysis: Jian Liu, Xi Chen and Liguo Zhang. Wrote the paper: Anliang Yao and Xiaojun Zhang.
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1.Bistulfi G, Affronti HC, Foster BA, Karasik E, Gillard B, Morrison C, Mohler J, Phillips JG, Smiraglia DJ. The essential role of methylthioadenosine phosphorylase in prostate cancer. Oncotarget. 2016;7:14380–14393. doi: 10.18632/oncotarget.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh JJ, Byun SS, Lee SE, Hong SK, Jeong CW, Kim D, Kim HJ, Myung SC. Genetic variations in VDR associated with prostate cancer risk and progression in a Korean population. Gene. 2014;533:86–93. doi: 10.1016/j.gene.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 3.Galunska B, Gerova D, Kosev P, Anakievski D, Hinev A. Serum 25-hydroxy vitamin D levels in Bulgarian patients with prostate cancer: a pilot study. Clinical laboratory. 2015;61:329–335. doi: 10.7754/clin.lab.2014.140802. [DOI] [PubMed] [Google Scholar]
- 4.Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, Redfern CH, Fehrenbacher L, Saleh MN, Waterhouse DM, Carducci MA, Vicario D, Dreicer R, et al. ASCENT Investigators. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborti CK. Vitamin D as a promising anticancer agent. Indian J Pharmacology. 2011;43:113–120. doi: 10.4103/0253-7613.77335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tayeb MT, Clark C, Haites NE, Sharp L, Murray GI, McLeod HL. Vitamin D receptor, HER-2 polymorphisms and risk of prostate cancer in men with benign prostate hyperplasia. Saudi Med J. 2004;25:447–451. [PubMed] [Google Scholar]
- 7.Cheteri MB, Stanford JL, Friedrichsen DM, Peters MA, Iwasaki L, Langlois MC, Feng Z, Ostrander EA. Vitamin D receptor gene polymorphisms and prostate cancer risk. Prostate. 2004;59:409–418. doi: 10.1002/pros.20001. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, Haussler CA, Galligan MA, Thatcher ML, Encinas Dominguez C, Haussler MR. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cellular Endocrinol. 2001;177:145–159. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 9.Yousaf N, Afzal S, Hayat T, Shah J, Ahmad N, Abbasi R, Ramzan K, Jan R, Khan I, Ahmed J, Siraj S. Association of vitamin D receptor gene polymorphisms with prostate cancer risk in the Pakistani population. Asian Pac J Cancer Prev. 2014;15:10009–10013. doi: 10.7314/apjcp.2014.15.22.10009. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohning D. Meta-analysis: a unifying meta-likelihood approach framing unobserved heterogeneity, study covariates, publication bias, and study quality. Methods Inf Med. 2005;44:127–135. [PubMed] [Google Scholar]
- 12.Atoum MF, AlKateeb D, AlHaj Mahmoud SA. The Fok1 vitamin D receptor gene polymorphism and 25(OH) D serum levels and prostate cancer among Jordanian men. Asian Pac J Cancer Prev. 2015;16:2227–2230. doi: 10.7314/apjcp.2015.16.6.2227. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Yu Y, Yu B, Ge J, Ji J, Lu H, Wei J, Weng Z, Tao Z, Lu J. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet. 2009;10:125. doi: 10.1186/1471-2350-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodiwala D, Luscombe CJ, French ME, Liu S, Saxby MF, Jones PW, Fryer AA, Strange RC. Polymorphisms in the vitamin D receptor gene, ultraviolet radiation, and susceptibility to prostate cancer. Environ Mol Mutagen. 2004;43:121–127. doi: 10.1002/em.20000. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZQ, Deng LS, Lin WJ. The association of vitamin D receptor genotypes and risk of prostate cancer. Chin J Lab Med Clin Sci. 2001;2:60–62. [Google Scholar]
- 16.Chokkalingam AP, McGlynn KA, Gao YT, Pollak M, Deng J, Sesterhenn IA, Mostofi FK, Fraumeni JF, Jr, Hsing AW. Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2001;61:4333–4336. [PubMed] [Google Scholar]
- 17.Cicek MS, Liu X, Schumacher FR, Casey G, Witte JS. Vitamin D receptor genotypes/haplotypes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2549–2552. doi: 10.1158/1055-9965.EPI-06-0409. [DOI] [PubMed] [Google Scholar]
- 18.Correa-Cerro L, Berthon P, Haussler J, Bochum S, Drelon E, Mangin P, Fournier G, Paiss T, Cussenot O, Vogel W. Vitamin D receptor polymorphisms as markers in prostate cancer. Hum Genet. 1999;105:281–287. doi: 10.1007/s004390051102. [DOI] [PubMed] [Google Scholar]
- 19.Spina CS, Ton L, Yao M, Maehr H, Wolfe MM, Uskokovic M, Adorini L, Holick MF. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103:757–762. doi: 10.1016/j.jsbmb.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Holt SK, Kwon EM, Peters U, Ostrander EA, Stanford JL. Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1929–1933. doi: 10.1158/1055-9965.EPI-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SP, Huang CY, Wu WJ, Pu YS, Chen J, Chen YY, Yu CC, Wu TT, Wang JS, Lee YH, Huang JK, Huang CH, Wu MT. Association of vitamin D receptor FokI polymorphism with prostate cancer risk, clinicopathological features and recurrence of prostate specific antigen after radical prostatectomy. Int J Cancer. 2006;119:1902–1907. doi: 10.1002/ijc.22053. [DOI] [PubMed] [Google Scholar]
- 22.Berndt SI, Dodson JL, Huang WY, Nicodemus KK. A systematic review of vitamin D receptor gene polymorphisms and prostate cancer risk. J Urol. 2006;175:1613–1623. doi: 10.1016/S0022-5347(05)00958-4. [DOI] [PubMed] [Google Scholar]
- 23.John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470–5479. doi: 10.1158/0008-5472.CAN-04-3134. [DOI] [PubMed] [Google Scholar]
- 24.Luscombe CJ, French ME, Liu S, Saxby MF, Jones PW, Fryer AA, Strange RC. Prostate cancer risk: associations with ultraviolet radiation, tyrosinase and melanocortin-1 receptor genotypes. Br J Cancer. 2001;85:1504–1509. doi: 10.1054/bjoc.2001.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate. 2007;67:911–923. doi: 10.1002/pros.20570. [DOI] [PubMed] [Google Scholar]
- 26.Mishra DK, Bid HK, Srivastava DS, Mandhani A, Mittal RD. Association of vitamin D receptor gene polymorphism and risk of prostate cancer in India. Urol Int. 2005;74:315–318. doi: 10.1159/000084429. [DOI] [PubMed] [Google Scholar]
- 27.Oakley-Girvan I, Feldman D, Eccleshall TR, Gallagher RP, Wu AH, Kolonel LN, Halpern J, Balise RR, West DW, Paffenbarger RS, Jr, Whittemore AS. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev. 2004;13:1325–1330. [PubMed] [Google Scholar]
- 28.Rowland GW, Schwartz GG, John EM, Ingles SA. Protective effects of low calcium intake and low calcium absorption vitamin D receptor genotype in the California Collaborative Prostate Cancer Study. Cancer Epidemiol Biomarkers Prev. 2013;22:16–24. doi: 10.1158/1055-9965.EPI-12-0922-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rukin NJ, Luscombe C, Moon S, Bodiwala D, Liu S, Saxby MF, Fryer AA, Alldersea J, Hoban PR, Strange RC. Prostate cancer susceptibility is mediated by interactions between exposure to ultraviolet radiation and polymorphisms in the 5′ haplotype block of the vitamin D receptor gene. Cancer Lett. 2007;247:328–335. doi: 10.1016/j.canlet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Ruan L, Li ZM, Li G. Relationship between SNP of vitamin D receptor start codon and prostate cancer. Int Med Health Guidance News. 2009;15:12–14. [Google Scholar]
- 31.Torkko KC, van Bokhoven A, Mai P, Beuten J, Balic I, Byers TE, Hokanson JE, Norris JM, Baron AE, Lucia MS, Thompson IM, Leach RJ. VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res. 2008;14:3223–3229. doi: 10.1158/1078-0432.CCR-07-4894. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Wang S, Ye Z, Yang W. [Association of single nucleotide polymorphism of vitamin D receptor gene start codon and the susceptibility to prostate cancer in the Han nationality in Hubei area] Zhonghua Nan Ke Xue. 2004;10:411–414. [PubMed] [Google Scholar]
- 33.Hu J, Qiu Z, Zhang L, Cui F. Kallikrein 3 and vitamin D receptor polymorphisms: potentials environmental risk factors for prostate cancer. Diagn Pathol. 2014;9:84. doi: 10.1186/1746-1596-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, He B, Pan Y, Deng Q, Sun H, Li R, Gao T, Song G, Wang S. Systematic review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Tumour Biol. 2014;35:4153–4169. doi: 10.1007/s13277-013-1544-y. [DOI] [PubMed] [Google Scholar]
- 35.Yan Y, Luo YC, Wan HY, Wang J, Zhang PP, Liu M, Li X, Li S, Tang H. MicroRNA-10a is involved in the metastatic process by regulating Eph tyrosine kinase receptor A4-mediated epithelial-mesenchymal transition and adhesion in hepatoma cells. Hepatology. 2013;57:667–677. doi: 10.1002/hep.26071. [DOI] [PubMed] [Google Scholar]
- 36.Risio M, Venesio T, Kolomoets E, Armaroli P, Gallo F, Balsamo A, Muto G, D'Urso L, Puppo P, Naselli A, Segnan N, Group BEW. Genetic polymorphisms of CYP17A1, vitamin D receptor and androgen receptor in Italian heredo-familial and sporadic prostate cancers. Cancer Epidemiol. 2011;35:e18–24. doi: 10.1016/j.canep.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Guo Z, Wen J, Kan Q, Huang S, Liu X, Sun N, Li Z. Lack of association between vitamin D receptor gene FokI and BsmI polymorphisms and prostate cancer risk: an updated meta-analysis involving 21,756 subjects. Tumour Biol. 2013;34:3189–3200. doi: 10.1007/s13277-013-0889-6. [DOI] [PubMed] [Google Scholar]
- 38.V ON, Asani FF, Jeffery TJ, Saccone DS, Bornman L. Vitamin D Receptor Gene Expression and Function in a South African Population: Ethnicity, Vitamin D and I. PloS one. 2013;8:e67663. doi: 10.1371/journal.pone.0067663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30:1170–1180. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Alvero R. Racial and ethnic differences in physiology and clinical symptoms of polycystic ovary syndrome. Semin Reprod Med. 2013;31:365–369. doi: 10.1055/s-0033-1348895. [DOI] [PubMed] [Google Scholar]
- 41.Rejnmark L, Jorgensen ME, Pedersen MB, Hansen JC, Heickendorff L, Lauridsen AL, Mulvad G, Siggaard C, Skjoldborg H, Sorensen TB, Pedersen EB, Mosekilde L. Vitamin D insufficiency in Greenlanders on a westernized fare: ethnic differences in calcitropic hormones between Greenlanders and Danes. Calcif Tissue Int. 2004;74:255–263. doi: 10.1007/s00223-003-0110-9. [DOI] [PubMed] [Google Scholar]
- 42.Lunet N, Azevedo A. On the comparability of population-based and hospital-based case-control studies. Gac Sanit. 2009;23:564. doi: 10.1016/j.gaceta.2009.02.014. author reply 565. [DOI] [PubMed] [Google Scholar]
- 43.Chiang CH, Chen KK, Chang LS, Hong CJ. The impact of polymorphism on prostate specific antigen gene on the risk, tumor volume and pathological stage of prostate cancer. J Urol. 2004;171:1529–1532. doi: 10.1097/01.ju.0000116538.15995.93. [DOI] [PubMed] [Google Scholar]
- 44.Yin M, Wei S, Wei Q. Vitamin D Receptor Genetic Polymorphisms and Prostate Cancer Risk: A Meta-analysis of 36 Published Studies. Int J Clin Exp Med. 2009;2:159–175. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Q, Shan Y. Genetic polymorphisms of vitamin D receptor and the risk of prostate cancer: a meta-analysis. J BUON. 2013;18:961–969. [PubMed] [Google Scholar]
- 46.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]


